Abstract
Nitrogen containing bisphosphonates (NBPs) are taken by millions for bone disorders but may cause serious inflammatory reactions. Here, we utilized a murine peritonitis model to characterize the inflammatory mechanisms of these agents. At dosages comparable to those used in humans, injection of NBPs into the peritoneum caused recruitment of neutrophils, followed by an influx of monocytes. These cellular changes corresponded to an initial increase in IL-1α, which preceded a rise in multiple other proinflammatory cytokines. IL-1 receptor, IL-1α, and IL-1β were required for neutrophil recruitment, whereas other MyD88-dependent signaling pathways were needed for the monocyte influx. Mice deficient in mast cells, but not mice lacking lymphocytes, were resistant to NBP-induced inflammation and reconstitution of these mice with mast cells restored sensitivity to NBPs. These results document the critical role of mast cells and IL-1 in NBP mediated inflammatory reactions.
INTRODUCTION
Alendronate, pamidronate, and zoledronate are Food and Drug Administration (FDA) approved nitrogen-containing bisphosphonates (NBPs) that are used as oral or intravenous treatments for osteoporosis and cancer related bone disorders(1). Bisphosphonates bind to the bone surface, have a very long half-life, and thus can attain high local concentrations. They prevent osteoclast mediated bone degradation through inhibition of the mevalonate pathway, which leads to altered protein prenylation(2). Several inflammatory reactions have been observed in patients treated with NBPs, of which osteonecrosis of the jaw is the most ominous(3, 4). NBPs can modulate immunity as a result of inhibition of the mevalonate pathway, leading to an accumulation of isopentenyl-5-pyrophospate (IPP) in monocytes, which then activate γδT-cells(5). However, γδT-cells have not been conclusively shown to be the major cause of NBP-induced inflammatory reactions in vivo. Pretreatment of mice with alendronate increases IL-1 production in response to lipopolysaccharide (LPS) (6, 7) and sensitizes cells to MyD88-dependent signaling(8); however, a more precise mechanism of NBP immune modulation has remained elusive. Hence, the goal of the studies presented here was to dissect the role of different cell types and mediators in NBP-induced inflammation.
MATERIALS AND METHODS
Mice
C57BL/6,Rag1−/−, Beige (Lystbg-J/Lystbg-J), Il1r1−/−, P2x7R−/−, and c-kitW/Wv (W/Wv) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Nlrp3−/− mice were gifts from Dr. H.Hoffman (University of California San Diego, CA -personal communication)(9), Casp1−/− and Myd88−/−mice were gifts from Dr. R.Flavell (Yale University, New Haven, CT) (10), and Dr. S.Akira (Osaka University, Japan)(11) respectively and were maintained on a C57BL/6 backgroundat UCSD. Pretty2 mice were a gift from Dr. Bruce Beutler(The Scripps Research Institute, La Jolla, CA) (12). Il1α−/− and Il1β−/−mice were obtained from Yoichiro Iwakura, University of Tokyo, Japan(13) and maintained on a C57BL/6 background.Mast cell engraftment to W/Wv mice was performed as described previously (14).All procedures and protocols were approved by the InstitutionalAnimal Care and Use Committee.
Reagents
Alendronate (ALD), pamidronate (PMD), clodronate (CLD), cromolyn, compound 48/80, and lovastatin were purchased from Sigma (St. Louis, MO) and zoledronate (ZLD)was purchased from AK Scientific (Mountain View, CA). Bisphosphonates were endotoxin free as determined by the Limulus Amebocyte Lysate test (Charles River, Wilmington MA). PBS was used as the vehicle control.
In vivo peritonitis experiments
Mice (8–12week old)were injected i.p. with 0.8 mg/mouse ALDor other bisphosphonates as indicated. Peritoneal cells were recovered with 3mL PBS and total cell numbersin peritoneal lavage fluids were determined with a Guava Personal Cytometer (Millipore, Danvers MA) and cells were differentiated by Wright-Giemsa staining. The levels of cytokines and chemokines in serum and lavage fluid were analyzed with Luminex beads (Invitrogen, Carlsbad CA). Cromolyn and compound 48/80 were i.p. injected as previously described(14).
