Abstract
Background
Complex regional pain syndrome patients have increased tryptase in the skin of the affected extremity indicating mast cell (MC) accumulation and degranulation, processes known to be mediated by substance P (SP). The dysregulation of SP release from primary afferent neurons is characteristic of complex regional pain syndrome. We hypothesized that SP acting through the NK1 receptor results in mast cell accumulation, degranulation and nociceptive sensitization in a rat model of complex regional pain syndrome.
Methods
Groups of 6 to 10 rats underwent tibia fracture and hindlimb casting for 4 weeks, and the hindpaw skin was harvested for histological and immunohistochemical analysis. The effects of a selective NK1 receptor antagonist (LY303870) and of direct SP intraplantar injection were measured. Dermal MC degranulation induced by sciatic nerve stimulation and the effects of LY303870 on this process were investigated. Finally, the antinociceptive effects of acute and chronic treatment with a MC degranulator (48/80) were tested.
Results
We observed that 1) fracture caused MC accumulation, activation, and degranulation which were inhibited by LY303870, 2) the percentage of MCs in close proximity to peptidergic nerve fibers increased after fracture, 3) electrical stimulation caused MC activation and degranulation, which was blocked by LY303870, 4) intraplantar SP-induced MC degranulation, and 5) acute administration of 48/80 caused MC degranulation and enhanced postfracture nociception, but MCs depleted animals showed less sensitization.
Conclusions
These results indicate that facilitated peptidergic neuron-MC signaling after fracture can cause MC accumulation, activation and degranulation in the injured limb resulting in nociceptive sensitization.
Introduction
Complex regional pain syndrome (CRPS) is a painful, chronic and often disabling condition affecting the extremities. Type I CRPS, which does not involve primary nerve injury, is a frequent sequelae of distal tibia1 and radius fractures2. Recently, we developed a CRPS model in rats involving tibial fracture and cast immobilization that exhibits chronic unilateral hindlimb warmth, edema, facilitated spontaneous protein extravasation, allodynia, unweighting, and periarticular osteoporosis3. This constellation of post-fracture changes closely resembles the clinical presentation of acute or “warm” CRPS4. In this model, we observed the up-regulation of inflammatory cytokines such as interleukin-1β, interleukin-6, tumor necrosis factor-alpha (TNFα) and nerve growth factor in the epidermal keratinocytes of hindpaw skin, and we demonstrated that inhibition of cytokine and nerve growth factor signaling during cast immobilization prevents allodynia and attenuates unweighting5–10. We also demonstrated that the neuropeptide substance P (SP) can initiate an interleukin-1β response in skin acting through neurokinin-1 (NK1) receptors which are themselves up-regulated in skin after fracture and immobilization6,11. While epidermal keratinocytes have received the most attention in investigations involving neuro-cutaneous signaling, they are not the only cells in skin expressing NK1 receptors, or the only cells capable of producing nociceptive mediators.
Cutaneous mast cells are a type of innate immune cell that resides in the dermis. They are characterized by abundant secretory granules that contain numerous preformed inflammatory mediators. They are intimately associated with cutaneous sensory nerves which can control degranulation12–15. When activated during tissue injury, mast cells are capable of releasing histamine along with various inflammatory mediators including cytokines, prostaglandin D2, proteases and other substances into the skin16 that promote plasma protein leakage, vasodilation and pain, characteristic of neurogenic inflammation. Making matters more complex, histamine has been shown to act through H1, H3 and H4 receptors in skin to cause pain and nociceptive sensitization in various models17–19. Mast cells are also major cellular participants in the development of chronic inflammatory skin diseases such as psoriasis, atopic dermatitis and palmoplantar pustulosis14,16,20–21. The morphological contacts between neurofilament-positive sensory nerves and tryptase-positive mast cells are more numerous in these skin diseases than in normal skin14,20–22, suggesting that the interaction between sensory nerves and mast cells plays a role in these diseases' pathogenesis.
It has been shown that CRPS patients have increased tryptase in the skin of the affected extremity indicating increased mast cell activation and degranulation23, and it is well known that cutaneous mast cells express NK1 receptor24. Based on these data and our observations concerning the increase of SP and NK1 protein in the injured hindlimb after fracture, we hypothesized that mast cell inward migration, activation and degranulation may occur upon release of SP in the rat tibia fracture model of CRPS, and that NK1 mediated mast cell degranulation can cause nociceptive sensitization. The demonstration of such a pathway would be novel to our understanding of the pathogenesis of CRPS, and would further support the role of neurocutaneous signaling in this condition.
2. Materials and methods
These experiments were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, California) and followed the animal subjects guidelines of the International Association for the Study of Pain25. Adult (9-month-old) male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA) were used in all experiments. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding and were given food and water ad libitum. During the experimental period the animals were fed Lab Diet 5012 (PMI Nutrition Institute, Richmond, IN), which contains 1.0% calcium, 0.5% phosphorus, and 3.3 IU/g of vitamin D3, and were kept under standard conditions with a 12-h light-dark cycle.
2.1 Surgery
Tibia fracture was performed under 2–4% isoflurane to maintain surgical anesthesia as we have previously described3. The right hindlimb was wrapped in stockinet (2.5 cm wide) and the distal tibia was fractured using pliers with an adjustable stop (Visegrip, Petersen Manufacturing, Dewitt, NE) that had been modified with a 3-point jaw. The hindlimb was wrapped in casting tape (Delta-Lite, Johnson & Johnson, New Brunswick, NJ) so the hip, knee and ankle were flexed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. The cast over the paw was applied only to the plantar surface; a window was left open over the dorsum of the paw and ankle to prevent constriction when postfracture edema developed. To prevent the animals from chewing at their casts, the cast material was wrapped in galvanized wire mesh. The rats were given subcutaneous saline and buprenorphine immediately after procedure (0.03 mg/kg) and on the next day after fracture for postoperative hydration and analgesia. At 4 weeks the rats were anesthetized with isoflurane and the cast removed with a vibrating cast saw. All rats used in this study had union at the fracture site after 4 weeks of casting.
2.2 Histological analysis of mast cell number and degranulation
Animals were euthanized and perfused with 4% paraformaldehyde in phosphate buffered saline (PBS), pH 7.4, via the ascending aorta; the dorsal hindpaw skin including subdermal layers was removed and postfixed in 4% paraformaldehyde for 2 h, then the tissues were treated with 30% sucrose in PBS at 4°C before embedding in optimum cutting temperature embedding compound (Sakura Finetek USA, Torrance, CA). Following embedding, 10-μm thick slices were made using a cryostat, mounted onto Superfrost microscope slides (Fisher Scientific, USA, Pittsburgh, PA), and stored at −70°C until use in histological analysis and immunofluorescence confocal microscopy.
For histological analysis of dermal mast cells, the sections were stained with toluidine blue and eosin26. After staining, randomly selected sections were examined under light microscopy. Based upon the degree of degranulation and metachromasia, it is possible to identify 3 types of mast cells27, each representing a different stage in mast cell degranulation: (1) intact mast cells show a deep blue (orthochromatic) staining; (2) cells from which some granules have been extruded, but where the cell outline is largely intact and in which the granules demonstrate purple-red metachromasia; and (3) cells in which degranulation is more extensive and widespread in which the granules show metachromasia. A mast cell was considered to be degranulating if one or more extruded granules were visible adjacent to the cell. Since there is an element of subjectivity in distinguishing between types 2 and 3, the mast cells were divided into only 2 types, the intact and degranulating, encompassing types 2 and 3. For each skin sample, the total, intact and degranulating mast cell numbers per 10 high power fields (magnification × 400) were counted in the upper dermis. Each high power field had an area of 10,000 um2 and the total area examined per specimen was 100,000 um2.
