Abstract
Metabolic oligosaccharide engineering is an emerging technology wherein non-natural monosaccharide analogs are exogenously supplied to living cells and are biosynthetically incorporated into cell surface glycans. A recently reported application of this methodology employs fluorinated analogs of ManNAc, GlcNAc and GalNAc to modulate selectin-mediated adhesion associated with leukocyte extravasation and cancer cell metastasis. This monograph outlines possible mechanisms underlying the altered adhesion observed in analog-treated cells; these range from the most straightforward explanation (e.g., structural changes to the selectin ligands ablate interaction with their receptors) to the alternative mechanism where the analogs inhibit or otherwise perturb ligand production to more indirect mechanisms (e.g., changes to the biophysical properties of the selectin binding partner, the nanoenviroment of the binding partners, or the entire cell surface).
Keywords: ManNAc GlcNAc, GalNAc, and Neu5Ac analog, glycan engineering, biomechanical and biophysical properties of the glycocalyx
Introduction
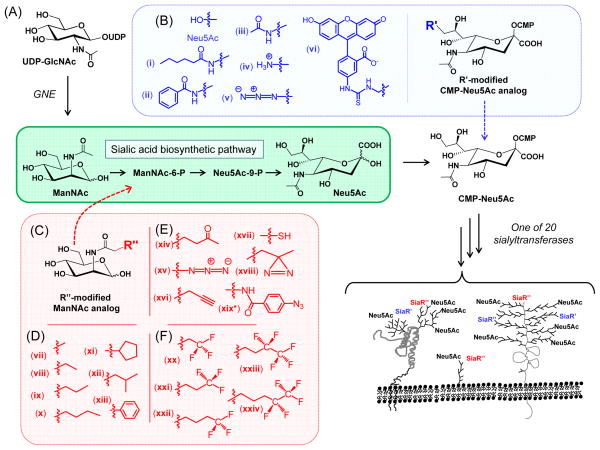
Metabolic oligosaccharide engineering (MOE) is a maturing technology that had its origins over two decades ago when the sialyltransferases were found to have the capacity to add modified sialic acids (Fig. 1A) – even those bearing groups as large as fluoroscein (cpd vi in Fig. 1B) – to glycoconjugates.1, 2 This technology took a dramatic step forward when the Reutter group showed that by using modified ManNAc analogs (Fig. 1C),3 rather than the cell-impermeant CMP-sialic acids used in the initial experiments just mentioned, MOE could be used in living cells and animals. By using ManNAc analogs with elongated N-acyl side chains such as ManNProp, ManNBut, and ManNPent (cpds vii, viii, and ix in Fig. 1D), the corresponding N-acyl-modified sialic acids could be installed on the cell surface. Another advance came when the Bertozzi group showed that bioorthogonal chemical functional groups such as the ketone (cpd xiv in Fig. 1E)4 and azide (xv)5 could be installed in the glycocalyx via MOE; recently the trend towards increasing the chemical repertoire of this technology has resulted in the development of analogs with alkyne (xvi),6 thiol (xvii),7 diazarine (xiii),8 and arylazide (xix)9 functional groups.10 From a practical standpoint, an early limitation of MOE was the low efficiency by which monosaccharide analogs were uptaken by cells. This problem has been confronted and largely overcome by using short chain fatty acid (SCFA)-derivatized analogs, first with acetate11 and more recently with n-butryate.12, 13
Figure 1. Overview of sialic acid-based MOE.
(A) Sialic acid biosynthesis begins with UDP-GlcNAc, which is converted to CMP-Neu5Ac; in humans Neu5Ac is subsequently installed into cell surface glycans by one of 20 sialyltransferases. (B) Various “R’” modified sialosides can be introduced into glycoconjugates by using the indicated CMP-Neu5Ac analogs. (C) Alternately, the sialic acid pathway can be intercepted with ManNAc analogs bearing (D) non-reactive alkyl N-acyl groups, (E) bioorthogonal functional groups, or (F) fluorinated substituents.
With over a decade of successful targeting of the sialic acid pathway in hand, efforts in the past few years have expanded MOE to fucose,14 GalNAc,15 and GlcNAc16 (incorporation of the latter analog is believed to be limited to nucleocytosolic “O-GlcNAc” while being excluded from N-glycans).10 Up to now, MOE has been a largely chemistry-driven enterprise, but there have been several intriguing biological consequences of this methodology reported. For example, the early analogs with elongated N-acyl alkyl chains reported by Reutter’s group altered viral binding and infectivity17 and they modulated the fate of embryonic neuronal cells;18 similarly, thiolated sialic acid analogs used in concert with a high affinity gold growth substrate also directed neuronal differentiation.7 For treatment of human disease, because of the abundance of sialic acid on many types of cancer MOE has been often applied to treatment of malignant disease with efforts used to direct therapeutic agents (such as ricin 4 or doxorubicin19) to over-expressed metabolically engineered sialosides or to use this method to enhance the immunogenicity of tumor associated carbohydrate antigens.20 A full discussion of the emerging biological applications of MOE is beyond the scope of this monograph (an interested reader can consult review articles10, 17, 21); instead we will focus on early indications that this technology can alter leukocyte (and, by extension, cancer cell) extravasation via modulation of selectin mediated adhesion22 (Fig. 2A).
