Abstract
Although impairment of episodic memory is common after traumatic brain injury (TBI), the complex nature of human memory suggests the need to study more than recall alone. For this reason, we are presenting an extension with additional analyses of persons reported in a previous publication (Russell et al., 2011). We examined both the encoding and recognition components of an episodic memory paradigm containing both word and letter string blocks using fMRI and neuropsychological testing. This paradigm was completed by 12 persons with complicated mild, moderate, or severe TBI and 12 matched uninjured controls. Comparisons were made between groups and stimulus types. While task behavioral performance was not significantly different between groups, imaging results showed greater activation for the TBI group during the encoding portion of the task, while the control group exhibited more activation on the recognition portion. Observed areas of activation suggest that the TBI group may have used a less effective, but more automatic verbal strategy for encoding the non-pronounceable letter strings, while controls may have opted for more of a recognition-focused strategy. Group differences in CVLT-II performance supported these ideas and further neuropsychological testing also suggested limitations in executive functioning in the TBI group that may have influenced performance. Implications for intervention are discussed.
Keywords: traumatic brain injury, magnetic resonance imaging, episodic memory, encoding, recognition
Introduction
Traumatic Brain Injury (TBI) is the leading cause of disability in working-age adults in the United States (Faul, et al., 2010; Finkelstein, et al., 2006), and memory impairment is among the most reported cognitive consequences (Corrigan, et al., 2004; Raskin, 2000). Episodic memory, which is defined as memory for discrete events, is known to be impaired following TBI (Himanen, et al., 2006; Levin et al., 1990; Millis & Ricker, 1994; Ricker, et al., 2001; Wiegner & Donders, 1999; Wright, et al., 2010). In order for episodic memory to be functional, information must be attended to, encoded, stored, accessed effectively, managed properly upon retrieval, and used accurately in response to prompts. Breakdowns may occur at any processing stage, resulting in functional impairment. In addition, studies have suggested that many facets of memory, including modality, learning and forgetting rates, and recall delay may cause problems for persons with TBI (see Vakil, 2005). While it is not possible to study all aspects of episodic memory in a single study, if a goal is to improve treatments for individuals with episodic memory deficits after TBI, focusing simply on one part of the process (e.g., recall) is limiting. For this reason, it is important to take a multimodal approach. Ultimately, memory interventions will need to be personalized to meet the needs of each individual, and to reach this personalization we need to develop methods that will yield additional insights into memory functioning after TBI.
There have been few neuroimaging studies of episodic memory after TBI, but these studies have added to our understanding of the substrates associated its impairment. Two such studies involving the evaluation of retrieval processes are notable. Ricker and colleagues (2001) examined free recall, cued recall, and recognition of word stimuli using O-15 PET in persons with TBI and in control participants. While brain activation during recognition was similar for both groups, differences were found during recall, suggesting a different strategy was used by the TBI group (Ricker et al., 2001). Levine and colleagues (2002) also studied episodic memory after TBI using O-15 PET. Although identical scanning data were collected during both encoding and retrieval conditions, encoding scans were treated as a baseline for recall and not separately evaluated. That study yielded activation similarities between control and TBI groups, but some evidence of less lateralized, diffuse, and more intense areas of activation in the TBI group was indicated. Most recently, Russell, et al., (2011) used fMRI to evaluate episodic memory during both encoding and recognition phases of verbal and visuospatial stimuli. While the behavioral performance of persons with TBI was comparable to matched controls, fMRI results indicated increased activation, as well as increased bilaterality of activation for persons with TBI.
An additional consideration may be differences in the strategies used when encoding or recalling information. For example, it has been shown that memory is impacted by the deployment of different encoding strategies (Mangels, et al., 2002; Strangman et al., 2009), and that changes in executive functioning may impact the ability of those with TBI to employ commonly used strategies (Turner & Levine, 2008). Strangman and colleagues (2008) found that strategic verbal learning was associated with activity in the left prefrontal area. Decreased learning was associated with both under- and over-activation of the ventrolateral prefrontal cortex (VLPFC) in individuals with TBI. While it is clear that the TBI population is not homogeneous (Vakil, 2005), studying memory deficits may allow for subgrouping and the development of individualized treatments.
Though there have been a handful of studies using functional imaging to examine episodic memory after TBI, to our knowledge only our group (Russell et al., 2011) has provided imaging results for both the encoding and recognition aspects of episodic memory. The present set of analyses extends the previous paper by concentrating on detailed differences observed during the encoding and recognition of letters and words during fMRI. In addition, extra-scanner neuropsychological testing results from these participants are analyzed in the present manuscript.
Method
Participants
The participants have been previously described in Russell, et al. (2011). Fifteen persons with TBI and 14 uninjured controls participated. Technical difficulties and subject movement rendered some sessions unusable, resulting in 12 controls (2 F) and 12 persons with TBI (3 F) comprising the current data set. Exclusion criteria included left-handedness, history of neurological impairment (except for TBI in the injury group), pre-injury psychiatric illness, and substance abuse. Standard MRI exclusionary criteria were also followed. All consent and method procedures were approved by the university institutional review board (IRB).