2.3. Immunofluorescence confocal microscopy
Selective mast cell markers and immunofluorescence confocal microscopy were used to determine dermal mast cell activation and NK1 receptor protein expression in mast cells. To assess mast cell proximity to cutaneous sensory nerves in the rat CRPS model, sections of hindpaw skin were immunolabeled for a mast cell marker and costained for either SP or calcitonin gene-related peptide (CGRP). Briefly, frozen sections were permeabilized and blocked with PBS containing 10% donkey serum and 0.3% Triton X-100 prior to primary antibody incubation. Sections were incubated with primary antibody diluted in PBS containing 2% serum at 4° C overnight. After washing in PBS, the sections were incubated with fluorophore-conjugated secondary antibody. For double labeling experiments, primary antibody from a different species against the second antigen was applied to the sections and visualized using an alternative fluorophore-conjugated secondary antibody. After three washes, the sections were mounted with anti-fade mounting medium (Invitrogen, Eugene, OR). Images were visualized using a confocal microscope (Zeiss LSM510 Upright 2 photon; Carl Zeiss, Germany). The primary antibodies used were rabbit anti-rat lysosome-associated membrane glycoprotein 1 (LAMP1), 1:1000 (LifeSpan Biosciences, Seattle, WA), fluorescein isothiocyanate -labeled rabbit anti-rat mast cell, 1:400 (Cedarlane Laboratories, Burlington, NC), rabbit anti-rat NK1 receptor, 1:8000 (Sigma-Aldrich, St Louis, MO), monoclonal mouse anti-rat keratin, 1:50 (clone AE1/AE3, Thermo Fisher Scientific, Fremont, CA), rabbit anti-rat SP, 1:6000 (Peninsula Laboratories Inc., San Carlos, CA), and rabbit anti-rat CGRP, 1:8000 (Sigma-Aldrich) were used. Double and triple labeling immunofluorescence was performed with donkey anti-mouse immunoglobulin G (1:500) conjugated with Dylight 549, donkey anti-rabbit immunoglobulin G (1:500) conjugated with Dylight 488, and donkey anti-rabbit immunoglobulin G (1:500) conjugated with Dylight 649 secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Control experiments included incubation of slices in primary and secondary antibody-free solutions both of which led to low intensity nonspecific staining patterns in preliminary experiments (data not shown). Quantitative studies were based on four or more replicates. Mast cells were counted per high-power field (400×) in the dermis of fracture and control rats. The numbers of mast cells in close proximity to nerve fibers (defined as ≤2 μm) were counted separately. All slides were coded and counted under blinded conditions.
2.4 Drug treatments
To test the hypothesis that SP signaling might regulate mast cell activity in the CRPS model, fracture rats were treated with either an NK-1 receptor antagonist LY303870 (Eli Lilly Co., Indianapolis, IN) at a dose of 20 mg/kg/day intraperitoneally or saline for 8 days before euthanization. The dose was chosen on the basis of our previous studies28. We also tested the local effects of intraplantar SP injection in control rats on mast cell degranulation. The intact animals were treated with either SP (Sigma-Aldrich) at a dose of 25μg/50μl intraplantar or with saline. The dose was chosen on the basis of preliminary studies. The animals were euthanized and dorsal hindpaw skin was harvested at 1, 3, and 6 h postinjection for histological analysis of mast cell degranulation as mentioned in section 2.2.
To test the hypothesis that mast cells mediate the nociceptive and vascular changes observed after tibia fracture in rats, a mast cell degranulator, secretagogue compound 48/80 (Sigma-Aldrich,) was administered by intraperitoneally For acute experiments, 400μg 48/80 in sterile saline vehicle (0.9% NaCl) or vehicle alone was administered. Second, we tested the effects of 48/80 mediated mast sell depletion on nociceptive and vascular changes using escalating doses of 48/80: 50 μg (intraperitoneally) on the first day, 120 μg on the second day, 250 μg on the third day, and two injections of 400 μg on the fourth day (a total of 1.22 mg), chosen on the basis of our preliminary studies and the reports of others29–30. Control rats received vehicle alone. Additional animals were treated with 48/80 or saline prior to immunohistochemical analysis.
To assess if histamine supported warmth, edema, allodynia and unweighting in the CRPS model, fracture rats were treated with either a highly selective H1 histamine receptor blocker, cetirizine (Sigma-Aldrich) at a dose of 5 mg/kg intraperitoneally or saline vehicle.
2.5. Electrical stimulation of sciatic nerve and the effects of LY 303870
We evaluated 1) if electrical stimulation (ES) of sciatic nerves could induce mast cell degranulation and nociceptive sensitization in hindlimb skin and 2) if ES caused mast cell degranulation is mediated by neuropeptide SP. Sixteen normal rats were divided into three cohorts: the first cohort of rats (n = 4) served as control was sham-operated (sciatic nerve exposure without ES). Second cohort (n = 6) was subjected to 30 min of sciatic nerve ES (5 Hz, 0.5 ms pulse duration, 10 mA) under isoflurane anesthesia as described previously11. The third cohort (n = 6) was treated with LY303870 (intraperitoneally, 30 mg – 40 mg/kg) 60 min prior to the ES. Nociceptive testing was performed at baseline and at 3 h after ES as described in Section 2.6. The animals were euthanized and perfused with 4% paraformaldehyde in PBS, pH 7.4, via the ascending aorta, at 3 h after ES chosen on the basis of preliminary studies; the dorsal hindpaw skin including subdermal layers was harvested to assess mast cell degranulation by histological analysis and immunofluorescence confocal microscopy as described in Section 2.2 and 2.3.
2.6. Hindpaw nociception
To measure mechanical allodynia in the rats, an up-down von Frey testing paradigm was used as we have previously described 3,31.
An incapacitance device (IITC Inc. Life Science, Woodland Hills, CA) was used to measure hindpaw unweighting. The rats were manually held in a vertical position over the apparatus with the hindpaws resting on separate metal scale plates and the entire weight of the rat was supported on the hindpaws. The duration of each measurement was 6 s and 10 consecutive measurements were taken at 60-s intervals. Eight readings (excluding the highest and lowest ones) were averaged to calculate the bilateral hindpaw weight bearing values3,31.
2.7. Hindpaw volume
A laser sensor technique was used to determine the dorsal-ventral thickness of the hindpaw, as we have previously described3,28,31.
2.8. Hindpaw temperature
For these experiments room temperature was maintained at 23°C and humidity ranged between 25% and 45%. The temperature of the hindpaw was measured using a fine wire thermocouple (Omega) applied to the paw skin, as previously described3,28,31.
2.9. Statistical analysis
Statistical analysis was performed using a two-way analysis of variance (ANOVA) followed by Bonferroni post hoc testing to compare the time course between the control cohort (fracture only) and the fracture rat cohorts that were injected with mast cell degranulator 48/80 or histamine receptor 1 antagonist cetirizine treated fracture rats. One-way ANOVA was employed followed by post hoc Newman-Keuls multiple-comparison testing to compare means of three samples. For simple comparisons of two means, unpaired Student's t-test was performed. All data are presented as the mean ± SE of the mean, and differences are considered significant at a p value less than 0.05 (Prism 5, GraphPad Software, San Diego, CA).