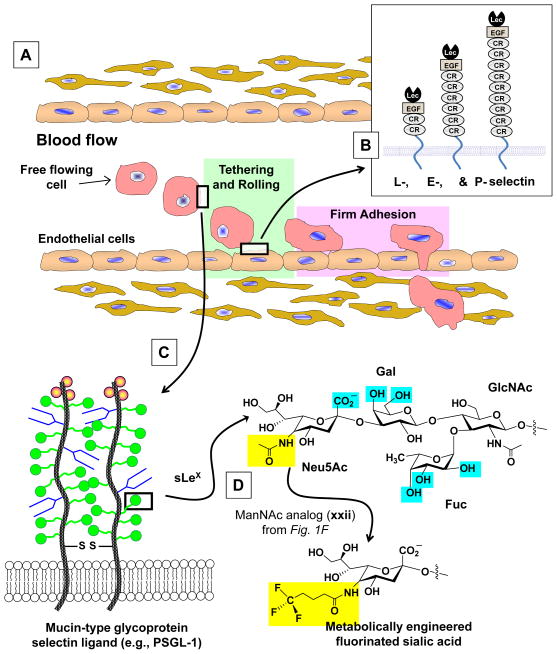
Figure 2. Overview of leukocyte extravasation.
(A) Free flowing cells in the bloodstream exit the vascular by a two stage process; selectin-mediated tethering and rolling followed by integrin-assisted firm adhesion. (B) Schematic representation of L-, E-, and P-selectin and (C) the P-selectin binding partner PSGL-1. (D) The structure of sLeX showing binding determinant critical for selectin recognition in blue and the N-acyl group modified by ManNAc-based MOE in yellow.
In a series of ground breaking experiments, Dafik and coworkers showed that fluorinated analogs that are incorporated into cell surface sialosides (Fig. 1F) reduced the adhesion of tumor cells to E- and P-selectin.23, 24 Superficially, a comparison of fluorinated hydrocarbons to Teflon offers a trivial explanation for this result (i.e., that the cell surface has been endowed with “non-stick” properties). However, upon deeper reflection, the mechanisms by which fluorinated analogs inhibit selectin-based adhesion involved in leukocyte extravasation and cancer cell metastasis is certainly remarkably more complex. For example, by increasing van der Waals interactions, fluorination affects the separation tendency of fluorinated and nonfluorinated molecules thus potentially altering the size, distance, and inner ligand density of cluster patches that are important for cell rolling.25 Accordingly, this monograph explores several possible ways by which MOE can, at least in theory, modulate the biophysical properties of the cell surface and by extension selectin-mediated adhesion. Once properly understood we anticipate that MOE will provide a lucrative new tool for modulation of not only selectin adhesion and leukocyte extravasation that are the focus of this report, but virtually any biological process affected by cell surface glycans.
“Obvious” mechanisms by which MOE modulates selectin-based interactions
Direct analog incorporation into selectin ligands and subsequent interference with binding
Based on the primary goal of metabolic oligosaccharide engineering, which is the incorporation of non-natural monosaccharides into the glycocalyx, the most obvious way by which fluorinated ManNAc and Neu5Ac analogs alter selectin-mediated adhesion is through the replacement of natural Neu5Ac found within sLeX with non-natural forms of this sugar. The MOE-installed non-natural sialic acids create modified ligands that no longer properly interact with the lectin domains of selectins. For example, modifications made by MOE at the C9 (e.g., via the CMP-Neu5Ac analogs shown in Fig. 1B or other C9 modified analogs reported in the literature26) or the N-acyl position of Neu5Ac (via the ManNAc analogs shown in Fig. 1D–F) in theory could render the sLeX structure too bulky to fit into the binding pocket of a selectin lectin domain.
Molecular level insight into how sLeX interacts with selectins was first deduced from a “glycomimetics” analysis that indicated that the underlying Lewis X trisaccharide made several contacts with the selectin binding pocket (Fig. 2D) while the only part of sialic acid that was required for binding was the carboxylic acid moiety of this monosaccharide.27 Subsequent crystal structures of sLeX bound to P- and E-selectin confirmed that selectins have a shallow binding pocket with much of the sialic acid residue oriented away from the binding site into empty space.28 Accordingly, it is unlikely that MOE-mediated modifications made to Neu5Ac directly interfere in a substantial way with the binding of sLeX to the lectin domain of a selectin. A counterargument to this premise, however, can be made based on recent molecular dynamics simulations that indicate that changes to one part of sLeX can be structurally reflected in other parts of the molecule in ways that could alter binding to a selectin.29 Consequently the presence of a modified sialic acid installed in sLeX via MOE could affect bond strain and submolecular electronic features throughout the tetrasaccharide epitope thus altering the relative positions of the critical underlying contact points that it makes with a selectin, thereby diminishing binding.
