Abstract
Background & Aims
Little information is available about what factors determine serum levels of α-fetoprotein (AFP) levels (e.g., demographic, virological, or clinical features) among individuals who do not develop hepatocellular cacrcinoma (HCC). This information might improve AFP-based algorithms for HCC detection.
Methods
We examined data from patients in the national Veterans’ Affairs Hepatitis C Virus (HCV) Clinical Case Registry who received at least 1 AFP test (258,275 AFP tests in 76,357 patients; 1.9% developed HCC). We constructed hierarchical multivariate models of AFP levels. Potential predictors of AFP values included patients’ sex, race, cirrhosis status, model for end-stage liver disease (MELD) score, HCV genotype, level of alanine aminotransferase (ALT) within 30 days before the AFP test, time to diagnosis of HCC, and time elapsed from the HCV index date.
Results
Significant determinants for increased levels of AFP included presence of cirrhosis, higher MELD scores and increased levels of ALT. AFP levels were also affected by the interaction between ALT levels and the presence and time to development of HCC. Among patients that did not have HCC, the AFP level increased with the level of ALT; the AFP values in the presence of ALT (37–56 U/L), ALT (57–92 U/L), or ALT > 92 U/L were 16%, 35%, and 68% higher, respectively, than AFP values at ALT (0–36 U/L). However, patients who developed HCC within 30 days of receiving the AFP test had a lower rate of increase in AFP with each higher category of ALT level, with increases of 31%, 39% and 37% for the same respective ALT categories.
Conclusions
In patients with chronic HCV infection, AFP and ALT values correlate; however, among patients with HCC, levels of AFP increase disproportionately to, or unaccompanied by, increases in levels of ALT. The prognostic and diagnostic value of AFP levels might be increased by adjusting for ALT values.
Keywords: biomarker, risk, liver cancer, disease progression
Introduction
Alpha-fetoprotein (AFP) is a glycoprotein used as a marker for the detection of hepatocellular carcinoma (HCC). AFP is produced by the fetal liver and becomes normally undetectable 8–12 months after birth, but is known to be elevated in germ cell cancer, liver disease and HCC1, 2. Although it has been used as tumor marker, there is variable AFP test performance in the surveillance and detection of HCC3, 4. These variations may be attributed to different study designs and populations including underlying etiology of liver disease (i.e. hepatitis B or C)5, 6. AFP test performance even differs among studies of similar design as well as study populations. For example, a systematic review of 5 high quality studies that used a > 20 ng/mL cutoff value of a positive AFP test showed the sensitivity for detecting HCC was 41% to 65%; specificity, 80% to 94%; positive likelihood ratios, 3.1 to 6.8; and negative likelihood ratios, 0.4 to 0.6 3. These variations may be related to other factors beyond study design and liver disease etiology. For instance, serum AFP levels are also higher in patients with advanced fibrosis7–9. Furthermore, AFP cut-off value is fluctuant in different ethnic groups. The best threshold value of AFP for HCC detection in surveillance has been reported to be 30 ng/mL in a U.S. population (sensitivity of 66%)10 and in a Sicilian population (sensitivity of 65%, specificity of 89%) compared with 200 ng/mL cutoff (sensitivity of 70%, specificity of 100%) in a Burmese population11. Therefore, there seem to be patient factors that influence AFP test characteristics and the ability to detect HCC.
There is reason to believe that other demographic, virologic or clinical variables influence AFP levels12. However, apart from cirrhosis and HCC, relatively little is known about the determinants of serum AFP levels. A U.S. study reported a raised serum AFP in association with female sex, Black race, decreased platelet count, increased serum AST/ALT ratio, and serum ferritin among 1,145 HCV-infected patients with advanced hepatic fibrosis enrolled in a randomized controlled trial of HCV treatment8. Other non US studies have shown age, decreased platelet count, ALT and AST elevation to be associated with elevated serum AFP levels7, 9. Incorporation of these factors may improve the predictive value of serum AFP level-based algorithms for the diagnosis of HCC. Therefore, we examined several possible determinants of serum AFP values obtained among members of a large cohort with HCV-infected patients. The goal of this study was the construction of models that predict the serum AFP values.
