Abstract
OBJECTIVE
To identify treatment patterns and predictors of receiving multimodality therapy in patients with locally advanced prostate cancer (LAPC).
PATIENTS AND METHODS
The cohort comprised patients ≥ 66 years with clinical stage T3 or T4 non-metastatic prostate cancer diagnosed between 1998 and 2005 identified from the Surveillance, Epidemiology and End Results (SEER) cancer registry records linked with Medicare claims.
Treatments were classified as radical prostatectomy (RP), radiation therapy (RT) and androgen deprivation therapy (ADT) received within 6 and 24 months of diagnosis.
We assessed trends over time and used multivariable logistic regression to identify predictors of multimodality treatment.
RESULTS
Within the first 6 months of diagnosis, 1060 of 3095 patients (34%) were treated with a combination of RT and ADT, 1486 (48%) received monotherapy (RT alone, ADT alone or RP alone), and 461 (15%) received no active treatment.
The proportion of patients who received RP increased, exceeding 10% in 2005 .
Use of combined RT and ADT and use of ADT alone fluctuated throughout the study period.
In all 6% of patients received RT alone in 2005.
Multimodality therapy was less common in patients who were older, African American, unmarried, who lived in the south, and who had co-morbidities or stage T4 disease.
CONCLUSIONS
Treatment of LAPC varies widely, and treatment patterns shifted during the study period.
The slightly increased use of multimodality therapy since 2003 is encouraging, but further work is needed to increase combination therapy in appropriate patients and to define the role of RP.
Keywords: prostate cancer, locally advanced, treatment, SEER, practice patterns
INTRODUCTION
More than 200,000 men will be diagnosed with prostate cancer in the United States this year and up to 10% will have locally advanced disease (clinical stage T3 or T4) at presentation [1,2]. Numerous modalities, alone and in combination, have been advocated for treating these patients, but consensus guidelines are lacking. Mounting evidence supports the use of a multimodality approach to treat locally advanced prostate cancer (LAPC), including some combination of radiation therapy (RT) with androgen deprivation therapy (ADT) or radical prostatectomy (RP) with adjuvant RT. Indeed, multiple randomized controlled trials have demonstrated a survival advantage to combined RT and ADT compared with either modality alone [3–8]. Furthermore, adjuvant RT or ADT after RP in select patients with pathologically advanced prostate cancer confers a significant survival advantage [2,9–11].
The role of radical surgery for these patients has not been investigated systemically. Traditionally RP has not been routinely used in LAPC except in patients with low-volume, clinically staged T3 prostate cancer. Recent evidence suggests that patients with higher-risk prostate cancer treated initially with RP could have lower risks of metastatic progression and prostate cancer-specific death than those treated with RT initially [12]. Attempts to reduce the likelihood of biochemical recurrence after RP by using up to 8 months of neoadjuvant ADT have been unsuccessful [13–15]. The management of other clinically localized, high-risk solid tumours, such as breast and colon cancer, frequently combines surgery with other treatment modalities [16,17]. Such an approach has had limited success in prostate cancer. However, with refinements in RP technique and a reduced risk of perioperative complication rates, the role of surgery in combination with RT, chemotherapy or ADT for patients with LAPC is evolving.
On a population level, surprisingly little is known about LAPC treatment patterns and the proportion of patients receiving various treatment modalities. There is a poor understanding of which factors influence the type of treatment these patients receive and why some receive monotherapy while others are treated with multimodal strategies. Our objective was to characterize treatment patterns for clinically staged T3 and T4 prostate cancer in a population-based patient cohort and to identify predictors of multimodality therapy.
SUBJECTS AND METHODS
Data were obtained from the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) programme and linked Medicare claims and enrolment information [18]. The SEER-Medicare files were used in accordance with a data-use agreement from NCI, and the study was approved by the Institutional Review Board at Memorial Sloan-Kettering Cancer Center.
Patients ≥ 66 years with an incident diagnosis of clinically staged T3 or T4 prostate cancer between 1998 and 2005 were included in the study. Those diagnosed only at the time of death, who had a history of another malignancy or who had metastatic prostate cancer at diagnosis were excluded.