Participants with TBI were included if they had a moderate, severe, or complicated mild injury in the previous 1–3 years (M = 1.7, SD = 0.6, range = 1.06–2.61). This time range was chosen to represent persons in the early chronic phase of injury, but to avoid hemodynamic changes not associated with cognitive activity (see Yamaki, et al., 1996). The lowest Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974) score in the first 24 hours after injury was used to classify injury severity (severe injury GCS = 3–8; moderate injury = 9–12). “Complicated mild” injuries were defined as a GCS ≥ 13, with documentation of positive neuroradiological findings attributable to TBI. Persons with complicated mild TBI were included because they have been shown to have outcomes similar to persons with moderate TBI (Kashluba, et al., 2008; Williams, et al., 1990). Initial GCS scores ranged from 3–15 (M = 8.9, SD = 5.5), and best GCS scores in the first 24 hours ranged from 7–15 (M = 12.2, SD = 3.4). Structural MRIs acquired at the time of study participation were examined in both groups. The TBI group was found to have mostly diffuse injury. Three persons had residual contusions, but not in consistent locations (i.e., 1 bifrontal, 1 right temporal, and 1 basal ganglia). More details of the demographic information for the participants with TBI are provided in Table 1. Scans of control participants did not yield significant findings and were within normal limits for age.
Table 1.
Participant Demographics for the TBI Group
| Gender | Age | Yrs. Educ. | Initial GCS | Best GCS/24 hrs. | Yrs. Since Injury | Injury Cause |
|---|---|---|---|---|---|---|
| F | 20 | 15 | 12 | 15 | 2.4 | Fall |
| F | 21 | 12 | 15 | 15 | 1.9 | MVA |
| F | 24 | 16 | 4 | 14 | 1.1 | MVA |
| M | 18 | 12 | 15 | 15 | 1.1 | Sports |
| M | 23 | 14 | 3(TP) | 11 | 2.2 | MVA |
| M | 30 | 16 | 13 | 15 | 2.0 | MCA |
| M | 31 | 15 | 3(T) | 7 | 2.2 | MCA |
| M | 35 | 12 | 3(T) | 7 | 1.9 | MVA |
| M | 41 | 18 | 7 | 11 | 1.1 | Falling object |
| M | 47 | 16 | 15 | 15 | 1.1 | Bicycle |
| M | 53 | 16 | 14 | 14 | 2.6 | Fall |
| M | 54 | 16 | 3(TP) | 7 | 1.1 | MCA |
GCS = Glasgow Coma Score, T = intubated, P = given paralytic medication, MVA = motor vehicle accident, MCA = motorcycle accident
Matching controls to persons with TBI was based on age, gender, and years of education. Age matching was within five-year strata. Education was matched as closely to one year as possible. Groups did not differ in either age (controls: M = 26.5, SD = 8.7, range = 19–50; TBI: M = 33.1, SD = 12.9, range = 18–54; t(22) = −1.46, p = 0.16), or education (controls: M = 16.2, SD =2.8, range = 12–22; TBI: M = 14.8, SD = 1.9, range = 12–18; t(22) = 1.37, p = 0.19).
Materials and Design
A block design was used for this study. Sets of paired encoding and recognition blocks displaying four types of stimulus items were presented. Stimuli used were line drawings of objects, line drawings of shapes in arrays, words, and unpronounceable letter strings. All were presented as black on a white background. For each stimulus type, the corresponding encoding block immediately preceded the recognition block, but the orders of the four stimulus types were counterbalanced by participant. The line drawings were from Snodgrass & Vanderwart (1980), and the words were developed from their original set of stimuli (as used by Nolde, et al., 1998; Raye, et al., 2000). Items were of high linguistic frequency, and easy to name and visualize. Shape and letter stimuli were not easy to verbally mediate: in the shape condition, randomly generated groupings of basic shapes were presented, while the letter condition was made up of unpronounceable 4–8 letter strings. For this paper, we focus on the word and letter conditions. Contrasts with the pictographic data were reported previously (Russell, et al., 2011).
Each block comprised a 6-minute fMRI run, with only one type of stimulus. During encoding blocks, participants were asked to press one button if a stimulus was “pleasant,” and another if not, in an attempt to enhance attention and encoding. In recognition blocks, participants pressed buttons to indicate if they had seen the stimulus before. Half of the items in the recognition blocks were new. A single trial during a block was comprised of a 500 ms fixation screen, followed by stimulus presentation for 2500 ms, and then a blank screen presentation for 1000, 3000, or 5000 ms. Presentation was controlled by E-prime software (www.pstnet.com), and items were randomly ordered within blocks.
Procedure
The session began after informed consent was obtained, with questionnaires and neuropsychological assessments taking place before imaging. Tests included the Token Test (DeRenzi & Vignolo, 1962; Spreen & Benton, 1969), Wechsler Test of Adult Reading (WTAR; Wechsler, 2001), Brief Symptom Inventory (BSI; Derogatis, 1993), Michigan Alcoholism Screening Test (MAST; Selzer, 1971), and California Verbal Learning Test –II (CVLT-II; Delis, et al., 2000). Token Test screening confirmed that participants were able to follow verbal commands. Scores on the WTAR were in the high average range for controls (M = 114, SD = 10.2) and the average range for persons with TBI (M = 101, SD = 9.3). No participants were excluded with the BSI or the MAST. The CVLT-II was used to ensure that participants with TBI exhibited some degree of memory impairment, and that control participants did not. We believe that including only those TBI participants with persisting memory impairment improves the potential generalizability of the findings to those individuals that one would typically see clinically for neuropsychological assessment As severity of injury may have variable impact on memory functioning, choosing to screen in this manner also increases homogeneity within the TBI group, despite the inclusion of participants with a range of documented injury severity. In order to screen in this manner, only persons with TBI who performed at least 1 standard deviation below norms (on any CVLT-II index) were included in the study. In contrast, control participants were only included if they scored no lower than 1 standard deviation below normative standards in all categories (indicating “normal” performance on the episodic memory task). Also administered were five executive control measures: Wisconsin Card Sorting Task (WCST; Grant & Berg, 1948; Milner, 1963), Trail Making Test (TMT; Army Individual Test Battery, 1944), Controlled Oral Word Association Test (COWAT—Benton & Hamsher, 1976), Similarities from the Wechsler Adult Intelligence Scale –III (WAIS-III; Wechsler, 1997), and the Stroop test (Golden & Freshwater, 2002). After neuropsychological testing, pre-scanning practice of the episodic memory task was completed, followed by the scanning session.