Mast cell preparation and in vitro assays
Bone marrow-derived mast cells (BMMC) were prepared in culture with 3ng/mL IL-3 (BD Biosciences, San Jose, CA) for 6 weeks (14). Cultures were confirmed to be >99% mast cells with toluidine blue staining and FACS analysis for FcεR1 and c-kit (BioLegend, San Diego, CA). Mesothelial cells were isolated as previously described (15) and were plated at a density of 104 cells/well in 96 well plates. Mast cells (105 cells/well) were added and co-cultured with themesothelial cells (1:10 ratio) in appropriate media with 50ng/mL murine stem cell factor (Sigma) (17). IL-1α and IL-6 levels in the culture supernatants were measured by ELISA (BioLegend, san Diego CA, and BD Biosciences, respectively). Mast cell degranulation was measured by treating cells with 100μM of the indicated compounds for 1.5 h, and measuringβ-hexosaminidase release in a colorimetricassay, as previously described (16).
Statistics
Statistical analyses for multiple comparisons were performed using one-way ANOVA with Dunnett's or Bonferroni's test as indicated. A p<0.05 was considered significant.
RESULTS AND DISCUSSION
Characterization of nitrogen-bisphosphonate-induced peritonitis
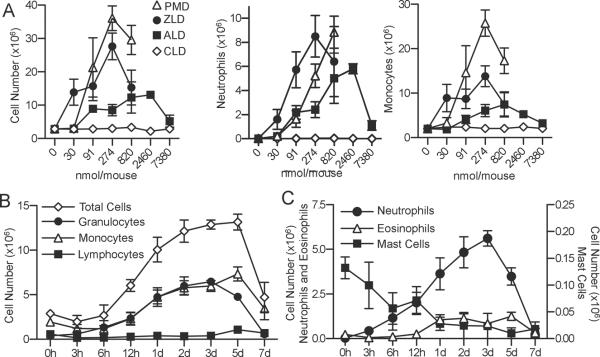
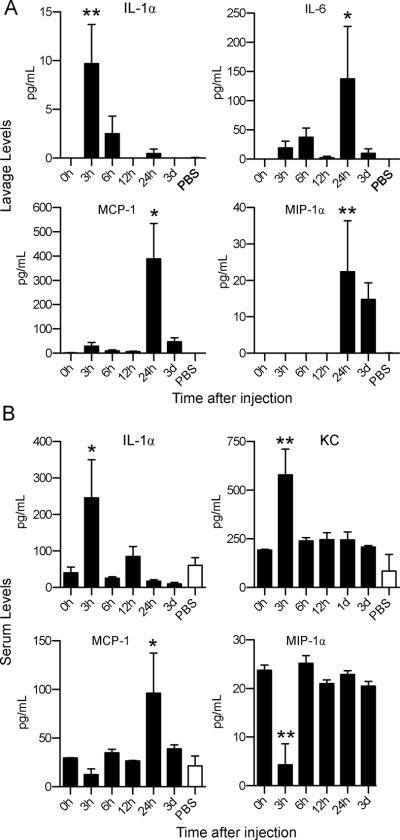
Alendronate (ALD), pamidronate (PMD), and zoledronate (ZLD) all caused profound inflammation after injection into the peritoneal cavity of mice. In contrast, the non-nitrogen-bisphosphonate clodronate (CLD) did not cause peritonitis (Figure 1A). The proinflammatory effect of NBPs, but not CLD, validated this model, since NBPs can cause inflammatory side effects in humans, while CLD does not(17). In subsequent experiments, ALD was used as a prototypical NBP to elucidate the cell types mediating the inflammation (18). Intraperitoneal administration of as little as 91 nmol (29 μg) mouse induced an increase in leukocyte infiltration (Figure 1A). Time course experiments demonstrated that neutrophils appeared in the peritoneal cavity after ALD injection, followed by a large-scale influx of monocytes (Figure 1B) and neutrophils (Figure 1C). The results indicated that there were two distinct phases of inflammation caused by ALD (Figure 1B and 1C). The increase in neutrophils correlated with a peak in IL-1α levels in the peritoneum, and in both IL-1α and KC levels in the serum (p< 0.05Figure 2). The later influx of monocytes at 24–72h correlated with a peak in MCP-1, MIP-1α, and IL-6 levels in the peritoneum and MCP-1 in the serum (Figure 2).
