Abstract
Vaccine strategies aimed at generating CD8+ T cells memory responses are likely to show augmented efficacy against chronic challenges like tumor. Since, a variety of CD8+ T cell memory responses have been described, it would be important to develop new vaccine strategies to generate different memory responses and evaluate them for tumor efficacy. We demonstrate that by varying the regimen of rapamycin treatment, we can regulate virus vaccination induced CD8+ T cell responses for quantitative and qualitatively distinct memory functions and differential tumor efficacy. Strikingly, a short-course of high dose; but not low dose rapamycin treatment, transiently blocks viral vaccination induced mTOR activity in CD8+ T cells, leading to dampened activation and type 1 effector maturation but enhanced persistence and antigen-recall responses, which was abrogated by prolonged rapamycin administration. Moreover, high dose rapamycin generated CD8+ T cell memory responses were independent of IL-15 and showed early programing for sustenance and greater tumor efficacy than low dose rapamycin. These results demonstrate that the regimen of rapamycin treatment can profoundly influence vaccine induced CD8+ T cell responses and the application of rapamycin to tune mTOR activity can be useful to augment vaccine efficacy.
Key words/phrases: mTOR, dose, duration, CD8+ T cell, immunization, effector, IL-15 and memory
Introduction
The CD8+ T cells play an important role in host defense against intracellular infections and cancer. Their inherent ability to localize, recognize, kill affected cells, as well as form memory, makes them an excellent target for vaccination against tumors and intracellular infections (1–3). Based on our understanding of CD8+ T cell responses to infectious agents, several vaccination strategies have been designed to generate type 1 effector responses and they have been evaluated for tumor efficacy (4, 5). Although, viral vaccination can successfully produce tumor-antigen specific CD8+ T cell clonal expansion and type 1 effector maturation, their anti-tumor efficacy has been less than desirable (6–8). It has been suggested that the generation of CD8+ T cell memory responses may be required for protection against indolent challenges like chronic infections and/or cancer (9, 10). Recent advances have identified the role of various cell extrinsic factors in generating a wide variety of CD8+ T cell memory responses (11, 12); although vaccine strategies to produce varying CD8+ memory responses and their efficacy to indolent challenges like cancer remain uncharacterized. Nevertheless, there is considerable interest in applying new mechanistic insights regulating CD8+ T cell memory generation towards the development of vaccines against tumor and intracellular infections.
The mammalian target of rapamycin (mTOR) is a highly conserved serine/threonine kinase that integrates cell extrinsic signals to control several key functions like transcription, translation, cell size, division and migration (13). The activation of the mTOR kinase causes phosphorylation of the downstream targets S6 kinase/S6 and 4E-BP1, that mediate protein translation, cell cycle and growth (14). Early studies had identified the mTORC1 specific inhibitor; rapamycin, as an immunosuppressive agent and was thus used to treat allo-rejection reactions encountered during solid organ transplantation (15). However, due to its modest efficacy, it is currently used mainly in combination with cyclosporine A and corticosteroids (16). Previously, it was demonstrated that rapamycin treatment induced CD4+ T cell anergy, FoxP3+ regulatory cells and/or deviation to the type 2 effector phenotype, thus causing immune regulation (17–19). Recent studies have uncovered a novel role for mTORC1 in determining functional fates in both CD4+ and CD8+ T cells and the ability of rapamycin treatment to facilitate transition of effector CD8+ T cell responses to memory (20–23). Strikingly, the ability of in vivo rapamycin administration to augment CD8+ T cell memory responses to viral challenge was cell-autonomous (21) and was mediated by causing a shift from T-bet to Eomesodermin dominated transcription program (23). Although, it was previously reported that rapamycin regimen can affect virus induced CD8+ T cell memory response (21), the study was not designed to characterize the cellular mechanisms underpinning the impact dose and duration dependent of rapamycin treatment on vaccine induced CD8+ T cell responses. Moreover, the ability of rapamycin mediated CD8+ memory responses to affect tumor growth was not tested. Since, rapamycin administration can cause tolerance (17, 24), it is imperative that careful studies to understand the impact of rapamycin treatment on vaccine induced CD8+ T cell responses should be conducted prior to further exploration in the clinic.
A vaccination strategy that can consistently generates tumor-antigen specific CD8+ T cell responses of required quality, magnitude and duration is highly desirable and exploiting the emerging information on the central role of mTOR in regulating antigen specific CD8+ T cell responses is particularly attractive due to ease of translation. In this study, by monitoring vaccine induced CD8+ T cells we characterize the impact of dose and duration of rapamycin treatment on the quantity and quality of CD8+ memory responses induced by viral vaccination and their ability to afford durable tumor protection.
