Abstract
Total knee arthroplasty (TKA) is associated with persistent quadriceps dysfunction. Since quadriceps dysfunction impairs functional performance, minimizing quadriceps dysfunction by attenuating central activation deficits early after surgery may improve function later in life. Rehabilitation strategies incorporating neuromuscular electrical stimulation and early, aggressive quadriceps strengthening may prove beneficial. Further, surgical approaches such as minimally invasive TKA may minimize post-operative quadriceps dysfunction.
Keywords: Strength, muscle inhibition, rehabilitation, neuromuscular electrical stimulation, joint replacement
INTRODUCTION
Over 650,000 total knee arthroplasties (TKAs) are performed annually in the United States to alleviate knee osteoarthritis (OA)-related pain and disability.(1) This number is expected to increase to 3.48 million per year by 2030.(12) While TKA reliably reduces pain and improves self-reported function, quadriceps strength fails to recover to levels of healthy, age-matched adults even years after surgery. Quadriceps muscle weakness has profound functional consequences and is associated with decreased gait speed(4), balance(17), stair-climbing(14), and chair rise(25) ability, as well as increased risk for falls.(16) Therefore, it is not surprising that physical function, like quadriceps weakness, remains impaired in over 50% of patients one year after TKA.(19) As such, attenuating quadriceps strength deficits is imperative to limiting disability later in life.
Long-term deficits in quadriceps strength may stem from pre-operative weakness and postoperative strength loss. Individuals with tibiofemoral OA demonstrate quadriceps weakness compared to persons without radiographic evidence of the disease.(20) Post-operatively, patients experience a 60% decrease in quadriceps strength 3–4 weeks after surgery from pre-operative levels.(15) Both pre- and post-operative strength loss may be attributable to impaired quadriceps activation. Muscle atrophy may further explain post-operative quadriceps weakness (15); however, central activation deficits (CAD) reportedly account for twice the strength loss as muscle atrophy early after surgery.(15)
Quadriceps CAD are prevalent in individuals with knee OA, though CAD are magnified after TKA (15), resulting in more severe muscle weakness than prior to surgery. Although recent evidence suggests that quadriceps CAD largely resolve within the first post-operative year(21), these early deficits and related muscle weakness may substantially contribute to the long-term muscle weakness documented in these patients. The presence of quadriceps CAD may contribute to early muscle atrophy and also hinder the ability to restore muscle mass, further impairing quadriceps strength long-term. As such, we contend that reducing CAD early after TKA is imperative to countering quadriceps weakness and more effectively restoring functional performance. In this article, we provide several strategies for attenuating CAD following TKA as well as directions for future research to further understand and treat post-operative CAD.
CENTRAL ACTIVATION DEFICITS
Origins
The neurophysiologic mechanisms underlying CAD are not fully understood, though CAD may be due, at least in part, to altered afferent feedback following joint damage. Altered afference may lead to reduced quadriceps alpha motoneuron excitability, ultimately decreasing volitional force output. Cortical pathways may also contribute to reduced alpha motoneuron excitability, though how these pathways are involved is not well understood.
In addition to joint damage, the presence of joint effusion and pain may further contribute to CAD. (29) Pain-free, experimental knee joint effusions with as little as 20–30 ml of saline have been shown to produce quadriceps CAD.(29) The presence of joint effusion may activate several gating mechanisms within the central nervous system, including both pre- and post-synaptic inhibition, ultimately reducing excitatory input to the muscles surrounding the effused joint. Further, the presence of joint effusion may activate Ruffini endings, which contribute to the regulation of muscle tone and movement by their influence on the Golgi tendon organ to regulate joint stiffness and stability.(9) Ruffini endings may then activate inhibitory interneurons, thereby reducing alpha motoneuron excitability. Experimental muscle pain has also been found to reduce voluntary muscle activation due to central mechanisms.(13) Inhibition of spinal neurons receiving nociceptive, afferent inflow through descending pathways is well established in feline experiments and may be a potential source of quadriceps CAD with OA and early after TKA. Although these investigations do not completely explain the underlying neurophysiologic mechanisms for CAD, collectively, they do suggest the involvement of a central mechanism in regulating the excitability of the motoneuron pool responsible for CAD.