3. Results
3.1 Tibia fracture induces mast cell activation and degranulation in hindpaw skin
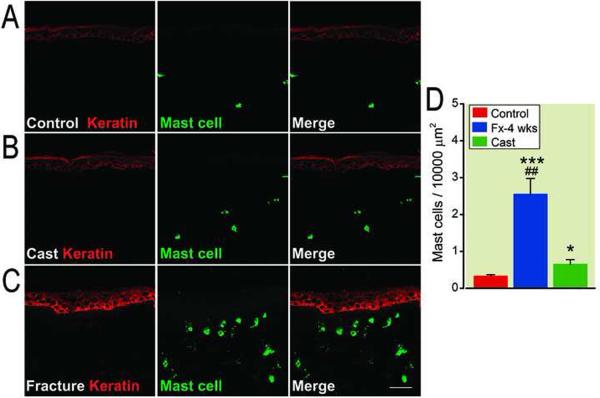
We first assessed mast cell numbers and degranulation in hindpaw skin harvested from cast immobilization, fracture with cast and control rats. Skin sections were incubated with an antibody directed against the rat keratin Pan-Ab1 antigen and co-stained with a selective mast cell marker, which recognizes both activated and inactivated mast cells. We observed only a few mast cells in the superficial dermis in control rats (fig. 1A), and a small increase in mast cell number and degranulation in the upper dermis in cast rats (fig. 1B), while the number of detectable mast cells and degranulation were dramatically increased in the upper dermis adjacent to basal membrane 4 weeks post-fracture (fig. 1C). Figure 1D illustrates a 7-, and 4-fold increase in positive mast cells in the skin of the fractured hindlimb compared with control and cast animals respectively, suggesting that the changes in mast cells is largely produced by the distal tibia fracture.
Figure 1.
Fluorescence photomicrographs of co-staining of mast cells and keratinocytes in hindpaw skin 4 weeks post-fracture. In skin from control rats only a few mast cells were present in the superficial dermis (A). The number of detectable mast cells and degranulation were slightly increased in the upper dermis at 4 weeks post-cast (B), while dramatically increased in the upper dermis adjacent to the epidermal basement membrane 4 weeks post-fracture (C). Scale bar = 50μm. D: Quantification of mast cell-positive cells in the upper-middle dermis. ***p < 0.001 for ipsilateral fracture (n = 9 rats) vs control (n = 5) values, *p < 0.05 for ipsilateral cast (n = 5 rats) vs control (n = 5) values, ## p < 0.01 for ipsialateral to fracture (n = 5) vs ipsilateral to cast (n = 5). Fx: fracture.
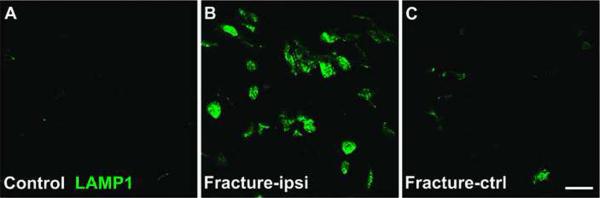
Next, mast cell activation in dermis of post-fracture rats was confirmed by immunostaining of lysosome-associated membrane glycoprotein 1 (LAMP-1, i.e., CD107a), a selective active mast cell marker32. No LAMP-1 positive cells were observed in normal hindpaw skin (fig. 2A), whereas at 4 weeks post-fracture large numbers of LAMP-1 immunoreactive cells were present in the dermis of the hindpaw ipsilateral to fracture (fig. 2B), but only a few LAMP-1 immunostained cells were observed in the contralateral hindpaw (fig. 2C), indicating that long-term activation of dermal mast cells was restricted to the injured limb.
Figure 2.
Fluorescence photomicrographs of immunostaining of lysosome-associated membrane glycoprotein (LAMP1), an activated mast cell marker in dermis of hindpaw skin 4 weeks post-fracture. In control skin, active mast cells are absent in the dermis (A), while the large numbers of active mast cells were observed in the ipsilateral hindpaw dermis at 4 weeks post-fracture (B). Scattered activated mast cell profiles were observed in the dermis of skin from paws contralateral to fracture (C). ipsi: ipsilateral, ctrl: contralateral. Scale bar =20 μm.
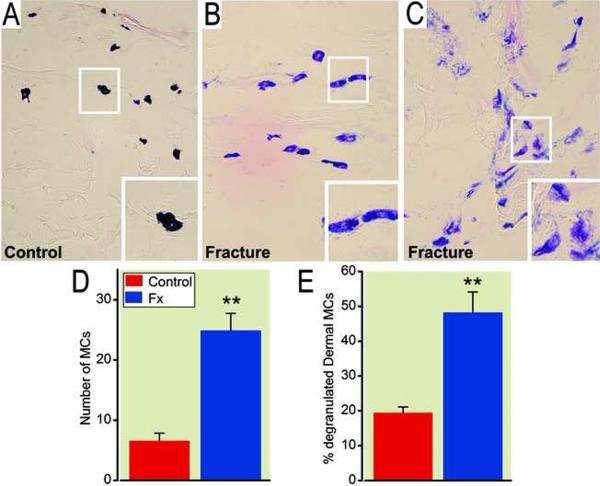
Finally, dermal mast cell degranulation after fracture was confirmed using histological analysis of the hindlimb sections with toluidine blue and eosin staining. Figure 3A shows representative light micrographs of histological staining for mast cells from control rats and from the skin of hindpaws ipsilateral to fracture at 4 weeks postinjury. In normal rats, dermal mast cells were intact with dark blue staining (fig. 3A), while degranulating mast cells appearing purple-red were observed 4 weeks postfracture (fig. 3B and 3C). There was a 390% increase in mast cell number (fig. 3D) and 250% increase in the percentage of degranulated mast cells (fig. 3E) in the upper dermis of fractured hindlimb compared with controls. Degranulation was prominent in the region of the dermal-epidermal boundary.
Figure 3.
Photomicrographs of toluidine blue and eosin staining of mast cells in the dermis of hindpaw skin at 4 weeks post-fracture. Panel A illustrates that dermal mast cells are intact in control rats, showing a dark blue staining, while degranulating mast cells appearing purple-red are observed 4 weeks post-fracture (B and C). The degranulation can be divided into two types: slight discharge of granules (B) and extensive disruption and discharge of granules (C). The number of mast cells (D) and the percentage of degranulated mast cells (E) were dramatically increased in the dermis at 4 weeks post-fracture (n = 6 per cohort). ** p < 0.01, compared to control animals. MCs: Mast cells, FX: fracture.
3.2 SP signaling mediates the post-fracture increase in mast cell activation and degranulation in hindpaw skin
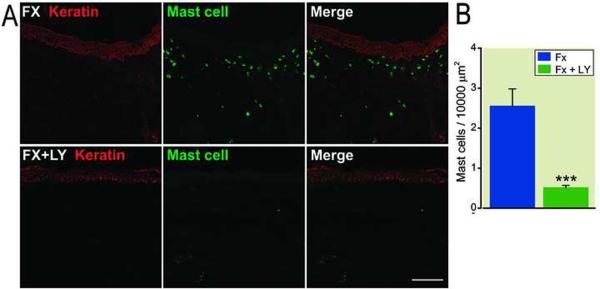
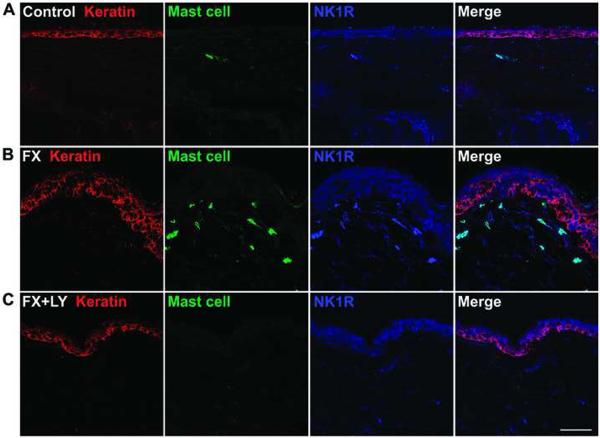
We next hypothesized that increased SP signaling after fracture causes mast cell activation and degranulation in the skin. To test the hypothesis, fracture rats were treated with either an NK-1 receptor antagonist (LY303870) or saline for 8 days prior to cast removal. Figure 4A lower panel shows representative confocal immunofluorescence microscopy results for mast cells in dorsal hindpaw skin from LY303870 treated fracture animals, and figure 4B presents quantification of mast cells in the hindpaw dermis. These results demonstrate that treatment after fracture with LY303870 completely reversed the fracture-induced increases in mast cell number.