Methods
Data sources and study population
We identified adult patients in the VA HCV Clinical Case Registry13 who were diagnosed with HCV during 1998–2005, and had at least one AFP test after HCV diagnosis. Details of the overall cohort were previously published14. The date of first occurrence of a positive HCV antibody test or HCV ICD-9-CM code served as the HCV index date. Patients who developed HCC or died within 12 months following HCV index date were excluded. HCC was defined using a previously validated algorithm based on the presence of ICD-9-CM code 155.0 and the absence of ICD-9-CM code 155.115. We did not exclude patients with additional causes of liver disease (e.g., alcohol or hepatitis B). We obtained information on patient demographics including age, race, and sex. Cirrhosis was identified by one of several previously validated ICD-9-CM codes (571.2, 571.5, or 571.6)16 and assigned a cirrhosis index date based on the first appearance of a cirrhosis codes. Liver disease severity was assessed with the Model for End Stage Liver Disease (MELD) score, which was calculated using laboratory values within 6 months prior to or following the HCV (or cirrhosis) index date17. Laboratory data were used to determine AFP levels in ng/ml, alanine aminotransferase (ALT) levels in U/L and aspartate aminotransferase (AST) levels in U/L.
Data Analysis
AFP levels
We constructed a hierarchical multivariate model to predict the logarithmic values of the AFP levels. The logarithmic transformation was performed to correct for the skewed distribution of the AFP variable.
Predictors
Potential predictors of AFP fell into two categories: fixed baseline factors associated with average AFP levels and time-varying factors associated with changes in AFP level over time. The baseline factors included cirrhosis status, MELD score at HCV index, HCV genotype, race, sex and age at HCV diagnosis. Time-varying factors included most recent ALT level measured within 30 days prior to AFP test, time to HCC diagnosis (if any), and time elapsed from HCV index. HCV genotype was categorized into 1 or 4, 2 or 3, no result or no test done. ALT results were categorized into 5 levels: quartiles of the ALT values (with thresholds at 36, 56 and 92) and a “no ALT test within 30 days of AFP”. MELD score was categorized into 4 levels (<15, 15–20, > 20 and missing). Time to HCC diagnosis was categorized into 3 levels: HCC within 30 days of AFP test, HCC within 31–365 days and no HCC within 365 days. Time elapsed from HCV index to AFP test was included in the model to adjust for the year-by-year effect of unmeasured time-varying effects on AFP level. Likewise, modification of ALT effect by patient cirrhosis status was modeled by cirrhosis-ALT interaction terms. Correlation of within-patient AFP values due to fixed patient level attributes was adjusted for by inclusion of a normally distributed patient-level intercept random effect.
Model construction
In the model building process, predictors or covariates were considered in which Wald univariate p-values were < 0.15. At each subsequent step, variables were retained on the basis of significance tests and effect size. Significance was assessed by Wald tests of class effects, adjusted by the Bonferroni and Benjamini–Hochberg–Yekutieli methods to control for the family-wide error rate and the false discovery rate (FDR), respectively. Predictors and covariates were required to attain Wald p-values ≤0.05 and show at least 10% difference in AFP among the variable levels. Effects which showed a difference in AFP level of at least 20% were considered to be important predictors.
Results
We analyzed 258,275 AFP tests in a cohort of 76,347 HCV-infected patients who had at least one AFP test after HCV diagnosis (Table 1); the median age of these patients was 50 (IQR 46–54) and 54.3% were Caucasian. Of these patients, 41,077 (53.8%) of these were tested in the first year following HCV diagnosis and an additional 12,123 (15.8%) were tested in the second year. The characteristics of this cohort and the subcohort of patients who developed HCC were previously published14, 18. The diagnosis of cirrhosis was recorded in 12,675 (16.6%) patients and 1,480 (1.9%) developed HCC. The mean duration of time between the HCV index date and the HCC diagnosis date was 3.86 years (SD ± 1.96).
