Abstract
Background
Microparticles bud from cellular elements during inflammation and are associated with vascular dysfunction related to type 2 diabetes. Although weight loss is known to reduce inflammation, the metabolic effects of bariatric surgery on microparticle concentration and composition are not known.
Objectives
To determine the effect of bariatric surgery on microparticle concentration and correlate these changes with clinical parameters.
Setting
Multispecialty group practice
Methods
We studied 14 obese subjects with type 2 diabetes two weeks before and at one and 12 months following bariatric surgery. Nine of the patients underwent Roux-en-Y gastric bypass and 5 received gastric restrictive surgery.
Results
One month following surgery, body mass index was reduced by ~10%, glycemic control improved dramatically (P < 0.01), and there was a >60% reduction in endothelial, platelet microparticles and CRP levels (P < 0.05). Tissue factor microparticles reduced by 40% ( p = 0.1). Twelve months following surgery, BMI was reduced by ~20%, glycemic control was maintained (P < 0.01), and there was a >50% reduction in monocyte microparticles compared to pre-surgery. The reduction in monocyte microparticles one month after surgery was strongly associated with the reduction in hemoglobin A1c (P < 0.05). The reduction in monocyte microparticles 12 months following surgery correlated strongly with the reduction in body mass index (P < 0.05).
Conclusion
The reduction in microparticles after bariatric surgery in patients with type 2 diabetes reflects an attenuation of inflammation and this mechanism may contribute to normalization of glycemic control.
Keywords: microparticles, tissue factor, inflammation, obesity, bariatric surgery, type 2 diabetes
Introduction
Obesity and type 2 diabetes mellitus are closely inter-related and associated with an increased risk for cardiovascular disease. Although the pathogenesis of vascular complications is complex in diabetes mellitus, substantial evidence points to the role of inflammation and oxidative stress as a result of hyperglycemia and insulin resistance(1). Recent studies have identified cellular microparticles, membrane fragments that bud from normal cells such as leukocytes, platelets and vascular endothelial cells during activation or apoptosis, and serve as markers of vascular dysfunction characterized by altered signaling processes and changes in membrane lipids(2). Microparticles are increased in patients with obesity and type 2 diabetes compared to lean healthy individuals(2–5). In particular, platelet-derived, monocyte-derived and endothelial cell-derived microparticles are increased in such patients with retinopathy, nephropathy and cardiovascular disease(2–5). Microparticles express pro-coagulant activity in that they bind to cells via specific adhesion receptors, thereby stimulating these cells to produce both tissue factor and cytokines that further promote coagulation, pro-inflammation and endothelial dysfunction(6).
Obesity is a key driving factor for diabetes and cardiovascular disease development. At present, bariatric surgery is emerging as the treatment of choice for severely obese patients with type 2 diabetes. Rapid improvements in glycemic control are observed days to weeks following surgery, and are not completely attributable to weight loss. Moreover, supra-normal improvements in insulin sensitivity have been noted acutely following surgeries, and also do not parallel the degree of weight loss(7). After bariatric surgery, about 87% of patients with type 2 diabetes achieve enhanced glycemic control with fewer anti-diabetes medications and 78% achieve euglycemia(8). However, the metabolic effects of bariatric surgery on microparticle concentration and composition as they relate to changes in clinical status in patients with type 2 diabetes are not known.
We hypothesized that improved glycemic control as a result of bariatric surgery would have potent effects to reduce total microparticle concentration and alter the cellular origin of microparticles in patients with type 2 diabetes. We expected that this improvement would correlate directly with the improvement in glycemic control and insulin sensitivity rather than weight loss conferred by surgery.
Subjects and Methods
Subjects
Fourteen patients with type 2 diabetes mellitus attending our Bariatric and Metabolic Institute Clinic participated in the study. There were seven males and seven females, mean age of 52 ± 13 years, mean body mass index of 47.3 ± 9 kg/m2, fasting plasma glucose of 141 ± 43 mg/dL and hemoglobin A1c of 7.2 ± 1%. Nine patients underwent Roux-en-Y gastric bypass (RYGB) and five patients underwent gastric restrictive surgery. The Cleveland Clinic Institutional Review Board approved the protocol, and all participants provided written informed consent.
