Abstract
The successful clinical implementation of adoptive cell therapeutics, including bone marrow transplantation and other stem cell-based treatments, depends critically on the ability to deliver cells to sites where they are needed. E-selectin, an endothelial C-type lectin, binds sialofucosylated carbohydrate determinants on its pertinent ligands. This molecule is expressed in a constitutive manner on bone marrow and dermal microvascular endothelium, and inducibly on post-capillary venules at all sites of tissue injury. Engagement of E-selectin with relevant ligand(s) expressed on circulating cells mediates initial “tethering/rolling” endothelial adhesive interactions prerequisite for extravasation of blood-borne cells at any target tissue. Most mammalian cells express high levels of a transmembrane glycoprotein known as CD44. A specialized glycoform of CD44 called “Hematopoietic Cell E-/L-selectin Ligand” (HCELL) is a potent E-selectin ligand expressed on human cells. Under native conditions, HCELL expression is restricted to human hematopoietic stem/progenitor cells. We have developed a technology called “Glycosyltransferase-Programmed Stereosubstitution” (GPS) for custom-modifying CD44 glycans to create HCELL on the surface of living cells. GPS-based glycoengineering of HCELL endows cell migration to endothelial beds expressing E-selectin. Enforced HCELL expression targets human mesenchymal stem cell homing to marrow, licensing transendothelial migration without chemokine signaling via a VLA-4/VCAM-1-dependent “Step 2-bypass pathway”. This review presents an historical framework of the homing receptor concept, and will describe the discovery of HCELL, its function as the bone marrow homing receptor, and how enforced expression of this molecule via chemical engineering of CD44 glycans could enable stem cell-based regenerative medicine and other adoptive cell therapeutics.
Key terms: Multi-step paradigm, selectin, selectin ligand, E-selectin, L-selectin, mesenchymal stem cell, hematopoietic stem cell, adoptive cell therapeutics, Step 2-bypass pathway, glycosyltransferase-programmed stereosubstitution
INTRODUCTION
The success of stem cell-based regenerative medicine and, in general, of adoptive cell therapeutics, critically hinges on the ability to deliver relevant cells to sites of tissue injury/inflammation. Delivery of cells can be achieved in two ways: direct (i.e., local) injection or intravascular administration. Though seemingly straight-forward, direct injection is laden with obstacles: (1) It is applicable only for organs/tissues with well-defined anatomic boundaries (e.g., the heart, but not the lung); (2) It is an invasive procedure, thus potentially augmenting target tissue damage and/or instigating collateral tissue damage; (3) The procedure itself could be labor-intensive and could require use of high-tech imaging and delivery systems, especially for relatively inaccessible and/or fragile organs/tissues (e.g., the central nervous system); (4) By introducing pertinent cells under hydrostatic pressure and suspended in (characteristically) crystalloid solutions, the procedure could harm the delivered cells and, furthermore, could exacerbate the inflammatory process in situ. Beyond these numerous fundamental considerations, many degenerative and inflammatory conditions are multifocal in nature (e.g., osteoporosis, inflammatory bowel disease, multiple sclerosis, etc), and thus direct injection is impractical. The vascular route of administration is indicated for these and all “systemic” disorders, especially for extensively involved tissues with problematic access and/or anatomy not amenable to local injection (e.g., the pancreas in diabetes, the bone in osteoporosis). Therefore, achieving the enormous promise of adoptive cell therapies depends on the creation of methodologies to optimize the expression/activity of molecular effectors directing the physiologic migration of intravascularly administered cells.
Recruitment of circulating cells to a specific anatomic site is initiated by discrete adhesive interactions between cells in flow and vascular endothelium at the target tissue(s). The molecules that mediate these adhesive contacts are called “homing receptors”, and, as defined historically, these structures pilot tropism of cells in blood to the respective target tissue. Thus, the need to maintain and/or enforce expression of homing receptors is common to all attempts to deliver cells via the vasculature. To this end, this review will focus on the homing receptor known as Hematopoietic Cell E-/L-selectin ligand (HCELL), reflecting on its discovery and unique functional properties, and on the development of an effective strategy to enforce its expression for adoptive cell therapeutics.
THE HOMING RECEPTOR CONCEPT: HISTORICAL BASIS
The term “homing receptor” dates back several decades, a phrase initially proposed to explain observations made regarding the extensive flux of lymphocytes between blood and lymph. In this physiologic process, known as “lymphocyte recirculation”, lymphocytes migrate from blood to lymph nodes, exit nodes via efferent lymphatics, and thereafter drain back into the bloodstream. The most prominent conduit for lymphocyte reentry to the circulation is via the thoracic duct, a large lymph vessel that accumulates efferent lymphatic flow from lymphoid tissues below the diaphragm and drains into the systemic circulation via an anastomosis at the left brachiocephalic vein. The conspicuously nonrandom pattern of lymphocyte migration to lymphoid organs led to the hypothesis in the 1950s that a homing molecule existed on the surface of lymphocytes that directed trafficking to lymph nodes. This notion received considerable experimental support based on both physiologic and electron microscopic studies of James L. Gowans and colleagues 1–4, showing that lymphocyte migration to lymph nodes occurred in a highly regulated fashion with lymphocytes specifically adhering at defined vascular regions of nodes consisting of plump, cuboidal endothelial cells known as “high endothelial venules” (HEV). Of note, the electron microscopic studies of Gowans and Vincent T. Marchesi 3 showing lymphocyte adherence to HEV were prompted by prior microscopic studies of leukocyte migration by Howard W. Florey (who was Gowans’ post-doctoral mentor in the late 1940s, and who was the recipient of the 1946 Nobel Prize for Medicine, shared with Alexander Fleming and Boris Chain for discovery of penicillin and its clinical application). In contrast to lymphocyte recruitment at HEV which occurred under steady-state conditions, Florey’s studies showed that leukocyte extravasation at sites of inflammation was a dynamic process, occurring within two hours of injury at discrete post-capillary venules displaying adhesive properties (postulated as “sticky” substance) that supported leukocyte adherence and ensuing endothelial transmigration 5, 6. Subsequent studies in the 1960s by Bertram M. Gesner (who performed post-doctoral training with Gowans) with his then post-doctoral fellow, Judith J. Woodruff, showed that lymphocyte migration to lymph nodes was mediated by a trypsin-sensitive lymphocyte surface molecule(s), thus focusing the search for this “homing” protein 7.