Assessment Techniques
Quadriceps CAD are measured by superimposing percutaneous electrical stimuli on a maximal voluntary isometric contraction (MVIC). There are two common methods by which CAD are assessed. The burst superimposition technique utilizes a train of electrical stimuli superimposed on a maximal voluntary muscle contraction (Figure 1a). In the presence of CAD, an increase in torque is observed when the train of stimuli is delivered. A common alternative is the interpolation technique. This technique involves the superimposition of an electrical stimulus, most commonly a single pulse, both at rest and during a maximal voluntary contraction. In the presence of CAD, an increase in torque is observed when the stimulus is delivered. With the interpolation technique, the increased torque is normalized to the torque produced when the stimulus is delivered to the muscle at rest to account for differences in tissue impedance (Figure 1b).
Figure 1.
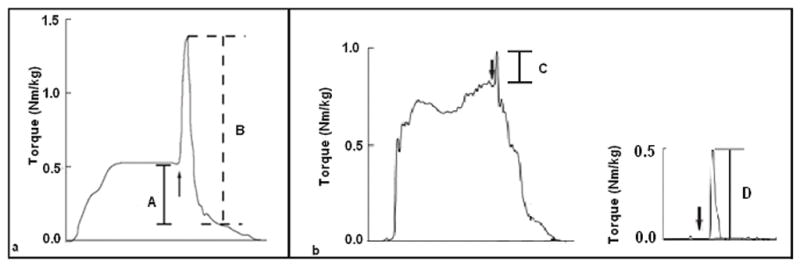
Schematic of how central activation deficits (CAD) are calculated. a) Using the burst superimposition technique, CAD are determined by the equation: (A/B)*100. b) Using the interpolation technique, CAD are determined by the equation (1-(C/D))*100. The arrows indicate delivery of the electrical stimulus.
ATTENUATING DEFICITS
Neuromuscular Electrical Stimulation
The presence of large CAD may impair the ability to train the quadriceps at sufficient intensities to promote strength gains.(30) Neuromuscular electrical stimulation (NMES) may override CAD, thereby allowing for restoration of normal quadriceps muscle function more effectively than voluntary exercise alone.
How NMES improves muscle strength is unclear, though some theories have emerged. First, the intensity of the muscle contraction produced during stimulation may be greater than that without NMES. Training programs require intensities of at least 30–50% of maximal voluntary effort to overload the muscle sufficiently to induce strength gains(27), which may not be possible volitionally in muscles with CAD. Similar to higher intensity voluntary muscle contractions, electrically-elicited muscle contractions at high intensities produce muscle hypertrophy and corresponding increases in force production if used for a sufficient length of time. Second, NMES may alter motor recruitment. Electrically-elicited muscle contractions allow for activation of a greater proportion of type II muscle fibers than volitional exercise at comparable intensity. Type II muscle fibers are larger than type I, so greater activation of type II fibers maximizes force production, though these fibers are typically only activated during higher intensity voluntary contractions. With voluntary contractions, smaller motoneurons have lower activation thresholds than larger motoneurons; therefore, smaller motoneurons and type I muscle fibers are recruited before larger motoneurons and type II muscle fibers. With electrically-elicited muscle contractions, factors such as the size of the axonal branches and their orientation to the current field influence motor unit at lower contraction intensities. Finally, NMES may also influence functional measures of motor performance via peripheral afferent inputs that alter motor cortex excitability. Stimulation of peripheral afferent nerves can induce prolonged changes in the excitability of the human motor cortex. A study investigating the effects of stimulation of the hand afferents on cortical activity after sub-threshold peripheral stimulation in healthy individuals found an increase in fMRI signal intensity in the primary and secondary motor and somatosensory areas.(6) Similar results have been demonstrated in individuals following stroke, further supporting the notion that NMES may play an important role in enhancing cortical excitability to allow for improved motor function.
NMES has been used effectively in a variety of patient populations, including individuals following stroke, to both re-educate muscle and facilitate hypertrophy.(18) In individuals following stroke, a 77% improvement in quadriceps force and nearly 20% improvement in motor unit recruitment were achieved through NMES treatment compared to only 31% improvement and no change, respectively, without NMES.(18)Similarly, NMES may improve quadriceps strength in individuals with knee osteoarthritis. Specifically, Tablot et al.(34) noted a 9% increase in muscle strength in patients receiving NMES compared to a 7% loss of strength in patients who did not receive NMES.