Study design
Patients were studied one week prior to surgery and at one and twelve months following surgery in a prospective, longitudinal design with repeated measures. During each visit, vital signs including body mass index were obtained. Metabolic parameters including insulin sensitivity were assessed during a mixed meal tolerance test as previously described at each visit(9). Fasting plasma samples obtained for each study visit were aliquoted for microparticle analysis and the inflammatory marker, C-reactive protein. Complete blood counts were performed in a subset of subjects (n = 11) for clinical care. Oral anti-diabetic agents were discontinued 24 hours prior to the procedures.
Microparticle (MP) measurement and characterization of cellular of origin
Collected specimens were centrifuged for 15 minutes at 1,500g (2700 rpm) and 22°C to isolate platelet rich plasma. Platelet rich plasma was then centrifuged at 13,000g for 2 minutes to prepare platelet free plasma, which was aliquotted (0.5mL/tube) and frozen at −80°C. Fifty microliters of platelet free plasma was separately incubated for 30 minutes in the dark at room temperature with either no additions, 5 μL of FITC-labeled annexin V, or 5 μL of each of the following conjugated monoclonal antibodies: Isotype control murine IgG-PE; CD 144-PE (endothelial cells); CD 14-PE (monocytes); tissue factor, isotype control IgG-PECy5 and CD 41–PECy5 (platelets). After 30 minutes of incubation, samples were re-suspended in 400 μL of a PBS solution for analysis. Samples were analyzed using a BD LSR II Flow Cytometer, with an analysis gate determined using 1 μm fluorescent beads (Sigma, St Louis, MO). Prior to analysis, a known amount of 2.5 μm fluorescent beads (Invitrogen, Carlsbad, CA) were added to the sample. These beads caused a signal outside of the analysis gate for the microparticles, but allowed the collection to be terminated after a fixed and consistent volume of the microparticle suspension was analyzed. Thus, numbers of microparticles between samples could be accurately compared.
Analytic Determinations
Blood glucose was measured immediately using the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH). Plasma insulin was assayed by a double-antibody radioimmunoassay (RIA, Linco Research, St. Charles, MO). The intra and interassay coefficient of variation was 2.6% and 3.0% respectively for level 1, and 1.6% and 4.0% for level 2 respectively. A high sensitivity CRP immunoturbidimetric assay was run on a Roche Modular platform (Roche Diagnostics, Indianapolis, IN). This assay involves anti-CRP mouse monoclonal antibodies coupled to latex microparticles that react with the antigen to form an antigen/antibody complex. The analytical sensitivity is >0.425 mg/L.
Statistical analysis
Differences in microparticle concentration before and after surgery were analyzed using a repeated measures analysis of variance. We determined the relationship between microparticle composition and concentration (endothelial, platelet, and monocyte) and metabolic parameters, including insulin sensitivity, using linear regression analysis. All data are expressed as mean ± SE.
Results
Clinical and metabolic parameters after surgery (Table 1)
Table 1.
Effects of bariatric surgery on body mass index and metabolic outcomes.
| Baseline | 1 month after surgery | 12 months after surgery | |
|---|---|---|---|
| Body mass index, kg/m2 | 47±9 | 42±8* | 36±8* |
| Leptin, ugm/L | 21.4±6 | 16.4±4* | 10.9±4 |
| Fasting plasma glucose, mg/dL | 141±43 | 103±21* | 93±19* |
| Hemoglobin A1c, % | 7.2±1 | 6.2±0.6* | 5.7±0.5* |
| Matsuda Index | 4.6 | 7.5* | 13.2* |
| CRP, mg/L | 9.7±2.5 | 7.6±1.2* | 3.2±0.9* |
Data represents mean ± S.E.M.
indicates, P<0.01 as compared to baseline value.
Following bariatric surgery, body mass index was reduced by ~10% (P < 0.01) at one month and ~20% (P < 0.01) by twelve months. As expected, leptin decreased at one month with further reductions at 12 months. Fasting plasma glucose declined markedly (P < 0.01) at one month and at 12 months (P < 0.01). Hemoglobin A1c levels also decreased significantly at one (P < 0.01) and 12 months (P <0.01) after surgery. Insulin sensitivity represented by the Matsuda Index (19) increased 2-fold (P < 0.01) at one month and three-fold (P < 0.01) at 12 months following surgery. C-reactive protein levels reduced by ~40% at one month and ~ 65% at twelve months. Complete blood count at 1 month showed no change in white blood cells (5.7 ± 1.2 vs. 6.9 ± 1.4 × 103/uL, P = NS) or platelet count (233 ± 53 vs. 253 ± 60 × 103/uL, P = NS). No significant change in white blood count (6.0 ± 1.1 × 103/uL) or platelet count (242 ± 57 × 103/uL) was noted at 12 months ( P = NS). No significant changes in blood pressure or cholesterol levels were noted at 1 or 12 month following surgery (data not shown).