fMRI Parameters
A 3-Tesla Siemens Allegra head-dedicated scanner was used. The functional images were sets of 39 contiguous 3mm axial slices (TR = 2000ms, TE = 25ms, 64×64 matrix, FOV = 200mm, flip angle = 79°). Two sets of structural scans were also acquired: axial T2 weighted images (39 contiguous 3mm slices, TR = 6440 ms, TE = 73 ms, 256 × 256 matrix, FOV = 200mm, flip angle = 150°) and a sagittal 3-D MPRAGE sequence (224 contiguous 0.78 mm slices, TR = 1680ms, TE = 2.48ms, 256×256 matrix, FOV = 200mm, flip angle = 8°).
fMRI Analysis
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) was used for pre-processing and data analysis. Pre-processing steps included motion correction, coregistration of structural and functional images, segmentation, normalization, and smoothing. Explicit masks were created with the ART program (http://www.nitrc.org/projects/artifact_detect). A voxel-based approach to data analysis was employed, with individual first-level analyses entered into two-sample t-tests, and with subsequent contrasts completed to provide direct group comparisons, as described below. As previous fMRI studies of TBI have shown activation outside typical regions of interest, we chose a voxel-based approach in order to ensure that such activation would be captured. Talairach coordinates (Talairach & Torneau, 1998) were created from the MNI coordinates (Mazziotta, et al., 1995) using the icbm2tal algorithm (Lancaster, et al., 2007). The Talairach Client (www.talairach.org/client.html Lancaster, et al., 2000; Lancaster, et al., 1997) was used to localize the coordinates.
Results
Neuropsychological Data
Values from the above executive control measures were converted to T scores, and the average of the five measures comprised the composite score. Scores were compared between groups and controls; the TBI group performed more poorly than controls (t (22) = 2.47, p = .022). When compared on single scales, the TBI group had fewer total words on the COWAT (t (20) = 3.00; p = 0.007), greater interference on the Stroop (t (20) = 2.80, p = 0.015), and lower scores on Similarities (t (20) = 2.45, p = 0.023). Interestingly, TMT-B completion time and WCST perseverative errors were not significantly different between groups.
The CVLT-II was used for initial screening, but an additional in-depth examination was made to characterize the strategies used by the two groups. One TBI participant scored greater than 2 standard deviations above the TBI mean on both Trials 1–5 and semantic categorization 1–5, and was thus excluded from the rest of the analyses in this section as a clear outlier. No other participants in either group met this criterion. Performance on individual trials 1–5 was compared between the groups and it was found that mean number of words recalled was lower in the TBI group for trials 3, 4, and 5 (see Table 2 for more details on the comparisons presented in this section), and not significant for trial 1. These findings suggest that while initial performance was equivalent to controls, it did not progress in the same manner. This pattern may represent the inability to use (or choose) the same strategies employed by controls. Two pre-defined strategies on the CVLT-II are semantic clustering and serial clustering, with the former of these possibly requiring more explicit employment while the latter may be a more implicitly used strategy. Two-tailed t tests comparing scores for each strategy on each trial were performed to determine if both groups utilized these at the same rate. Semantic clustering rates were significantly different between controls and persons with TBI for trials 2–5, suggesting controls were either choosing to use this strategy more, or were able to use it more effectively. Serial clustering was not significantly different between groups on any of the 5 initial trials (all ps > 0.1).
Table 2.