Results of studies applying NMES to the quadriceps muscle of patients after TKA are promising. Gotlin et al.(7) noted that NMES applied within the first week after TKA reduced the knee extensor lag (i.e., deficit in the ability to actively extend the knee through the full available range of motion) from 7.5° to 5.7° compared with control subjects who had an increase in extensor lag from 5.3° to 8.3° in the same timeframe. As such, early NMES treatment after TKA may translate to better quadriceps function. Similarly, Avramidis et al.(2) demonstrated a significant increase in walking speed in patients following six weeks of daily NMES treatment (4 hours per day) compared with controls 6 weeks after TKA. There was a carry-over in faster walking speed with NMES at 12 weeks postoperatively, which is likely secondary to an initially faster recovery of quadriceps muscle force and subsequent ability to participate more fully in the voluntary exercise program.(2) Finally, when unilateral NMES was initiated 3–4 weeks after bilateral TKA and continued for six weeks, quadriceps muscle activation and force increased 431% in the limbs that received NMES plus voluntary exercise and only 182% in the contralateral limbs which received voluntary exercise alone.(31) Despite the augmented response, force production in NMES-treated limbs remained below expected levels for healthy adults six months after surgery.(31)
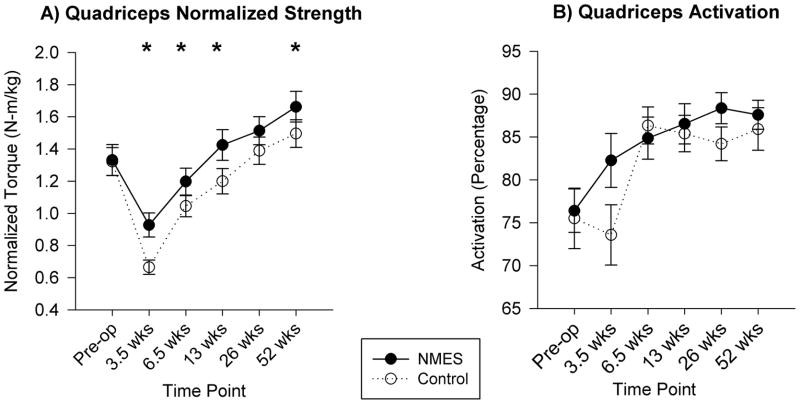
A recent investigation in our laboratory examined the effects of NMES delivered twice per day (15 repetitions per session; biphasic waveform at 50 pps; 250 s pulse duration; duty cycle 15 s on and 45 s off). Treatment began within 48 hours after surgery and continued for six weeks. Patients also received standardized rehabilitation for 8 weeks (inpatient, home, and outpatient). The control group received only standardized rehabilitation. Results indicated that individuals receiving NMES demonstrated greater quadriceps and hamstrings strength, knee range of motion (ROM), and functional performance than controls (Figure 2).(30) Three and a half weeks after TKA, activation trended towards greater improvements with NMES treatment compared to pre-operative values (Figure 2). Furthermore, strength and functional performance differences between groups were maintained 52 weeks post-operatively.(30) Early restoration of quadriceps strength by countering activation deficits likely contributed to the long-term improvement in functional performance in patients receiving NMES.
Figure 2.
Differences in quadriceps strength (A) and central activation deficits (B) between neuromuscular electrical stimulation and control groups. Data are mean±standard deviation.
* indicates statistically significant difference in quadriceps strength between groups (P<0.05). [Adapted from (30) PTJ. November 17, 2011; doi: 10.2522/PTJ.201101224; [epub ahead of print], with permission of the American Physical Therapy Association. This material is copyrighted, and any further reproduction or distribution requires written permission from APTA.]
Though the results of several investigations indicate NMES may be beneficial following TKA, a recent randomized controlled trial comparing 1) exercise and 2) exercise + NMES suggests that NMES (10 contractions, twice per week for 6 weeks) initiated one month post-operatively may not be any more beneficial than exercise alone.(22) Specifically, the authors noted no differences between the exercise and exercise + NMES groups in quadriceps strength, CAD, or function three or 12 months post-operatively.(22) Both groups, however, had better strength, CAD, and function 12 months after TKA compared to a cohort receiving less intensive rehabilitation in the community.(22) These results suggest that the timing and frequency of NMES treatment may be critical to patient outcomes. Specifically, early use of NMES (i.e., before one month after TKA) and NMES delivered greater than twice per week may be necessary.