The primary outcome of interest was cancer treatment received within 6 months of diagnosis of LAPC. For descriptive purposes, patients were classified into four, mutually exclusive categories based on the most aggressive treatment received within this initial period: RP (open, minimally invasive or perineal); RT (external beam, brachytherapy or both; ADT (luteinizing hormone-releasing hormone agonist or orchidectomy); and (4) no active treatment (see the Appendix). Additional outcomes were single modality vs multimodality therapy, and treatments received within the first 24 months after diagnosis.
APPENDIX.
HCPCS AND ICD-9 CODES FOR TREATMENT OF LOCALLY ADVANCED PROSTATE CANCER
| HCPCS | ICD-9 | |
|---|---|---|
| Prostate cancer | 185, 233.4, 236.5 | |
| Radical prostatectomy | 60.5, 60.62 | 55801, 55810, 55812, 55815, 55821, 55831, 55840, 55842, 55845, 55866 |
| Radiation | ||
| External beam | 92.21, 92.22, 92.23 92.24, 92.25, 92.26 |
77400,77401,77402,77403,77404,77405,77406, 77407,77408,77409,77410,77411,77412,77413, 77414, 77416, 77417, 77418, 77419, 77420, 77425, 77427, 77430, 77431, 77432, 77470, 77520, 77522, 77523, 77525, 77789 |
| Brachytherapy | 92.27 | 55859,77750,77761,77762,77763,77776,77777, 77778, 77781, 77782,77783,77784,C2632,Q3001 |
| Androgen deprivation | ||
| Medical | 99.24 | 11980, J0970, J1000, J1380, J1390, J1950, J3315, J9202, J9217,J9218,J9219,S9560 |
| Surgical | 62.3,62.4, 62.41, 62.42 | 54520,54521,54522,54530,54535 |
Abbreviations: HCPCS, Healthcare Common Procedural Coding System; ICD, International Classification Of Diseases
Demographic characteristics included patient age, race, marital status, geographic location and residence in a metropolitan vs a non-metropolitan county. Median income in the census tract of residence was used as a marker of socioeconomic status. Clinical characteristics included clinical tumour stage, biopsy Gleason score and year of diagnosis. Comorbidity was estimated using the Charlson comorbidity index based on inpatient claims in the 12 months before prostate cancer diagnosis [19].
For statistical analysis, we characterized the cohort and their treatment patterns using descriptive statistics and used multivariable logistic regression to evaluate the impact of demographic and clinical characteristics on the likelihood of receiving multimodality therapy. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
In all, 3095 patients were identified with clinical stage T3 or T4, non-metastatic prostate cancer diagnosed between1998 and 2005 in the SEER-Medicare dataset; 48% of patients were ≥ 75 years and 82% of the cohort were white (Table 1). Seventy-nine per cent of patients were classified as clinical stage T3, and 21% as clinical stage T4. More than 60% of patients had a Gleason score ≥ 8. Patients treated with RP tended to be younger, have lower-staged tumours and less comorbidity than the other treatment categories. Patients who had ADT or no active treatment tended to be older and more likely to have clinically staged T4 LAPC.
TABLE 1.