Materials and Methods
Mice and reagents
The C57BL/6 (B6) mice, CD8+ TCR transgenic mice with Thy1.1 congenic marker (OT-1) were bred and housed at Roswell Park Cancer Institute (RPCI). Act-OVA B6 mice (ACTB-OVA) were purchased from the Jackson Laboratory (Bar Harbor, ME) (25). The IL-15 deficient B6 (B6-IL-15−/−) mice were purchased from Taconic (Germantown, NY). All animals were used according to the IACUC guidelines of RPCI. Rapamycin was purchased from ChemieTek (Indianapolis, IN). The rapamycin was diluted with PBS and used at 0.075 mg/kg/day or 0.75 mg/kg/day in vivo by intraperitoneal (i.p.) injection. Phorbol ester PMA, ionomycin and Brefeldin A were purchased from Sigma-Aldrich.
Adoptive transfer and virus immunization
Purified naïve OT-1 cells (2×106) labeled with or without 5 μM CFSE (Invitrogen) were (i.v.) adoptively transferred into syngeneic B6 recipients. B6 recipients were immunized with recombinant poxvirus expressing chicken ovalbumin-mLFA-3/mICAM/mB7.1 (designated Tricom, 2 × 107 pfu) or control virus (no antigen) on day 0 (26). All viruses were a kind gift from Sanofi Pasteur (Toronto, Canada). In some experiments, the anti-IL-7Rα (100 μg per mouse twice a week) was injected so that IL-7 blockade in vivo could be achieved. The hybridoma secreting anti-IL-7Rα (clone SB199) was kindly provided by Dr. P. Kincade (University of Oklahoma).
Abs and flow cytometry
All Ab’s used for flow cytometry were purchased from BD PharMingen except anti-IL-7Rα (A7R34), anti-Eomesodermin (Eomes, Dan11mag), anti-T-bet (eBio4B10) and anti-Granzyme B (16G6) from eBioscience, Annexin V-conjugated with FITC and propiodium iodide (PI) was obtained from BD PharMingen. Anti-pS6 (Ser 235/236) was obtained from Cell Signaling. Intracellular staining (ICS) and flow cytometry for IFN-γ, T-bet, Eomes, Granzyme B (Gzm B), and pS6 was performed as described (27). Expression of IFN-γ was determined after a 5 hr antigen re-stimulation. Single-cell suspensions from spleens were analyzed by flow cytometry. Donor OT-1 cells were detected as CD8α and Thy1.1 double positive and gated for further analysis. LSR II and FACSCalibur (Becton Dickinson) were used for flow cytometry event collection and events were analyzed with FlowJo (Tree star) and CellQuest software (BD Biosciences).
Evaluation of in vivo OT-1 cells responses
Mice were sacrificed and the total number of adoptively transferred OT-1 cells (CD8α+/Thy1.1+) detected by flow cytometry and numbers calculated by multiplying the total cell counts by the percentage of CD8α+/Thy1.1+ T cells. The phenotype and CFSE analysis was performed on OT-1 gated cells (>10,000 events).
Tumor challenge and survival
Intact B6 (n = 10) challenged with EL4 thymoma or EG.7-OVA thymoma cells (28) by ip (3 × 106 cells per mouse) on day −10 were adoptively transferred with naïve OT-1 cells (2 × 106) on day −2. In some experiments, Act-OVA B6 (n = 10) were challenged with EG.7 tumor cells by ip (3 × 106 cells per mouse) on day −10 without OT-1 cells transfer and challenged mice were immunized with by s.c. injection of Tricom (2 × 107 pfu) or empty control virus (2 × 107 pfu) on day 0. Rapamycin (0.075 mg/Kg/day or 0.75 mg/Kg/day) was given by ip injection from day 0 – 7 or day 0 – 39 and monitored thereafter for tumor growth and morbidity. To demonstrate durable protection by programmed OT-1 cells, they were isolated by anti-CD90.1 MicroBeads; on a MiniMACS Separator (Miltenyi Biotec) on day 8 from various animals and 1 × 106 OT-1 cells (thy1.1+) were adoptively transferred into 10 days inoculated EG.7 tumor-bearing hosts. The tumor-free survival was monitored.
Statistical analysis
For statistical analysis, the unpaired Student’s t test was applied. Tumor survival between various groups was compared using Kaplan Meier survival curves and log-rank statistics. Significance was set at p < 0.05.