Perturbation of metabolic flux
The incorporation of fluorinated ManNAc analogs into cell surface sialosides23 is somewhat of an anomaly insofar as fluorinated metabolic intermediates have long been employed in drug development to inhibit a targeted enzyme or metabolic pathway. In fact, one of the very first – albeit unintended MOE experiments – occurred when 4-F-ManNAc was used to inhibit sialic acid production in cancer cells but was instead incorporated into surface elements.30, 31 By contrast to fluorinated ManNAc, the use of other fluorinated hexosamines has proved more successful at inhibiting glycosylation, both in general and in ways that specifically apply to the modulation of selectin binding interactions. In a recent example, fluorinated GlcNAc (where the -OH at the C4 position is replaced with -F) successfully reduced the amount of the E-selectin ligand sLeX produced by human T and leukemic cells25 by reducing UDP-GlcNAc levels.32 A drawback of targeting GlcNAc, however, is that this sugar is involved in multiple facets of human glycan production beyond sLeX by forming the attachment point for N-glycans to proteins, by appearing in O-glycans (e.g., as part of sLeX, Fig. 2), and by constituting the nucleocytosolic “O-GlcNAc” post-translational modification.33
The impact of 4-F-GlcNAc reaches beyond the inhibition of the incorporation of GlcNAc into glycans by altering GalNAc metabolism through changes to the equilibrium between UDP-GlcNAc and UDP-GalNAc.34 On one hand, because of the appearance of selectin ligands in both O- and N-glycans35, 36 a compound such as 4-F-GlcNAc that impinges upon both types of glycans logically should have maximal efficacy at disrupting selectin-mediated adhesion. However, to avoid unintended side effects from broadly altering glycosylation, more precise tools would be advantageous. For example, 4-F-GalNAc, which has the potential to specifically target O-glycans, reduced selectin ligand abundance on HL-60 cells resulting in reduced leukocyte migration in a murine model of thioglycolate-induced peritonitis.37
Interestingly, in addition to inhibiting the production of certain glycan epitopes, 4-F-GalNAc was also reported to be incorporated into the oligosaccharides of the selectin ligand PSGL-1.37 Similarly, the fluorinated ManNAc analogs mentioned above that reduced selectin mediated adhesion did not inhibit flux through the sialic acid pathway but instead replaced a proportion of the natural Neu5Ac-containing epitopes with those bearing fluorinated N-acyl groups.24 Consequently, monosaccharide analogs that perturb glycosylation can result in extraordinarily complex and difficult to unravel changes to glycan production leaving open the possibility that explanations other than just the reduction or structural alteration of the sLeX (or other glycan-based) selectin ligands is needed to account for the diminished selectin adhesion in cells treated with fluorinated hexosamine analogs.
Limitations and perplexing aspects of “direct” mechanism
As discussed above, it is possible although not yet confirmed that direct interactions between the modified sLeX ligands presented on cells subject to MOE and their selectin binding partners play a major role in dampening selectin-mediated adhesion. For example, fluorinated GlcNAc and GalNAc analogs that inhibit glycosylation result in complex global changes to glycan patterns that have yet to be fully characterized. Even fluorinated ManNAc analogs, which more precisely target a subset of the glycosylation machinery (i.e., Neu5Ac-bearing glycans, because there are few interconnections between sialic acid biosynthesis and the rest of the glycosylation enzymes, as discussed in more detail elsewhere10, 38) and thus are unlikely to dramatically perturb the overall repertoire of cells surface glycans, remain puzzling. In particular, these compounds can leave the number of native sLeX ligands relatively unchanged while installing additional non-natural ligands.24 The native selectin ligands remaining on the analog-treated cells should, at least in theory, offset the anti-adhesive effects of the modified sLeX ligands. Several possibilities by which the MOE can indirectly modulate adhesion and thereby offer insights into this conundrum are presented next.
Indirect mechanisms putatively involved in selectin-based adhesion
Indirect mechanisms – i.e., those that do not occur by inhibiting the production of sLeX or incorporation of non-natural monosaccharides into this epitope by MOE – by which MOE could regulate leukocyte extravasation occur on a hierarchy of size scales, which will be discussed from smallest to largest. At the smallest scale lies the “direct” submolecular mechanism discussed above where the larger N-acyl group of the modified analogs provided steric hindrance to binding. Next, are the molecular (e.g., the entire selectin ligand), the nano (e.g., the effect on multiple proteins or assemblages such as the “glycosynapse”), and the micro (e.g., the bulk properties of the plasma membrane) scales. In addition, although the primary purpose of MOE is to modulate glycan structures, increasing evidence is accumulating that this technology affects cell signaling and gene expression in ways that could impact leukocyte extravasation. Each of these possibilities will now be discussed in more detail.
Molecular scale mechanisms
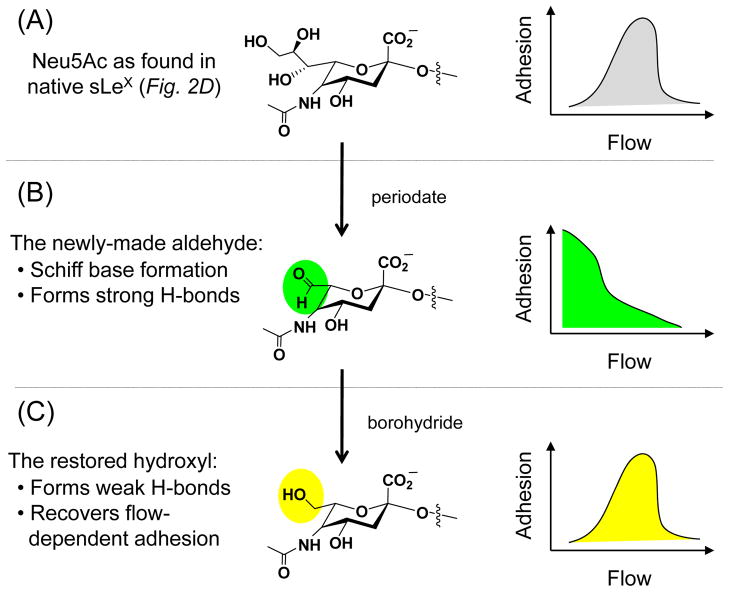
Increasing evidence suggests that to be effective, selectin ligands must have a modicum of flexibility39 that – significantly – resides outside of a single sLeX structure. This possibility was first raised by periodate treatment of sLeX-expressing cells, which ablated characteristic threshold shear dependent adhesion (Fig. 3).40 Schiff base formation wherein the aldehyde of the modified sialic acid formed a covalent bond with an amine located in the lectin domain of a selectin offered a possible explanation for how adhesion was achieved in the absence of flow for periodate treated cells (Fig. 3B). This idea was supported by further treatment with borohydride, which reduced the aldehyde to a hydroxyl group and restored characteristic flow dependent binding (Fig. 3C).40 This mechanism, however, was later debunked by AFM experiments that showed no difference in binding between periodate-treated and native sLeX indicating that the key molecular change enacted by periodate treatment lay outside of single selectin ligand.41 An intriguing hypothesis to explain this discrepancy is that the collective effect of small chemical changes to numerous sialic acids can alter the flexibility of a selectin ligand and thereby modulate leukocyte tethering and rolling.