CVLT-II performance, by trial
|
Control |
TBI |
||
|---|---|---|---|
| Trial | Mean (SD) | Mean (SD) | T-statistic (p-value)* |
| Total # of Words Recalled | |||
| 1 | 7.00 (2.37) | 6.00 (1.55) | 1.18 (0.25) |
| 2 | 10.08 (2.97) | 8.00 (2.19) | 1.90 (0.071) |
| 3 | 12.17 (2.04) | 9.00 (2.32) | 3.48 (0.002) |
| 4 | 13.25 (2.45) | 9.18 (2.27) | 4.11 (< 0.001) |
| 5 | 14.17 (1.64) | 9.18 (1.94) | 6.67 (< 0.001) |
|
| |||
| Semantic Clustering | |||
| 1 | 1.08 (1.16) | 0.73 (0.90) | 0.81 (0.43) |
| 2 | 2.33 (1.83) | 1.09 (0.83) | 2.07 (0.051) |
| 3 | 2.50 (1.51) | 1.36 (0.92) | 2.15 (0.043) |
| 4 | 4.17 (2.79) | 1.55 (0.82) | 2.99 (0.007) |
| 5 | 5.00 (4.33) | 1.45 (1.69) | 2.54 (0.019) |
|
| |||
| Serial Clustering | |||
| 1 | 2.42 (2.35) | 2.18 (1.66) | 0.27 (0.79) |
| 2 | 2.75 (1.22) | 1.73 (1.62) | 1.72 (0.099) |
| 3 | 3.58 (1.98) | 2.55 (1.37) | 1.45 (0.16) |
| 4 | 3.58 (2.31) | 2.27 (1.79) | 1.51 (0.15) |
| 5 | 3.33 (2.42) | 2.00 (1.79) | 1.49 (0.15) |
degrees of freedom for all tests = 21
Behavioral Data
A 2×8 analysis of variance (ANOVA) was conducted on the response time data with Group (Control, TBI) and Condition (encoding and recognition, for each of the four stimulus types) as factors. While the factor of condition exhibited a significant main effect (F (7, 154) = 14.71, p < 0.001), there was no main effect for group (F (1, 22) = 0.28, p = 0.60) and no interaction (F (7, 154) = 0.50, p = 0.83). The accuracy data again revealed a main effect for condition (F (3, 66) = 130.36, p < 0.001) and no effect of group (F (1, 22) = 0.60., p = 0.45) or group by condition interaction (F (3, 66) = 1.11, p = 0.35).
Across all participants, words were responded to faster and more accurately than letters (Word RT: M = 1194, ms SD = 136 ms; Letter RT: M = 1355 ms, SD = 244 ms; Word Accuracy: M = 82%, SD = 11%; Letter Accuracy: M = 60%, SD = 12%). These differences were significant (RT: t (23) = 3.18, p = 0.004, Accuracy: t (23) = −10.56, p < 0.001, two-tailed, as are all t-tests reported here), and held in nearly all cases when the tests were repeated for each group alone (Controls, RT: t (11) = 1.86, p = 0.09; TBI, RT: t (11) = 2.56, p = 0.027; Controls, ACC: t (11) = −6.80, p < 0.001; TBI, ACC: t (11) = −7.99, p < 0.001). Note that the RT values are collapsed across encoding and recognition conditions, while the accuracy values include only the recognition conditions (the pleasantness task in encoding conditions was subjective and therefore no accuracy scores were computed).
Because accuracy in the letter recognition condition was relatively poor, tests were conducted to determine whether accuracy percentages were different than would be expected by chance, but scores did turn out to be significantly greater than chance for both groups (control: z = 3.72, p < 0.001; TBI: z = 2.09, p = 0.037). No significant differences in accuracy were observed between groups (t (22) = 1.30, p = 0.21). Controls were also not faster to respond during either letter encoding (t (22) = −.201, p = 0.84) or letter recognition (t (22) = −1.004, p = 0.22). Both groups were, however, faster (controls: t (11) = 4.29, p = .001; TBI: t(11) = 3.56, p = 0.004) and more accurate for word recognition as compared to letter recognition. No group differences were found for average response times between the associated encoding conditions (ps > 0.27).
Imaging Data
Models of contrasts comparing encoding and recognition of letters and words were run first for each individual, and the maps generated by these analyses were entered into a group comparison. Each of the following paragraphs reports the results of the between-group contrasts. Since there was variation even among individuals in the same group, initial consideration of the data from the group comparison was conducted using a threshold of p = 0.01 uncorrected, with a cluster size > 10 voxels, (see Miller, et al., 2009 for a discussion of individual differences in fMRI). More restrictive thresholding (p < 0.001, uncorrected, cluster size > 10 voxels) was then implemented.
In comparing components of the letter encoding task, we initially evaluated activation during letter encoding as compared to letter recognition. As shown in Table 3, while controls did not show any additional activation for letter encoding over letter recognition, there were many additional areas initially shown in the TBI group. These areas included some typically associated with a wide range of cognitive systems, including language, memory, and executive functioning. The largest series of areas involved bilateral cingulate cortex (Brodmann Area (BA) 24 and 32), with the posterior portion represented only on the left (BA 36). Activation was also seen in parahippocampal gyri bilaterally, but in different areas (left—BA 36, right—BA27). Finally, there was a left-lateralized area of activation in BA 38, with peaks in both the superior and middle temporal gyri. This set of active areas might suggest that a verbal strategy is being employed by the TBI group, perhaps as an automatic response to language-related processing, and that attempts were being made to inhibit this processing, as it is not a useful strategy under these circumstances. This hypothesis will be considered in more detail below. Unfortunately, these areas did not survive the more restrictive thresholding step.
Table 3.