The identification of the lymphocyte homing receptor was enabled by the creation of an in vitro assay by Woodruff and Hugh B. Stamper in the mid-1970s that mimicked physiologic lymphocyte adherence to HEV (the “Stamper-Woodruff assay”) 8. This assay consists of overlaying suspensions of lymphocytes onto thin sections of lymph nodes in the cold (4°–7°C) under shear conditions (as originally described, fluid shear delivered by a rotatory platform). It was fortuitous that the assay was performed in the cold, as engagement of several (confounding) adhesion molecules, particularly integrins, is blunted under cold conditions 9. These investigators accurately surmised that because adherence was occurring under blood flow conditions, the binding of lymphocytes to HEV necessitated shear stress. This assay was highly specific, allowing reproducible analysis of the avid adhesion between lymphocytes and HEV. Indeed, in their initial description of the assay and results derived therefrom, the authors were the first to refer to the lymphocyte homing molecule as a surface “receptor” for HEV 8. This powerful assay then allowed for development of monoclonal antibody reagents in the early 1980s that could neutralize the function of the receptor, initially described by two independent investigators, Yee Hon Chin, then a post-doctoral fellow working under Woodruff 10–12, and by W. Michael Gallatin 13; the Chin mAb was directed against the rat homing receptor (known as A.11), whereas the Gallatin mAb was directed against the mouse homologue (known as MEL-14). Notably, the Stamper-Woodruff assay also allowed for development of a mAb known as MECA79 that neutralizes the capacity of HEV to support lymphocyte adherence 14, and this mAb was instrumental in defining a family of sulfated, sialofucosylated glycoproteins that serve as L-selectin ligands on HEV, known collectively as “peripheral lymph node addressins” (for review see 15).
The identity of the authentic lymph node homing receptor was muddled in the mid-1980s by various conflicting, and still rather bewildering, results. Studies of the human “Hermes” antigen, both by biochemical and Stamper-Woodruff assay approaches, led some to believe that this molecule was the human lymph node homing receptor 16–18, and immunologic cross-reactivity between Hermes and MEL-14 proteins was reported 18. Furthermore, early immunoprecipitation studies using the MEL-14 mAb indicated that the target antigen was ubiquitin 19–21. The confusion in molecular features of the lymph node homing receptor engendered by these reports stymied publication of the cDNA sequence encoding the genuine rat homing receptor obtained by the author in 1987 while a post-doctoral fellow in the lab of Chin; this sequence was generated by probing a λgt11 phage expression library of rat thoracic duct lymphocyte cDNA using polyclonal antiserum raised against the A.11 protein (data reviewed in 22). Reports of cloning of the lymph node homing receptor by several groups in the late 1980s and early 1990s showed that the molecule belonged to a family of C-type lectins, subsequently called “selectins” 23, 24. These cloning data definitively separated this molecule, known now as L-selectin, from the Hermes antigen, which was revealed to be CD44 25–27. Importantly, the logic for choosing shear conditions to detect L-selectin in the Stamper-Woodruff assay was subsequently validated (in the mid-1990s) by hydrodynamic assays using the parallel plate flow chamber, which showed that L-selectin receptor/ligand interactions require a threshold level of fluid shear stress 28–30. In contrast, binding of CD44 (“Hermes”) to its principal ligand, hyaluronic acid, is not dependent on shear and occurs readily under static conditions. It was thus presumed that the molecular basis of lymphocyte trafficking to lymph nodes had been deciphered: a single molecule, L-selectin, was the mediator of homing to lymph node.
Despite the abundant data supporting a key role for L-selectin in directing cell migration to lymph nodes, it was soon clear that a variety of cells that do not typically home to lymph nodes express L-selectin, including mature myeloid cells and hematopoietic progenitor cells (reviewed in 31). Indeed, adherence assays under fluid shear conditions showed that L-selectin expressed on granulocytes was capable of binding to HEV ligands 32. These findings raised a key challenge to the homing receptor concept: How could a structure serve as a homing receptor on one cell and not another? This conundrum was solved by the observation that physiologic migration of circulating cells into tissues requires a coordinated sequence of steps. Thus, it became clear that homing receptors are a necessary feature – but not necessarily sufficient – for cell migration.
THE MULTISTEP PARADIGM OF CELL MIGRATION
A variety of independent lines of evidence obtained throughout the 1980s and 1990s revealed that cell migration involves chemoattractants characteristically operating through G-protein coupled receptors (GPCRs). Engagement of these receptors results in intracellular signaling (“inside-out” signaling) that activates adhesiveness of cell surface integrins such as LFA-1 and VLA-4 to their respective endothelial ligands, ICAM-1 and VCAM-1 (reviewed in 33). The most important of these chemoattractants consist of a family of small molecular weight glycoproteins known as chemokines, some of which are distributed in a tissue-specific manner and others which are expressed in response to inflammatory insults. From these data, combined with results of studies of homing receptors, a generalized model was proposed whereby cell migration is encoded by a cascade of overlapping steps. According to this paradigm, from a biophysical perspective, a homing receptor functions as a molecular brake, effecting initial tethering then sustained rolling contacts of cells in blood flow onto the target tissue vascular endothelium at velocities below that of the prevailing bloodstream (Step 1) (reviewed in 34). These rolling interactions occur secondary to fast on-off binding kinetics between homing receptors with their pertinent endothelial counterreceptors, translating into cellular torque under the action of fluid shear forces 30. Thereafter, a cascade of events evolve, typically potentiated by chemokines engaging their cognate ligand(s) on the surface of the blood-borne cells, resulting in G-protein-coupled activation of integrin adhesiveness (Step 2). The ensuing integrin attachment to endothelial coreceptors results in firm adherence (Step 3), followed by endothelial transmigration (Step 4) 33. This “multi-step paradigm” holds that tissue-specific migration is regulated by a discrete combination of homing receptor and chemokine receptor expression on a given circulating cell, allowing for recognition of a pertinent “traffic signal” displayed by the relevant vascular adhesive ligands and chemokines expressed within target endothelium in an organ-specific manner. Thus, circulating cells expressing the relevant coreceptors for the displayed endothelial adhesion molecules and chemoattractants will be recruited to the target. Though recent insights have expanded the original cascade steps into subcomponents and have suggested alternative pathways of integrin activation (reviewed in 35), one aspect of the model remains unchanged: expression of homing receptors are indispensable for cell migration, as tethering and rolling adhesive interactions are obligatory to allow recognition of chemoattractants and elaboration of other downstream events.