Results
Rapamycin administration regulates vaccine-induced early CD8+ T cell responses in a dose-dependent manner
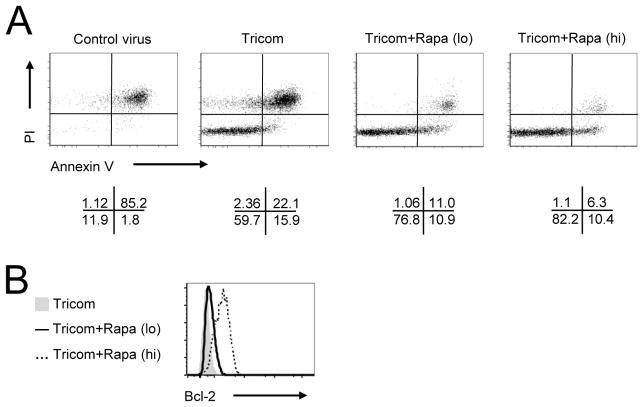
To study the impact of varying rapamycin treatment on viral vaccine induced CD8+ T cell responses, we monitored adoptively transferred naïve TCR transgenic CD8+ T cells (OT-1) in thy 1 congenic B6 recipients. The OT-1 recipients were typically vaccinated on day 0 with canary poxvirus expressing chicken ovalbumin-mLFA-3/mICAM/mB7.1 (Tricom) or control virus (no antigen) (26) and some recipients were given mTOR inhibitor; rapamycin, at dose of either 0.075 mg/kg/day (lo) or 0.75 mg/kg/day (hi) from day 0–7/everyday. The lymph node and spleen cells from animals treated with Tricom or Tricom plus rapamycin at either dose were stained, gated for CD8+Thy1.1+ and analyzed by flow cytometry on day 4. The intracytoplasmic phosphorylation of ribosomal protein S6 (pS6); an mTOR kinase target (29), was increased in Tricom immunized animals (Fig. 1A). Surprisingly, low dose of rapamycin treatment produced no reduction in pS6, but substantial decreases were noted in OT-1 cells derived from high dose rapamycin treated animals (Fig. 1A). In congruence, high dose rapamycin treatment dampened activation; CD44 and CD122 low, CD62L and IL-7Rα high (Fig. 1A), proliferation; lower CFSE dilution (Fig. 1B) and clonal expansion (Fig. 1C, 1D), whereas animals receiving low dose rapamycin showed only marginal reduction in OT-1 activation (surface phenotype CD44hi, CD62Llo-mid, IL-7Rαlo, and CD122hi) and proliferation (CFSE dilution), but produced enhanced clonal expansion; due to increased Bcl-2 expression and survival (Fig. 1A–D). The OT-1 responses detected in vaccinated mice were antigen specific as control virus failed to increase the frequency and number of OT-1 cells (data not shown). The reduction in pS6 and dampened OT-1 responses by high dose rapamycin was transient, as withdrawal of rapamycin on day 8 rapidly restored mTOR activity along with their activation phenotype, proliferation and clonal expansion (Supplemental Fig. 1A–D). The ability of low dose rapamycin treatment to produce higher clonal expansion along with reduced cell proliferation of viral vaccine induced OT-1 responses on day 4 (Fig. 1B–D; CFSE dilution and Numbers of OT-1 cells), led to test the impact of rapamycin dose on virus vaccine induced CD8+ T cell apoptosis by evaluating the Annexin V/PI staining and cytoplasmic expression of Bcl-2 protein by OT-1 cells. As anticipated, the addition of rapamycin at either doses dramatically decreased cell death (Annexin V+PI+ OT-1 cells) (Fig. 2A) with concomitant increases in Bcl-2 expression in a dose dependent manner (Fig. 2B). Thus confirming the ability of low dose rapamycin treatment to enhance vaccine induced CD8+ T cell expansion was due in part to increased Bcl-2 expression and reduction in apoptotic cell death (Fig. 2A, 2B), but not enhanced proliferation (CFSE dilution) (Fig. 1B). Since the relative expression levels of the transcriptional factors; T-bet and Eomesodermin, determine the functional fate of CD8+ T cells (30), we next evaluated the impact of rapamycin dose on expression of T-bet and/or Eomes by intracytoplasmic staining and flow cytometry of OT-1 cells on day 4 and 8 post vaccination. As shown in Fig. 3, high dose rapamycin reduced expression of T-bet and IFN-γ, but only produced a marginal change in Granzyme B and Eomes expression on day 4 post transfer. Interestingly, low dose rapamycin showed no reduction in T-bet and IFN-γ expression relative to Tricom alone, which is in agreement with the levels of CD122 expression (Fig. 1A); as CD122 is a direct T-bet target (30). Similar to our observed rebound in early pS6 and CD8+ T cell responses after cessation of rapamycin treatment, the levels of T-bet and IFN-γ expression were rapidly restored in OT-1 cells obtained from high dose rapamycin treated animals and in fact the IFN-γ and Granzyme B expression was greater in high dose compared to low dose or rapamycin untreated viral vaccinated animals on day 8 post transfer (Supplemental Fig. 2). Therefore, the inhibition of mTOR activity, activation, proliferation, and effector maturation by rapamycin treatment is dose dependent and produces distinct regulation of vaccine induced early CD8+ T cell responses.