Summary of Letter Encoding and Letter Recognition Activation
| Process | Group | Side | x | y | z | BA | Location | # voxels | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Encoding (LE > LR) | Control | no significant voxels | |||||||
| TBI | L | −30 | 6 | −35 | 38 | superior temporal gyrus | 78 | 0.001 | |
| L | −38 | 4 | −36 | 38 | middle temporal gyrus | … | 0.003 | ||
| L | −20 | −48 | 14 | 30 | posterior cingulate | 64 | 0.001 | ||
| L | −3 | 0 | 22 | 24 | cingulate gyrus | 73 | 0.002 | ||
| L | −45 | 40 | −3 | 10 | sub-gyral gray | 42 | 0.004 | ||
| L | −11 | 25 | 35 | 32 | cingulate gyrus | 16 | 0.004 | ||
| L | −40 | −22 | −15 | 36 | parahippocampal gyrus | 11 | 0.006 | ||
| R | 23 | −29 | −2 | 27 | parahippocampal gyrus | 37 | 0.002 | ||
| R | 12 | 13 | 40 | 32 | cingulate gyrus | 43 | 0.003 | ||
| R | 45 | −19 | −17 | 20 | sub-gyral gray | 13 | 0.004 | ||
| R | 23 | 20 | 12 | NA | claustrum | 23 | 0.005 | ||
| R | 23 | 11 | 42 | 24 | cingulate gyrus | 10 | 0.008 | ||
| Recognition (LR > LE) | Control | L | −7 | −42 | 16 | 29 | posterior cingulate | 7872 | <0.001 |
| L | −40 | 28 | 24 | 46 | middle frontal gyrus | … | <0.001 | ||
| L | −16 | −40 | 31 | 31 | cingulate gyrus | … | <0.001 | ||
| L | −18 | −66 | −28 | NA | cerebellum-pyramis | 120 | 0.002 | ||
| L | −12 | −59 | −23 | NA | cerebellum-dentate | … | 0.005 | ||
| L | −37 | −49 | 24 | 22 | superior temporal gyrus | 44 | 0.003 | ||
| R | 21 | −59 | 28 | 7 | precuneus | 2494 | <0.001 | ||
| R | 35 | −56 | 37 | 40 | inferior parietal lobule | … | <0.001 | ||
| R | 39 | −50 | 33 | 40 | supramarginal gyrus | … | <0.001 | ||
| R | 47 | −9 | 20 | 13 | insula | 76 | 0.001 | ||
| R | 49 | −23 | −13 | 20 | inferior temporal gyrus | 31 | 0.002 | ||
| R | 43 | −35 | 13 | 41 | superior temporal gyrus | 92 | 0.003 | ||
| R | 13 | −68 | 47 | 7 | precuneus | 95 | 0.003 | ||
| R | 17 | −63 | 51 | 7 | superior parietal lobule | … | 0.003 | ||
| R | 7 | −64 | 43 | 7 | precuneus | … | 0.009 | ||
| R | 26 | −19 | 21 | NA | claustrum | 61 | 0.004 | ||
| R | 28 | −22 | 14 | NA | claustrum | … | 0.007 | ||
| R | 19 | −22 | 15 | NA | thalamus | … | 0.008 | ||
| R | 38 | 2 | 21 | 13 | insula | 28 | 0.004 | ||
| R | 14 | −10 | −4 | NA | subthalamic nucleus | 17 | 0.004 | ||
| R | 12 | −66 | −27 | NA | cerebellum-pyramis | 15 | 0.007 | ||
| TBI | L | −38 | −34 | 29 | 40 | inferior parietal lobule | 50 | 0.002 | |
| R | 22 | −31 | 51 | 7 | precuneus | 12 | 0.005 | ||
| R | 2 | −54 | 33 | 31 | precuneus | 11 | 0.007 | ||
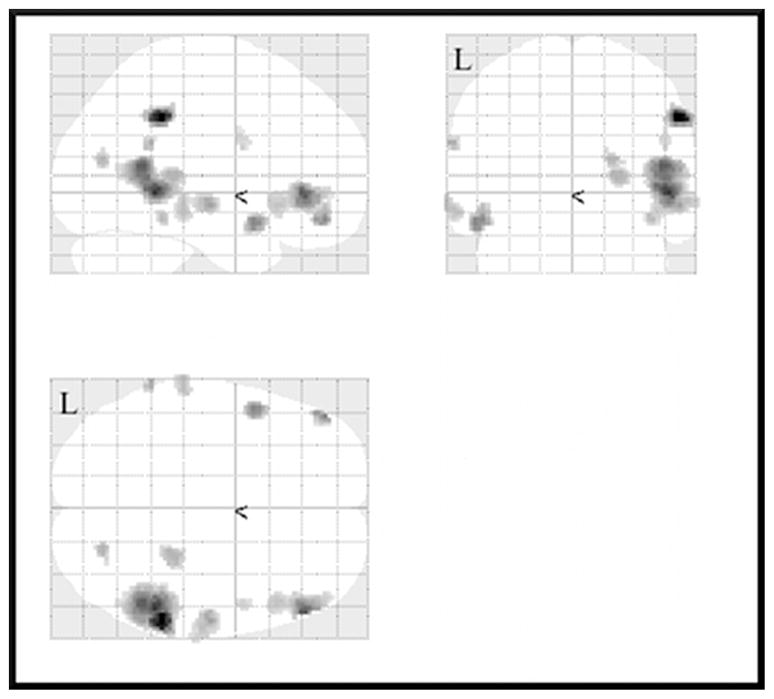
After comparing letter encoding with letter recognition, our next comparison was an evaluation of activation during letter encoding as compared to word encoding. Of these comparisons, the word encoding condition did not yield significant voxels over the letter encoding condition for either controls or persons with TBI. The only area above the lower threshold for controls for letter encoding was the right precentral gyrus (BA 6), but the TBI group had multiple areas of activation in this comparison, across both hemispheres, again being evidenced in areas associated with multiple cognitive skills. Areas shown to be active for the TBI group at the p < 0.001 level include the superior and middle temporal gyri in both hemispheres (BA 38 and 21 on the left and 39 and 22 on the right), inferior frontal gyri on both sides (10 on the left and 45/47 on the right), as well as BA 40 bilaterally. Some additional right-lateralized activation was seen in the right thalamus, cuneus (BA 23), fusiform gyrus (37), and precentral gyrus (6). See Figure 1 for a graphical representation of these areas of activation.