THE SELECTINS: SWEET ADHESION
Studies in the 1990s and 2000s established that the selectins and their ligands are the most efficient effectors of Step 1 interactions. This family is comprised of three proteins, E-, P- and L-selectin 24. As the name implies, selectins are lectins that bind to specialized carbohydrate determinants, consisting of sialofucosylations containing an α(2,3)-linked sialic acid substitution(s) and an α(1,3)-linked fucose modification(s) prototypically displayed as the tetrasaccharide sialyl Lewis X 36, 37. E- and P-selectin are expressed on vascular endothelium (P-selectin also on platelets), and, as noted above, L-selectin is expressed on circulating leukocytes and on hematopoietic progenitor cells 34. P-selectin is stored in granules of platelets and endothelial cells, where it is translocated to the membrane rapidly in response to agonists such as thrombin. In addition, P-selectin and E-selectin are each inducible endothelial membrane molecules that are prominently expressed at sites of tissue injury and inflammation, characteristically upregulated by inflammatory cytokines such as IL-1 and TNF. In this regard, it is important to draw distinction between rodents and primates: whereas IL-1 and TNF each induce transcription of mRNA encoding P-selectin and E-selectin in rodents, the P-selectin promoter of primates lacks the relevant response elements for these cytokines and only E-selectin is transcriptionally induced 38. Indeed, in transgenic mice bearing the human P-selectin gene on a murine P-selectin knock-out background, TNF administration actually decreased human P-selectin expression (i.e., decreased P-selectin mRNA levels), whereas it increased murine P-selectin expression in wild type animals 39; a similar pattern of decreased human P-selectin mRNA was observed in skin of the transgenic mice undergoing contact hypersensitivity reactions. This physiologic difference in regulation of P-selectin expression has important implications for drawing relevance of rodent findings to human biology, indicating that results obtained from both steady-state and inflammatory models in mice may over-emphasize the contribution(s) of P-selectin, and under-emphasize the contribution(s) of E-selectin, compared to clinical reality.
Though most endothelial beds do not natively express vascular selectins, the microvasculature of bone marrow and of skin constitutively expresses these selectins in mice 40, 41, and constitutive E-selectin expression, but not P-selectin, has been consistently documented in noninflamed human bone marrow and skin microvessels 42–44. Such differences in steady-state expression of P- and E-selectin between mice and humans indicates that cell recruitment to skin and marrow in humans is dependent on E-selectin-binding homing receptors more so than in mice: as expected, intravital microscopy studies in transgenic mice bearing the human P-selectin gene have shown that E-selectin dominates basal rolling interactions in the skin of the transgenic mice (analogously to humans), whereas P-selectin does so in wild-type animals 39. Notably, the ability of lymphocytes to migrate to skin in humans is critically dependent on expression of the “skin homing receptor”, a structure known as “Cutaneous Lymphocyte Antigen” (CLA), which is recognized by a rat IgM mAb known as HECA-452 45. This mAb recognizes sialofucosylated structures such as sLex and its isomer sLea 46. Early studies showed that most human skin-resident lymphocytes are reactive with HECA-452 (thus, CLA+) 45. Later studies showed that CLA serves as a ligand for E-selectin 47, and biochemical studies then showed that CLA is a specialized glycoform of PSGL-1, a leukocyte molecule that serves as the principal ligand for P-selectin and that also binds L-selectin. Therefore, on human lymphocytes, the CLA molecule is defined as PSGL-1 reactive with mAb HECA-452 (that recognizes sLex). In particular, CLA is prominently expressed on restricted subsets of effector T cells that migrate to skin, and other leukocyte membrane structures may carry HECA-452-reactive glycans on non-PSGL-1 scaffolds that can serve as ligands for E-selectin and thereby promote dermatotropism, including CD43 48 and glycolipids 49. Lymphocyte trafficking to lymph node and lymphocyte trafficking to skin are thus operationally linked as functions of selectins: L-selectin mediates homing of lymphocytes to lymph node by binding to ligands bearing relevant sialofucosylated structures recognized by mAb MECA79, whereas CLA and other HECA-452 reactive structures on relevant lymphocytes (e.g., skin-homing effector memory cells) engages E-selectin that is permanently expressed on dermal microvasculature.