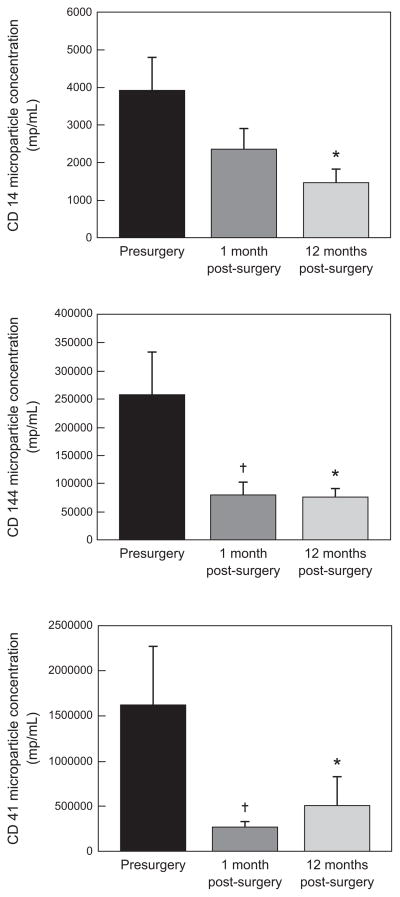
Microparticle concentration after surgery (Figure 1)
Figure 1. Microparticle concentrations before and after surgery.
CD 14, monocyte microparticles. CD 144, endothelial microparticles. CD 41, platelet microparticles.
†Presurgery vs. 1 month after surgery (p <0.05).
*Presurgery vs. 12 months after surgery (p <0.05).
Platelet microparticles (CD 41) and endothelial microparticles (CD 144) were reduced significantly (P < 0.05) one month following bariatric surgery. Additionally, there was a trend for tissue factor microparticles to be reduced by 40% (730 ± 215 vs. 1195 ± 445 MP/ml, P = 0.1) at one month that did not persist at 12 months (745 ±465 MP/ml, P = NS). This reduction persisted for 12 months following surgery with no further reduction noted as compared to the levels at one month. There was a decrease in monocyte (CD14) microparticle concentration at one month, though this did not reach statistical significance until 12 months following surgery (P < 0.05).
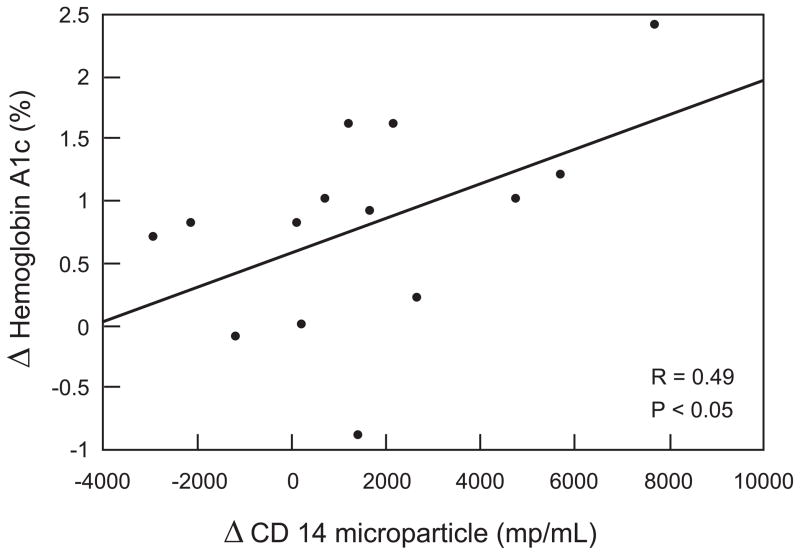
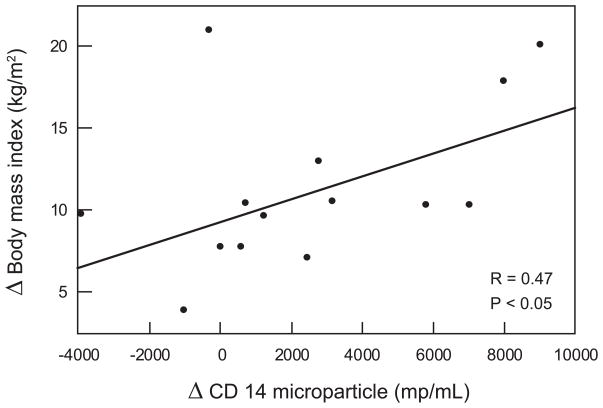
Correlation between microparticles and metabolic parameters (Figure 2a and 2b)
Figure 2.