Figure 1.
Activation for letter encoding over word encoding for the traumatic brain injury (TBI) group as compared to the control
group at p < .001, uncorrected, excluding clusters with fewer than 10 contiguous voxels.
After comparisons to evaluate differences of letter encoding as compared to related tasks, we then focused on letter recognition. First we evaluated differences in activation during letter recognition as compared to letter encoding. Whereas the TBI group exhibited more activation during letter encoding, it is the control group that showed additional activation during letter recognition. The TBI group showed only a single area on the left (inferior parietal lobule—BA 40), and a nearby area on the right (precuneus—BA 7, 31), neither of which survived the more restrictive threshold. The controls showed activation in multiple areas on the left and right, especially in areas related to memory and executive control. After more restrictive thresholding, several active areas remained (see Figure 2), including clusters in both left and right parietal areas (left: superior parietal lobule and precuneus (both in BA 7) and right: precuneus (BA 7), inferior parietal lobule, and supramarginal gyrus (both in BA 40)) and cingulate gyrus/posterior cingulate (left: BA 29, 31; right: BA 29, 32, 24). One final active area was seen in the middle frontal gyrus on the left (BA 46). These results, together with the encoding findings, suggest that controls exert more effort with letter strings at recognition than during encoding.
Figure 2.
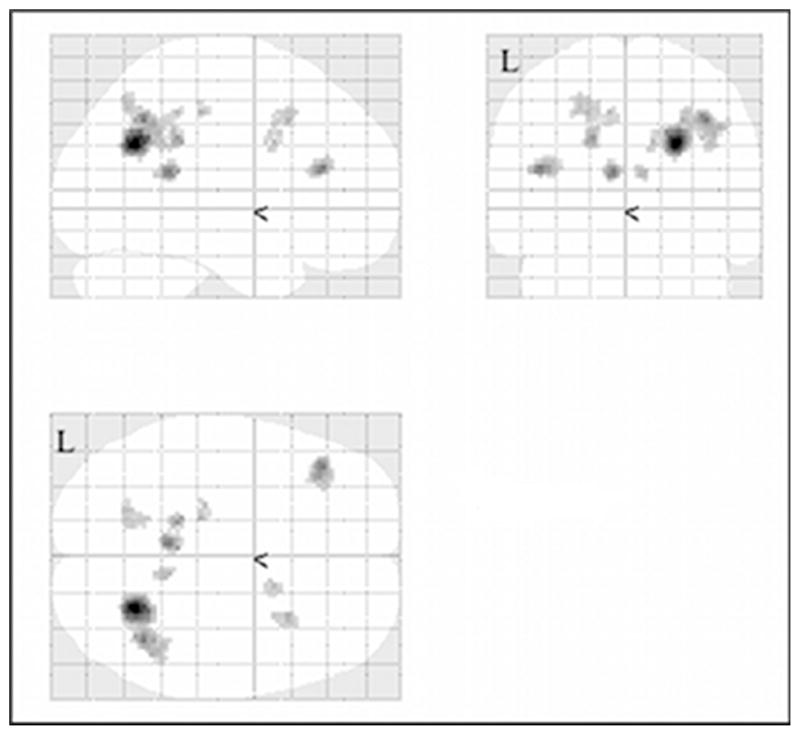
Activation for letter recognition over letter encoding for the control group as compared to the traumatic brain injury (TBI)
group at p < .001, uncorrected, excluding clusters with fewer than 10 contiguous voxels.
Finally, to complete the evaluation of letter recognition activation, we compared activation observed during letter recognition in contrast to what was observed during word recognition. For the comparison of word recognition over letter recognition, there was minimal activation in the TBI group, with nothing surviving the second thresholding, and nothing passing even the lower threshold in the control group. The activation seen in the TBI group was bilateral and subcortical (thalamus and caudate), though a small cluster of activation was found in left parahippocampal gyrus (BA 30). For letter recognition over word recognition, there was activation for each group at the lower threshold, which did not survive higher thresholding in the TBI group. For the control group at the higher threshold, the letter recognition condition led to completely left-lateralized activation in BA 40 and BA 6, with the former localized in the inferior parietal lobule and middle frontal gyrus, and the latter in the precentral gyrus. For either group, it seems that letter recognition generated more areas of activation as compared to word recognition, though some areas did not pass the more restrictive threshold. This finding suggests an increased difficulty associated with the recognition of the letter strings.
Discussion
In this study, we examined persons with TBI who specifically had an episodic memory deficit (as indexed by the CVLT-II). We noted that the ability of individuals with TBI to freely recall words appeared to be related to differences in the process of encoding the words. Specifically, we noted that while persons with TBI did not differ significantly from controls on the number of words recalled during the first of the five learning trials, by the third, fourth, and fifth learning trials, they recalled fewer words as compared to controls. In essence, the slope of the learning curve was flattened in the TBI group. In addition, it was noted that there was no significant difference between groups in serial clustering (which is an implicit strategy), but the TBI group did not appear to utilize (or was less able to utilize) semantic or categorical clustering. Since semantic clustering is considered to require more executive skills to implement, and the TBI group also scored significantly lower on measures of executive functioning, it is possible that the ability to process and encode the words at this higher level was impacted by injury. As executive functioning is also well known to be compromised by TBI, the fact that it may also compromise episodic memory functioning is not surprising.