Figure 1. Characterization of the murine peritonitis model induced by nitrogen-bisphosphonates.
A)C57BL/6 mice (n= 5–6) werei.p. injected with different doses of NBPs pamidronate (PMD), zoledronate (ZLD), and alendronate (ALD) as well as the non-nitrogen-bisphosphonate clodronate (CLD). Infiltrating cells were recovered 3 days after administration. B)Mice (n=5) were injected i.p. with 0.8mg/mL ALD and peritoneal infiltrating cells were recovered at different time points. C) The granulocytes populations were further differentiated into neutrophils, eosinophils, and mast cells based on morphology. Data are represented as means±SEMof pooled two independent experiments.
Figure 2. Alendronate causes a rapid increase and IL-1 α and KC.
Peritoneal lavage fluid and serum were collected at indicated time points from mice (n=5) i.p. administered 0.8 mg ALD (black bars) or vehicle (white bars). The levels of chemokines and cytokines in lavage (A) and serum (B) were analyzed by Luminex bead assay. Data are represented as means+SEM. * p<0.05, and ** = p<0.01 compared to 0h or vehicle controls (at the time point of significance with ALD) by one-way ANOVA with Dunnett's test.
Alendronate-induced peritonitis is dependent upon mast cells
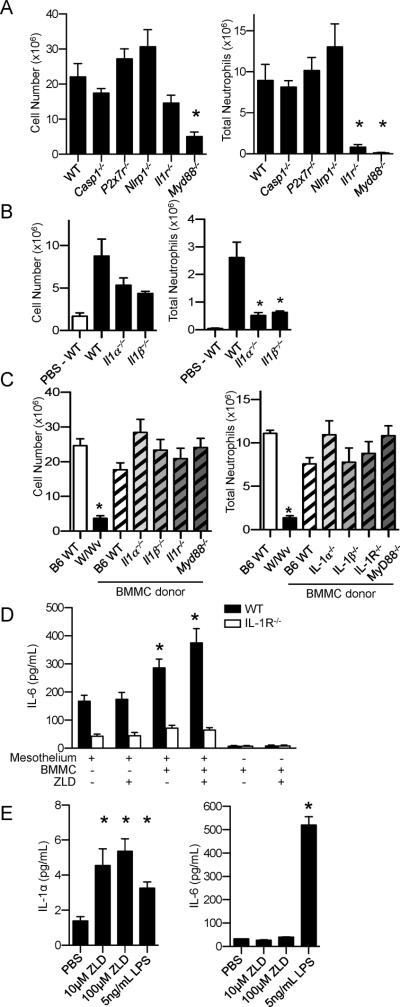
Mice deficient in specific cell types were injected with ALD to determine which cells are responsible for the inflammatory effects of the drug. Rag1−/− mice lack mature lymphocytes, including γδT-cells(17),but developed ALD-induced peritonitis indistinguishable from WT mice (Figure 3A). Similarly, Beige (BgJ) mice, which lack natural killer cells, had an unimpaired inflammatory response to ALD. These results indicated that γδT-cells are dispensable for NBP-induced peritonitis in mice. In addition, pretreatment of mice with i.p. administration of lovastatin (2.46μmol/mouse on days −1, 0, 1, and 2) to prevent ALD induced accumulation of IPP(19), did not inhibit the ALD-induced leukocyte infiltration (leukocyte number ×106±SEM: ALD – 22.6±6.3, lovastatin + ALD – 25.2±2.1; n=4, p=0.7), indicating further that the pyrophosphate metabolites that activate γδT-cells(5) are not exclusively responsible for NBP-induced peritoneal inflammation. Pretty2 and W/Wvmice are deficient in melanocytes and mast cells due to mutations in the c-kit gene(12, 14, 20).ALD did not cause a significant influx of cells into the peritoneum of the two mast cell deficient mouse strains (Figure 3A and B). Moreover, reconstitution of W/Wv mice with WT mast cells restored their ALD responsiveness (Figure 3B). These results confirm a role for mast cells in ALD-induced peritonitis. Mast cell degranulation was not required for the inflammatory actions of ALD, because inhibition of degranulation with cromolyn, or exhaustive prior degranulation with compound 48/80, did not attenuate ALD-induced peritonitis in WT mice (Figure 3C). In addition, ALD and ZLD did not induce most cell degranulation in vitro (Figure 3D).