Figure 3. Effect of chemical modifications to sialic acid on flow-dependent adhesion.
(A) The natural form of sLeX supports flow dependent selectin adhesion, which is depicted schematically on the right. As an aside, it might be noted that under non-physiological conditions, (e.g., at very high ligand density39 selectin mediated adhesion can also occur in the absence of flow.76 (B) Periodate treatment creates an aldehyde at the C7 position of sialic acid, which ablates the usual requirement for shear stress allowing adhesion to take place in the absence of flow (again depicted schematically). (C). Borohydride reduces the aldehyde to a hydroxyl, thereby restoring characteristic flow dependent adhesion.
To illustrate this concept, the P-selectin ligand PSGL-1 (illustrated in Fig. 2) or the L-selectin ligand CD34 (illustrated in Fig. 3 or described in the literature42, 43) each are 70 to 80% carbohydrate. The resultant >100 kD mass of carbohydrates represents at least 50 oligosaccharide chains (probably more, considering that most are relatively small O-glycan structures), each of which can be terminated with one or more sialic acid residues. The aldehyde resulting from periodate oxidation of the C7–C8 bond (Fig. 3B) can form stronger hydrogen bonds (4–7 kcal/mol) compared to hydroxyl groups (0–3 kcal/mol), which are restored upon borohydride treatment (Fig. 3C). Summed over the entire molecule, which contains dozens of sialic acids, the stronger aldehyde-mediated hydrogen bonding could add 100 kcal or more of “stability” (e.g., similar to two or more disulfide bonds) to the macromolecule. These back-of-the-envelope calculations indicate that it is plausible that selectin ligand flexibility could be altered by periodate treatment. This premise, combined with the elegant series of experiments reported by the McEver group and their collaborators that establish that selectin ligand flexibility is critical for the apparent catch bond properties of these molecules that contribute to the flow-dependent adhesion sketched in Figure 3,44, 45 suggests that MOE could be used to alter tethering and rolling. At present, no clear information is available to guide MOE experiments as to whether selectin ligands should be rendered more or less flexible to optimally disrupt leukocyte extravasation, or the precise analogs to use for either endpoint; nevertheless this almost entirely unexplored research area opens many intriguing possibilities for the manipulation of surface molecules and adhesion.
Nanoscale multi-molecular assemblies
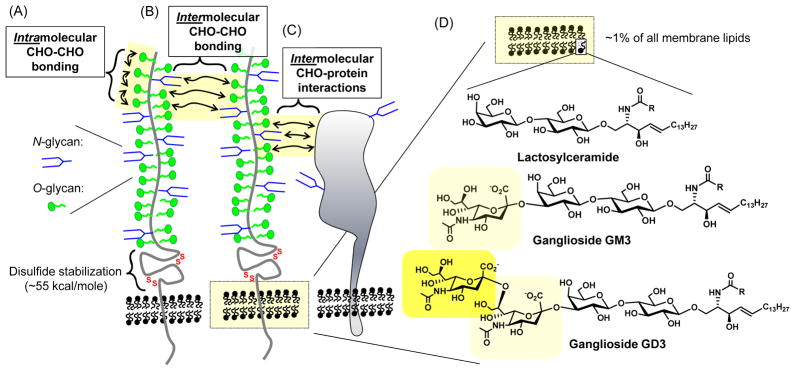
The typical selectin ligand such as PSGL-1 or CD34 is a large biomacromolecule, consisting of polypeptide backbone with dozens of attached oligosaccharides; one way to alter the flexibility of as discussed in the previous paragraph would be through intramolecular carbohydrate-carbohydrate interactions (Fig. 4A). Evidence for direct interactions of one carbohydrate with another (including sLeX-sLeX)46 has solidified in recent years;47, 48 in a slight variation water can act as a bivalent “bridging” molecule between two carbohydrates via hydrogen bonding (in fact one explanation proposed for some of the effects of ethanol is that it disrupts this bridging allowing only one H-bond to form49, 50). Furthermore, because selectin ligands such as PSGL-1 often exist in a dimeric form, carbohydrate-carbohydrate interactions can also occur intermolecularly between glycans on different peptide chains (Fig. 4B); an example of this type of interaction, is how antibody affinity can be modulated.51 Finally protein-carbohydrate interactions (Fig. 4C) can affect the aggregation of multimeric protein complexes;52 of particular relevance to this discussion, MOE-based methods using photoactivatable functional groups (e.g,. diazarine (cpd xviii in Fig. 1E) 9 and arylazide (xix) 8) have already been demonstrated to capture carbohydrate-protein interactions made in living cells.
Figure 4. Modulation of multimeric molecular complexes by carbohydrates.
CD34 is illustrated schematically, showing how carbohydrate (CHO)-carboydrate interactions can take place between glycans on the same polypeptide (A) or between adjacent glycoproteins (B); in addition carbohydrate-protein interactions are possible (C). (D) Glycoproteins embedded in the plasma membrane are surrounded by glycosphingolipids which include lactosylceramide and gangliosides GM3 and GD3.