Characteristics of cohort by most aggressive primary treatment within 6 months of Diagnosis
| All patients | RP ± RT, ADT | RT ± ADT | ADT | No active treatment | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 3095 (−) | 234 (8) | 1275 (41) | 1125 (36) | 461 (15) |
|
| |||||
| Age at diagnosis, years | |||||
| 66–69 | 681 (22) | 135 (58) | 277 (22) | 169 (15) | 100 (22) |
| 70–74 | 916 (30) | 70 (30) | 474 (37) | 265 (24) | 107 (23) |
| 75–79 | 806 (26) | 25 (11) | 369 (29) | 306 (27) | 106 (23) |
| 80–84 | 435 (14) | * (≤ 1) | 124 (10) | 225 (20) | 83 (18) |
| 85+ | 257 (8) | * (≤ 1) | 31 (2) | 160 (14) | 65 (14) |
|
| |||||
| Race | |||||
| White | 2530 (82) | 184 (79) | 1073 (84) | 918 (82) | 355 (77) |
| Black | 326 (11) | 29 (12) | 104 (8) | 120 (11) | 73 (16) |
| Other | 237 (8) | 21 (9) | 97 (8) | 86 8) | 33 (7) |
| Unknown | * (1) | * (≤ 1) | * (≤1) | * (1≤) | * (≤ 1) |
|
| |||||
| Census tract median income | |||||
| First quartile | 764 (25) | * (≤ 21) | 257 (20) | 317 (28) | 141 (31) |
| Second quartile | 765 (25) | 52 (22) | 326 (26) | 278 (25) | 109 (24) |
| Third quartile | 765 (25) | 57 (24) | 332 (26) | 272 (24) | 104 (23) |
| Fourth quartile | 764 (25) | 73 (31) | 346 (27) | 247 (22) | * (≤ 21) |
| Unknown | 37 (1) | * (≤ 1) | 14 (1) | 11 (1) | * (≤ 2) |
|
| |||||
| Urban-rural residence | |||||
| Metropolitan | 2534 (82) | 193 (82) | 1064 (83) | 895 (80) | 382 (83) |
| Non-metropolitan | 561 (18) | 41 (18) | 211 (17) | 230 (20) | 79 (17) |
|
| |||||
| Region | |||||
| North-east | 1512 (49) | 82 (35) | 657 (52) | 523 (46) | 250 (54) |
| South | 526 (17) | 28 (12) | 231 (18) | 199 (18) | 68 (15) |
| Midwest | 460 (15) | 53 (23) | 159 (12) | 187 (17) | 61 (13) |
| West | 597 (19) | 71 (30) | 228 (18) | 216 (19) | 82 (18) |
|
| |||||
| Married | |||||
| Yes | 2072 (67) | 180 (77) | 938 (74) | 685 (61) | 269 (58) |
| No | 765 (25) | 38 (16) | 282 (22) | 291 (26) | 154 (33) |
| Unknown | 258 (8) | 16 (7) | 55 (4) | 149 (13) | 38 (8) |
|
| |||||
| Clinical stage | |||||
| T3a | 604 (20) | 70 (30) | 304 (24) | 150 (13) | 80 (17) |
| T3b | 766 (25) | 75 (32) | 366 (29) | 217 (19) | 108 (23) |
| T3,nos | 1061 (34) | 63 (27) | 439 (34) | 413 (37) | 146 (32) |
| T4 | 664 (21) | 26 (11) | 166 (13) | 345 (31) | 127 (28) |
|
| |||||
| Preoperative PSA level | |||||
| Elevated | 2426 (78) | 168 (72) | 1040 (82) | 885 (79) | 333 (72) |
| Borderline | 135 (4) | * (≤ 9) | 70 (5) | 24 (2) | * (≤ 4) |
| Normal | 81 (3) | * (≤ 4) | 50 (4) | 15 (1) | * (≤ 2) |
| Unknown | 453 (15) | 36 (15) | 115 (9) | 201 (18) | 101 (22) |
|
| |||||
| Gleason score | |||||
| 2–4 | 30 (1) | * (≤ 1) | * (≤ 1) | * (≤ 1) | 11 (2) |
| 5–7 | 1031 (33) | 78 (33) | 492 (39) | 296 (26) | 165 (36) |
| 8–10 | 1933 (62) | 144 (62) | 753 (59) | 779 (69) | 257 (56) |
| Unknown | 101 (3) | * (≤ 4) | * (≤ 2) | * (≤ 4) | 28 (6) |
|
| |||||
| Year of diagnosis | |||||
| 1998 | 299 (10) | 14 (6) | 133 (10) | 100 (9) | 52 (11) |
| 1999 | 287 (9) | 16 (7) | 139 (11) | 92 (8) | 40 (9) |
| 2000 | 515 (17) | 43 (18) | 201 (16) | 185 (16) | 86 (19) |
| 2001 | 440 (14) | 24 (10) | 217 (17) | 140 (12) | 59 (13) |
| 2002 | 451 (15) | 17 (7) | 197 (15) | 171 (15) | 66 (14) |
| 2003 | 400 (13) | 30 (13) | 125 (10) | 183 (16) | 62 (13) |
| 2004 | 359 (12) | 37 (16) | 139 (11) | 137 (12) | 46 (10) |
| 2005 | 344 (11) | 53 (23) | 124 (10) | 117 (10) | 50 (11) |
|
| |||||
| Charlson comorbidity score | |||||
| 0 | 1348 (44) | 116 (50) | 618 (48) | 393 (35) | 221 (48) |
| 1 | 594 (19) | 64 (27) | 246 (19) | 212 (19) | 72 (16) |
| 2+ | 1153 (37) | 54 (23) | 411 (32) | 520 (46) | 168 (36) |
Cells with counts ≤ 11 and relevant adjacent cells are not shown, in adherence with SEER-Medicare Data Use Agreement.