Figure 4.
Post-fracture accumulation of mast cells (green) in the dermis of the hindpaw skin, and the effects of the Substance P (neurokinin-1) receptor antagonist. Keratin (red) is a marker for epidermal keratinocytes. Panel A: Systemic treatment with the neurokinin-1 receptor antagonist LY303870 for 8 days blocked fracture-induced increases in the dermal mast cells. Scale bar = 100μm. Panel B: Quantification of immunostained mast cells in the dermis of saline vehicle (n = 9) and LY303870-treated fracture rats (n = 6). *** p < 0.001, vs. saline treated. Fx: fracture, LY: LY303870.
We also looked at the change of NK1 receptor expression in hindpaw skin in untreated and in LY303870 treated fracture rats. Figure 5 demonstrates NK1 receptor expression in dermal mast cells and epidermal keratinocytes, with a clear increase in NK1 receptor expression in both types of cells at 4 weeks after fracture (fig. 5A and 5B), whereas LY 303870 treatment prevented these fracture-induced changes (fig. 5C). We previously had observed a similar increase in epidermal keratinocyte NK1 expression in the rat fracture model11.
Figure 5.
Fluorescence photomicrographs showing co-localization of keratinocytes (red), mast cells (green), and neurokinin-1 (NK-1) receptors (blue) in hindpaw skin 4 weeks post-fracture. Compared with control animals (A), the NK-1 receptor expression is upregulated in dermal mast cells at 4 weeks post-fracture (B). Treatment with LY303870 blocked the accumulation of mast cells and the up-regulation of NK-1 receptors (C). Scale bar = 50μm. Fx: fracture, LY: LY303870, NK1R: NK-1 receptors.
3.3 Tibia fracture induces close associations between mast cells and SP and CGRP positive nerves in hindpaw skin
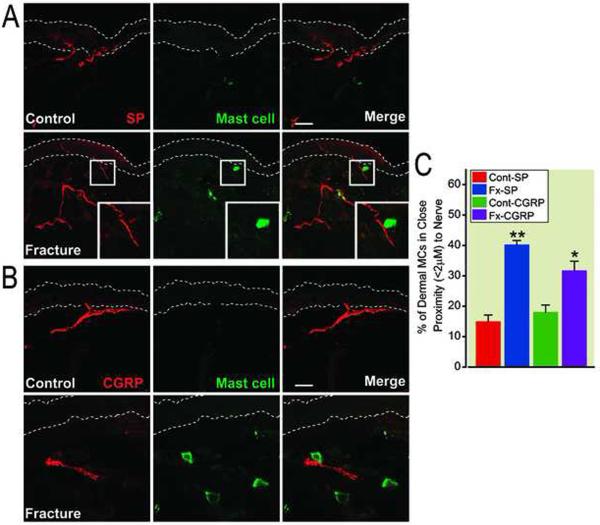
Functional peripheral neuron-mast cell communication in skin has been inferred based on increased nerve fiber-mast cell proximity in other disease models, and we examined fracture rat skin for evidence of such changes. Sections of hindpaw skin from control rats and 4 weeks post-fracture rats were stained with anti-SP, anti-CGRP and anti-mast cell antibodies, to identify peptidergic nerves and mast cells respectively. As showed in figure 6A and 6B, we observed close associations between mast cells and SP and CGRP positive nerves in hindpaw skin at 4 weeks postfracture. When expressed as the percentage of dermal mast cells that occurred within 2 μm of the nearest nerve fiber, we found that the percentage of mast cells in close proximity with SP and/or CGRP nerve fibers was significantly increased in the hindpaw skin at 4 weeks postfracture (fig. 6C).
Figure 6.
Close associations between neuropeptide expressing nerve fibers and mast cells in hindpaw dermis at 4 weeks post-fracture. Panel A: Immunohistochemical staining of substance P (SP) expressing nerve fibers (red) and mast cells (green) in hindpaw dermis from control and fracture rats. The dotted lines define the limits of the epidermis. Panel B: Immunohistochemical staining of calcitonin gene-related peptide (CGRP) expressing nerve fibers (red) and mast cells (green) in hindpaw dermis from control and fracture rats. Scale bars = 40 um (Panel A) and 20 um (Panel B). Panel C: The percentage of mast cells in close proximity (≤ 2 um) to SP and CGRP positive nerve fibers was significantly increased in hindpaw dermis at 4 weeks post-fracture. **p < 0.01 and *p < 0.05 for fracture vs. control values (n = 5 per cohort). FX: fracture, Cont: control.
3.4 Sciatic nerve electrical stimulation depletes mast cells, and this effect is mediated by substance P.
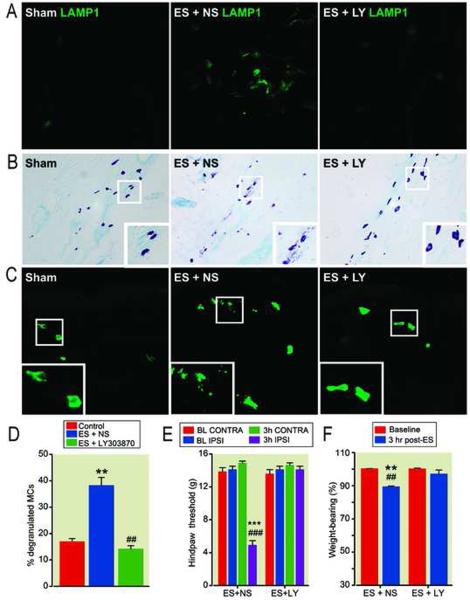
The findings in section 3.3 are suggestive of functional communication between neuropeptide containing nerve fibers and mast cells. To further evaluate this view, we investigated whether ES of sciatic nerve could induce mast cell activation, degranulation and nociceptive sensitization as well as whether an NK1 receptor antagonist (LY303870) could block any possible ES-induced effects. Our data showed that antidromic ES of sciatic nerve induced mast cell activation (fig. 7A), degranulation (fig. 7B by toluidine blue-eosine staining and fig. 7C by a mast cell marker) and nociceptive sensitization (figs. 7E and 7F). There was a 210% increase in the percentage of degranulated mast cells in the dermis at 3 hours after electrical stimulation compared with controls (fig. 7D). The antagonist LY 303870 blocked these changes (fig. 7).
Figure 7.