In comparison with free recall, recognition memory paradigms are considered to be “easier.” While we did find differences on free recall measures between groups on the CVLT-II, as discussed above, there were no significant recognition memory differences found. Our fMRI design used a recognition memory paradigm to test episodic memory. In part, the use of recognition is necessitated by the requirements of fMRI, during which movements much greater than finger presses in response (i.e., movements of the head caused by verbal responding) can cause motion artifacts which may negatively impact the quality of the data. As a result, the episodic memory task completed in the scanner should have been significantly easier than the free recall required by the CVLT-II. Our findings during the fMRI task support this as the TBI group did not perform less accurately or more slowly than the control group, and both groups showed the same pattern of increased difficulty with letters as compared to words, both in terms of accuracy and speed of responding. In this sense, then, we have a group with known episodic memory deficits, but we have provided a task which they are able to perform at the same level as those in the control group. As a result, differences observed on fMRI should be due to differences in how each group is processing the task, as opposed to differences due to difficulty. In fact, our fMRI findings indicated differences in location and intensity of brain activation in individuals with TBI as compared to controls. Specifically, when encountering more difficult stimuli (letters), fMRI data for the TBI group showed greater activation during the encoding phases of memory as compared to the recognition component. This was true both in comparing letter encoding to word encoding, as well as comparing letter encoding to letter recognition. In contrast, the control group did not display evidence of increased activation for encoding in either comparison. This suggests that perhaps the TBI group is exerting greater cognitive effort during encoding to ultimately achieve the same level as controls over the course of these tasks. When looking at this in concert with neuropsychological testing findings, this seems to correlate with the findings which indicated increased difficulty over the course of the encoding portion of the CVLT-II for individuals in the TBI group.
Interestingly, when viewing comparisons of letter recognition over letter encoding (again, recall that this was the more difficult of the tasks presented), it was the control group which had increased fMRI activation at recognition as compared to encoding of letters, where the TBI group did not display significant activation. When considering that ultimately the two groups did not perform significantly differently, this suggests that the control group may be expending more effort or energy on recognition as compared to TBI group.
When evaluating word encoding or recognition over the corresponding letter condition, neither group displayed significant activation. This set of results reflects the fact that letters were more difficult for both groups, and suggests that the groups may not have significant differences. Taken together, these patterns suggest that, when the task is relatively simple (i.e., word processing), there is not much difference between groups in terms of performance or activation. However, when a less automatic task is introduced (i.e., letter processing), the groups diverge in terms of where greatest effort is concentrated. For the TBI group, the greatest cognitive effort appears to be expended during the encoding portion of the letter task, while the control group appears to expend greater effort on the recognition task.
The TBI group had more areas of activation during the letter encoding condition. This was true whether compared to controls, or in a within-group comparison with another fMRI condition. The letter encoding condition seems to elicit much more activation in the TBI group. The locations of the extra activation were in multiple areas, including areas commonly conceptualized as involved in language, executive control, and memory. These results suggest that the TBI group is using a different strategy for encoding these items. It is not clear what this strategy is, but since there are language areas active in the TBI group that are not active for controls, it is possible that the strategy used involves a linguistic component. Given the nature of the stimuli (i.e., that the letter strings are unpronounceable and thus consist of 4–8 items that are difficult to “chunk”), as well as the experimental pacing, it would be nearly impossible for a verbal rehearsal strategy to work. Despite this, it is possible that the tendency to employ a linguistic strategy may be more automatic when presented with letters from one’s native language. Given the observed limitations in executive functioning indicated by neuropsychological testing for the TBI group, it is possible that this group had increased difficulty inhibiting a more automatic process. In comparison, based on activation patterns of those in the control group, it is possible that the controls may have had a greater ability to recognize that such a strategy would not work for the task, and then employ a different encoding strategy. As seen in Table 3, controls had greater right hemisphere activation as compared to left, which would further support use of a non-liguistic strategy. While our current study may not allow us to pinpoint the strategy used by the control group, there was suggestion that they focused more cognitive resources during recognition compared to encoding.
There are additional considerations for future studies. The letter condition, while yielding some interesting results, was quite difficult and did not function as a control condition in the way we had originally anticipated. It may be useful to intentionally employ this as an experimental condition (either with or without explicit instructions on what strategy to attempt for encoding) and find a more suitable control condition. The other issue which may have influenced data interpretation was individual variability. To ensure adequate recruitment, we included persons with differences in age, education level, time since injury, etc., and these may have contributed to the variation we saw when looking at the individual activation maps. Given that enrolling participants that are more restricted in demographics and other characteristics could decrease generalizability of the results, it may be preferable to explore less group-oriented analysis paths in the future. While still preliminary, the present results further clarify the mechanisms of post-TBI memory impairment, and by doing so may positively influence the development of enhanced and empirically supported approaches to memory compensation or strategy training in the future.
Acknowledgments
This study was supported in part by a grant (NIH-NINDS R01NS048178-01) awarded to J.H. Ricker.
Footnotes
Author Disclosure Statement
The authors have no conflicts of interest to report. No competing financial interests exist.