FIGURE 1. Regimen of rapamycin regulates vaccine induced effector CD8+ T cell responses.
B6 hosts received CFSE-labeled or CFSE-unlabeled naïve OT-1 cells (2 × 106 cells) on day −1 were immunized with Tricom (2 × 107 pfu) or empty control virus on day 0. Rapamycin at low (0.075mg/Kg/day, lo) or high dose (0.75mg/Kg/day, hi) was given by ip daily injection. Spleens were isolated on day 4. The CD8α+Thy1.1+ cells were gated and evaluated by flow cytometry for phenotyping and proliferation (CFSE dilution) of OT-1 cells; A, The expression of pS6, CD44, CD62L, IL-7Rα, and CD122 on gated OT-1+ cells; B, The CFSE dilution of OT-1+ cells; C, The frequency of OT-1+ cells. The box identifies the OT-1 population and the numbers indicate the percent frequency; D, The absolute number of OT-1+ cells. The results shown are representative of three independent experiments with similar results. * p < 0.05, ** p < 0.01.
FIGURE 2. Rapamycin decreases vaccine-induced apoptosis of CD8+ T cells.
Tricom or control virus immunized OT-1 recipients injected with low or high dose of rapamycin as indicated in Fig. 1. On day 4 after transfer, spleen and lymph node were isolated. A, Annexin V and PI profiles were analyzed on gated OT-1+ cells. B, Intracellular Bcl-2 expression was analyzed on gated OT-1+ cells. The results represent one of three independent experiments.
FIGURE 3. Vaccine-induced T-bet expression and effector function were inhibited in response to high dose of rapamycin treatment.
B6 recipients received OT-1 cells were daily injected with either no, low or high dose of rapamycin as indicated above. Spleen cells harvested on day 4 after start of rapamycin treatment were stained; surface and intracellular, gated and analyzed by flow cytometry for T-bet, Eomes, IFN-γ, and Granzyme B. Data representing three experiments with identical outcomes is shown.
High dose rapamycin enhances vaccination induced CD8+ T cell memory responses
Instructions provided early during antigen stimulation produce a programmed long-term CD8+ T cell response (31). To determine whether the rapamycin dose dependent effects on vaccine induced CD8+ T cells also regulated long-term responses, we evaluated OT-1 persistence till day 40 after vaccination and tested their ability to mount antigen-recall responses (re-challenge on day 40–43). The persistence of viral vaccine induced OT-1 responses was significantly enhanced by rapamycin treatment in a dose-dependent manner. The short-duration of high dose rapamycin treatment produced greater OT-1 cell persistence than lower dose of rapamycin treatment (Fig. 4A, 4B). Surprisingly, the surface phenotype of the OT-1 cells in all treatment groups was similar; CD44hi, CD62Lhi, and IL-7Rαhi, except for the expression of T-bet and CD122 (Fig. 4C). In contrast to OT-1 cells from Tricom vaccinated mice, OT-1 cells from mice vaccinated with Tricom plus rapamycin demonstrated heightened antigen-recall responses 3 days after re-challenge (day 43 absolute OT-1 number, the production of IFN-γ and Granzyme B), with high dose rapamycin treatment showing significantly greater memory response (Fig. 4D). It was also noted that although both low and high dose of rapamycin treatment augmented Tricom vaccine mediated antigen-specific recall response, the fold expansion observed with high dose rapamycin treatment was inferior to low dose of rapamycin treatment (Fig. 4D-recall response), which may reflect the differences in the type of CD8+ memory generated (11). These observations indicate that regulation of vaccine induced mTOR activity by dose of rapamycin administration can produce quantitatively and qualitatively distinct CD8+ T cell memory responses.
FIGURE 4. Transient mTOR blockade enhances vaccine-induced CD8+ T cell memory.
Recipients of OT-1 cells were challenged with Tricom or control virus on day 0 followed by no, low (0.075 mg/Kg/day) or high dose (0.75 mg/Kg/day) rapamycin treatment every day from day 0 – 7. The spleen cells were harvested, stained and evaluated on day 40 for persistence and antigen-recall responses on day 43 (3 days post-immunization). A, The percentage of OT-1+ (CD8α+Thy1.1+) cells on day 40; B, The absolute numbers of OT-1 cells on day 40; C, The expression of CD44, CD62L, IL-7Rα, CD122 and T-bet on day 40; D, The left panel, the absolute numbers of OT-1 cells on day 43. The number in parenthesis indicates the fold increase in OT-1 post-immunization; the right panels, the expression of IFN-γ and Granzyme B on gated OT-1 cells. Values represent means plus or minus SD of three independent experiments. * p < 0.05, ** p < 0.01.