Figure 3. Alendronate requires mast cells to induce peritonitis.
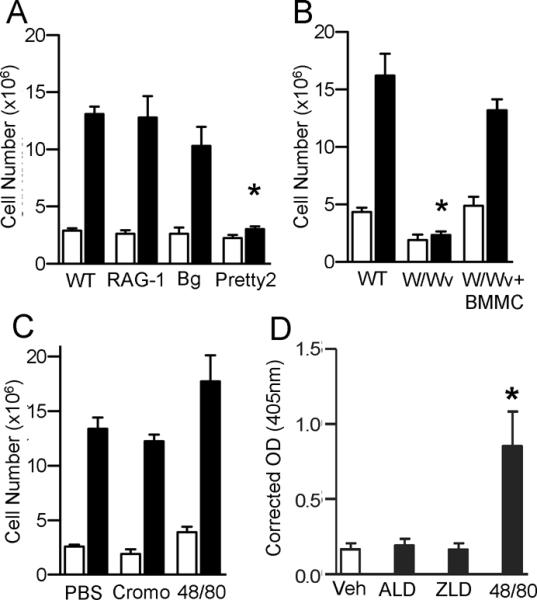
A) WT C57BL/6 (n=6/group), Rag1−/−(n=7/group), beige (BgJ, n=5–6/group) or Pretty2 (n=3/group) mice were injected with 0.8 mg ALD (black bar) or vehicle (white bar). The peritoneal lavage was collected after 3 days. B) W/Wv mice, littermate WT mice, and W/Wv mice reconstituted BMMC received ALD (black) or vehicle (white). The peritoneal lavage was collected and total cell number was determined. C) ALD was injected in WT mice (n=10–12/group) treated with cromolyn (Cromo, n=7–8/group) or compound 48/80 (48/80, n=5/group). Peritoneal infiltrating cells were collected 3 days after ALD (black) or vehicle (white) injection.D)Degranulation of BMMC treated with 100μM each compound for 1.5h measured by β-hexosaminidase released in supernatant (Å405). For all panels data is the pooled means+SEM from 2–3 independent experiments, *p<0.05 by one-way ANOVA with Dunnett's test.
IL-1 is necessary for the NPB-induced inflammation
NBP-induced total leukocyte and neutrophil infiltration into the peritoneal cavity were both dependent on MyD88 signaling, while IL-1R deficiency significantly diminished only the infiltration of neutrophils (Figures 4A and 4B). IL-1α and IL-1β deficiencies similarly decreased the numbers of neutrophils in ALD-induced peritonitis (Figure 4B). Mice deficient in factors involved in inflammasome activation did not have attenuated responses to ALD (Figure 4A), supporting earlier findings (21). Taken together, these data reveal that IL-1α and IL-1β are involved in NBP-induced neutrophil infiltration, independent of inflammasome activation.
Figure 4. Mast cells and IL-1 were involved in NPB-induced peritonitis.