Radiation therapy with or without other treatment modalities was the most common primary treatment within 6 months of diagnosis (41%), followed by ADT alone (36%), no active treatment (15%) and RP with or without other treatment modalities (8%) (Table 2).
TABLE 2.
Treatments received within 6 and 24 months after prostate cancer diagnosis
| Received within 6 months | Received within 24 months | |
|---|---|---|
| Treatment | N (%) | N (%) |
| ADT only | 1125 (36%) | 939 (30%) |
| RT + ADT | 1060 (34%) | 1352 (44%) |
| No active treatment | 461 (15%) | 346 (11%) |
| RT only | 215 (7%) | 212 (7%) |
| RP only | 146 (5%) | 109 (4%) |
| RP + ADT | 63 (2%) | 76 (2%) |
| RP + RT + ADT | * (≤ 1%) | 42 (1%) |
| RP + RT | * (≤ 1%) | 19 (1%) |
Cells with counts ≤ 11 and relevant adjacent cells are not shown, in adherence with SEER-Medicare Data Use Agreement.
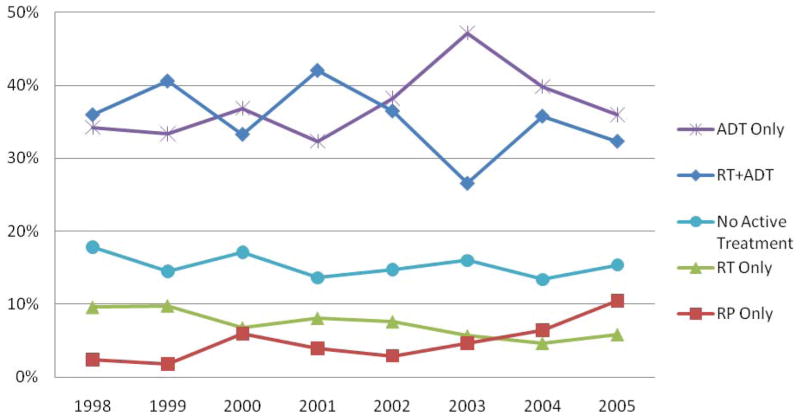
There was some variation over time in the frequency of the five most common treatments (Fig. 1). After 2003 there was a pronounced decrease in the use of ADT as monotherapy and an overall increase in the use of combined RT and ADT therapy. The proportion of patients who received both RT and ADT rose from 26% in 2003 to 32% in 2005, still slightly less than the 34% in 1998. The percentage treated with ADT alone decreased from 47% in 2003 to 36% in 2005. The use of RP alone increased from 2% in 1998 to 10% in 2005. Of the 234 patients who underwent RP, 188 (80%) received a pelvic lymph node dissection and, of those, 30 (16%) had positive lymph nodes on pathological evaluation.
FIG. 1.
Trends in five of the most common primary treatments given within 6 months of diagnosis of LAPC.
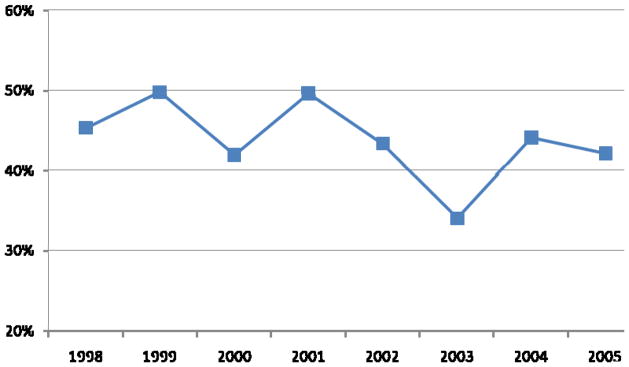
Over the entire study period, in the first 6 months after diagnosis single modality therapy was most common (48%), followed by multimodality therapy (37%) and no active treatment (15%). Of patients who had any active treatment, 42% received multimodality therapy in 2005, slightly fewer than the 45% observed in 1998, although this proportion fluctuated over the study period (Fig. 2). The increase in multimodality treatment since 2003 was due to the increased use of combined ADT and RT.