Persistent inhibition of mTOR suppressed CD8+ memory responses
Previously, persistent low dose rapamycin treatment has been shown to enhance virus specific CD8+ T cell memory responses (21). Based on our observations above, we reasoned that persistent dampening of vaccine induced activation, proliferation and effector maturation would further enhance CD8+ T cell memory responses. To test this notion, we extended the high dose rapamycin treatment from day 0 to day 39 and evaluated its impact on long-term OT-1 responses. Surprisingly, prolonged high dose (0.75 mg/Kg/day) rapamycin administration abrogated OT-1 persistence (day 40) produced by short-course high dose rapamycin treatment (Fig. 5A). Notably, the low frequency of OT-1 cells in day 0–39 rapamycin treated animals produced a weak antigen-recall response on day 43, albeit with high IFN-γ but no Granzyme B expression (Fig. 5B, right panel). Thus demonstrating that long-term mTOR blockade was not suitable for generating robust memory CD8+ T cell responses and emphasized the importance of mTOR re-induction for enhanced memory responses with both IFN-γ and Granzyme B production.
FIGURE 5. Persistent mTOR inhibition abrogates vaccine induced CD8+ T cell responses.
Naïve OT-1 cell recipients were challenged with Tricom (2 × 107 pfu) or control virus at day 0. Rapamycin (high dose, 0.75 mg/Kg/day) was administered from day 0 – 7 or day 0 – 39. Spleen cells harvested on day 40; persistence or day 43 (3 days post-immunization) antigen-recall response, were stained, gated and evaluated by flow cytometry. A, The absolute number of OT-1 cells; day 40; B, The absolute number of OT-1 cells and IFN-γ and Granzyme B expression; day 43. The numbers in parenthesis indicate the fold-increase in OT-1 numbers post-immunization. All data are representative of two independently performed experiments. * p < 0.05, ** p < 0.01.
Dose of rapamycin treatment determines IL-15 requirement for CD8+ T cell memory
The common gamma chain cytokine IL-15 has been shown to be crucial for CD8+ T cell memory generation (32). Previously we had reported the ability of rapamycin to augment lymphopenia-induced CD8+ T memory responses in an IL-15 independent manner (27). Since, the dose of rapamycin treatment differentially affected T-bet and CD122 expression by OT-1 cells (Fig. 3, Fig. 1A), we predicted that rapamycin in a dose dependent manner generates qualitatively distinct CD8+ memory responses in terms of their dependence on cell extrinsic factors like IL-15. In agreement with previous reports, OT-1 cells transferred into IL-15−/− hosts and vaccinated with Tricom failed to produce persistent responses; day 40 (Fig. 6A) and generated poor memory responses (Fig. 6B). Interestingly, rapamycin treatment rescued Tricom induced OT-1 cells in IL-15−/− recipients; both persistence (Fig. 6A, day 40) and antigen recall (Fig. 6B, day 43). However, only the high dose of rapamycin which blocked mTOR at early time-points (Tricom+Rapa (hi)> Tricom+Rapa (lo)), was able to produce CD8+ memory in a cell-autonomous manner. Although, it was noted that no significant differences in effector molecule expression; IFN-γ and Granzyme B, was observed during antigen-recall with either doses of rapamycin used (Fig. 6B, right panel). Furthermore, the memory responses observed with high dose rapamycin treatment were also maintained in the presence of IL-7 blockade in vivo (Supplemental Fig. 3), however, the persistence was slightly reduced. Thus implying that short but complete mTOR blockade facilitates transition of effector cells to memory and rapamycin dose variation affects the dependence on cell extrinsic factors for CD8+ memory generation.
FIGURE 6. Rapamycin promotes IL-15 independent CD8+ T cell memory in a dose dependent manner.
WT or IL-15−/− OT-1 recipients immunized with Tricom or control virus were either treated with no, low dose rapamycin (0.075 mg/Kg/day) or high dose rapamycin (0.75 mg/Kg/day) from day 0 – 7. Spleen cells harvested on day 40; persistence or day 43; antigen-recall, were stained, gated and evaluated by flow cytometry. A, The absolute numbers of OT-1 cells on day 40; B, Antigen-recall response of OT-1 cells on day 43. The left panel, the absolute number of OT-1 cells. The numbers in parenthesis indicate the fold-increase in OT-1 numbers post-immunization. The right panel, the expression of IFN-γ+ and Granzyme B+ by gated OT-1 cells. The error bars are SD of values obtained from three animals/group and a representative of two independent experiments with identical outcomes is shown. *, p<0.05; **, p<0.01; ***, p<0.001.