A) WT C57BL/6 (n=10/group), Casp1−/−(n=8 /group), Nlrp3−/− (n=8/group), P2x7−/−(n=6/group) or Il1r−/− (n=7/group) mice wereinjected with 0.8mg ALD. The number of infiltrating cells (left) and neutrophil number (right) in the peritoneal lavage were determined 3 days after ALD administration.B) Five-6 week old WT C57BL/6 (n=6/group), Il1α−/− (n=9/group), and Il1β−/− (n=6/group) mice werei.p. injected with 0.8mg ALD and WT C57BL/6 (n=4/group) mice were injected with PBS. The number of infiltrating cells (left) and neutrophil number (right) in the peritoneal lavage were determined 24h after ALD administration. Data are pooled means+SEM from two independent experiments. C) W/Wvmice were reconstituted with mast cells derived from WT, and Myd88−/−, Il1r−/−, Il1α−/−, and Il1β−/− mice (n=8–9/group). The infiltrating cells were collected 3 days after administration of ALD. A–C) Data are pooled means+SEM from 2 independent experiments *p<0.05 by one-way ANOVA with Dunnett's test compared to WT ALD treated mice. D) IL-6 secretion in co-cultures of BMMC and peritoneal mesothelial cells (from WT or Il1r−/− mice). BMMC derived from WT mice were cultured with mesothelial cells from WT or Il1r−/− mice for 6 h in the presence and absence of ZLD (10 μM). IL-6 in the supernatant was quantified by ELISA. Data are mean+SEM of a representative experiment (n =3) performed in triplicate. *p<0.05 by one-way ANOVA with Bonferroni's post-hoc test. E) IL-1α and IL-6 release from BMMC 16h after treatment with ZLD or LPS. Data are mean+SEM of a representative experiment (n =2) performed in triplicate. *p<0.05 compared to vehicle by one-way ANOVA with Bonferroni's test.
ALD did not induce peritonitis in mice lacking mast cells (W/Wv) (Figure 3B). Mast cell reconstitution studies showed that the proinflammatory effects of the mast cells were not diminished by individual loss of IL-1α, IL-1β, IL-1R or MyD88 in mast cells (Figure 4C). Thus, the data are consistent with a model in which mast cells are a pre-existing source of IL-1α or IL-1β, which are released after NBP exposure. Peritoneal mesothelial cells are known to be IL-1 responsive (22). IL-1α and IL-1β have redundant function in this regard because individual loss of either IL-1α or IL-1β did not attenuate the proinflammatory effects of NBP (Figure 4C). Co-cultivation of mast cells with peritoneal mesothelial cells induced an increase in IL-6 production (Figure 4D and 4E). Furthermore, when ZLD, an NBP more potent than ALD, was added to the mast cell-mesothelial cell co-cultures, additional IL-6 was released, which required IL-1R expression by the mesothelial cells (Figure 4D). Treatment of isolated mast cells with ZLD caused a selective release of IL-1α, compared to IL-6 (Figure 4E), suggesting that NBPs stimulated mast cells to release IL-1, which in turn activated theIL-1R on the mesothelial cells.
In summary, NBP-induced peritoneal inflammation depends upon mast cells and IL-1R signaling for neutrophil recruitment. The NPBs are rapidly cleared from plasma and sequestered in bone (23).The half life of ALD in bone is at least 200 days in rodents and over 1000 days in dogs (23). Hence, ALD and other NPB concentrations in bone progressively rise with time and can attain high local levels. Thus the pharmacokinetics of NBPs, and their ability to recruit neutrophils, as demonstrated here, may explain the inflammation and osteonecrosis that can occur in humans after chronic administration.
Acknowledgments
JTN was supported by T32CA121938 Postdoctoral Cancer Therapeutics Training grant. In addition, this work was supported by the Arthritis Foundation (MPC), by CA23100 (DAC) from the NIH/NCI and HHSN272200900034C (DAC) from DEA/NIH/NIAID.
We thank Shiyin Yao, Christine Gray, Lisa Ronacher, and Richard Mathewson of UCSD for technical assistance.