FIG. 2.
Trends in multimodality therapy (vs monotherapy) given within 6 months of diagnosis of LAPC (N = 2634; excludes patients receiving no active therapy [N = 461]).
In multivariable analysis, age, race, geographic region, marital status, clinical stage, Charlson comorbidity score and year of diagnosis were all significant predictors of receiving multimodality therapy rather than monotherapy (Table 3). Older and non-white patients were more likely to receive monotherapy than combination treatment, controlling for other characteristics. Black patients had 33% lower odds of receiving multimodality therapy than white patients (odds ratio [OR], 0.67; 95% CI, 0.50–0.91; P < 0.05). Clinically staged T4 patients had half the odds of receiving combination therapy compared with stageT3 patients (OR, 0.50; 95% CI, 0.40–0.62; P < 0.001).
TABLE 3.
Multivariable analysis of predictors of multimodality therapy versus monotherapy for treatment of locally advanced prostate cancer within 6 months following diagnosis (N = 2532)
| Characteristic | Adjusted OR (95% CI) | P value |
|---|---|---|
| Age at diagnosis, years | ||
| 66–69 | Reference | < 0.001 |
| 70–74 | 1.08 (0.86–1.35) | |
| 75–79 | 0.83 (0.66–1.05) | |
| 80–84 | 0.49 (0.36–0.66) | |
| 85+ | 0.21 (0.13–0.33) | |
|
| ||
| Race | ||
| White | Reference | 0.02 |
| Black | 0.67 (0.50–0.91) | |
| Other | 0.83 (0.61–1.14) | |
|
| ||
| Urban-rural residence | ||
| Metropolitan | Reference | 0.23 |
| Non-metropolitan | 0.87 (0.69–1.09) | |
|
| ||
| Region | ||
| Northeast | Reference | 0.001 |
| South | 0.77 (0.57–1.05) | |
| Midwest | 1.11 (0.83–1.47) | |
| West | 1.37 (1.08–1.72) | |
|
| ||
| Married | ||
| Yes | Reference | < 0.001 |
| No | 0.76 (0.62–0.94) | |
| Unknown | 0.41 (0.29–0.57) | |
|
| ||
| Clinical stage | ||
| T3 | Reference < 0.001 | |
| T4 | 0.50 (0.40–0.62) | |
|
| ||
| Gleason score | ||
| 5–7 | Reference | 0.34 |
| 2–4 | 1.05 (0.40–2.72) | |
| 8–10 | 1.15 (0.96–1.37) | |
|
| ||
| Charlson comorbidity score | ||
| 0 | Reference | 0.001 |
| 1 | 0.81 (0.65–1.01) | |
| 2+ | 0.69 (0.57–0.83) | |
| Year of diagnosis | 0.94 (0.90–0.98) | 0.002 |
Patients with missing race or Gleason score were excluded from analysis.
DISCUSSION
The National Comprehensive Cancer Network lists three initial treatment options for LAPC: combined RT and ADT, ADT alone and RP alone [20]. Although evidence from randomized controlled trials suggests that combining RT and ADT for LAPC is superior to either given as monotherapy [3–8], we found that 48% of patients in this population-based cohort were treated with monotherapy, 34% received a combination of RT and ADT, and 15% received no active treatment within 6 months of diagnosis. Throughout the study period, combined RT and ADT and ADT alone were the two most common treatment strategies, and a number of demographic and health characteristics impacted on receipt of multimodality therapy.
The urological literature is replete with studies describing treatment patterns for localized prostate cancer, but less is known about treatment patterns for locally advanced (clinical stage T3 or T4) disease. Several previous population-based studies described treatment patterns that differ somewhat from the findings of the present study [21–23]. Using SEER data alone, one analysis found that by 2001, 60% of patients with clinically staged T3 prostate cancer received RT, compared with 40% in 1995; RP utilization decreased from 18% in 1995 to 9% in 2001 [21]. Using the same database, another study focused on RP in clinically staged T4 patients and found that most of their cohort was treated with ADT or expectant management (62%) and only 7% had RP [22].