SEARCHING FOR THE BONE MARROW HOMING RECEPTOR: THE DISCOVERY OF HCELL
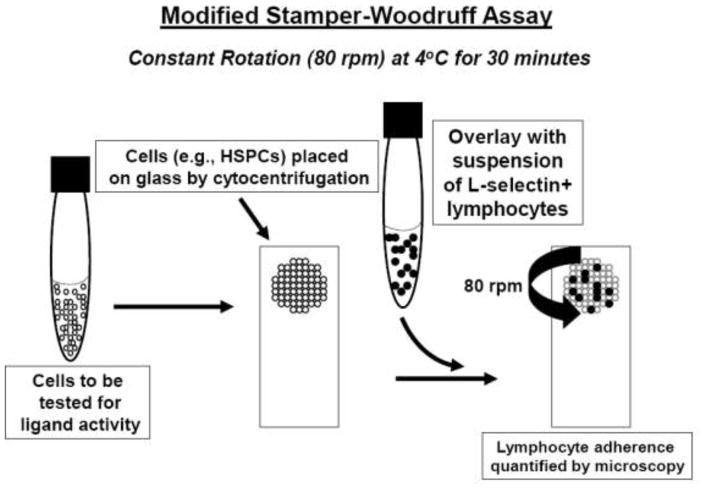
Various studies in the late 1980s and early 1990s revealed that L-selectin is expressed on normal human bone marrow cells in a tightly regulated fashion 50, 51. In particular, studies of human marrow cells demonstrated that L-selectin expression is conspicuously bimodal: hematopoietic stem/progenitor cells (“HSPCs”, defined as cells expressing the CD34 antigen but lacking markers associated with lineage commitment, i.e., CD34+/lin-cells) and terminally differentiated marrow lymphoid and myeloid cells were found to characteristically display L-selectin, but the protein was consistently absent on the surface of all erythroid and megakaryocytic progenitors and on all cells in intermediate stages of leukocyte development, both lymphoid and myeloid (reviewed in 31). The striking expression among human HSPCs raised speculation for a role of L-selectin in early hematopoietic events, such as in homing and/or lodgment of the cells to marrow microenvironmental growth niches. This notion received support from studies of human HSPCs in the early 1990s showing that incubation of the cells in vitro with anti-L-selectin antibody blocked clonogenic outgrowth of cells in both long-term (stromal) and short-term (methylcellulose) assays 52. Moreover, prior studies in mice had highlighted a role for cell surface lectins in homing to marrow 53, 54, supporting speculation that L-selectin could serve as a “bone marrow homing receptor”. Beyond these facts, the presumptive link between L-selectin and hematopoiesis was heightened considerably in the early 1990s with reports that a glyoform of CD34 expressed on murine lymph node HEV was reactive with L-selectin and with the MECA79 antibody 55, 56. Although these studies had been performed solely in mice, the findings drew immediate attention to the possibility that CD34 expressed on human marrow endothelium and/or on human HSPCs could function to promote HSPC homing to, and lodgment in, marrow, respectively. To formally explore the latter possibility, we undertook Stamper-Woodruff assays of native hematopoietic cells from human marrow and of cells from various human hematopoietic cell lines 57. To this end, we modified the conventional Stamper-Woodruff assay to assess the capacity of single cells to express L-selectin ligand activity by employing a cytocentrifuge apparatus to place relevant human hematopoietic cells on glass slides (see Figure 1). The assay was otherwise performed exactly as had been originally described by Stamper and Woodruff, with isolated human peripheral blood lymphocytes and rat thoracic duct lymphocytes each serving as input cells, and using rat lymph node sections as control 58. We found that a large subset of normal human CD34+ cells supported L-selectin-dependent lymphocyte adherence. To further define the identity of this L-selectin ligand, we performed Stamper-Woodruff assays on a panel of hematopoietic cell lines, including cell lines that did and did not express the CD34 antigen. Moreover, we sorted CD34+ cells, obtained from both normal human marrow and from CD34+ cell lines, into separate populations expressing high and low amounts of CD34 surface protein. We also analyzed binding of cells that had been transfected with CD34 cDNA and expressed robust levels of CD34. The results of all these studies showed the presence of a potent glycoprotein L-selectin ligand on normal humans HSPCs and on the human hematopoietic cell line KG1a, but the observed L-selectin ligand activity was not attributable to CD34 57. These findings were the first to demonstrate the existence of an L-selectin ligand on a non-endothelial cell type. Several years later, it was determined that PSGL-1 binds both P-selectin and L-selectin, with an overlapping N-terminal binding site requiring O-linked sialofucoylsations and tyrosine sulfation (for review see 59). In sharp contrast, though the L-selectin ligand detected by Stamper-Woodruff assay on HSPCs required characteristic sialofucosylation(s) for activity, extensive biochemical studies throughout the 1990s showed that the ligand was not reactive with MECA79, that it possessed sulfation-independent binding activity, and, most importantly, that relevant sialofucosylated binding determinants were displayed on N-linked glycans 60, 61. Notably, despite the fact that PSGL-1 is expressed on HSPCs, this molecule did not engage L-selectin under the shear conditions imposed by the Stamper-Woodruff assay 61. Altogether, these operational biochemical features were novel in scope, clearly distinguishing the identified HSPC L-selectin ligand from all previously characterized L-selectin ligands, including those expressed on HEVs.
Figure 1. Schematic of the modified Stamper-Woodruff Assay.
Cells to be tested for L-selectin ligand activity are placed within a defined region of the glass slide by cytocentrifugation. All other steps, including fixation (typically with glutaraldehyde), are performed similarly to conventional Stamper-Woodruff assays (which utilize frozen sections of tissue, e.g., lymph node). Shear stress is created by rotation of the slide platform at 80 rpm.
Studies of the HSPC L-selectin ligand in parallel plate flow chamber assays showed that binding to L-selectin required a minimum of 1.0 dyne/cm2 shear stress. Due to this shear dependency, efforts in our lab to immunoprecipitate the ligand with L-selectin-immunoglobulin chimeric construct (L-selectin-Ig) consistently failed. Though it was clear that sialofucosylations displayed as sLex were critical to binding activity, no inhibition of binding was observed by incubation of cells with HECA-452 mAb, and imunoprecipitation of lysates of HSPCs and KG1a cells with HECA-452 yielded a complex mixture of proteins, one of which was PSGL-1. However, a critical clue emerged from our studies using N-glycanase to assess the relevant carbohydrate linkage of L-selectin binding determinants: preparations of membranes from HSPCs and KG1a cells retained L-selectin ligand activity despite being subjected to SDS and β-mercaptoethanol denaturing conditions used in the N-glycanase buffer 61. This finding suggested that the ligand’s binding activity would withstand SDS-polyacrylamide gel electrophoresis conditions and the subsequent transfer of protein onto PVDF membranes. We thus performed western blot of KG1a membrane preparations and placed the PVDF sheet into the parallel plate flow chamber. This technology, which we named the “blot rolling assay” (for technical details, see 62), revealed reproducible and robust L-selectin-dependent lymphocyte rolling on a ~90–100kD band 63. The band was excised, and mass spectrometry showed that the protein consisted of a novel sialofucosylated glycoform of CD44 63. Blot rolling assays were then performed using Chinese hamster ovary (CHO) cells transduced to express E-selectin (CHO-E cells). These studies showed that the same band strongly supported E-selectin binding, but not P-selectin binding 64. Further biochemical and functional studies showed that this CD44 glycoform, named “Hematopoietic Cell E-/L-selectin Ligand”, is the most potent (i.e., confers the slowest rolling velocity and the highest resistance to detachment under fluid shear stress) of all the E- and L-selectin ligands expressed natively on human cells64–66.