Electrical stimulation (ES) of the sciatic nerve on dermal mast cell activation, degranulation, nociceptive and vascular changes, and the effects of an NK1 receptor antagonist. A: lysosome-associated membrane glycoprotein (LAMP1) immunostaining (green) showing that ES caused mast cell activation, and treatment with LY303870 blocked this response. B: Toluidine blue-eosin staining showing that ES caused mast cell degranulation in the dermis of a rat hindpaw skin, and LY303870 treatment blocked the ES-induced response. C: ES-induced mast cell degranulation and the blocking effects of LY303870 were confirmed by immunohistochemical studies using a broad-spectrum mast cell marker (green). Scale bars = 50 um (A, C) and 100 um (B). D: The percentage of degranulated mast cells in the upper-middle dermis. ** p < 0.01 for ES (n = 6 rats) vs control (n = 5) values, ##p < 0.01 for LY303870 (n = 6) vs ES (n = 6). E and F: Effects of ES, LY303870 plus ES, on hindlimb hyperalgesia (E) and weight bearing (F) at 3 hours post-ES. ***p < 0.001 and ** p < 0.01 for ES (n = 6 rats) vs control (n = 6) values, ###p < 0.001 and ##p < 0.01 for ES (n = 6) vs ES+LY303870 (n = 6). MCs: mast cells, LY: LY303870, NS: normal saline. BL: baseline, CONTRA: contralateral to ES, IPSI: ipsilateral to ES.
3.5 Intraplantar SP induces mast cell activation and degranulation in intact rats
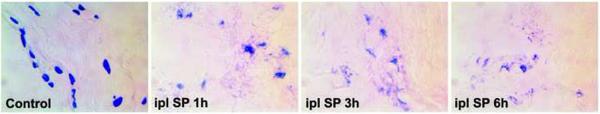
Next, we examined the local effects of intraplantar SP injection in control nonfractured rats on mast cell degranulation. As showed in figure 8, intraplantar SP injection induced extensive mast cell degranulation in the plantar hindpaw skin at 1, 3, and 6 h postinjection.
Figure 8.
Intraplantar Substance P (SP) injection induced extensive mast cell degranulation in the plantar hindpaw skin of rats at 1, 3, and 6 hours post-injection (n = 3 per cohort). Ipl: intraplantar injection.
3.6 Acute administration of a mast cell degranulator enhances fracture-induced nociceptive behavior
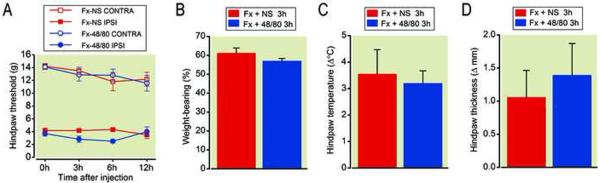
Since mast cell degranulation releases variety of inflammatory mediators such as histamine, cytokines, prostaglandin D2, and tryptase into the skin, and ES of sciatic nerve induces mast cell degraulation and hindlimb nociceptive changes, we hypothesized that mast cell degranulation can exacerbate nociceptive sensitization in the fracture CRPS model. To test this hypothesis, a mast cell degranulator and secretagogue compound 48/80 was administered systemically at 4 weeks postfracture. We observed that acute administration of 48/80 (400 ug/rat, intraperitoneally) caused additional mast cell degranulation (data not shown) and enhanced postfracture allodynia (fig. 9A), but had no additional effects on weight-bearing (fig. 9B) or warmth (fig. 9C) at 4 weeks postfracture compared with vehicle treated fracture rats. There was a trend towards elevation of hindpaw thickness at 3 h postinjection of 48/80 (fig. 9D). The acute administration of 48/80 had no effects on paws contralateral to fracture.
Figure 9.
Acute administration of the mast cell degranulating compound 48/80 (400 μg/rat once, i.p.) caused an exacerbation of hindpaw allodynia at 3 and 6 hours post-injection (A), but had no effect on hindpaw unweighting (B), warmth (C), or edema (D) in rats at 4 weeks post-fracture (n =10 per cohort). Unweighting measurements (B) represent weight bearing on the fractured hindlimb as a ration to 50% of the total bilateral hindlimb loading. Thus a percentage lowers than 100% represents hindpaw unweighting. Hindpaw temperature (C) and thickness (D) measurements represent the difference between the fracture side and the contralateral paw, thus a positive value represents an increase in temperature or thickness on the fracture side. #p < 0.05, ###p < 0.001 for 48/80 (n=10) vs. saline (n=8) treatment. FX: fracture, NS: normal saline, Contra: contralateral to fracture, Ipsi: ipsilateral to fracture.
3.7 Chronic administration of a mast cell degranulator attenuates fracture-induced nociceptive behavior
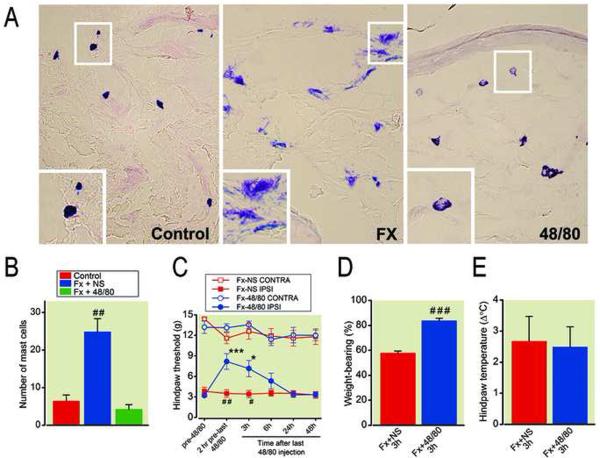
To further evaluate our hypothesis that mast cells mediate the nociceptive and vascular changes observed after tibia fracture in rats, ascending doses of compound 48/80 were administered systemically for 4 days before testing and skin harvest at 4 weeks postfracture. Figure 10 presents the effects of chronic 48/80 treatment (1.22 mg/rat, intraperitoneally total) on mast cell degranulation, nociceptive and vascular changes at 4 weeks postfracture. Fracture caused a 4-fold increase in mast cell numbers, and chronic 48/80 treatment reversed this increase (figs. 10A, 10B). Furthermore, chronic 48/80 treatment partially reversed the hindpaw allodynia and unweighting that developed in the fracture rats (figs. 10C, 10D), but no effects were observed on hindpaw warmth (fig. 10E). Collectively, these results strongly support our hypothesis that mast cells play a role in the nociceptive changes observed after tibia fracture.
Figure 10.
Chronic 48/80 treatment (1.22 mg/rat over 4 days, i.p.) reduced hindpaw mast cell accumulation, allodynia, and unweighting in rats at 4 weeks post-fracture. The histological profile of degranulation as well as the absolute number of mast cells were reduced in fracture rats after 4 days of compound 48/80 administration (A, B). Hindpaw allodynia (C) and unweighting (D) were attenuated were attenuated, but warmth (E) was not altered when mast cells were depleted after 4 days of 48/80 treatment in fracture rats. ## p < 0.01 for fracture saline treated (n=5) vs. intact control (n=5) rats (panel B); * p <0.05, *** p <0.001 for post-48/80 treatment (n=9) vs. pre-48/80 treatment (n=9), #p < 0.05, ##p < 0.01, and ###p < 0.001 for 48/80 (n=9) vs saline treated (n=7) (panel C); ###p < 0.001 for 48/80 (n=9) vs saline treated (n=7) (panel D). FX: fracture, NS: normal saline, Contra: contralateral to fracture, Ipsi: ipsilateral to fracture.