Limitations and future directions
The effectiveness of NMES is limited by patient tolerance to the treatment. Data from our laboratory and others examining the use of NMES following anterior cruciate ligament reconstruction (26) indicate that higher intensity stimuli (i.e., a greater dose of the treatment) are needed to achieve greater gains in strength and activation (Figure 3). Yet, the ideal intensity and frequency of NMES treatment are unclear across studies and patient populations. Following TKA, our results suggest that doses as little as 10–20% of daily maximal voluntary isometric contraction may be effective when applied early (2 days after surgery) and frequently after TKA (2 times/day)(30). We found a significant association between NMES dose and change in quadriceps strength at 3.5 weeks (R2=0.68) at 6.5 weeks (R2=0.25) after TKA. In contrast, less frequent NMES application after TKA (2 times/week) has not been effective even with a minimum 30% treatment dose.(22) Even though high intensities result in greater gains in strength and activation, stimuli delivered at high intensities are often uncomfortable for patients. Further, both electrode size and placement need to be considered during NMES treatment to improve patient comfort. Smaller electrodes have a higher current density and, thus, may increase patient discomfort compared to larger electrodes. Current density is also influenced by the distance between electrodes, with closer distances yielding greater current densities. As patient tolerance is critical to the success of NMES, future investigations would benefit from determining stimulation parameters and doses to optimize comfort and attenuate CAD. Further, while some patients do not tolerate NMES well, others are capable of exceeding the maximal capability of the stimulators, which also limits the effectiveness of NMES application. NMES may also have limited effects in patients without CAD, since studies of NMES applications in healthy individuals demonstrate fewer benefits than applications in patient populations with activation deficits. As will be discussed in the next section, patients with limited activation deficits may benefit from other forms of treatment, including aggressive voluntary strengthening exercises. A better understanding of who will benefit most from NMES treatment is still necessary to target the most appropriate patients.
Figure 3.
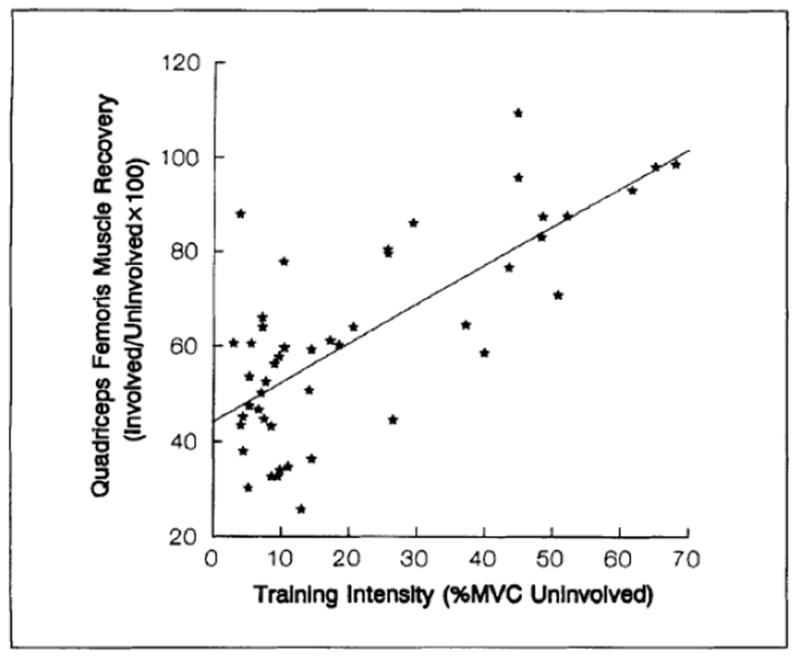
Relation between dose of neuromuscular electrical stimulation treatment and quadriceps strength recovery following anterior cruciate ligament reconstruction. %MVIC=percentage of maximal voluntary contraction. (Reprinted from [Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74(10):901–7.], with permission of the American Physical Therapy Association. This material is copyrighted, and any further reproduction or distribution requires written permission from APTA.)