CD44 and HCELL display an inverse functional relationship: CD44 is a lectin (i.e., it is the primary receptor for the glycosaminoglycan hyaluronic acid), whereas HCELL is a lectin ligand. Accordingly, it is inaccurate to state that “CD44 is a selectin ligand”. In particular, CD44 is an extremely polymorphic molecule with multiple protein isoforms generated by alternative splicing, with commensurate remarkable functional pleiotropism, highlighting the need to use clarifying nomenclature to delineate discrete CD44 properties (for review, see 67). The term “HCELL” refers to a specialized CD44 glycoform that binds E- and/or L-selectin; on human HSPCs, HCELL is expressed predominantly on the “standard” CD44 isoform (called “CD44s”) lacking any peptide products of splice sequences. The key glycan structural features of HCELL were revealed by early studies showing that inhibition of N-glycosylation, enzymatic removal of N-glycans, and α(2,3)-sialidase or α(1,3)-fucosidase digestion in each case eliminated ligand activity, thereby highlighting the functional dependency on N-linked terminal sialofucosylations displayed as sLex motifs 57, 61, 64. Notably, in Stamper-Woodruff assays, we consistently observed that L-selectin ligand activity was unaffected on human HSPCs and KG1a cells treated by the crosslinking fixative glutaraldehyde, which markedly alters protein conformation but does not affect glycan structure 57. Moreover, as described above, we also observed that HCELL activity is maintained under SDS-PAGE and other protein denaturing conditions. Collectively, these results indicate that the CD44 protein is essentially inert with regard to HCELL activity, i.e., the working end of HCELL is not the protein, it is the carbohydrates.
HCELL: ROLE IN BONE MARROW HOMING AND INTEGRIN ACTIVATION
During the 1990s and early 2000s, a variety of investigative approaches, principally based in mouse models and also utilizing xenogeneic transplants of human HSPC in immunocompromised mice, elucidated the molecular basis of HSPC homing to marrow (reviewed in 68). Numerous studies showed that homing to marrow involved HSPC surface expression of E-selectin ligand(s), together with the chemokine receptor CXCR4 and the β1 integrin VLA-4. Intravital microscopy studies in mice definitively showed that migration of HSPCs to marrow is mediated by engagement of E-selectin expressed on marrow microvessels, colocalized discretely with the chemokine CXCL12 (the ligand for CXCR4, also known as “SDF-1”), specifically at endothelial beds that are the sites of recruitment of HSPCs 41. Intravital microscopy also showed that marrow vasculature constitutively displays the ligand for VLA-4, VCAM-1, in an overlapping but more general endothelial distribution compared with that of E-selectin 41. Thus, a multistep model of HSPC homing to marrow was established, whereby E-selectin receptor/ligand interactions mediate Step 1, allowing engagement of co-localized CXCL12 to CXCR4, resulting in activation of VLA-4 (Step 2), followed by VLA-4 firm adherence on VCAM-1 (Step 3) and subsequent transmigration (Step 4).
The extremely high avidity of HCELL for E-selectin led us to reason that this molecule could serve as the authentic human bone marrow homing receptor. To test this notion, we created a platform technology called “glycosyltransferase-programmed stereosubstitution” (GPS) for glycoengineering the surface of living cells (reviewed in 37; for technical details see 69), and utilized a target cell devoid of step 1 effectors, human mesenchymal stem cells (MSCs). MSC represent a small population of cells present within normal marrow, but they can be readily expanded in culture. The human MSC used in our studies expressed VLA-4 but not CXCR4 70. Biochemical studies also showed that the cells displayed high levels of a sialylated glycoform of CD44, bearing N-linked terminal α(2,3) sialylated lactosaminyl glycans that were lacking only α(1,3)-fucosylation at N-acetylglucosamine to complete the sLex motif 70. We thus converted the native CD44 into HCELL by treating human MSC with an α(1,3)-specific fucosyltransferase, fucosyltransferase VI (FTVI), specifically formulated (with attendant reaction conditions) to avoid cell toxicity. Notably, following enforced surface α(1,3)-fucosylation ex vivo, western blot showed that the only membrane glycoprotein staining with HECA-452 (i.e., expressing sLex) was CD44, indicating that CD44 was the predominant target of exofucosylation 70. Parallel plate flow chamber studies on cytokine stimulated HUVEC monolayers showed that buffer treated (HCELL-) human MSC did not interact with endothelial cells, but enforced HCELL expression yielded robust E-selectin-dependent binding interactions under hydrodynamic shear conditions, with organized rolling persisting at upwards of 30 dyns/cm2. As visualized by intravital microscopy in immunodeficient mouse hosts, intravenously infused HCELL+ human MSC displayed robust tethering and rolling interactions on marrow vessels, with copious parenchymal infiltrates evident within 1 hour after injection. In comparison, HCELL-MSC showed minimal interactions with marrow vessels, with a paucity of marrow infiltrates observed even at 48 hours post-injection. Most importantly, extravasated HCELL+ MSC lodged within marrow endosteal surfaces and created human osteoid in mouse marrow 70. Thus, enforced HCELL expression programmed osteotropism of human MSCs, with phenotype and viability preserved, yielding characteristic osteoblast progeny that lodged in the pertinent microenvironment(s).