Continuing in the vein of multimeric protein assemblies, adhesion molecules of the type involved in leukocyte extravasation are surrounded by nanoscale raft-type assembly of glycosphingolipids whose structure, biophysical properties, and ultimate function are dominated by carbohydrates.53 Approximately 1% of the ~100 billion phospholipids that comprise cellular membranes are glycosylated; a common glycosphingolipid is lactosylceramide (shown in Fig. 4D). Importantly, the glycolipids are not uniformly distributed throughout the plasma membrane but are instead clustered into lipid raft assemblies that are often associated with functional membrane proteins. An outstanding example of how glycolipids, in particular the sialic acid residues of gangliosides (gangliosides are sialic acid-derivatized glycolipids, represented by GM3 and GD3 in Fig. 4D), can modulate the activity of membrane components is provided by ion channels.54, 55 Another well established example is provided by integrins, which mediate the “firm adhesion” stage of leukocyte extravasation (Fig. 1A) and also exemplify “The Glycosynapse” concept.56 Although less is known about the interaction of selectins or their ligands with glycolipids located within their native nanoenviroment in living cells, intriguing membrane model systems in which the alkyl moieties sLeX-derivatized lipids were fluorinated revealed that the clustering of these E-selectin ligands was disrupted thereby impeding cell tethering and rolling.57 It is intriguing to speculate that incorporation of fluorinated moieties into the glycan portion of selectin binding partners can similarly impact ligand clustering and thereby contribute to the aforementioned reduced adhesion observed in cells treated with fluorinated ManNAc23, 24 or GalNAc37 analogs.
Global cell scale mechanisms
Moving beyond the nanoscale (e.g., the “glycosynapse” discussed above), glycosylation can affect the overall biophysical properties of the cell membrane via the “galectin lattice” 58, 59 in ways that have an impact on cell adhesion involved in cancer60 and many other biological processes.61 To our knowledge the impact on selectin adhesion has not been tested, but considering that the receptors must be localized at the tips of the microvilli, altering the galactin lattice could stabilize them in position, enhancing adhesion, or it could slow them from localizing properly, thereby impeding adhesion. The relevance of MOE to the galectin lattice has been established by studies that detail how the activities of MGAT4/5 (the glycosyltransferases responsible for highly branched N-glycans required for lattice formation) require very high intracellular levels of UDP-GlcNAc (i.e., 10 mM or higher). In a manner reminiscent of sialic acid-based MOE outlined in Figure 1, but with a completely natural monosaccharide, galectin lattice formation can be augmented with exogenously-supplied GlcNAc that increases flux through the hexosamine biosynthetic pathway and increases UDP-GlcNAc levels.62 Alternately, 4-F-GlcNAc, which decreases UDP-GlcNAc levels as discussed above, would be expected to diminish the galectin lattice. Finally, it is noteworthy that sialic acid can play a yin-yang role in galectin lattice formation;63 specifically, by masking the penultimate galactose/GalNAc residues lattice formation is weakened thereby reversing the effects of MGAT4/5 and increased flux through the HBP.64 Consequently, the galectin lattice can be dampened by using ManNAc analogs that enhance sialic acid production in contrast to strengthening it via GlcNAc; in summary MOE promises exquisite control over this determinant of membrane properties.
Non-glycan based mechanisms
It has long been known that simple sugars can be signaling molecules in the context of the lac operon in bacteria. In mammals, this concept has been slower to be solidified, but recently analogs of the type used in MOE (e.g., ManNAc itself, as well as ManNProp, cpd vii) now have been shown to alter gene expression.65 Short chain fatty acid (SCFA)-modified analogs also have the ability to act as signaling molecules and alter gene expression, in part through the HDACi properties of hydrolyzed SCFA 66 but also through “whole molecule” effects that suppress NF-κB activity.67–69 Again, the impact of these mechanisms on leukocyte extravasation remains unknown, but to fully elucidate the mechanism by which MOE impacts selectin adhesion they will need to be addressed.
Technical obstacles and practical matters
This monograph, by providing a brief overview of “unknowns” inherent in MOE-based efforts to manipulate selectin-based adhesion was not able to fully explore many practical issues. For example, one important issue not addressed in adequate detail is the exact metabolic intermediate at which to intercept a biosynthetic pathway. For example, as we discuss elsewhere in detail,70 flux into the sialic acid pathway potentially can be augmented with GlcNAc, ManNAc, Neu5Ac, and CMP-Neu5Ac analogs. Although most extant MOE experiments now utilize ManNAc analogs,10 fluorinated Neu5Ac analogs are more robustly displayed on the cell surface23 possibly by overcoming the bottleneck comprised of sialic acid synthase.71, 72 In a similar technical vein, it has been long known that analog incorporation is highly cell type dependent both in cell culture73 and in vivo;74 these considerations exquisitely apply to E-selectin ligands, which were suppressed by 4-F-GlcNAc on T cells but not on natural killer (NK) cells.75 Clearly, future opportunities to exploit MOE to modulate selectin based adhesion in a meaningful way will require not only a fundamental understanding of the mechanisms in play, but also more mundane, but equally important technical issues such as analog design and cell specificity.
Summary and concluding comments
In pioneering experiments, MOE has successfully modulated selectin-mediated adhesion by employing ManNAc and Neu5Ac analogs with fluorinated N-acyl alkyl groups. These compounds presumably gained efficacy by replacing natural selectin ligands with ineffective counterparts because the overall level of sialic acids on the cell surface was not reduced by analog treatment. By contrast, fluorinated GalNAc and GlcNAc analogs appear to primarily function as inhibitors of certain enzymes involved in glycosylation and thus gain effectiveness primarily by reducing the number of selectin ligands on treated cells. As outlined in this article, the precise mechanisms through which monosaccharide analogs modulate selectin-mediated adhesion remain to be elucidated, but once they are, many opportunities will exist to further exploit this technology. For example, additional chemical modifications to the N-acyl group of analogs that target the sialic acid pathway (as shown in Fig. 1) may be more effective than currently tested compounds. Alternately, as-of-yet unexplored glycosylation pathways may be lucrative; for example metabolic replacement of fucose14 (which is a critical part of the sLeX epitope for selectin binding, Fig. 2D) may prove to be an ideal way to exploit MOE to control leukocyte extravasation.