Transient mTOR blockade augments for tumor efficacy of vaccine-induced CD8+ T cells
To determine the efficacy of rapamycin generated distinct CD8+ T cell memory responses; low versus high dose regimen, we vaccinated day 10 tumor bearing (i.p. EG.7) mice with either Tricom alone, Tricom plus low or high dose rapamycin (day 10–17) and monitored their tumor growth/survival. As shown in Fig. 7A, EG.7 bearing mice vaccinated with Tricom alone showed marginal increase in survival over non-antigen expressing virus, which was enhanced by low dose rapamycin treatment, but animals vaccinated with Tricom and treated with high dose rapamycin showed remarkable increases in survival (~30% > day 100 survivors), the effects of rapamycin required antigen induced CD8+ T cell responses as non-antigen expressing virus plus high dose rapamycin treated animals showed no survival benefits (data not shown). In congruence with the loss of CD8+ T cell memory due to longer duration of high dose rapamycin administration, we also failed to observe increased survival (Fig. 7B). The observed impact of dose of rapamycin treatment on tumor immunity was identical when the modality was tested in a fully syngeneic (Actin-OVA transgenic mouse) tumor challenge model without adoptive transfer of TCR transgenic CD8+ (OT-1) cells (Fig. 7C). Although, some differences in the extent of tumor free survival was observed, the overall conclusions reached were identical. Thus demonstrating the benefit of transient mTOR inhibition by high dose rapamycin treatment for viral vaccine induced CD8+ T cell memory responses and tumor efficacy. To confirm that the rapamycin induced early changes; day 8, in CD8+ T cells were responsible for the augmented CD8+ memory and tumor efficacy, we isolated OT-1 cells (thy1.1+) from either Tricom alone or Tricom plus high dose of rapamycin (day 0–7) treated animals on day 8 and equal numbers of purified OT-1 cells were re-transferred into day −10 EG.7 or EL-4 (non-antigen parental tumor) tumor bearing recipients and their impact on tumor growth/survival monitored. Remarkably, the OT-1 cells obtained from Tricom and high dose rapamycin (day 0–7) treated mice produced greater (60% EG.7; but not EL4) tumor-free survival (Fig. 8A) and demonstrated greater memory functions (Fig. 8B). Thus demonstrating that a short-course of high dose rapamycin treatment programs vaccine induced CD8+ T cells early for cell autonomous memory and tumor efficacy.
FIGURE 7. A short-course of high dose rapamycin treatment enhances anti-tumor vaccine efficacy.
B6 mice challenged with 3 × 106 EG.7 by i.p. injection on day −10 received 2×106 naïve OT-1 cells on day −1 following by s.c injection of Tricom (dose as indicated above) or control virus on day 0. A, Vaccinated animals were treated either with rapamycin from day 0 – 7 at low dose (0.075 mg/kg/day) or high dose (0.75 mg/kg/day) (A) or with rapamycin from day 0 – 7 or day 0 – 39, rapamycin at high dose (0.75 mg/kg/day) (B). C, Act-OVA B6 mice were inoculated with 3 × 106 EG.7 by i.p. injection on day −10 and treated with Tricom and rapamycin (dose and duration as indicated above). A – C, survival curve of tumor-free animals. The results of A and B are the summation from three independent experiments with 10 mice/experimental group/experiment. The results of C are the summation of two independent experiments with 10 mice/experimental group. The outcomes of all experiments were similar.
FIGURE 8. A short-course of high dose rapamycin treatment programs vaccine-induced CD8+ T cells for durable anti-tumor response.

The congenic OT-1+ cells; Thy1.1+ (1 × 106) isolated on day 8 form animals treated with either Tricom alone or Tricom plus rapamycin(day 0–7 at 0.75 mg/kg/day) were adoptively transferred into recipients bearing day10 EL4 (non-antigen control) or EG.7 tumors (A) or intact B6 mice (B). A, Tumor-free survival. B, persistence; day 40 and antigen-recall responses; day 43. The left panel, the absolute number of OT-1+ cells on day 40. The right panel, the absolute number of OT-1+ cells on day 43 after re-challenge with antigen (IFA/OVA) on day 40. The numbers in parenthesis indicate the fold-increase in OT-1 numbers post-immunization. The results shown are representative of two independent experiments with identical outcomes.