Using the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database, White et al. [24] investigated quality-of-life issues in patients undergoing treatment for LAPC. They describe 608 patients with clinical stage T3 or T4 prostate cancer (representing 4.4% of the CaPSURE cohort) and their primary treatment. In stark contrast to the findings of the present study, these authors report that 43% received ADT, 24% RT, 17% RP and 8% cryotherapy (percentages calculated from their table 1 of that study) [24]. Brachytherapy accounted for 84% of their RT treatment. Differences between these findings and the current study are likely attributable to differences in the study cohorts. The CaPSURE database, a provider-based registry of patients with prostate cancer from a number of community-based urology practices across the US, reflects the experience of patients in all age groups who are seen by CaPSURE urologists. A separate CaPSURE study focusing on localized prostate cancer (clinical stage ≤ T3a) found substantial treatment variation and concern for undertreatment of patients with high-risk disease as defined by the D’Amico risk groups and Cancer of the Prostate Risk Assessment (CAPRA) score.(25)
In the present study, the use of multimodality therapy increased from 2003 to 2005. While reports of improved survival and local control with combined ADT and RT vs RT alone for patients with LAPC appeared as early as 1997 [4], we found that 6% of patients in 2005 still received RT alone. Other randomized controlled trials investigating the use of RT with and without ADT in patients with LAPC were reported in the early 2000s and coincided with an increase in the use of combination RT and ADT and a decrease in the use of RT alone [3,26,27]. An optimistic explanation for these trends is the practice of evidence-based medicine, with providers changing disease management strategies as new, high-quality evidence emerges. Trends in LAPC treatment could also have been influenced by other factors. For example, the Medicare Modernization Act of 2003 drastically reduced physician payments for the administration of medical ADT starting in 2004 and probably contributed to its decreasing use [28]. Although we observed a slight absolute decrease in the use of RT alone, the percentage of patients who received this therapy remained relatively stable over the study period, despite evidence from randomized trials supporting the addition of ADT. Although combining ADT with RT confers a survival advantage to patients with LAPC over RT alone, side-effects from ADT could have a detrimental impact on quality of life. Concerns about adverse cardiac and skeletal events, cognitive and metabolic changes, and sexual side-effects might preclude some patients from receiving ADT with RT.
The role for RP alone or in combination with RT or ADT remains uncertain for patients with LAPC. Only 8% of patients in this cohort had RP with or without RT or ADT. Recent retrospective analysis of patients treated at one large academic cancer centre suggests that patients with higher-risk prostate cancer treated initially with RP could have a lower risk of metastatic progression and prostate cancer-specific death than those treated with RT initially. Adjusting for clinical variables, RP was associated with a reduced risk of metastasis (hazard ratio [HR], 0.35; 95% CI, 0.19–0.65; P < 0.001) and prostate cancer-specific mortality (HR, 0.32; 95% CI, 0.13–0.80; P = 0.01) [12]. Although there are no adequately powered randomized controlled trials comparing RT (with or without ADT) and RP (with or without ADT), combination therapy involving RT has become the predominant treatment for LAPC. Several single-institution, retrospective studies have described their surgical experience with LAPC. Researchers from Memorial Sloan-Kettering Cancer Center reported a 10-year actuarial probability of freedom from biochemical recurrence of 44% after RP alone for selected clinical stage T3 patients.(29) Likewise, the Mayo Clinic reported a 10-year recurrence-free rate of 43% for patients with T3 LAPC undergoing RP, showing that selected patients can be cured with surgery [30].
The present study includes a large, population-based cohort with detailed information about treatment, comorbidity and other important patient characteristics. Since prostate cancer is primarily a disease of the elderly, our findings in a population-based cohort of patients aged ≥ 66 years should be generalizable to most patients with LAPC. Caution is warranted in drawing inferences about the relationship between patient characteristics and the use of specific therapies, as unmeasured confounders could bias results. For example, information about functional status, patient preference and physician recommendations are not available in the SEER data set or in Medicare claims. In addition, SEER did not record numeric PSA values and exact Gleason scores until 2004, thus limiting our ability to control for those factors in multivariable analysis of treatment predictors.