Abbreviations
- NBP
nitrogen bisphosphonates
- ALD
alendronate
- ZLD
zoledronate
- CLD
clodronate
- PMD
pamidronate
- IPP
isopentyl farnesyl pyrophosphate
- FDA
Food and Drug Administration
- KC
murine chemokine (C-X-C motif) ligand 1
Footnotes
CONFLICTS OF INTEREST None
REFERENCES
- 1.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- 2.Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. Bisphosphonates: Molecular Mechanisms of Action and Effects on Bone Cells, Monocytes and Macrophages. Curr Pharm Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 3.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 4.Lesclous P, Abi Najm S, Carrel JP, Baroukh B, Lombardi T, Willi JP, Rizzoli R, Saffar JL, Samson J. Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45:843–852. doi: 10.1016/j.bone.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi K, Motegi K, Iwakura Y, Endo Y. Involvement of interleukin-1 in the inflammatory actions of aminobisphosphonates in mice. Br J Pharmacol. 2000;130:1646–1654. doi: 10.1038/sj.bjp.0703460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng X, Yu Z, Funayama H, Shoji N, Sasano T, Iwakura Y, Sugawara S, Endo Y. Mutual augmentation of the induction of the histamine-forming enzyme, histidine decarboxylase, between alendronate and immuno-stimulants (IL-1, TNF, and LPS), and its prevention by clodronate. Toxicol Appl Pharmacol. 2006;213:64–73. doi: 10.1016/j.taap.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Norton JT, Hayashi T, Crain B, Corr M, Carson DA. Role of IL-1 receptor-associated kinase-M (IRAK-M) in priming of immune and inflammatory responses by nitrogen bisphosphonates. Proc Natl Acad Sci U S A. 108:11163–11168. doi: 10.1073/pnas.1107899108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, Soroosh P, Watford WT, O'Shea JJ, Kastner DL, Hoffman HM. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coban C, Igari Y, Yagi M, Reimer T, Koyama S, Aoshi T, Ohata K, Tsukui T, Takeshita F, Sakurai K, Ikegami T, Nakagawa A, Horii T, Nunez G, Ishii KJ, Akira S. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe. 2010;7:50–61. doi: 10.1016/j.chom.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Guma M, Kashiwakura J, Crain B, Kawakami Y, Beutler B, Firestein GS, Kawakami T, Karin M, Corr M. JNK1 controls mast cell degranulation and IL-1{beta} production in inflammatory arthritis. Proc Natl Acad Sci U S A. 107:22122–22127. doi: 10.1073/pnas.1016401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi T, Cottam HB, Chan M, Jin G, Tawatao RI, Crain B, Ronacher L, Messer K, Carson DA, Corr M. Mast cell-dependent anorexia and hypothermia induced by mucosal activation of Toll-like receptor 7. Am J Physiol Regul Integr Comp Physiol. 2008;295:R123–132. doi: 10.1152/ajpregu.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bot J, Whitaker D, Vivian J, Lake R, Yao V, McCauley R. Culturing mouse peritoneal mesothelial cells. Pathol Res Pract. 2003;199:341–344. doi: 10.1078/0344-0338-00427. [DOI] [PubMed] [Google Scholar]
- 16.Kuehn HS, Radinger M, Gilfillan AM. Curr Protoc Immunol Chapter 7:Unit7. 2010. Measuring mast cell mediator release; p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mombaerts P. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int Rev Immunol. 1995;13:43–63. doi: 10.3109/08830189509061737. [DOI] [PubMed] [Google Scholar]
- 18.Deng X, Yu Z, Funayama H, Yamaguchi K, Sasano T, Sugawara S, Endo Y. Histidine decarboxylase-stimulating and inflammatory effects of alendronate in mice: involvement of mevalonate pathway, TNFalpha, macrophages, and T-cells. Int Immunopharmacol. 2007;7:152–161. doi: 10.1016/j.intimp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Thompson K, Roelofs AJ, Jauhiainen M, Monkkonen H, Monkkonen J, Rogers MJ. Activation of gammadelta T Cells by Bisphosphonates. Adv Exp Med Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 20.Oda A, Wakao H, Fujita H. Calpain is a signal transducer and activator of transcription (STAT) 3 and STAT5 protease. Blood. 2002;99:1850–1852. doi: 10.1182/blood.v99.5.1850. [DOI] [PubMed] [Google Scholar]
- 21.Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JH, Duggan DE, Chen IW, Ellsworth RL. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate, in laboratory animals. Drug Metab Dispos. 1991;19:926–932. [PubMed] [Google Scholar]