3.8 Cetirizine treatment failed to block fracture-induced nociceptive behavior
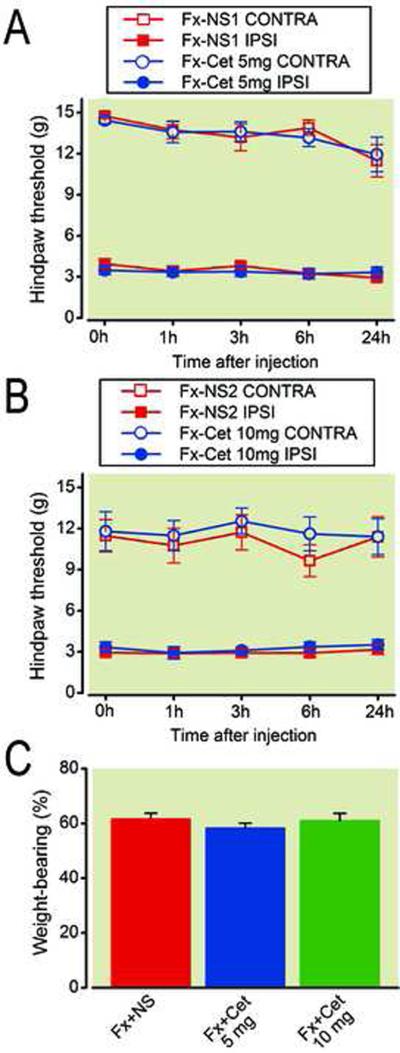
Histamine is a commonly studied preformed mediator in mast cell secretory granules which is released upon activation, e.g., by SP or an allergen, and it is able to induce nociceptive responses via histamine receptors, most notably of the H1 subtype33–37. We therefore tested if histamine acting through H1 receptors is required for the warmth, edema, allodynia and unweighting in the CRPS model. Fracture rats were treated with either a highly selective H1 histamine receptor blocker, cetirizine at a dose of 5 and 10 mg/kg intraperitoneally or saline. Unweighting, temperature, edema and mechanical thresholds were tested in the hindpaws at 1, 3, 6 and 24 h after cetirizine injection. Figure 11 demonstrates that acute administration of cetirizine failed to block nociceptive and vascular changes (data not shown) in the CRPS model.
Figure 11.
Effects of acute administration of cetirizine, a selective histamine H1 receptor blocker, on mast cell mediated nociceptive and vascular changes at 4 weeks post-fracture. Acute administration of 5 mg (A) and 10 mg (B) of cetirizine failed to block hindpaw allodynia or unweighting (C) in the CRPS model (n = 10 per cohort). NS: saline, Cet: cetirizine, Ipsi: ipsilateral to fracture, Contra: contralateral to fracture.
4. Discussion
CRPS patients have increased tryptase in the skin of the affected extremity, indicating increased mast cell accumulation and degranulation23. Mast cells release a number of inflammatory mediators such as histamine, cytokines, prostaglandin D2, and tryptase into the skin, and we hypothesized that mast cell degranulation contributes to nociceptive sensitization in a rat tibia fracture model of CRPS. Furthermore, after tibia fracture SP signaling in the injured hindlimb skin is up-regulated, and we observed previously that treating fracture rats with an NK1 receptor antagonist (LY303870) attenuates nociceptive sensitization in the hindpaw3. It is well known that mast cells express NK1 receptors and are capable of responding to SP24. We therefore pursued the hypothesis that increased SP signaling through NK1 receptors after fracture causes mast cell accumulation, activation and degranulation in the skin resulting in nociceptive sensitization. Finally we tested if H1 histamine receptors contribute to the nociceptive sensitization after tibia fracture.
In this study, using a polyclonal anti-mast cell antibody that recognizes both active and inactive mast cells, we showed that only a few mast cells were present in the superficial dermis of control unfractured rats, and only a small increase in mast cell number was observed in the upper dermis 4 weeks postcast in unfractured animals. However, the number of detectable mast cells was dramatically increased in the upper dermis in the combined fracture/cast animals which most faithfully replicate the signs of CRPS in humans3. Using LAMP-1, an activated mast cell marker32, we demonstrated that LAMP-1 positive cells were absent in normal skin, whereas at 4 weeks post-fracture there was a dramatic increase in LAMP-1 immunoreactive cells in the dermis, suggesting the activation of mast cells after fracture. Consistent with the LAMP-1 immunoreactivity were the direct histological observations of degranulation using toluidine blue-eosin staining. Collectively, these data indicate that tibia fracture leads to both the accumulation and, perhaps more importantly, the activation of degranulation in hindpaw skin. This finding is in agreement with the previous work of Huygen et al.23 in CRPS patients where enhanced tryptase levels were observed in the fluid from suction blisters made in the involved versus uninvolved CRPS extremity. Mast cell activation has also been detected in different painful conditions such as in wound healing38, nerve injury37, migraine headache39–40, interstitial cystitis41, chronic pancreatitis42–43, inflammatory arthritis44 and in chronic inflammatory skin diseases like psoriasis, atopic dermatitis and palmoplantar pustulosis14,16,20–21. The aforementioned studies provided evidence for a range of mediators being responsible for the mast cell activation, e.g., through endogenous proteins such as TNF, tryptase, complement component 5a, SP and vasoactive intestinal polypeptide as well as by histamine release.
One key question is how fracture can cause cutaneous mast cell accumulation, activation and degranulation. While leukocyte mediated inflammation is absent in CRPS affected skin and joints, facilitated neurogenic signaling and inflammation has been demonstrated in several studies45–47. Similarly, after tibia fracture and immobilization in rats, increased SP signaling and NK1 receptor expression are observed in sensory neurons and epidermal keratinocytes in the fractured limb. These changes lead to keratinocyte activation, proliferation and overexpression of pro-inflammatory mediators3,5,28. Neuroimmune signaling involving the peripheral nervous system and mast calls is also well documented. For example, mast cells in close proximity to neurites are known to form “synapse-like” structures involving the cell adhesion molecule N-cadherin48. Cutaneous mast cell degranulation has been shown to rely on the integrity of such synapse-like structures formed with peptidergic neurons; disruption of these N-cadherin containing structures by deletion of the gene coding for the membrane-type 5 matrix metalloproteinase alters mast cell degranulation and nociceptive sensitization49.
The close association of sensory nerves and mast cells is implicated in several inflammatory skin diseases12–14 and in wound healing38. We thus hypothesized that facilitated neuro-cutaneous signaling also contributes to the dermal mast cell activation and degranulation after fracture. Our data demonstrate that close associations between mast cells and neuropeptide nerve fibers were present in hindpaw skin at 4 weeks postfracture, and the percentage of mast cells in close proximity with SP and/or CGRP nerve fibers was significantly increased in the hindpaw skin after fracture. These findings are suggestive of functional communication between peptidergic neuron and mast cells, to support this view, we first detected that mast cells present in skin after fracture express NK1 receptors, and that an NK1 antagonist blocked the fracture-induced increase in mast cell degranulation. We then observed that ES of the sciatic nerve significantly degranulated dermal mast cells and induced nociceptive responses. However, the ES-induced mast cell degranulation and nociceptive response were blocked in rats pretreated with a NK1 antagonist. Moreover, we showed that intraplantar SP injection induces mast cell degranulation. It should be noted that elevated skin levels of SP have been measured in the CRPS model as has augmented NK1 receptor dependent neurogenic edema11. Others have reported that ES of sciatic nerve can cause a significant increase in dermal mast cell degranulation and histamine release in rat hindpaws50–51. However, this response was significantly blocked in rats pretreated in the neonatal period with capsaicin to reduce afferent neuropeptide signaling50. Thus, our observations that the number of mast cells increases and the proximity of the mast cells to neuropeptide expressing nerve fibers is likely functionally meaningful.