Table 1.
Characteristics of 76,347 patients with HCV and at least one AFP test following HCV index date.
| variable | value |
|---|---|
| age at HCV index
| |
| median (IQR) | 50 (46–54) |
|
| |
| gender
| |
| female | 2145 (2.8%) |
| male | 74202 (97.2%) |
|
| |
| race
| |
| white | 41451 (54.3%) |
| black | 24228 (31.7%) |
| other | 5279 (6.9%) |
| unreported | 5389 (7.1%) |
|
| |
| cirrhosis
| |
| no | 63672 (83.4%) |
| yes | 12675 (16.6%) |
|
| |
| MELD category
| |
| <15 | 33044 (43.3%) |
| 15–20 | 879 (1.1%) |
| >20 | 282 (0.4%) |
| missing | 42142 (55.2%) |
|
| |
| HCV genotype
| |
| 1 | 35671 (46.7%) |
| 2 | 5401 (7.1%) |
| 3 | 3380 (4.4%) |
| 4 | 426 (0.6%) |
| no result | 5660 (7.4%) |
| not done | 25809 (33.8%) |
|
| |
| HCC
| |
| no | 74859 (98.1%) |
| yes | 1488 (1.9%) |
|
| |
| highest AFP ng/ml
| |
| median (IQR) | 4.6 (3.0–8.4) |
|
| |
| highest ALT U/L
| |
| median (IQR) | 65.0 (40.–110.0) |
|
| |
| highest AST U/L
| |
| median (IQR) | 53.0 (34.0–93.0) |
There were several important predictive correlates of elevated AFP levels, which were statistically significant and met the 20% effect size criterion in both unadjusted and adjusted analyses. Fixed baseline factors including presence of cirrhosis, HCV genotype 1 or 4, lower MELD scores and Black race were associated with higher AFP levels. AFP values for MELD > 20 were 20 % lower than those corresponding to MELD < 15. Black race was associated with a 24.8% increase in AFP value over white race and HCV genotype 2 or 3 with 24.2% reduced AFP levels over genotype 1 or 4. We also found a significant race-HCV genotype interaction that can be summarized as follows: blacks with genotype 1 or 4 had an AFP ratio that is 97% higher than blacks with genotype 2 or 3 and 26% higher than non blacks with genotype 1 or 4, and 57% higher than non blacks with genotypes 2 or 3.
Time varying effects that were associated with an elevated AFP were an HCC diagnosis within 365 days of AFP test and elevated ALT levels. Other significant covariates, whose effect sizes did not meet the 20% criterion, included duration of time elapsed from HCV index and older age. Effects for all of these covariates except the time effect between HCV and AFP test and MELD had overall significance p<.0001 even with FDR and Bonferroni adjustments. Patient sex was not significantly correlated with AFP levels.
ALT-HCC interactions
AFP levels were affected by a strong interaction between ALT levels and time to HCC; thus the AFP-ALT relation differed by proximity to HCC (Table 2 for unadjusted estimates and Table 3 and Figure 1 for model adjusted estimates). In the absence of HCC within 365 days of AFP test, there was a steady increase in AFP trend by each higher ALT quartile: 16.5%, 35.0% and 68.5% from quartile 1 to 2, 2 to 3 and 3 to 4 (higher AFP with higher ALT). However, in the presence of HCC within 365 days of AFP test, the AFP-ALT trend shows a stronger initial increase followed by a weaker increase; the increases from the first ALT quartile to the higher quartiles are 22.3%, 40.3% and 54.5%. For AFP and ALT tests within 30 days before or after HCC diagnosis, a strong initial increase is followed by a flat trend; the changes are +31.1%, +39.5% and +36.6%. Thus, in the presence of HCC, the AFP and ALT levels are nearly independent of one another. The disassociation between AFP and ALT levels in the presence of HCC is in sharp contrast with the strong positive correlation between AFP and ALT at all levels in the absence of HCC.