Figure 2a : Correlation between CD14 (monocyte microparticles) concentration and hemoglobin A1c one month after surgery
Figure 2b : Correlation between CD 14 (monocyte microparticles) concentration and body mass index 12 months after surgery
The reduction in CD14 monocyte microparticles one month after bariatric surgery were strongly associated with the reduction in hemoglobin A1c (P < 0.05) but not with weight loss (R=0.12, P = NS), insulin sensitivity (R =0.09, P=NS), total cholesterol (R= 0.054, P= NS), or blood pressure parameters. The reduction in CD14 monocyte microparticles at 12 months after bariatric surgery correlated linearly with the reduction in body mass index, but not with improved glycemic control (R=0.04, P= NS), insulin sensitivity (R=0.13, P=NS), total cholesterol ( R = 0.12, P = NS), or blood pressure (data not shown). The reduction in C-reactive protein at one month was strongly associated with the reduction in hemoglobin A1c (R = 0.39, P < 0.05) and with the improvement in insulin sensitivity (R = 0.45, P < 0.05). At twelve months, the reduction in C-reactive protein was associated with the reduction in body mass index (R = 0.45, P < 0.05). No association was seen between CD144 and hemoglobin A1c (R = 0.12, P = NS), total cholesterol (R = 0.045, P=NS) or insulin sensitivity (R = 0.018, P = NS). Similarly, no association were noted with CD41 microparticles with hemoglobin A1c (R= 0.13, P = NS), total cholesterol (R= 0.0023, P = NS) or insulin sensitivity (R = 0.046, P = NS).
Discussion
Given the recognized effects of obesity on metabolic stress and inflammation that could enhance the secretion of inflammatory microparticles from activated tissues, we evaluated for the first time the effects of surgical weight loss on microparticle concentrations in a type 2 diabetes cohort. Acutely following bariatric surgery, reductions in C-reactive protein and platelet-, tissue factor-, and endothelial-derived microparticles were noted. Following sustained weight loss and improved glycemic control at 12 months, a further reduction in C-reactive protein and monocyte microparticle concentration was noted and correlated with the reduction in body mass index. Given the importance of adiposity in the generation of inflammation (largely mediated by C-reactive protein)(10), and the role of cellular microparticles to promote the expression of tissue factor-mediated athero-thrombotic vascular injury(1–3), our data suggest that surgical weight loss restores vascular health and stabilizes thrombotic activity by reduction of tissue factor, monocyte, platelet and endothelial microparticles.
Our data indicate a dramatic reduction in platelet and endothelial microparticle concentrations and a modest effect on tissue factor microparticles acutely following surgery. Furthermore, a positive relationship between the decrease in monocyte microparticles with the rapid reduction in hyperglycemia (ie. hemoglobin A1c). Short-term weight loss with very-low calorie diet in obese non-diabetic females was similarly noted to result in diminished release of platelet and leukocyte-derived particles in addition to plasminogen-activator inhibitor-1 (PAI-1)(11). Leukocyte-derived microparticles were demonstrated to induce endothelial dysfunction and promote the expression of tissue factor, the main initiator of blood coagulation(11,12). In asymptomatic subjects, leukocyte-derived MPs were found to be related with the component of metabolic syndrome and were predictive of the extent of intra-clinical atheroma independently of traditional risk factors(12). These data suggest that short-term diet and surgical induced weight loss as well as glycemic control reduce microparticle release that could contribute to both inflammatory and thrombogenic propensity described in obesity. We have previously demonstrated in this cohort that the acute reduction of glucose levels 4 weeks following gastric bypass surgery is linked to improved insulin sensitivity and insulin secretion related in part to incretin stimulation(9). Although insulin sensitivity is acutely restored following gastric bypass surgery, and is linked to reduced levels of inflammation such as tumor necrosis factor α, C-reactive protein and interleukins(10), no association between insulin sensitivity and microparticle levels were noted in our cohort. Our data lends support to the known ability of hyperglycemia to promote microvascular damage and suggests that reduced microparticle concentrations following bariatric surgery may provide a potentially important mechanism by which surgery may acutely reduce progression of vascular dysfunction underlying complications related to type 2 diabetes mellitus.