High Intensity Rehabilitation
While NMES offers a promising strategy to target quadriceps CAD, it is also possible that utilizing a more intensive, progressive rehabilitation program may also be effective. Evidence from a variety of sources, including studies conducted on individuals following periods of both detraining and immobilization, supports the possibility that strength and CAD can be improved by progressive resistance exercise. Henwood and Taaffe(8), for example, demonstrated a 17% improvement in strength following retraining exercise in older adults. Similarly, individuals demonstrated improvements in both muscle strength and functional performance following rehabilitation after immobilization subsequent to ankle fracture.(24, 32) Suetta et al.(33) induced CAD in older and younger males via cast immobilization. Following cast removal and four weeks of rehabilitation, quadriceps strength improved by 23% in older males and 32% in younger males.(33) Further, older males demonstrated a 10% increase in central activation while younger males improved their activation by 5%.(33) Finally, previous research suggests that strengthening exercises for individuals on bed rest may attenuate strength loss and decrease CAD.(10)
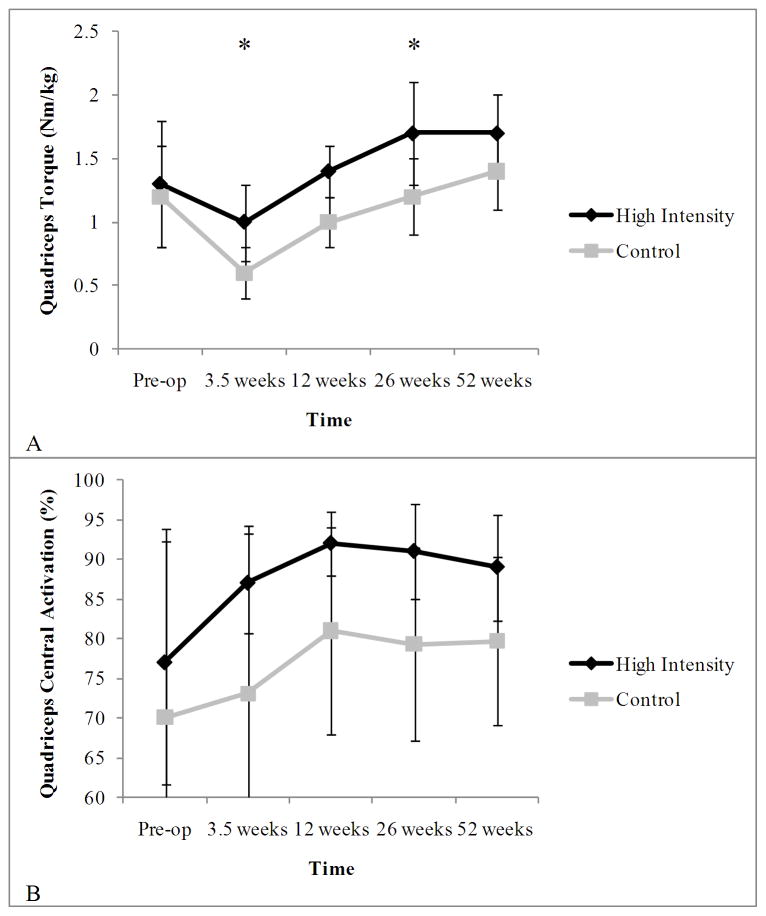
Despite this evidence suggesting activation deficits can be improved with intensive, progressive resistance training, less information is available for patients after TKA. In the presence of profound CAD after TKA, initial evidence suggests that progressive exercise may help attenuate quadriceps CAD. A recent investigation in our laboratory compared a high intensity rehabilitation program with a lower intensity rehabilitation program following TKA. High intensity training consisted of 22 outpatient physical therapy visits beginning within one week after surgery. Exercises included general lower extremity exercises with emphasis placed on the quadriceps. These exercises were progressed from seated and side lying exercises to more aggressive, eccentrically focused functional exercises. Resistance was increased from ankle weights to machine-based exercises. Patients progressed through exercises if they could complete two sets of 8 repetitions without fatigue and with pain less than 5/10 using a numeric pain rating scale (0–10). The lower intensity training group participated in 10 outpatient physical therapy sessions over six weeks, beginning 2 weeks after surgery. Exercises consisted of seated and side lying hip and knee muscle strengthening exercises as well as step-ups, step-downs, and wall slides. The maximal weight used by patients in the lower intensity training group was 4.5 kg. Results indicated that high intensity rehabilitation produced greater quadriceps strength and a trend toward smaller quadriceps CAD compared to lower intensity rehabilitation (Figure 4).(3)
Figure 4.
Quadriceps strength (A) and central activation deficits (B) following high-intensity and traditional rehabilitation. Data are presented pre-operatively and 3.5, 12, 26, and 52 weeks post-operatively. Data are mean±standard deviation.
* indicates statistically significant from pre-operative time point (P<0.05)
Limitations and Future directions
Although high intensity rehabilitation has been shown to be beneficial after TKA, its benefits may be more limited in patients whose weakness is centrally mediated. These patients may require NMES or other modalities aimed at over-riding CAD before they can optimally benefit from strength training. Future studies should investigate the specific causes (e.g., spinal, cortical, peripheral, etc.) of quadriceps weakness and CAD so that targeted interventions can be implemented. As the origins of weakness and CAD may not be the same in every patient, researchers need to develop simple, cost effective ways to determine the origins of these impairments within individual patients so that patient-specific rehabilitation strategies can be employed.