Table 2.
Mean AFP values (ng/ml), AFP ratios (AFP level at a given ALT level/AFP level at ALT 0–36) presented in categories of ALT quartiles and HCC status.
| AFP mean level (ng/ml) AFP ratio n (number of AFP tests) |
|||||
|---|---|---|---|---|---|
| ALT level (U/L) | 0–36 | 37–56 | 57–92 | 93+ | no ALT test |
| no HCC in 365 days | 3.55 | 4.80 | 6.38 | 8.68 | 5.55 |
| 1.00 | 1.35 | 1.79 | 2.44 | 1.56 | |
| n = 41,928 | n = 37,420 | n = 39,294 | n = 38,574 | n = 25,576 | |
|
| |||||
| HCC in 31–365 days | 25.21 | 40.17 | 46.61 | 56.36 | 57.39 |
| 1.00 | 1.59 | 1.85 | 2.24 | 2.28 | |
| n = 280 | n = 389 | n = 536 | n = 510 | n = 318 | |
|
| |||||
| HCC within 30 days | 42.37 | 63.60 | 69.67 | 69.56 | 71.61 |
| 1.00 | 1.50 | 1.64 | 1.64 | 1.69 | |
| n = 757 | n = 949 | n = 1086 | n = 1048 | n = 435 | |
Table 3.
Predictors of serum AFP levels. Results from the final linear multivariate regression model and AFP ratios are adjusted to all other variables in the model.
| effect | AFP ratio (95% CI) | p value |
|---|---|---|
| time from HCV index (as continuous growth rates by year) | ||
| year 1 | 1.03 (1.02–1.05) | <0.0001 |
| year 2 | 0.99 (0.98–1.00) | 0.16 |
| year 3 | 1.01 (0.99–1.02) | 0.37 |
| year 4 | 1.00 (0.99–1.02) | 0.64 |
| year 5 | 1.00 (0.99–1.02) | 0.69 |
| year 6 | 1.03 (1.01–1.06) | <0.01 |
| year 7 | 0.99 (0.97–1.02) | 0.43 |
| year 8 | 1.00 (0.97–1.04) | 0.89 |
| year 9 | 1.08 (1.02–1.16) | 0.02 |
|
| ||
| MELD score | ||
| <20 | ref | |
| 15–20 | 0.97 (0.92–1.02) | 0.26 |
| >20 | 0.79 (0.72–0.87) | <.0001 |
| missing | 1.00 (0.99–1.01) | 0.93 |
|
| ||
| race | ||
| white | ref | |
| black | 1.25 (1.23–1.27) | <.0001 |
| other | 1.13 (1.10–1.16) | <.0001 |
| unreported | 1.14 (1.12–1.17) | <.0001 |
|
| ||
| HCV genotype | ||
| 1 or 4 | ref | |
| 2 or 3 | 0.76 (0.74–0.77) | <.0001 |
| no result | 0.79 (0.77–0.81) | <.0001 |