Acknowledgments
Funding for the authors was provided by the NCI grants R01CA112314 (E.T. and M.P.M.) and R01CA101135 (R.T.A) and the NIBIB grant R01EB005692 and NCI grant 1U54CA143868 (K.J.Y).
References
- 1.Gross HJ, Brossmer R. Enzymatic introduction of a fluorescent sialic acid into oligosaccharide chains of glycoproteins. Eur J Biochem. 1988;177(3):583–589. doi: 10.1111/j.1432-1033.1988.tb14410.x. [DOI] [PubMed] [Google Scholar]
- 2.Gross HJ, Rose U, Krause JM, Paulson JC, Schmid K, Feeny RE, Brossmer R. Transfer of synthetic sialic acid analogues to N- and O-linked glycoprotein glycans using four different mammalian sialyltransferases. Biochemistry. 1989;28(18):7386–7392. doi: 10.1021/bi00444a036. [DOI] [PubMed] [Google Scholar]
- 3.Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem. 1992;267(24):16934–16938. [PubMed] [Google Scholar]
- 4.Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276(5315):1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 5.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287(5460):2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 6.Hsu TL, Hanson SR, Kishikawa K, Wang SK, Sawa M, Wong CH. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc Natl Acad Sci USA. 2007;104(8):2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampathkumar SG, Li AV, Jones MB, Sun Z, Yarema KJ. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat Chem Biol. 2006;2(3):149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Kohler JJ. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J Am Chem Soc. 2008;130(11):3278–3279. doi: 10.1021/ja7109772. [DOI] [PubMed] [Google Scholar]
- 9.Han S, Collins BE, Bengtson P, Paulson JC. Homo-multimeric complexes of CD22 revealed by in situ photoaffinity protein-glycan crosslinking. Nat Chem Biol. 2005;1(2):93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Meledeo MA, Wang Z, Khanna HS, Paruchuri VDP, Yarema KJ. Metabolic glycoengineering: sialic acid and beyond. Glycobiology. 2009;19(12):1382–1401. doi: 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemieux GA, Yarema KJ, Jacobs CL, Bertozzi CR. Exploiting differences in sialoside expression for selective targeting of MRI contrast reagents. J Am Chem Soc. 1999;121(17):4278–4279. [Google Scholar]
- 12.Kim EJ, Sampathkumar SG, Jones MB, Rhee JK, Baskaran G, Yarema KJ. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acetylmannosamine (ManNAc) analogs in Jurkat (human T-lymphoma-derived) cells. J Biol Chem. 2004;279(18):18342–18352. doi: 10.1074/jbc.M400205200. [DOI] [PubMed] [Google Scholar]
- 13.Aich U, Campbell CT, Elmouelhi N, Weier CA, Sampathkumar SG, Choi SS, Yarema KJ. Regioisomeric SCFA attachment to hexosamines separates metabolic flux from cytotoxicity and MUC1 suppression. ACS Chem Biol. 2008;3(4):230–240. doi: 10.1021/cb7002708. [DOI] [PubMed] [Google Scholar]
- 14.Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA. 2006;103(33):12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dube DH, Prescher JA, Quang CN, Bertozzi CR. Probing mucin-type O-linked glycosylation in living animals. Proc Natl Acad Sci USA. 2006;103(13):4819–4824. doi: 10.1073/pnas.0506855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, Bertozzi CR. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci USA. 2003;100(16):9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Biochemical engineering of the N-acyl side chain of sialic acid: biological implications. Glycobiology. 2001;11(2):11R–18R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C, Stehling P, Schnitzer J, Reutter W, Horstkorte R. Biochemical engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J Biol Chem. 1998;273(30):19146–19152. doi: 10.1074/jbc.273.30.19146. [DOI] [PubMed] [Google Scholar]
- 19.Nauman DA, Bertozzi CR. Kinetic parameters for small-molecule drug delivery by covalent cell surface targeting. Biochim Biophys Acta. 2001;1568(2):147–154. doi: 10.1016/s0304-4165(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 20.Chefalo P, Pan Y, Nagy N, Guo Z, Harding CV. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine. Biochemistry. 2006;45(11):3733–3739. doi: 10.1021/bi052161r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell CT, Sampathkumar SG, Weier C, Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Mol Biosyst. 2007;3(3):187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- 22.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11(11):1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dafik L, d’Alarcao M, Kumar K. Fluorination of mammalian cell surfaces via the sialic acid biosynthetic pathway. Bioorg Med Chem Lett. 2008;18(22):5945–5947. doi: 10.1016/j.bmcl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dafik L, d’Alarcao M, Kumar K. Modulation of cellular adhesion by glycoengineering. J Med Chem. 2010;53(10):4277–4284. doi: 10.1021/jm100374g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descheny L, Gainers ME, Walcheck B, Dimitroff CJ. Ameliorating skin-homing receptors on malignant T cells with a fluorosugar analog of N-acetylglucosamine: P-selectin ligand is a more sensitive target than E-selectin ligand. J Investig Dermatol. 2006;126(9):2065–2073. doi: 10.1038/sj.jid.5700364. [DOI] [PubMed] [Google Scholar]
- 26.Oetke C, Brossmer R, Mantey LR, Hinderlich S, Isecke R, Reutter W, Keppler OT, Pawlita M. Versatile biosynthetic engineering of sialic acid in living cells using synthetic sialic acid analogues. J Biol Chem. 2002;277(8):6688–6695. doi: 10.1074/jbc.M109973200. [DOI] [PubMed] [Google Scholar]