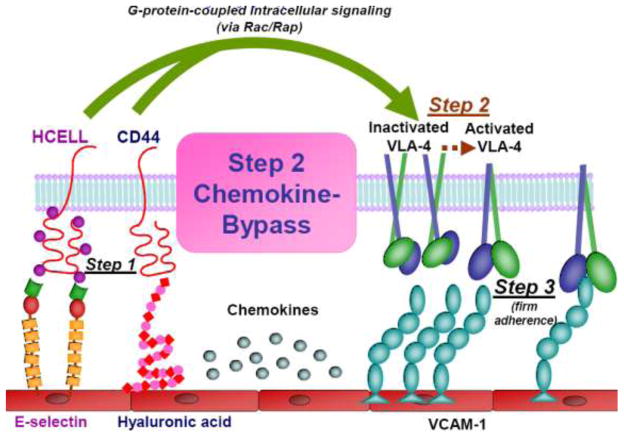
The finding that HCELL+ human MSC were capable of extravasation at marrow microvessels in absence of CXCR4 signaling prompted us to investigate whether MSC-endothelial interactions might be encoded by chemokine-independent pathways. Studies of human MSCs undertaken in both static and hydrodynamic flow conditions showed that engagement of CD44 with hyaluronic acid, or of (glycoengineered) HCELL with E-selectin, in each case triggers a Rac1/Rap1-GTPase signaling pathway, resulting in upregulation of VLA-4 firm adhesion to its ligands VCAM-1 and fibronectin. Consistent with these findings, human MSCs underwent transendothelial migration in the absence of exogenous chemokine input on cytokine-stimulated human endothelial monolayers expressing both E-selectin and VCAM-1 71. Interestingly, we observed that engagement of either CD44 or HCELL induces a bimolecular complex between CD44/HCELL and VLA-4, but that VLA-4 activation is dependent on G-protein-coupled signaling, not on the adhesion molecule co-association 71. Collectively, these findings provide a new dimension to the multistep paradigm, defining a novel integrin activation pathway coordinated by cross-talk between Step 1 and Step 3 effectors: this “Step 2-bypass pathway” is mediated by mechanosignaling induced by CD44 ligation, driving “inside-out” VLA-4 integrin activation, firm adherence, and transendothelial migration (see Figure 2).
Figure 2. The Step 2-bypass Pathway of Cell Migration.
Schematic representation of the surface structures of blood-borne cells (HCELL, CD44, VLA-4) and of endothelial cells (E-selectin, hyaluronic acid, and VCAM-1) licensing the Step 2-bypass pathway of cell migration. This process differs from the canonical multistep cascade in that Step 2 does not require chemokine input. Step 1 proceeds via HCELL binding to E-selectin and/or CD44 binding to endothelial HA, triggering mechanosignalling (Rac/Rap-mediated) yielding VLA-4 activation (Step 2), and subsequent firm adherence to endothelial VCAM-1 (Step 3).
HCELL EXPRESSION: OF MICE AND MEN
Our early studies of human hematopoietic cells showed that expression of HCELL was restricted to HSPCs. As noted above, the selectin binding carbohydrates of HCELL on HSPCs are expressed on CD44s, thus it is more appropriate to label this species as “HCELLs”. Subsequent studies of hematologic malignancies revealed that high expression of HCELLs is characteristic of leukemic blasts, especially among myeloid leukemias 61, 64. Studies in solid malignancies (e.g., colon cancer) also showed high level expression of HCELL 72, yet, conspicuously, the relevant selectin binding sialofucosylations are expressed not on N-glycans, but are on O-linked glycans distributed on splice variant sequences. Since CD44 isoforms containing peptide products of variant exons are collectively known as “CD44v”, this species of HCELL is designated as “HCELLv”. In contrast to HCELLs which binds only to E-selectin and L-selectin, there is evidence that HCELLv species may be capable of binding to P-selectin 73. HCELLv is as potent an E-selectin and L-selectin ligand as HCELLs, and current studies in our laboratory are focused on investigating its role as a homing receptor in mediating cancer metastasis.
Although an E-selectin binding glycoform of CD44 has been reported on mouse neutrophils by isolation of CD44 and clustering of the molecule on microbeads loaded with anti-CD44 mAb 74, it is worth remembering that binding of selectins to (putative) ligands displayed on artificial substrates is a function of ligand site density 75, 76; indeed, selectin-like adhesive interactions can be achieved on antibodies affixed to artificial substrates 77. Moreover, a comparative examination of the relevant potency of E-selectin ligands expressed on the surface of native mouse cells (i.e., not genetically altered to be deficient in selected adhesion molecules) has not been undertaken. To address this issue, we performed a comprehensive analysis of the E-selectin ligands expressed on mouse HSPCs isolated from normal (wild-type) marrow as compared to that of human HSPCs 66. Our studies in mice show that native mouse HSPCs do not express HCELL, however, surface expression of HCELL can be enforced by exofucosylation using FTVI. Importantly, though mice HSPCs display CLA and, also, a CD43 glycoform that binds E-selectin, exofucosylation only induced expression of HCELL (i.e., no other E-selectin ligands were created, and neither CLA nor CD43 were fucosylated). Enforced expression of HCELL markedly increased E-selectin ligand activity of mouse HSPCs (>4-fold), and, when injected intravenously into mice under steady-state conditions (i.e., not receiving any preparative radiation), HCELL+ mouse HSPCs displayed >3-fold more homing to marrow than HCELL-HSPCs 66. Together with studies of human MSC described above, these findings provide firm physiologic evidence of the capacity of HCELL to pilot tropism of intravascularly administered cells to bone marrow.
CONCLUSION
The original homing receptor hypothesis proposed that a single molecule on the surface of a cell would be sufficient to direct migration to a given target tissue. Though this notion was modified by the discovery of chemokines and their contributions in licensing transendothelial migration, the fact that engagement of HCELL can trigger integrin adhesiveness and transmigration in absence of chemokines provides validation of the fundamental concept. For migration to marrow, HCELL meets all the operational criteria to be considered a genuine “homing receptor”: it is expressed natively on pertinent cells that navigate to marrow (HSPCs), it binds potently to its cognate ligand (E-selectin) that is constitutively expressed on target tissue endothelium (i.e., marrow microvasculature), and enforced expression endows cells with the capacity to migrate to the intended anatomic site (i.e., MSC migration to marrow). As such, all evidence accumulated to date indicates that ex vivo glycoengineering of surface HCELL expression should prove useful in augmenting cell migration to marrow for clinical indications, including hematopoietic stem cell transplantation and MSC-based therapy of generalized bone diseases such as osteoporosis.