| not done | 0.93 (0.92–0.94) | <.0001 |
|
| ||
| age | ||
| <50 | 0.91 (0.90–0.92) | <.0001 |
| 50–64 | ref | |
| >64 | 1.06 (1.03–1.09) | <.0001 |
|
| ||
| cirrhosis | ||
| main effect | 1.60 (1.57–1.63) | <.0001 |
| interactions with ALT | ||
| ALT < 37 | ref | |
| ALT 37–56 | 1.07 (1.05–1.10) | <.0001 |
| ALT 57–92 | 1.17 (1.14–1.20) | <.0001 |
| ALT > 92 | 1.22 (1.19–1.25) | <.0001 |
| no ALT in 30 days | 1.24 (1.21–1.27) | <.0001 |
| interactions with HCC | ||
| no HCC in 365 days | ref | |
| HCC in 365 days | 0.74 (0.68–0.80) | <.0001 |
| HCC in 30 days | 0.47 (0.44–0.51) | <.0001 |
|
| ||
| HCC | ||
| main effect | ||
| no HCC in 365 days | ref | |
| HCC in 365 days | 5.01 (4.52–5.56) | <.0001 |
| HCC in 30 days | 12.03 (11.10–13.05) | <.0001 |
|
| ||
| ALT | ||
| main effect | ||
| ALT < 37 | ref | |
| ALT 37–56 | 1.17 (1.15–1.18) | <.0001 |
| ALT 57–92 | 1.36 (1.34–1.37) | <.0001 |
| ALT > 92 | 1.69 (1.66–1.71) | <.0001 |
| no ALT in 30 days | 1.26 (1.24–1.28) | <.0001 |
| interactions with HCC | ||
| ALT 37–56 by HCC in 365 days | 1.05 (0.96–1.15) | 0.31 |
| ALT 37–56 by HCC in 30 days | 1.13 (1.06–1.20) | <0.001 |
| ALT 57–92 by HCC in 365 days | 1.04 (0.95–1.14) | 0.41 |
| ALT 57–92 by HCC in 30 days | 1.03 (0.97–1.10) | 0.30 |
| ALT > 92 by HCC in 365 days | 0.92 (0.84–1.01) | 0.07 |
| ALT > 92 by HCC in 30 days | 0.81 (0.76–0.87) | <.0001 |
| no ALT 30 days by HCC in 365 days | 1.06 (0.96–1.17) | 0.25 |
| no ALT 30 days by HCC in 30 days | 1.09 (1.01–1.17) | 0.03 |
MELD: model for end stage liver disease
HCC: hepatocellular carcinoma
ALT: alanine aminotransferase
HCV: hepatitis C virus
Figure 1.
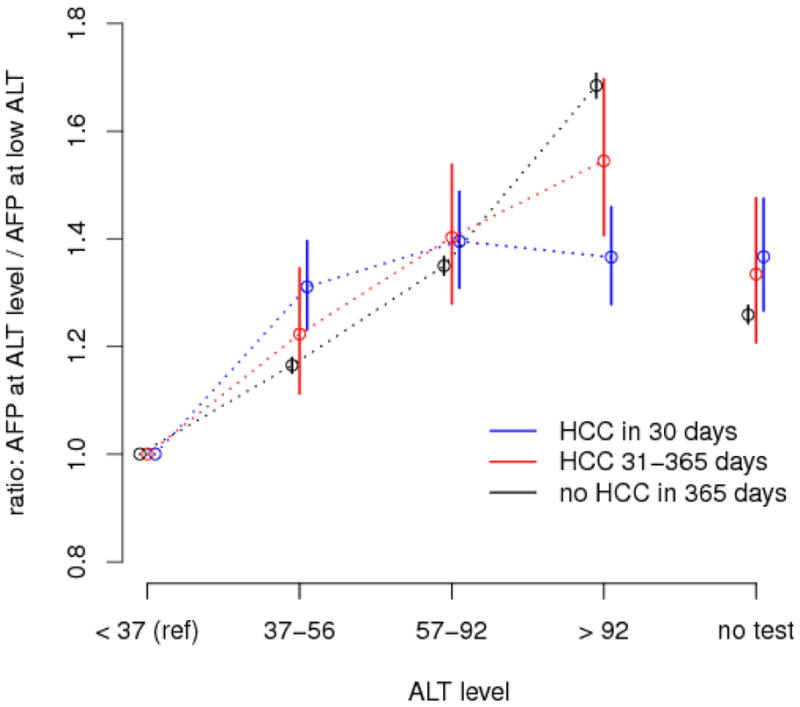
ALT effect modified by time to HCC diagnosis: effect, by HCC status, of ALT level on AFP level compared to reference (ALT < 37) derived from model estimates for ALT main effects and ALT-HCC interactions.