In conclusion, treatment of LAPC varies widely. In terms of oncological outcomes, level one evidence shows the superiority of a multimodality approach for treating LAPC. Future efforts should focus on further increasing the use of multimodality therapy for appropriate patients with LAPC and better defining the role of RP in this patient population.
Abbreviations
- ADT
androgen deprivation therapy
- NCI
National Cancer Institute
- CaPSURE
Cancer of the Prostate Strategic Urologic Research Endeavor
- RP
radical prostatectomy
- RT
radiation therapy
- SEER
Surveillance, Epidemiology and End Results programme
Footnotes
CONFLICT OF INTEREST
None declared. Source of funding: This work was supported in part by funds from the National Institutes of Health (T32-CA82088 to P.S. and W.L., 1RC1CA146516-01 to J.E., E.E., D.Y. and W.L.); the National Cancer Institute (P50-CA92629 SPORE to P.S., CA118189-01A2 to E.E.); Sidney Kimmel Center for Prostate and Urologic Cancers; and David H. Koch provided through the Prostate Cancer Foundation.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep–Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Jr, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006 Nov 15;296(19):2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002 Jul 13;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997 Jul 31;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008 Jan 23;299(3):289–95. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 6.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005 Apr 1;61(5):1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008 Feb 1;26(4):585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 8.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009 Jan 24;373(9660):301–8. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 9.Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005 Aug 13–19;366(9485):572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 10.Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006 Jun;7(6):472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009 Mar;181(3):956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010 Mar 20;28(9):1508–13. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fair WR, Rabbani F, Bastar A, Betancourt J. Neoadjuvant hormone therapy before radical prostatectomy: Update on the Memorial Sloan-Kettering Cancer Center trials. Mol Urol. 1999;3(3):253–60. [PubMed] [Google Scholar]
- 14.Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001 Aug;166(2):500–6. discussion 6–7. [PubMed] [Google Scholar]
- 15.Yee DS, Lowrance WT, Eastham JA, Maschino AC, Cronin AM, Rabbani F. Long-term follow-up of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010 Jan;105(2):185–90. doi: 10.1111/j.1464-410X.2009.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monga DK, O'Connell MJ. Surgical adjuvant therapy for colorectal cancer: current approaches and future directions. Ann Surg Oncol. 2006 Aug;13(8):1021–34. doi: 10.1245/ASO.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, et al. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40(4):321–9. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Prostate (Version 3.2010) http://www.nccn.org [October 16, 2010]; Available from: http://www.nccn.org.
- 21.Denberg TD, Glode LM, Steiner JF, Crawford ED, Hoffman RM. Trends and predictors of aggressive therapy for clinical locally advanced prostate carcinoma. BJU Int. 2006 Aug;98(2):335–40. doi: 10.1111/j.1464-410X.2006.06260.x. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone PA, Ward KC, Goodman M, Assikis V, Petros JA. Radical prostatectomy for clinical T4 prostate cancer. Cancer. 2006 Jun 15;106(12):2603–9. doi: 10.1002/cncr.21926. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer D, Egleston B, Abdalla I. Patterns of prostate cancer treatment by clinical stage and age. Am J Public Health. 2001 Jan;91(1):126–8. doi: 10.2105/ajph.91.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White WM, Sadetsky N, Waters WB, Carroll PR, Litwin MS. Quality of life in men with locally advanced adenocarcinoma of the prostate: an exploratory analysis using data from the CaPSURE database. J Urol. 2008 Dec;180(6):2409–13. doi: 10.1016/j.juro.2008.08.079. discussion 14. [DOI] [PubMed] [Google Scholar]
- 25.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010 Mar 1;28(7):1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003 Nov 1;21(21):3972–8. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001 Aug 1;50(5):1243–52. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 28.Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer. 2008 May 15;112(10):2195–201. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- 29.Carver BS, Bianco FJ, Jr, Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006 Aug;176(2):564–8. doi: 10.1016/j.juro.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 30.Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005 Apr;95(6):751–6. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]