- 27.Yarema KJ, Bertozzi CR. Chemical approaches to glycobiology and emerging carbohydrate-based therapeutic agents. Curr Opin Chem Biol. 1998;2(1):49–61. doi: 10.1016/s1367-5931(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 28.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to sLeX and PSGL-1. Cell. 2000;103(3):467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 29.Ishada T. Computational modeling of carbohydrate-recognition process in E-selectin complex: Structural mapping of sialyl Lewis X onto Ab Initio QM/MM free energy surface. J Phys Chem B. 2010;114(11):3950–3964. doi: 10.1021/jp905872t. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz EL, Hadfield AF, Brown AE, Sartorelli AC. Modification of sialic acid metabolism of murine erythroleukemia cells by analogs of N-acetylmannosamine. Biochim Biophys Acta. 1983;762(4):489–497. doi: 10.1016/0167-4889(83)90051-4. [DOI] [PubMed] [Google Scholar]
- 31.Hadfield AF, Mella SL, Sartorelli AC. N-acetyl-D-mannosamine analogues as potential inhibitors of sialic acid biosynthesis. J Pharm Sci. 1983;72(7):748–751. doi: 10.1002/jps.2600720709. [DOI] [PubMed] [Google Scholar]
- 32.Barthel SR, Antonopoulos A, Cedeno-Laurent F, Schaffer L, Hernandez G, Patil SA, North SJ, Dell A, Matta KL, Neelamegham S, Haslam SM, Dimitroff CJ. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J Biol Chem. 2011;286(24):21717–21731. doi: 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachara NE, Hart GW. The emerging significance of O-GlcNAc in cellular regulation. Chem Rev. 2002;102(2):431–438. doi: 10.1021/cr000406u. [DOI] [PubMed] [Google Scholar]
- 34.Nigro J, Wang A, Mukhopadhyay D, Lauer M, Midura RJ, Sackstein R, Hascall VC. Regulation of heparan sulfate and chondroitin sulfate glycosaminoglycan biosynthesis by 4-fluoro-glucosamine in murine airway smooth muscle cells. J Biol Chem. 2009;284(25):16832–16839. doi: 10.1074/jbc.M109.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, Konstantopoulos K. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J. 2006;20(2):337–339. doi: 10.1096/fj.05-4574fje. [DOI] [PubMed] [Google Scholar]
- 36.Alon R, Rosen S. Rolling on N-linked glycans: a new way to present L-selectin binding sites. Nat Immunol. 2007;8(4):339–341. doi: 10.1038/ni0407-339. [DOI] [PubMed] [Google Scholar]
- 37.Marathe DD, Buffone A, Jr, Chandrasekaran EV, Xue J, Locke RD, Nasirikenari M, Lau JTY, Matta KL, Neelamegham S. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood. 2010;115(6):1303–1312. doi: 10.1182/blood-2009-07-231480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchansky SJ, Yarema KJ, Takahashi S, Bertozzi CR. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J Biol Chem. 2003;278(10):8036–8042. doi: 10.1074/jbc.M212127200. [DOI] [PubMed] [Google Scholar]
- 39.Bakowsky U, Schumacher G, Gege C, Schmidt RR, Rothe U, Bendas G. Cooperation between lateral ligand mobility and accessibility for receptor recognition in selectin-induced cell rolling. Biochemistry. 2002;41(14):4704–4712. doi: 10.1021/bi0117596. [DOI] [PubMed] [Google Scholar]
- 40.Puri KD, Chen S, Springer TA. Modifying the mechanical property of and shear threshold of L-selectin adhesion independently of equilibrium properties. Nature. 1998;392(6679):930–933. doi: 10.1038/31954. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Bogorin DF, Moy VT. Molecular basis of the dynamic strength of the sialyl Lewis X--selectin interaction. ChemPhysChem. 2004;5(2):175–182. doi: 10.1002/cphc.200300813. [DOI] [PubMed] [Google Scholar]
- 42.Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: an update. J Biol Regul Homeost Agents. 2001;15(1):1–13. [PubMed] [Google Scholar]
- 43.Carlow DA, Kaus G, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230(1):75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 44.Fang Y, Wu J, McEver RP, Zhu C. Bending rigidities of cell surface molecules P-selectin and PSGL-1. 2009;42(3):303–307. doi: 10.1016/j.jbiomech.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 45.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneck E, Deme B, Gege C, Tanaka M. Membrane adhesion via homophilic saccharide-saccharide interactions investigated by neutron scattering. Biophys J. 2011;100(9):2151–2159. doi: 10.1016/j.bpj.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bucior I, Burger MM. Carbohydrate-carbohydrate interactions in cell recognition. Curr Opin Struct Biol. 2004;14(5):631–637. doi: 10.1016/j.sbi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 48.de la Fuente JM, Penades S. Understanding carbohydrate-carbohydrate interactions by means of glyconanotechnology. Glycoconjug J. 2004;21(3–4):149–163. doi: 10.1023/B:GLYC.0000044846.80014.cb. [DOI] [PubMed] [Google Scholar]
- 49.Klemm WR, Boyles R, Mathew J, Cherian L. Gangliosides, or sialic acid, antagonize ethanol intoxication. Life Sci. 1988;43(22):1837–1843. doi: 10.1016/0024-3205(88)90284-6. [DOI] [PubMed] [Google Scholar]
- 50.Klemm WR. Biological water and its role in the effects of alcohol. Alcohol. 1998;15(3):249–267. doi: 10.1016/s0741-8329(97)00130-4. [DOI] [PubMed] [Google Scholar]