The model-based estimates adjusted for covariates and within-patient correlations (Table 3) were qualitatively similar but slightly more conservative than the unadjusted estimates (Figure 1). For example, the unadjusted pooled AFP ratios in the absence of HCC within 365 days of AFP test were 1.35, 1.79 and 2.44 compared with the model-adjusted values of 1.16, 1.35 and 1.68. Similarly, the ratios in the presence of HCC with 30 days are 1.50, 1.64 and 1.64 before model adjustment and 1.31, 1.39 and 1.37 after model adjustment.
We also re-ran the multivariate analyses in the subset of patients with complete MELD data, and in the subset of patients with complete HCV genotype. In these two analyses, respectively, MELD effects ratios were altered by less than 1% and HCV genotype effect (genotype 1 or 4 vs. 2 or 3) was altered by less than 0.5%, compared with the findings of the model in the entire dataset. In these models, there were little changes in the ALT-HCC interactions described above.
Cirrhosis effects and interaction
In the absence of HCC within 365 days of AFP test, cirrhosis was associated with at least 60% higher levels of AFP and a strengthening of the AFP-ALT correlation as evidenced by the trend in cirrhosis-ALT interactions. However, in the presence of HCC, cirrhosis was associated with AFP values which were reduced by 50% compared to those in non-cirrhotic subjects as evidenced by the cirrhosis-HCC interaction estimates.
Discussion
The presence or impending development of HCC is the strongest correlate of serum AFP levels in HCV-infected patients. Some variables have an independent effect on AFP levels (cirrhosis status, ALT levels, HCV genotype, patient race, age, and MELD score), with interactions among ALT, HCC and cirrhosis. The main new finding of the study is the HCC-mediated relation between AFP and ALT values where within a patient’s history of ALT and AFP test values, a change in the trend of the relation of the two tests could foreshadow the future development of HCC. Correction for some of these variables could provide future ways to enhance the value of AFP tests for detection of HCC.
The study represents the largest analysis of AFP values to be published to date. Several findings are in agreement with previous studies7, 9, 19, 20, which supports the internal validity if the study. The sampling frame from the study is less likely to be affected by selection bias related to referral or survival than smaller or single center studies. Additional strengths include the uniformity of HCV diagnosis in the cohort, the relatively long follow-up, the presence of additional lab data. The presence of a realistically small proportion of HCC cases improves the generalizability of the findings as compared with studies in which most cases develop HCC. The absence of ALT tests within 30 days in 13.9% of all AFP tests in this study is a relative limitation. However, those AFP values are distributed similarly to those accompanied by ALT testing. Therefore, it should be possible to restrict an analysis of this type to AFP labs that are within 30 days of an ALT without loss of generalizability. Information on MELD score was absent on 55.2% and on HCV genotype in 41%; so we repeated the analysis on two subsample of patients with complete MELD and HCV genotype information, respectively and found no meaningful changes in the overall findings.
The other commonly measured liver test is AST. In preliminary univariate analyses (not shown), we found that the correlation between AST values and AFP values was similar but not identical to that between ALT and AFP. We also observed that the ALT and AST values closely tracked. Hence, whether the addition of information about AST would add further prognostic value about HCC merits further study.
There were possible limitations related to defining some of the study variables. The HCV index date is unlikely to correlate well with the timing of actual HCV infection. However, we adjusted for time elapsed since HCV index date as a way of adjusting for possible changes over time in practice patterns and possible changes in methods of testing and reporting of tests such as AFP or ALT. Cirrhosis diagnosis was based on the presence of several previously validated diagnostic codes. In a previous study of 257 patients with codes for cirrhosis and HCV (similar to the current study cohort definition) we found that compared to detailed review of clinical, radiological, and histological criteria that are available in the medical records, cirrhosis codes had a positive predictive value (probability that cirrhosis is present among those with a code) and negative predictive value (probability that cirrhosis is absent among those without a code) were 90% and 87% with 88% agreement and kappa = 0.7016. However, we acknowledge that an under-estimation of the prevalence of cirrhosis was still possible but such underestimation would only serve to make our findings related to cirrhosis even more conservative than they already are. Lastly, both African American race and genotype 1 were associated with increased AFP levels. Most (94%) of HCV in this study’s African Americans with a reported HCV genotype had genotype 1 (while only 25% of genotype 1 were African Americans) and while our multivariate model adjusted for this possible confounding it may not possible to confidently ascribe an association between African American race and AFP levels independent of HCV genotype given the relatively large proportion of patients missing genotype data.