Primary clinical principles dictate that the vasculature is the preferred route of administration of cells for adoptive therapeutics. The HCELL molecule is the most potent E-selectin ligand expressed on human cells, and is therefore a principal effector of cell migration to any site where E-selectin is expressed. Human endothelial beds upregulate expression of E-selectin and VCAM-1 in all inflammatory conditions 34, 78. The fact that HCELL (and CD44) forms a bimolecular complex with VLA-4, and that engagement of HCELL triggers upregulation of VLA-4 adhesiveness and transendothelial migration in absence of chemokine input, suggests that enforced HCELL expression could program delivery of cells to all sites of tissue injury/inflammation (via the Step 2-bypass pathway) regardless of chemokine receptor expression on pertinent cells. Importantly, the CD44 molecule is rather ubiquitously expressed, and VLA-4 expression is characteristic of adult stem cells derived from marrow (i.e., hematopoietic stem cells and mesenchymal stem cells) and from tissue sources (e.g., neural stem cells 79), and is also characteristic of lymphocytes 34. Accordingly, enforced HCELL expression could be exploited for applications of essentially all stem cell-based regenerative therapeutics, and for applications of lymphocyte-based immunotherapy such as in malignancy (e.g., delivering cytotoxic T cells) or in autoimmune disease (e.g., delivering regulatory T cells). In all cases, cells could be infused systemically by peripheral access, but, for some indications, vascular administration via angiographically guided catheters could be beneficial in attaining a “first pass” effect and thereby minimizing cell colonization at non-target tissues. Though the ideal vascular portal to achieve desired physiologic effects for adoptive cell therapeutics may ultimately depend on the administered cell type and the affected tissue(s), there is no doubt that GPS-based “sweet” manipulations to maintain and/or augment HCELL expression will open the pathways to effective and safe adoptive cellular therapeutics.
Acknowledgments
I thank all my talented and devoted co-workers for their invaluable assistance in elucidating the structure and biology of HCELL. This work was supported by the National Institutes of Health, in particular, the National Heart Lung Blood Institute (PO1 HL107146, RO1 HL60528, RO1 HL73714) and the National Cancer Institute (RO1 CA121335). According to National Institutes of Health policies and procedures, the Brigham & Women’s Hospital has assigned intellectual property rights regarding HCELL to the inventor (RS).
References
- 1.Gowans JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959;146:54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gesner BM, Gowans JL. The output of lymphocytes from the thoracic duct of unanaesthetized mice. Br J Exp Pathol. 1962;43:424–430. [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesi VT, Gowans JL. The Migration of Lymphocytes through the Endothelium of Venules in Lymph Nodes: an Electron Microscope Study. Proc R Soc Lond B Biol Sci. 1964;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 4.Gowans JL, Knight EJ. The Route of Re-Circulation of Lymphocytes in the Rat. Proc R Soc Lond B Biol Sci. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 5.Marchesi VT, Florey HW. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- 6.Florey HW, Grant LH. Leucocyte migration from small blood vessels stimulated with ultraviolet light: an electron-microscope study. J Pathol Bacteriol. 1961;82:13–17. doi: 10.1002/path.1700820103. [DOI] [PubMed] [Google Scholar]
- 7.Woodruff J, Gesner BM. Lymphocytes: circulation altered by trypsin. Science. 1968;161:176–178. doi: 10.1126/science.161.3837.176. [DOI] [PubMed] [Google Scholar]
- 8.Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertini O, Luscinskas FW, Kansas GS, Munro JM, Griffin JD, Gimbrone MA, Jr, Tedder TF. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991;147:2565–2573. [PubMed] [Google Scholar]
- 10.Chin YH, Carey GD, Woodruff JJ. Lymphocyte recognition of lymph node high endothelium. V. Isolation of adhesion molecules from lysates of rat lymphocytes. J Immunol. 1983;131:1368–1374. [PubMed] [Google Scholar]
- 11.Chin YH, Rasmussen R, Cakiroglu AG, Woodruff JJ. Lymphocyte recognition of lymph node high endothelium. VI. Evidence of distinct structures mediating binding to high endothelial cells of lymph nodes and Peyer’s patches. J Immunol. 1984;133:2961–2965. [PubMed] [Google Scholar]
- 12.Rasmussen RA, Chin YH, Woodruff JJ, Easton TG. Lymphocyte recognition of lymph node high endothelium. VII. Cell surface proteins involved in adhesion defined by monoclonal anti-HEBFLN (A.11) antibody. J Immunol. 1985;135:19–24. [PubMed] [Google Scholar]
- 13.Gallatin WM, I, Weissman L, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 14.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 16.Jalkanen ST, Bargatze RF, Herron LR, Butcher EC. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986;16:1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- 17.Jalkanen S, Reichert RA, Gallatin WM, Bargatze RF, Weissman IL, Butcher EC. Homing receptors and the control of lymphocyte migration. Immunol Rev. 1986;91:39–60. doi: 10.1111/j.1600-065x.1986.tb01483.x. [DOI] [PubMed] [Google Scholar]
- 18.Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987;105:983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegelman M, Bond MW, Gallatin WM, St John T, Smith HT, Fried VA, Weissman IL. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986;231:823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- 20.St John T, Gallatin WM, Siegelman M, Smith HT, Fried VA, Weissman IL. Expression cloning of a lymphocyte homing receptor cDNA: ubiquitin is the reactive species. Science. 1986;231:845–850. doi: 10.1126/science.3003914. [DOI] [PubMed] [Google Scholar]
- 21.Gallatin M, St John TP, Siegelman M, Reichert R, Butcher EC, Weissman IL. Lymphocyte homing receptors. Cell. 1986;44:673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- 22.Chin YH, Sackstein R, Cai JP. Lymphocyte-homing receptors and preferential migration pathways. Proc Soc Exp Biol Med. 1991;196:374–380. doi: 10.3181/00379727-196-43201a. [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua M, Butcher E, Furie B, Furie B, Gallatin M, Gimbrone M, Harlan J, Kishimoto K, Lasky L, McEver R, et al. Selectins: a family of adhesion receptors. Cell. 1991;67:233. doi: 10.1016/0092-8674(91)90174-w. [DOI] [PubMed] [Google Scholar]
- 24.Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91:379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picker LJ, De los Toyos J, Telen MJ, Haynes BF, Butcher EC. Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989;142:2046–2051. [PubMed] [Google Scholar]