- 51.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1108455108. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voynov V, Chennamsetty N, Kayser V, Helk B, Forrer K, Zhang H, Fritsch C, Heine H, Trout BL. Dynamic fluctuations of protein-carbohydrate interactions promote protein aggregation. PLoS ONE. 2009;4(12):e8425. doi: 10.1371/journal.pone.0008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hakomori S. Carbohydrate-to-carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconjug J. 2004;21(3–4):125–137. doi: 10.1023/B:GLYC.0000044844.95878.cf. [DOI] [PubMed] [Google Scholar]
- 54.Muthing J, Maurer U, Weber-Schurholz S. Glycosphingolipids of skeletal muscle: II. Modulation of Ca2+-flux in triad membranes by gangliosides. Carbohydr Res. 1998;307(1–2):147–157. doi: 10.1016/s0008-6215(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Tsui Z, Yang F. Antagonistic effect of ganglioside GM1 and GM3 on the activity and conformation of sarcoplasmic reticulum Ca 2+-ATPase. FEBS Lett. 1999;457(1):144–148. doi: 10.1016/s0014-5793(99)01024-8. [DOI] [PubMed] [Google Scholar]
- 56.Hakomori SI. The glycosynapse. Proc Natl Acad Sci USA. 2002;99(1):225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schumacher G, Bakowsky U, Gege C, Schmidt RR, Rothe U, Bendas G. Lessons learned from clustering of fluorinated glycolipids on selectin ligand function in cell rolling. Biochemistry. 2006;45(9):2894–2903. doi: 10.1021/bi052201r. [DOI] [PubMed] [Google Scholar]
- 58.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12(5):616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 59.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409(6821):733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 60.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18(10):750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 61.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23(4):383–392. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Dennis JW, I, Nabi R, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139(7):1229–1241. doi: 10.1016/j.cell.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278(9):7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 64.Cha S-K, Ortega B, Kurosu H, Rosenblatt KP, Kuro-o M, Huang C-L. Removal of sialic acid involving Klotho causescell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105(28):9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kontou M, Bauer C, Reutter W, Horstkorte R. Sialic acid metabolism is involved in the regulation of gene expression during neuronal differentiation of PC12 cells. Glycoconjug J. 2008;25(3):237–244. doi: 10.1007/s10719-008-9104-1. [DOI] [PubMed] [Google Scholar]
- 66.Sampathkumar SG, Jones MB, Meledeo MA, Campbell CT, Choi SS, Hida K, Gomutputra P, Sheh A, Gilmartin T, Head SR, Yarema KJ. Targeting glycosylation pathways and the cell cycle: sugar- dependent activity of butyrate-carbohydrate cancer prodrugs. Chem Biol. 2006;13(12):1265–1275. doi: 10.1016/j.chembiol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Elmouelhi N, Aich U, Paruchuri VDP, Meledeo MA, Campbell CT, Wang JJ, Srinivas R, Khanna HS, Yarema KJ. Hexosamine template. A platform for modulating gene expression and for sugar-based drug discovery. J Med Chem. 2009;52(8):2515–2530. doi: 10.1021/jm801661m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campbell CT, Aich U, Weier CA, Wang JJ, Choi SS, Wen MM, Maisel K, Sampathkumar SG, Yarema KJ. Targeting pro-invasive oncogenes with short chain fatty acid-hexosamine analogues inhibits the mobility of metastatic MDA-MB-231 breast cancer cells. J Med Chem. 2008;51(24):8135–8147. doi: 10.1021/jm800873k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Du J, Che PL, Meledeo MA, Yarema KJ. Hexosamine analogs: from metabolic glycoengineering to drug discovery. Curr Opin Chem Biol. 2009;13(5–6):565–572. doi: 10.1016/j.cbpa.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aich U, Yarema KJ. Non-natural sugar analogues: Chemical probes for metabolic oligosaccharide engineering. In: Fraser-Reid BO, Tatsuta K, Thiem J, editors. Glycoscience. Springer-Verlag; Berlin Heidelberg: 2008. pp. 2133–2190. [Google Scholar]
- 71.Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]
- 72.Viswanathan K, Lawrence S, Hinderlich S, Yarema KJ, Lee YC, Betenbaugh M. Engineering sialic acid synthetic ability into insect cells: Identifying metabolic bottlenecks and devising strategies to overcome them. Biochemistry. 2003;42(51):15215–15225. doi: 10.1021/bi034994s. [DOI] [PubMed] [Google Scholar]
- 73.Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J Biol Chem. 1998;273(47):31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- 74.Gagiannis D, Gossrau R, Reutter W, Zimmermann-Kordmann M, Horstkorte R. Engineering the sialic acid in organs of mice using N-propanoylmannosamine. Biochim Biophys Acta. 2007;1770(2):297–306. doi: 10.1016/j.bbagen.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 75.Gainers ME, Descheny L, Barthel SR, Liu L, Wurbel MA, Dimitroff CJ. Skin-homing receptors on effector leukocytes are differentially sensitive to glyco-metabolic antagonism in allergic contact dermatitis. J Immunol. 2007;179(12):8509–8518. doi: 10.4049/jimmunol.179.12.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barthel SR, Gavino JD, Wiese GK, Jaynes JM, Siddiqui J, Dimitroff CJ. Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology. 2008;18(10):807–816. doi: 10.1093/glycob/cwn070. [DOI] [PMC free article] [PubMed] [Google Scholar]