Minimally Invasive Total Knee Arthroplasty
In addition to rehabilitation strategies to attenuate quadriceps CAD following TKA, modifying the surgical technique may minimize post-operative quadriceps impairments. Compared to traditional approaches, minimally invasive surgery (MIS) for TKA employs smaller instrumentation and generates smaller incisions while avoiding patellar eversion and joint dislocation. Most importantly, this technique avoids disruption of the knee extensor mechanism and suprapatellar pouch and limits extreme knee flexion during surgery. Combined, these modifications have been postulated to minimize damage to the quadriceps muscle, thereby decreasing long-term, post-operative muscle weakness and functional impairments. Early results from retrospective, cohort comparisons indicated that MIS reduced hospital stays, decreased post-operative pain, and enabled patients to return to functional activities more quickly than traditional TKA.(35) However, more recent, small-scale randomized trials are not as universally supportive of MIS, with some studies noting that the benefits of MIS are temporary, while others have found no differences between surgical techniques post-operatively.(11) Within the first few days after surgery, Seon et al.(23) demonstrated that MIS yielded less pain, shorter time to achieve 90o knee flexion and straight leg raise, and a smaller knee extension lag, though these differences were not present two weeks post-operatively. Similarly, in individuals receiving simultaneous bilateral TKAs, with one knee receiving MIS and the other a conventional TKA, the knee receiving MIS had better Hospital for Special Surgery and Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) scores for up to six months postoperatively as well as lower WOMAC pain scores for up to 9 months post-operatively.(28) These differences were not present one year after surgery.
Recent results from a prospective, randomized controlled trial in our laboratory agree with those of other researchers. MIS resulted in greater hamstring strength and trends toward greater quadriceps strength four weeks after surgery; however, there were no differences between groups in quadriceps CAD, range of motion, functional performance, or WOMAC scores.(5) By 12 weeks post-operatively, strength was not significantly different between groups.(5) Additionally, 12 weeks after surgery, patients in the MIS group had better WOMAC scores, but differences between groups were smaller than previously established clinically meaningful differences. Further, differences between groups did not persist at 26 weeks.(5) Therefore, MIS led to a faster recovery of muscle strength in the first 4 weeks following TKA compared to traditional TKA, but this effect dissipated by 12 weeks following surgery. Importantly, functional performance was not influenced by surgical approach. Although the use of MIS may lead to faster recovery of strength in patients undergoing TKA, there is no apparent benefit of MIS on the longer-term recovery of strength or functional performance.
Limitations and future directions
MIS reduces visualization compared to traditional surgical approaches. As such, this technique requires specialized training to learn and perfect. An increasing body of evidence suggests that the purported benefits (e.g., reduced trauma to the knee joint complex) do not outweigh the cost of potentially longer operating times and advanced skills required to perform the surgery.(23, 28) Furthermore, the limited, short term benefits of MIS may not outweigh the risks associated with poor surgical visualization which could result in a greater number of surgical complications, such as poor alignment. Although research should continue to focus on advancing surgical techniques to avoid excess trauma to the extensor mechanism, techniques should be refined to improve visualization.
FUTURE DIRECTIONS
Reducing CAD early after TKA is essential for countering quadriceps weakness and more effectively restoring functional performance. Therefore, a deeper understanding of the mechanisms underlying quadriceps CAD is imperative to being able to successfully counter this impairment. Future studies should focus on differentiating the central (spinal and cortical) and peripheral contributions so that interventions targeted at the specific pathways involved can be implemented (Figure 5). Additionally, advances in operative techniques to reduce trauma during surgery ought to be explored.
Figure 5.