- 26.Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein LA, Zhou DF, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989;56:1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- 28.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 29.Alon R, Chen S, Puri KD, Finger EB, Springer TA. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence MB, Kansas GS, Kunkel EJ, Ley K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L, P, E) J Cell Biol. 1997;136:717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sackstein R. Expression of an L-selectin ligand on hematopoietic progenitor cells. Acta Haematol. 1997;97:22–28. doi: 10.1159/000203656. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 33.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 34.Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]
- 35.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 36.Polley MJ, Phillips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, Paulson JC. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991;88:6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sackstein R. Glycosyltransferase-programmed stereosubstitution (GPS) to create HCELL: engineering a roadmap for cell migration. Immunol Rev. 2009;230:51–74. doi: 10.1111/j.1600-065X.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao L, Setiadi H, Xia L, Laszik Z, Taylor FB, McEver RP. Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood. 1999;94:3820–3828. [PubMed] [Google Scholar]
- 39.Liu Z, Miner JJ, Yago T, Yao L, Lupu F, Xia L, McEver RP. Differential regulation of human and murine P-selectin expression and function in vivo. J Exp Med. 2010;207:2975–2987. doi: 10.1084/jem.20101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, von Andrian UH. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- 41.Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schweitzer KM, Drager AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, van der Schoot CE, Langenhuijsen MM. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 43.Chong BF, Murphy JE, Kupper TS, Fuhlbrigge RC. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- 44.Jung K, Linse F, Pals ST, Heller R, Moths C, Neumann C. Adhesion molecules in atopic dermatitis: patch tests elicited by house dust mite. Contact Dermatitis. 1997;37:163–172. doi: 10.1111/j.1600-0536.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 45.Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CJ, Butcher EC. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- 46.Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 47.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS, Tiemeyer M, Konstantopoulos K, Schnaar RL. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kansas GS, Dailey MO. Expression of adhesion structures during B cell development in man. J Immunol. 1989;142:3058–3062. [PubMed] [Google Scholar]
- 51.Terstappen LW, Huang S, Picker LJ. Flow cytometric assessment of human T-cell differentiation in thymus and bone marrow. Blood. 1992;79:666–677. [PubMed] [Google Scholar]
- 52.Gunji Y, Nakamura M, Hagiwara T, Hayakawa K, Matsushita H, Osawa H, Nagayoshi K, Nakauchi H, Yanagisawa M, Miura Y, et al. Expression and function of adhesion molecules on human hematopoietic stem cells: CD34+ LFA-1-cells are more primitive than CD34+ LFA-1+ cells. Blood. 1992;80:429–436. [PubMed] [Google Scholar]
- 53.Aizawa S, Tavassoli M. In vitro homing of hemopoietic stem cells is mediated by a recognition system with galactosyl and mannosyl specificities. Proc Natl Acad Sci U S A. 1987;84:4485–4489. doi: 10.1073/pnas.84.13.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardy CL, Tavassoli M. Homing of hemopoietic stem cells to hemopoietic stroma. Adv Exp Med Biol. 1988;241:129–133. doi: 10.1007/978-1-4684-5571-7_16. [DOI] [PubMed] [Google Scholar]
- 55.Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 56.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oxley SM, Sackstein R. Detection of an L-selectin ligand on a hematopoietic progenitor cell line. Blood. 1994;84:3299–3306. [PubMed] [Google Scholar]
- 58.Sackstein R, Borenstein M. The effects of corticosteroids on lymphocyte recirculation in humans: analysis of the mechanism of impaired lymphocyte migration to lymph node following methylprednisolone administration. J Investig Med. 1995;43:68–77. [PubMed] [Google Scholar]
- 59.Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230:75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 60.Sackstein R, Fu L, Allen KL. A hematopoietic cell L-selectin ligand exhibits sulfate-independent binding activity. Blood. 1997;89:2773–2781. [PubMed] [Google Scholar]
- 61.Sackstein R, Dimitroff CJ. A hematopoietic cell L-selectin ligand that is distinct from PSGL-1 and displays N-glycan-dependent binding activity. Blood. 2000;96:2765–2774. [PubMed] [Google Scholar]
- 62.Sackstein R, Fuhlbrigge R. Western blot analysis of adhesive interactions under fluid shear conditions: the blot rolling assay. Methods Mol Biol. 2009;536:343–354. doi: 10.1007/978-1-59745-542-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dimitroff CJ, Lee JY, Fuhlbrigge RC, Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci U S A. 2000;97:13841–13846. doi: 10.1073/pnas.250484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dimitroff CJ, Lee JY, Schor KS, Sandmaier BM, Sackstein R. differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J Biol Chem. 2001;276:47623–47631. doi: 10.1074/jbc.M105997200. [DOI] [PubMed] [Google Scholar]
- 66.Merzaban JS, Burdick MM, Gadhoum SZ, Dagia NM, Chu JT, Fuhlbrigge RC, Sackstein R. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood. 2011;118:1774–1783. doi: 10.1182/blood-2010-11-320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sackstein R. The biology of CD44 and HCELL in hematopoiesis: the ‘step 2-bypass pathway’ and other emerging perspectives. Curr Opin Hematol. 2011;18:239–248. doi: 10.1097/MOH.0b013e3283476140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122:1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 69.Sackstein R. Directing stem cell trafficking via GPS. Methods Enzymol. 2010;479:93–105. doi: 10.1016/S0076-6879(10)79005-4. [DOI] [PubMed] [Google Scholar]
- 70.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 71.Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2011;108:2258–2263. doi: 10.1073/pnas.1018064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanley WD, Burdick MM, Konstantopoulos K, Sackstein R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 2005;65:5812–5817. doi: 10.1158/0008-5472.CAN-04-4557. [DOI] [PubMed] [Google Scholar]
- 73.Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, Konstantopoulos K. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. Faseb J. 2006;20:337–339. doi: 10.1096/fj.05-4574fje. [DOI] [PubMed] [Google Scholar]
- 74.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunk DK, Hammer DA. Quantifying rolling adhesion with a cell-free assay: E-selectin and its carbohydrate ligands. Biophys J. 1997;72:2820–2833. doi: 10.1016/S0006-3495(97)78924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys J. 2003;85:2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen S, Alon R, Fuhlbrigge RC, Springer TA. Rolling and transient tethering of leukocytes on antibodies reveal specializations of selectins. Proc Natl Acad Sci U S A. 1997;94:3172–3177. doi: 10.1073/pnas.94.7.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- 79.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]