Adiposity is associated with monocyte/macrophage activation that causes adipogenic inflammation and apoptosis(13,15). Macrophage infiltration into adipose tissue in rodents is a key process related to TNFα and interleukin generation that hallmark sub-clinical inflammation underlying obesity and metabolic syndrome(13). Migration of monocytes into the sub-intimal space with lipid uptake is central to foam cell formation associated with atherogenesis. High fat diet and plasma elevations of nonesterified free fatty acids in humans are linked with monocyte activation of pro-inflammatory pathways mediated by NF kappa beta(14). Adipocyte apoptosis is linked to monocyte activation, inflammation and insulin resistance that contributes to hepatic steatosis(15). Reduced microparticle release may not only reflect reduced inflammation, but also decreased apoptosis, altered signaling pathways, changes in membrane lipid etc. Weight loss, regardless of the modality (dietary/surgical) even at modest levels, has been shown to reduce monocyte activation that underlies inflammation associated with diabetes related nephropathy and atherothrombotic risk. The significant association of monocyte microparticle reduction with reduced body mass index twelve months after bariatric surgery supports the view that sustained weight loss in addition to glycemic control may retard key inflammatory pathways mediating progression of diabetes via reductions in monocyte microparticles.
The limitations to our study include a small sample size. This limits our power to define a significant correlation between some of the reductions in microparticle subpopulations and other metabolic parameters. Due to our small sample size, we are not able to compare the effects of various types of bariatric surgery (i.e., gastric bypass vs. restrictive procedures) on microparticle concentration. Given that gastric bypass results in greater weight loss and superior effects on glucose regulation/insulin sensitivity, we would expect in larger scale studies greater effects of bypass surgery to restore microparticle reduction and athero-thombotic complications. Future large-scale studies comparing various types of bariatric procedures with conventional methods of weight loss on microparticle effects are warranted. In conclusion, the reduction in microparticles in these patients reflects an attenuation of inflammation and thrombotic factors and this mechanism may contribute to the improved cardiometabolic control that is widely observed after bariatric surgery.
Acknowledgments
This work was supported by National Institutes of Health, RO1 DK089547-01 NIDDK/NIH (SRK, JPK) and P50 HL081011 (R Silverstein, PI: K. McCrae, project 5).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabatier F, Darmon P, Hugel B, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–5. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 2.Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–30. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J. 2006;27:817–23. doi: 10.1093/eurheartj/ehi746. [DOI] [PubMed] [Google Scholar]
- 4.Ogata N, Imaizumi M, Nomura S, et al. Increased levels of platelet-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2005;68:193–201. doi: 10.1016/j.diabres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Omoto S, Nomura S, Shouzu A, Nishikawa M, Fukuhara S, Iwasaka T. Detection of monocyte-derived microparticles in patients with type II diabetes mellitus. Diabetologia. 2002;45:550–5. doi: 10.1007/s00125-001-0772-7. [DOI] [PubMed] [Google Scholar]
- 6.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–7. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 7.Bikman BT, Zheng D, Pories WJ, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93:4656–63. doi: 10.1210/jc.2008-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009;122:248, 256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–71. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belza A, Toubro S, Stender S, Astrup A. Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory merkers in obese subjects. Int J Obesity. 2009;33:456–64. doi: 10.1038/ijo.2009.27. [DOI] [PubMed] [Google Scholar]
- 11.Morel O, Luca F, Grunebaum L, et al. Short-term very low-calorie diet in obese females improves the haemostatic balance through the reduction of leptin levels, Pai-1 concentrations and a diminihed release of platelet and leukocyte-derived microparticles. Int J Obesity. 2011 Mar 8; doi: 10.1038/ijo.2011.19. (Epub) [DOI] [PubMed] [Google Scholar]
- 12.Chironi G, Simon A, Hugel B, et al. Circulating leukocyte-derived micropartciles predict subclinical atherosclerosis burden asymptomatic subjects. Arterioscler Thromb Vasc Biol. 2006;2:2775–80. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]
- 13.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reativity in healthy subjects. Diabetes. 2003;52:2882–7. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 15.Alkhouri N, Gornicka A, Berk MP, et al. Adipocyte apoptosis,a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–38. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]