Discussion
The paradigm shifting observations that mTOR inhibitor rapamycin can augment CD8+ T cell memory responses to lymphocytic choriomeningitis virus (LCMV) infection by acting directly on CD8+ T cells in vivo (21) and the central role of mTOR in regulating expression of master transcriptional factors; T-bet for type 1 effector functions and Eomesodermin for memory (23), have generated considerable interest for the use of rapamycin or mTOR inhibitors for generating memory CD8+ T cell responses. Herein, by characterizing the impact of the regimen of rapamycin treatment on vaccine induced CD8+ T cell activation, expansion and differentiation for type 1 effector and/or memory responses and relating these observations with their ability to promote efficacy against a syngeneic murine tumor, we provide new information to validate the use of rapamycin in vaccination. Our findings demonstrate that a short-course high dose of rapamycin treatment transiently blocks viral vaccination induced mTOR activity to dampen CD8+ T cells activation, proliferation, and effector differentiation. Strikingly, longer-course of rapamycin treatment abrogated the enhanced viral vaccination induced CD8+ memory generation; persistence and antigen-recall, generated by transient mTOR blockade. Although, the phenotype of antigen-specific CD8+ memory responses produced by vaccination alone or vaccination plus rapamycin appeared to be identical, they were quantitatively and qualitatively distinct and demonstrated different tumor efficacy.
These findings lend support to the notion that memory rather than effector CD8+ T cells are desirable for tumor efficacy. These results are the first to demonstrate the ability of rapamycin to regulate vaccine induced CD8+ T cell mTOR activity in a dose dependent manner and provide evidence of the impact differential mTOR inhibition has on early activation phenotype, proliferation/cell death for clonal expansion and T-bet mediated differentiation for type 1 effector functions. Although, some of these findings confirm previously noted changes in clonal pool of antigen specific CD8+ T cells generated in response to LCMV infection (21), the insights provided by characterizing cellular and molecular mechanisms underpinning the impact of dose and duration of rapamycin treatment on vaccine induced CD8+ T cell responses will allow judicious use of rapamycin treatment in a preclinical model and facilitate future clinical translation. The ability of low dose rapamycin treatment to significantly augment vaccine induced clonal expansion can be largely attributed to its reduction in apoptosis perhaps by augmenting Bcl-2 expression, which is in agreement with previous studies, that show the ability of rapamycin (10 μM) to reduce apoptosis of Th2 type cells due to reduced activation of capase 3 and 9 as well as Bim and Bid expression and simultaneously causing increased Bcl-XL expression (33). The mechanisms for this effect remains unclear, but it can be conjured that rapamycin treatment may reduce phosphorylation of p53 which in turn dampens the up regulation of pro-apoptotic protein of Bax and/or caspase activation for reduced apoptotic death (34, 35). Since high dose rapamycin treatment reduced vaccine induced OT-1 clonal expansion, our results demonstrate the relative balance between proliferation and cell death is regulated by the transient mTOR inhibition whereby determining the extent of clonal expansion achieved by immunization. Strikingly, withdrawal of rapamycin treatment on day 7 led to a rapid restoration of mTOR activity and resumption of cell proliferation which produced considerable clonal expansion by day 8 in animals treated by high dose rapamycin, although these observations seem to contradict previous studies (21), we contend that the differences may be due to the differences in experimental conditions such as the strength of antigen signal; LCMV versus Tricom, the characterization of blood rather than splenocytes and lymph node cells; which we have evaluated. It is also possible that the Tricom and LCMV infections produce varying levels of CD8+ T cell proliferation for clonal expansion and high dose of rapamycin shows greater ability to block higher mTOR activation state and favor transition of CD8+ T cell for memory, which is consistent with the role of mTOR in instructional activation and proliferation of CD8+ T cells (17, 36, 37). The new information on rapamycin dose dependent impact on vaccine induced clonal expansion by distinct cellular and molecular pathways is insightful and will be useful in designing preclinical studies for evaluating early responses and their predictive value in future clinical trials.
Analysis of memory CD8+ T cell formation after challenge vaccine plus low or high dose of rapamycin supports the following conclusions. First, the extent of mTOR inhibition produced by different doses of rapamycin administered after viral vaccination causes qualitative shift in CD8+ memory responses. The observed difference in CD122 and T-bet expression on day 40 by OT-1 cells in vaccinated animals treated with varying doses of rapamycin suggests that a transient early blockade of mTOR activity programs memory generation by cell-intrinsic mechanisms. Second, the dose of rapamycin determines whether extrinsic cytokine IL-15 and/or IL-7 is required for vaccine-induced memory CD8+ T cells. The ability of high dose rapamycin treatment to “rescue” the loss of CD8+ T cell memory responses in the absence of IL-15 or in the absence of functional IL-7 indicates the cell-intrinsic mechanisms of high dose of rapamycin induced memory CD8+ T cell generation. It is noteworthy that recovery of mTOR activity was necessary for the persistence of CD8+ T cells (Supplemental Fig. 1A), as long-term high dose of rapamycin fails to overcome the defects in memory CD8+ T cell generation in the absence of functional IL-7 and/or IL-15 signaling (27). These results indicate that rapamycin treatment mediated transient mTOR blockade promotes transition of vaccine induced effector cells for memory.