Pain and hyperalgesia are the most distressing symptoms in CRPS, which result, at least partially, from activation or sensitization of peripheral nociceptors52. Because of their large repertoire of inflammatory mediators such as histamine, cytokines, prostaglandins and proteases53, we speculated that mast cell degranulation could cause nociceptive sensitization in the CRPS model. To address this hypothesis, we next administered a mast cell degranulator, compound 48/80 at 4 weeks postfracture. The acute administration of 48/80 caused mast cell degranulation and enhanced postfracture nociception, but nociceptive behavior was attenuated when mast cells were depleted after 4 days of 48/80 treatment. These observations strongly suggest that mast cell degranulation after fracture can induce sensitization, thus providing new evidence for the importance of neurocutaneous signaling in the development of CRPS. Previous studies suggest that elevated mast cell number and degranulation are associated with the pathogenesis of chronic pain in inflammatory conditions30,36, following peripheral nerve injury37, and in several human diseases like migraine headache39–40, interstitial cystitis41 and chronic pancreatitis42–43.
We investigated the effects of cetirizine, a selective H1 histamine receptor blocker, on nociceptive and vascular changes in the CRPS model. Mast cell derived histamine may support sensitization in other pain models acting through the H1 receptor33–37,54. Surprisingly, our studies clearly show that acute administration of cetirizine failed to block nociceptive and vascular changes observed in the CRPS model. It should be noted that H1 blockade did not reduce the enhanced vascular leakage induced by the acute injection of 48/80 in a separate study55. We cannot rule out the possibility of histamine supporting sensitization via other histamine receptors as recent reports suggest the H3 and H4 receptors may mediate nociception in skin17–18.
Evidence from other groups suggests that mast cells may induce nociceptive sensitization through the action of nonhistamine granule contents. For example, nerve growth factor-β derived from mast cells sensitizes nociceptors and also increases expression of tryptase in mast cells56–57. We demonstrated previously that nerve growth factor is an active pain related mediator in the rat fracture model49. Substance P via activation of NK1 receptors on mast cells increases expression of TNF58–59, which in turn sensitizes nociceptive terminals via activation of the TNF receptors. Again, TNFα supports nociceptive changes in the model used here8. Finally, mast cells may facilitate nociceptive sensitization by activating surrounding cells like keratinocytes. There is growing evidence that keratinocytes express protease activated receptor 2 and histamine receptors, which can be activated by mast cell proteases and histamine respectively60–61, thus enhancing the expression of inflammatory cytokines, matrix metalloproteinases and antimicrobial peptides60,62–64. In this regard it is also notable that TRPA1 receptors are located in proximity to protease-activated receptor 2 receptors, and that activation of TRPA1 receptors by protease activated receptor 2 receptors may enhance the sensitivity of TRPA1 receptors during inflammation65. The observation of increased numbers of mast cells and apparent degranulation in the upper dermis in close proximity to the basal layer of the epidermis support this possible mechanism in this CRPS model.
In summary, these experiments indicate that facilitated sensory neuron-mast cell signaling after fracture can cause mast cell accumulation and degranulation into the skin of the injured limb and induce nociceptive sensitization. Future experiments are needed to determine which mast cell granule contents mediate pain and inflammation in this model. Given the diversity of mast cell granule contents it seems possible multiple parallel mechanisms are involved. An improved understanding of mast cell function in CRPS might lead to novel strategies for preventing CRPS in high-risk patients or reducing the severity of the condition once established.
Acknowledgments
This work was supported by grants F4516I and F7137R from Department of Veteran Affairs, Veterans Health Administration, Rehabilitation Research and Development Service (Washington, DC), and grants GM079126 and NS072143 from the National Institutes of Health (Bethesda, Maryland).
Footnotes
A part of contents was presented at the Annual Meeting of the Society for Neuroscience, San Diego, California, November 13–17, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75:450–2. doi: 10.1302/0301-620X.75B3.8496220. [DOI] [PubMed] [Google Scholar]
- 2.Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after Colles' fracture. J Bone Joint Surg Br. 1990;72:105–10. doi: 10.1302/0301-620X.72B1.2298766. [DOI] [PubMed] [Google Scholar]
- 3.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Wasner G, Schattschneider J, Heckmann K, Maier C, Baron R. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): Mechanisms and diagnostic value. Brain. 2001;124:587–99. doi: 10.1093/brain/124.3.587. [DOI] [PubMed] [Google Scholar]
- 5.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151:843–52. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, Clark JD. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain. 2009;147:277–86. doi: 10.1016/j.pain.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1β signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144:303–13. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137:507–19. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138:47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. Eur J Pain. 2009;13:253–62. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–86. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church MK, Lowman MA, Rees PH, Benyon RC. Mast cells, neuropeptides and inflammation. Agents Actions. 1989;27:8–16. doi: 10.1007/BF02222185. [DOI] [PubMed] [Google Scholar]
- 13.Harvima IT, Nilsson G, Naukkarinen A. Role of mast cells and sensory nerves in skin inflammation. G Ital Dermatol Venereol. 145:195–204. [PubMed] [Google Scholar]
- 14.Naukkarinen A, Harvima IT, Aalto ML, Harvima RJ, Horsmanheimo M. Quantitative analysis of contact sites between mast cells and sensory nerves in cutaneous psoriasis and lichen planus based on a histochemical double staining technique. Arch Dermatol Res. 1991;283:433–7. doi: 10.1007/BF00371778. [DOI] [PubMed] [Google Scholar]
- 15.Peters EM, Kuhlmei A, Tobin DJ, Muller-Rover S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–62. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Harvima IT, Nilsson G, Suttle MM, Naukkarinen A. Is there a role for mast cells in psoriasis? Arch Dermatol Res. 2008;300:461–78. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- 17.Cannon KE, Leurs R, Hough LB. Activation of peripheral and spinal histamine H3 receptors inhibits formalin-induced inflammation and nociception, respectively. Pharmacol Biochem Behav. 2007;88:122–9. doi: 10.1016/j.pbb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh GC, Chandran P, Salyers AK, Pai M, Zhu CZ, Wensink EJ, Witte DG, Miller TR, Mikusa JP, Baker SJ, Wetter JM, Marsh KC, Hancock AA, Cowart MD, Esbenshade TA, Brioni JD, Honore P. H4 receptor antagonism exhibits anti-nociceptive effects in inflammatory and neuropathic pain models in rats. Pharmacol Biochem Behav. 2010;95:41–50. doi: 10.1016/j.pbb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Schaffler K, Seibel K, Thomsen M, Edwards M. Effect of the new H1-antagonist ReN1869 on capsaicin-induced hyperalgesia in human skin/human phase-I trial using somatosensory evoked potentials induced by a CO2 laser. Arzneimittelforschung. 2004;54:187–91. doi: 10.1055/s-0031-1296957. [DOI] [PubMed] [Google Scholar]
- 20.Jarvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res. 2003;295:2–7. doi: 10.1007/s00403-002-0378-z. [DOI] [PubMed] [Google Scholar]
- 21.Naukkarinen A, Harvima I, Paukkonen K, Aalto ML, Horsmanheimo M. Immunohistochemical analysis of sensory nerves and neuropeptides, and their contacts with mast cells in developing and mature psoriatic lesions. Arch Dermatol Res. 1993;285:341–6. doi: 10.1007/BF00371834. [DOI] [PubMed] [Google Scholar]