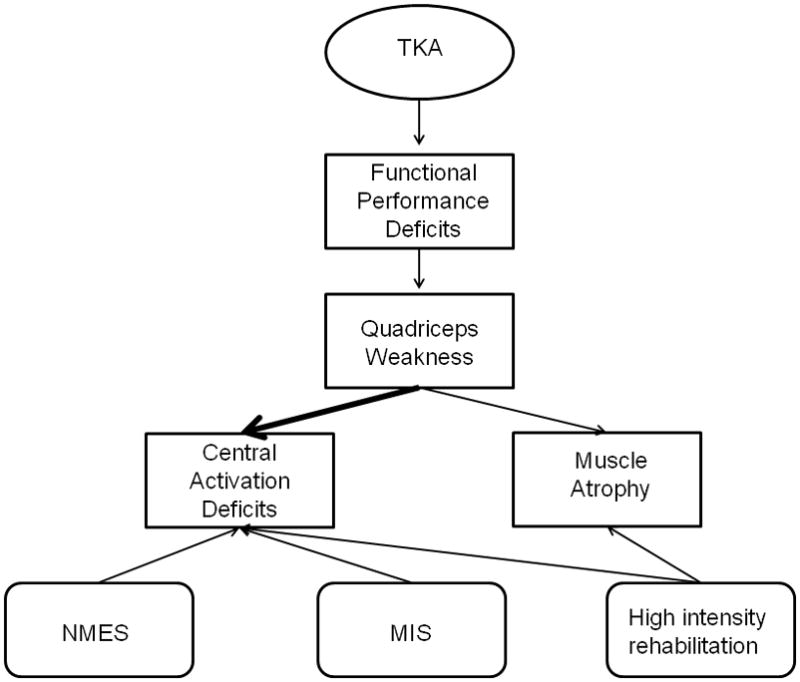
Schematic depicting deficits that arise following TKA and potential treatment strategies. TKA leads to functional performance deficits, which are largely attributable to pronounced quadriceps weakness. Quadriceps weakness results from a combination of central activation deficits and muscle atrophy, with activation deficits explaining more of the muscle weakness than atrophy. Quadriceps central activation deficits may be improved through NMES, altered surgical techniques (MIS) and high intensity rehabilitation. Muscle atrophy may also be mitigated effectively by high intensity rehabilitation. Ultimately, a combination of NMES, altered surgical techniques, and high intensity rehabilitation may be most effective in reducing deficits.
TKA= total knee arthroplasty; NMES= neuromuscular electrical stimulation; MIS= minimally invasive surgery.
SUMMARY
Targeting quadriceps CAD early after TKA, rather than waiting for deficits to resolve naturally, is both safe and effective. Further, this approach may allow for an earlier attenuation of quadriceps strength loss and related functional impairments to achieve better long term improvements in patient outcomes.
Acknowledgments
Funding: The studies described were supported by the National Institutes of Health (National Institute on Aging: K23-AG029978; National Institute of Arthritis and Musculoskeletal and Skin Diseases: R03-AR054538), an Arthritis Foundation New Investigator Award, and the American College of Rheumatology New Investigator Award to Dr. Stevens-Lapsley.
Footnotes
Conflict of Interest: None declared by Abbey C. Thomas. None declared by Jennifer E. Stevens-Lapsley.
References
- 1.American Academy of Orthopaedic Surgeons. [Accessed 07/24/2011];Most Commonly Performed Musculoskeletal-Related Procedures. Available from: http://www.aaos.org/research/stats/top_hospitalization_visits.pdf.
- 2.Avramidis K, Karachalios T, Popotonasios K, Sacorafas D, Papathanasiades AA, Malizos KN. Does electric stimulation of the vastus medialis muscle influence rehabilitation after total knee replacement? Orthopedics. 2011;34(3):175. doi: 10.3928/01477447-20110124-06. [DOI] [PubMed] [Google Scholar]
- 3.Bade MJ, Stevens-Lapsley JE. Early Progressive Rehabilitation Following Total Knee Arthroplasty Improves Outcomes. J Orthop Sports Phys Ther. doi: 10.2519/jospt.2011.3734. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):55–9. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 5.Dayton MR, Bade MJ, Shulman BC, Kohrt WM, Stevens-Lapsley JE. Minimally Invasive Total Knee Arthroplasty: Impact on Knee Strength and Functional Performance. Proceedings of the American Association of Hip and Knee Surgeons 21st Annual Meeting; November 4–6, 2011; Dallas, TX. [Google Scholar]
- 6.Golaszewski S, Kremser C, Wagner M, Felber S, Aichner F, Dimitrijevic MM. Functional magnetic resonance imaging of the human motor cortex before and after whole-hand afferent electrical stimulation. Scand J Rehabil Med. 1999;31(3):165–73. doi: 10.1080/003655099444506. [DOI] [PubMed] [Google Scholar]
- 7.Gotlin RS, Hershkowitz S, Juris PM, Gonzalez EG, Scott WN, Insall JN. Electrical stimulation effect on extensor lag and length of hospital stay after total knee arthroplasty. Arch Phys Med Rehabil. 1994 Sep;75(9):957–9. [PubMed] [Google Scholar]
- 8.Henwood TR, Taaffe DR. Detraining and retraining in older adults following long-term muscle power or muscle strength specific training. J Gerontol A Biol Sci Med Sci. 2008 Jul;63(7):751–8. doi: 10.1093/gerona/63.7.751. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9:135–59. [Google Scholar]
- 10.Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001 Jan-Feb;84(1–2):7–12. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- 11.Kolisek FR, Bonutti PM, Hozack WJ, Purtill J, Sharkey PF, Zelicof SB, Ragland PS, Kester M, Mont MA, Rothman RH. Clinical experience using a minimally invasive surgical approach for total knee arthroplasty: early results of a prospective randomized study compared to a standard approach. J Arthroplasty. 2007 Jan;22(1):8–13. doi: 10.1016/j.arth.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007 Apr;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 13.Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol. 2001 Sep;112(9):1633–41. doi: 10.1016/s1388-2457(01)00631-9. [DOI] [PubMed] [Google Scholar]