Testing the predictive value of AFP for the occurrence HCC in a longitudinal model on the basis of ALT and other predictors may seem like a more natural or clinically relevant approach than the one employed in this study (i.e. predicting AFP levels). However, the former approach is much less practical in the absence of specified hypotheses. For example, a Cox proportional hazard model would not yield usable quantitative estimates of hazards of HCC within fixed time windows.
Based on the knowledge of potential predictors of AFP levels and the ALT-HCC interaction gained in this study, we propose that future studies would test the possibility of “corrected” AFP values. Ideally, studies would utilize a longitudinal logistic model of the probability of presence of HCC or its occurrence in specified time windows. In these models, time-varying effects such as AFP values and recent ALT or AST would play the major predictive role. Such models have the potential of generating clinical useful predictive tools to enhance the predictive value of serum AFP in surveillance and diagnosis of HCC.
Footnotes
Conflict: None of the authors has any conflict of interest
Author contribution
Peter Richardson: analysis, writing
Zhigang Duan: analysis
Jennifer Kramer: design
Jessica A. Davila: design
Gia L. Tyson: writing
Hashem B. El-Serag: funding, design, writing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010;55(10):2744–2755. doi: 10.1007/s10620-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 2.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30(1):3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139(1):46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen TH, Chen CJ, Yen MF, et al. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int J Cancer. 2002;98(2):257–261. doi: 10.1002/ijc.10122. [DOI] [PubMed] [Google Scholar]
- 6.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112(1):44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 7.Chen TM, Huang PT, Tsai MH, et al. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22(5):669–675. doi: 10.1111/j.1440-1746.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- 8.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43(3):434–441. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Tai WC, Hu TH, Wang JH, et al. Clinical implications of alpha-fetoprotein in chronic hepatitis C. J Formos Med Assoc. 2009;108(3):210–218. doi: 10.1016/S0929-6646(09)60054-1. [DOI] [PubMed] [Google Scholar]
- 10.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137(1):110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soresi M, Magliarisi C, Campagna P, et al. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res. 2003;23(2C):1747–1753. [PubMed] [Google Scholar]
- 12.Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3(2):79–87. doi: 10.3233/cbm-2007-3202. [DOI] [PubMed] [Google Scholar]
- 13.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41(8):777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 16.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 17.US Government Web site. Health Resources and Services Administration, U.S. Department of Health & Human Services; 2011. [Accessed2011]. http://optntransplanthrsagov/resources/professionalResourcesasp?index=8. Available at: URL: http://www.unos.org/resources/meldpeldcalculator.asp. [Google Scholar]
- 18.El-Serag HB, Kramer JR, Chen GJ, Duan Z, Richardson PA, Davila JA. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011;60(7):992–997. doi: 10.1136/gut.2010.230508. [DOI] [PubMed] [Google Scholar]
- 19.Babali A, Cakal E, Purnak T, et al. Serum alpha-fetoprotein levels in liver steatosis. Hepatol Int. 2009 doi: 10.1007/s12072-009-9156-8. [Epub ahead of ptint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein NS, Blue DE, Hankin R, et al. Serum alpha-fetoprotein levels in patients with chronic hepatitis C. Relationships with serum alanine aminotransferase values, histologic activity index, and hepatocyte MIB-1 scores. Am J Clin Pathol. 1999;111(6):811–816. doi: 10.1093/ajcp/111.6.811. [DOI] [PubMed] [Google Scholar]