- 22.Hagforsen E, Nordlind K, Michaelsson G. Skin nerve fibres and their contacts with mast cells in patients with palmoplantar pustulosis. Arch Dermatol Res. 2000;292:269–74. doi: 10.1007/s004030000132. [DOI] [PubMed] [Google Scholar]
- 23.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett. 2004;91:147–54. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Hide M, Yanase Y, Greaves MW. Cutaneous mast cell receptors. Dermatol Clin. 2007;25:563–75. doi: 10.1016/j.det.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 26.Smith EW, Atkinson WB. Simple procedure for identification and rapid counting of mast cells in tissue sections. Science. 1956;123:941–2. doi: 10.1126/science.123.3204.941. [DOI] [PubMed] [Google Scholar]
- 27.Dyson M, Luke DA. Induction of mast cell degranulation in skin by ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 1986;33:194–201. doi: 10.1109/t-uffc.1986.26814. [DOI] [PubMed] [Google Scholar]
- 28.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121:158–67. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Coderre TJ, Basbaum AI, Levine JD. Neural control of vascular permeability: Interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989;62:48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- 30.Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J Neurosci. 1996;16:2716–23. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104:75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 32.Grutzkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, Henz BM. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A. 2004;61:62–8. doi: 10.1002/cyto.a.20068. [DOI] [PubMed] [Google Scholar]
- 33.Kajihara Y, Murakami M, Imagawa T, Otsuguro K, Ito S, Ohta T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience. 166:292–304. doi: 10.1016/j.neuroscience.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Bai ZT, Pang XY, Chai ZF, Jiang F, Ji YH. Degranulation of mast cells and histamine release involved in rat pain-related behaviors and edema induced by scorpion Buthus martensi Karch venom. Eur J Pharmacol. 2007;575:46–56. doi: 10.1016/j.ejphar.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 35.Massaad CA, Safieh-Garabedian B, Poole S, Atweh SF, Jabbur SJ, Saade NE. Involvement of substance P, CGRP and histamine in the hyperalgesia and cytokine upregulation induced by intraplantar injection of capsaicin in rats. J Neuroimmunol. 2004;153:171–82. doi: 10.1016/j.jneuroim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–44. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- 37.Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: A key role of mast cells. Pain. 2003;105:467–79. doi: 10.1016/S0304-3959(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 38.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. Faseb J. 2006;20:2366–8. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 39.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–40. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 40.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–76. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theoharides TC, Pang X, Letourneau R, Sant GR. Interstitial cystitis: A neuroimmunoendocrine disorder. Ann N Y Acad Sci. 1998;840:619–34. doi: 10.1111/j.1749-6632.1998.tb09601.x. [DOI] [PubMed] [Google Scholar]
- 42.Esposito I, Friess H, Kappeler A, Shrikhande S, Kleeff J, Ramesh H, Zimmermann A, Buchler MW. Mast cell distribution and activation in chronic pancreatitis. Hum Pathol. 2001;32:1174–83. doi: 10.1053/hupa.2001.28947. [DOI] [PubMed] [Google Scholar]
- 43.Hoogerwerf WA, Gondesen K, Xiao SY, Winston JH, Willis WD, Pasricha PJ. The role of mast cells in the pathogenesis of pain in chronic pancreatitis. BMC Gastroenterol. 2005;5 doi: 10.1186/1471-230X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woolley DE. The mast cell in inflammatory arthritis. N Engl J Med. 2003;348:1709–11. doi: 10.1056/NEJMcibr023206. [DOI] [PubMed] [Google Scholar]
- 45.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 46.Oyen WJ, Arntz IE, Claessens RM, Van der Meer JW, Corstens FH, Goris RJ. Reflex sympathetic dystrophy of the hand: An excessive inflammatory response? Pain. 1993;55:151–7. doi: 10.1016/0304-3959(93)90144-E. [DOI] [PubMed] [Google Scholar]
- 47.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–7. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki A, Suzuki R, Furuno T, Teshima R, Nakanishi M. N-cadherin plays a role in the synapse-like structures between mast cells and neurites. Biol Pharm Bull. 2004;27:1891–4. doi: 10.1248/bpb.27.1891. [DOI] [PubMed] [Google Scholar]
- 49.Folgueras AR, Valdes-Sanchez T, Llano E, Menendez L, Baamonde A, Denlinger BL, Belmonte C, Juarez L, Lastra A, Garcia-Suarez O, Astudillo A, Kirstein M, Pendas AM, Farinas I, Lopez-Otin C. Metalloproteinase MT5-MMP is an essential modulator of neuro-immune interactions in thermal pain stimulation. Proc Natl Acad Sci U S A. 2009;106:16451–6. doi: 10.1073/pnas.0908507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang ZL, Mochizuki T, Watanabe H, Maeyama K. Histamine release induced by immobilization, gentle handling and decapitation from mast cells and its inhibition by nedocromil in rats. Jpn J Pharmacol. 1999;80:255–62. doi: 10.1254/jjp.80.255. [DOI] [PubMed] [Google Scholar]
- 51.Kowalski ML, Kaliner MA. Neurogenic inflammation, vascular permeability, and mast cells. J Immunol. 1988;140:3905–11. [PubMed] [Google Scholar]
- 52.Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci Lett. 2008;437:199–202. doi: 10.1016/j.neulet.2008.03.081. [DOI] [PubMed] [Google Scholar]
- 53.Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990;11:458–64. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- 54.Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One. 2008;3:e2096. doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saria A, Hua X, Skofitsch G, Lundberg JM. Inhibition of compound 48/80--induced vascular protein leakage by pretreatment with capsaicin and a substance P antagonist. Naunyn Schmiedebergs Arch Pharmacol. 1984;328:9–15. doi: 10.1007/BF00496097. [DOI] [PubMed] [Google Scholar]
- 56.Groneberg DA, Serowka F, Peckenschneider N, Artuc M, Grutzkau A, Fischer A, Henz BM, Welker P. Gene expression and regulation of nerve growth factor in atopic dermatitis mast cells and the human mast cell line-1. J Neuroimmunol. 2005;161:87–92. doi: 10.1016/j.jneuroim.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 57.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–12. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 58.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–85. [PubMed] [Google Scholar]
- 59.Cocchiara R, Lampiasi N, Albeggiani G, Bongiovanni A, Azzolina A, Geraci D. Mast cell production of TNF-alpha induced by substance P evidence for a modulatory role of substance P-antagonists. J Neuroimmunol. 1999;101:128–36. doi: 10.1016/s0165-5728(99)00138-1. [DOI] [PubMed] [Google Scholar]
- 60.Kanda N, Watanabe S. Histamine enhances the production of granulocyte-macrophage colony-stimulating factor via protein kinase Calpha and extracellular signal-regulated kinase in human keratinocytes. J Invest Dermatol. 2004;122:863–72. doi: 10.1111/j.0022-202X.2004.22432.x. [DOI] [PubMed] [Google Scholar]
- 61.Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–73. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 62.Kanda N, Watanabe S. Histamine enhances the production of nerve growth factor in human keratinocytes. J Invest Dermatol. 2003;121:570–7. doi: 10.1046/j.1523-1747.2003.12428.x. [DOI] [PubMed] [Google Scholar]
- 63.Kohda F, Koga T, Uchi H, Urabe K, Furue M. Histamine-induced IL-6 and IL-8 production are differentially modulated by IFN-gamma and IL-4 in human keratinocytes. J Dermatol Sci. 2002;28:34–41. doi: 10.1016/s0923-1811(01)00147-5. [DOI] [PubMed] [Google Scholar]
- 64.Lee SE, Kim JM, Jeong SK, Jeon JE, Yoon HJ, Jeong MK, Lee SH. Protease-activated receptor-2 mediates the expression of inflammatory cytokines, antimicrobial peptides, and matrix metalloproteinases in keratinocytes in response to Propionibacterium acnes. Arch Dermatol Res. 2010;302:745–56. doi: 10.1007/s00403-010-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–87. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]