Several studies have found that inflammation generates “short-lived” effector CD8+ T cell due to increased T-bet expression (38–40). In this study, a short course of high dose of rapamycin treatment caused transient reduced T-bet expression without regulation of expression of Eomes. However, the relative ratio of Eomes to T-bet is higher in OT-1 cells from Tricom plus high dose of rapamycin treated animals (data not shown), which indicates that reduced T-bet expression and/or increased Eomes expression enhances vaccine induced CD8+ effector cells to survival. Nevertheless, the association of reduced T-bet expression or relative high ratio of Eomes vs T-bet and increases in persistence of OT-1 numbers in high dose rapamycin treated hosts suggests that regulation of T-bet and/or Eomes enhances transition of CD8+ effector cells to memory and CD122 expression whereby facilitating their transition to memory (40). These observations are congruent with the notion that robust activation and effector maturation is detrimental for persistence and memory generation.
Since, several studies have indicated that early instructions program CD8+ T cells for long-term responses, our observation that viral vaccination plus high dose rapamycin generated CD8+ T cells produced greater tumor efficacy upon re-transfer to tumor bearing untreated (vaccine and/or rapamycin) mice provides further evidence indicating the inherent programming of CD8+ T cells by day 8 which resulted in enhanced tumor efficacy in the absence of cell-extrinsic conditions. The fact that re-adoptive transfer of OT-1 cells educated with vaccine plus high dose rapamycin produced better tumor protection (~ 30% versus ~60% survivors), points to the potential impact rapamycin treatment may have on other cell types that may negatively influence CD8+ T cell tumor efficacy. These insights have important implications for developing new vaccination strategies for cancer and identify the use of mTOR inhibition regimens as an effective means to regulate CD8+ T cell functional fate for immunity.
Supplementary Material
Acknowledgments
We thank all members of the Shrikant and Gillander lab’s for their critical review, comments and discussions.
Footnotes
This study was supported in part by NIH (CA104645), Ovarian Cancer Research Fund, and Alliance Foundation of Roswell Park Cancer Institute (to P.A.S.).
References
- 1.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 2.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 4.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnjatic S, Jager E, Chen W, Altorki NK, Matsuo M, Lee SY, Chen Q, Nagata Y, Atanackovic D, Chen YT, Ritter G, Cebon J, Knuth A, Old LJ. CD8(+) T cell responses against a dominant cryptic HLA-A2 epitope after NY-ESO-1 peptide immunization of cancer patients. Proc Natl Acad Sci U S A. 2002;99:11813–11818. doi: 10.1073/pnas.142417699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8+ T cell responses tovaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route ofimmunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central-or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature reviews. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 15.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 16.Kahan BD. Fifteen years of clinical studies and clinical practice in renal transplantation: reviewing outcomes with de novo use of sirolimus in combination with cyclosporine. Transplant Proc. 2008;40:S17–20. doi: 10.1016/j.transproceed.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 18.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immun. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immun. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blazar BR, Taylor PA, Snover DC, Sehgal SN, Vallera DA. Murine recipients of fully mismatched donor marrow are protected from lethal graft-versus-host disease by the in vivo administration of rapamycin but develop an autoimmune-like syndrome. J Immunol. 1993;151:5726–5741. [PubMed] [Google Scholar]
- 25.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 26.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 27.Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, Shrikant PA. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 2011;34:541–553. doi: 10.1016/j.immuni.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 29.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immun. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 31.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immun. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 33.Mariotti J, Foley J, Jung U, Borenstein T, Kantardzic N, Han S, Hanson JT, Wong E, Buxhoeveden N, Trepel JB, Fojo AT, Telford W, Fowler DH. Ex vivo rapamycin generates apoptosis-resistant donor Th2 cells that persist in vivo and prevent hemopoietic stem cell graft rejection. J Immunol. 2008;180:89–105. doi: 10.4049/jimmunol.180.1.89. [DOI] [PubMed] [Google Scholar]
- 34.Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A, Nardacci R, Leduc P, Ingber DE, Druillennec S, Roques B, Leibovitch SA, Vilella-Bach M, Chen J, Este JA, Modjtahedi N, Piacentini M, Kroemer G. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. The EMBO journal. 2002;21:4070–4080. doi: 10.1093/emboj/cdf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castedo M, Ferri KF, Blanco J, Roumier T, Larochette N, Barretina J, Amendola A, Nardacci R, Metivier D, Este JA, Piacentini M, Kroemer G. Human immunodeficiency virus1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J Exp Med. 2001;194:1097–1110. doi: 10.1084/jem.194.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao RR, Li Q, Shrikant PA. Fine-tuning CD8(+) T cell functional responses: mTOR acts as a rheostat for regulating CD8(+) T cell proliferation, survival and differentiation? Cell cycle. 2010;9:2996–3001. doi: 10.4161/cc.9.15.12359. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 38.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immun. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 39.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.