References
- Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Benton AL, Hamsher K, editors. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- Corrigan JD, Whiteneck G, Mellick D. Perceived needs following traumatic brain injury. J Head Trauma Rehabil. 2004;19:205–216. doi: 10.1097/00001199-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- DeRenzi E, Vignolo LA. The Token Test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual. 4. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Golden CJ, Freshwater SM. Stroop Color and Word Test: Revised examiner’s manual. Wood Dale, IL: Stoelting Co; 2002. [Google Scholar]
- Grant AD, Berg EA. A behavioral analysis of reinforcement and ease of shifting to new responses in a Weigl-type card sorting. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Himanen L, Porthin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: A 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- Kashluba S, Hanks RA, Casey JE, Millis SR. Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch Phys Med Rehab. 2008;89:904–911. doi: 10.1016/j.apmr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Gary HE, Jr, Eisenberg HM, Ruff RM, Barth JT, Kreutzer J, High WM, Jr, Portman S, Foulkes MA, Jane JA, Marmarou A, Marshall LF. Neurobehavioral outcome 1 year after severe head injury: Experience of the Traumatic Coma Data Bank. J Neurosurg. 1990;73:699–709. doi: 10.3171/jns.1990.73.5.0699. [DOI] [PubMed] [Google Scholar]
- Levine B, Cabeza R, McIntosh AR, Black SE, Grady CL, Stuss DT. Functional reorganisation of memory after traumatic brain injury: A study with H2150 positron emission tomography. J Neurol Neurosurg Psychiatry. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels JA, Craik FI, Levine B, Schwartz ML, Stuss DT. Effects of divided attention on episodic memory in chronic traumatic brain injury: A function of severity and strategy. Neuropsychologia. 2002;40:2369–2385. doi: 10.1016/s0028-3932(02)00084-2. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: Theory and rationale for its development. Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Miller MB, Donovan CL, Van Horn JD, German E, Sokol-Hessner P, Wolford GL. Unique and persistent individual patterns of brain activity across different memory retrieval tasks. Neuroimage. 2009;48:625–635. doi: 10.1016/j.neuroimage.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SR, Ricker JH. Verbal learning patterns in moderate and severe traumatic brain injury. J Clin Exp Neuropsychol. 1994;16:498–507. doi: 10.1080/01688639408402661. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Nolde SF, Johnson MK, D’Esposito M. Left prefrontal activation during episodic remembering: An event-related fMRI study. Neuroreport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Raskin SA. Memory. In: Raskin SA, Mateer CA, editors. Neuropsychological management of mild traumatic brain injury. London: Oxford University Press; 2000. pp. 93–112. [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Nolde SF, D’Esposito M. FMRI investigations of left and right PFC to episodic remembering. Psychobiology. 2000;28:197–206. [Google Scholar]
- Ricker JH, Müller RA, Zafonte RD, Black KM, Millis SR, Chugani H. Verbal recall and recognition following traumatic brain injury: A [0–15] water positron emission tomography study. J Clin Exp Neuropsychol. 2001;23:196–206. doi: 10.1076/jcen.23.2.196.1204. [DOI] [PubMed] [Google Scholar]
- Russell KC, Arenth PM, Scanlon JM, Kessler LJ, Ricker JH. A functional magnetic resonance imaging investigation of episodic memory after traumatic brain injury. J Clin Exp Neuropsychol. 2011;33:538–547. doi: 10.1080/13803395.2010.537253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test (MAST): The quest for a new diagnostic instrument. Am J Psychiat. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn Mem. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton A. Neurosensory Center Comprehensive Examination for Aphasia. Victoria, BC: University of Victoria, Department of Psychology, Neuropsychology Laboratory; 1969. [Google Scholar]
- Strangman GE, Goldstein R, O’Neil-Pirozzi TM, Kelkar K, Supelana C, Burke D, Katz DI, Rauch SL, Savage CR, Glenn MB. Neurophysiological alterations during strategy-based verbal learning in traumatic brain injury. Neurorehab Neural Re. 2009;23:226–236. doi: 10.1177/1545968308324225. [DOI] [PubMed] [Google Scholar]
- Strangman GE, O’Neil-Pirozzi TM, Goldstein R, Kelkar K, Katz DI, Burke D, Rauch SL, Savage CR, Glenn MB. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Arch Phys Med Rehabil. 2008;89:974–981. doi: 10.1016/j.apmr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: a 3-dimensional proportional system, an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Teasdale G, Jennet B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:480–487. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levine B. Augmented neural activity during executive control processing following diffuse axonal injury. Neurology. 2008;71:812–818. doi: 10.1212/01.wnl.0000325640.18235.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: A selective review. J Clin Exp Neuropsychol. 2005;27:977–102. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III, WMS-III technical manual. New York: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. London: The Psychological Corporation; 2001. [Google Scholar]
- Wiegner S, Donders J. Performance on the California Verbal Learning Test after traumatic brain injury. J Clin Exp Neuropsychol. 1999;21:159–170. doi: 10.1076/jcen.21.2.159.925. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Schmitter-Edgecomb M, Woo E. Verbal memory impairment in severe closed head injury: The role of encoding and consolidation. J Clin Exp Neuropsychol. 2010;32:728–736. doi: 10.1080/13803390903512652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki T, Yoshino E, Fujimoto M, Ohmori Y, Imahori Y, Ueda S. Chronological positron emission tomographic study of severe diffuse brain injury in the chronic stage. J Trauma. 1996;40:50–56. doi: 10.1097/00005373-199601000-00010. [DOI] [PubMed] [Google Scholar]