- 14.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005 Jul;35(7):424–36. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 15.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005 May;87(5):1047–53. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004 Jul;52(7):1121–9. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 17.Moxley Scarborough D, Krebs DE, Harris BA. Quadriceps muscle strength and dynamic stability in elderly persons. Gait Posture. 1999 Sep;10(1):10–20. doi: 10.1016/s0966-6362(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 18.Newsam CJ, Baker LL. Effect of an electric stimulation facilitation program on quadriceps motor unit recruitment after stroke. Arch Phys Med Rehabil. 2004 Dec;85(12):2040–5. doi: 10.1016/j.apmr.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? Clin Orthop Relat Res. 2005 Feb;431:157–65. doi: 10.1097/01.blo.0000150130.03519.fb. [DOI] [PubMed] [Google Scholar]
- 20.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010 Jul;89(7):541–8. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petterson SC, Barrance P, Marmon AR, Handling T, Buchanan TS, Snyder-Mackler L. Time course of quad strength, area, and activation after knee arthroplasty and strength training. Med Sci Sports Exerc. Feb;43(2):225–31. doi: 10.1249/MSS.0b013e3181eb639a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petterson SC, Mizner RL, Stevens JE, Raisis L, Bodenstab A, Newcomb W, Snyder-Mackler L. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009 Feb 15;61(2):174–83. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 23.Seon JK, Song EK. Navigation-assisted less invasive total knee arthroplasty compared with conventional total knee arthroplasty: a randomized prospective trial. J Arthroplasty. 2006 Sep;21(6):777–82. doi: 10.1016/j.arth.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Shaffer MA, Okereke E, Esterhai JL, Jr, Elliott MA, Walker GA, Yim SH, Vandenborne K. Effects of immobilization on plantar-flexion torque, fatigue resistance, and functional ability following an ankle fracture. Phys Ther. 2000;80(8):769–80. [PubMed] [Google Scholar]
- 25.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994 Sep;23(5):371–7. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 26.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74(10):901–7. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 27.Snyder-Mackler L, Garrett M, Roberts M. A comparison of torque generating capabilities of three different electrical stimulating currents. J Orthop Sports Phys Ther. 1989;10(8):297–301. doi: 10.2519/jospt.1989.10.8.297. [DOI] [PubMed] [Google Scholar]
- 28.Song EK, Seon JK, Yoon TR, Park SJ, Bae BH, Cho SG. Functional results of navigated minimally invasive and conventional total knee arthroplasty: a comparison in bilateral cases. Orthopedics. 2006 Oct;29(10 Suppl):S145–7. [PubMed] [Google Scholar]
- 29.Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171–77. [PubMed] [Google Scholar]
- 30.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early Neuromuscular Electrical Stimulation to Improve Quadriceps Muscle Strength After Total Knee Arthroplasty: A Randomized Controlled Trial. Phys Ther. doi: 10.2522/ptj.20110124. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens JE, Mizner RL, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004 Jan;34(1):21–9. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 32.Stevens JE, Walter GA, Okereke E, Scarborough MT, Esterhai JL, George SZ, Kelley MJ, Tillman SM, Gibbs JD, Elliott MA, Frimel TN, Gibbs CP, Vandenborne K. Muscle adaptations with immobilization and rehabilitation after ankle fracture. Med Sci Sports Exerc. 2004 Oct;36(10):1695–701. doi: 10.1249/01.mss.0000142407.25188.05. [DOI] [PubMed] [Google Scholar]
- 33.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009 Oct;107(4):1172–80. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 34.Talbot LA, Gaines JM, Ling SM, Metter EJ. A home-based protocol of electrical muscle stimulation for quadriceps muscle strength in older adults with osteoarthritis of the knee. J Rheumatol. 2003 Jul;30(7):1571–8. [PubMed] [Google Scholar]
- 35.Tria AJ., Jr Advancements in minimally invasive total knee arthroplasty. Orthopedics. 2003 Aug;26(8 Suppl):s859–63. doi: 10.3928/0147-7447-20030802-07. [DOI] [PubMed] [Google Scholar]