Abstract
The CD20 mAb ofatumumab (OFA) induces complement-mediated lysis of B cells. In an investigator-initiated phase II trial of OFA plus chemotherapy for chronic lymphocytic leukemia (CLL), OFA treatment promoted partial CLL B cell depletion which coincided with reduced complement titers. Remaining CLL B cells circulated with bound OFA and covalently bound complement breakdown product C3d, indicative of ongoing complement activation. Presumably neither complement- nor effector cell-based mechanisms were sufficiently robust to clear these remaining B cells. Instead, almost all of the bound OFA as well as CD20 was removed from the cells, in accordance with previous clinical studies which demonstrated comparable loss of CD20 from B cells after treatment of CLL patients with rituximab. In vitro experiments with OFA and rituximab addressing these observations suggest that host effector mechanisms which support mAb-mediated lysis and tumor cell clearance are finite, and they can be saturated or exhausted at high B cell burdens, particularly at high mAb concentrations. Interestingly, only a fraction of available complement was required to kill cells with CD20 mAbs, and killing could be tuned by titrating the mAb concentration. Consequently, maximal B cell killing of an initial and secondary B cell challenge was achieved with intermediate mAb concentrations, whereas high concentrations promoted lower overall killing. Therefore mAb therapies that rely substantially on effector mechanisms subject to exhaustion, including complement, may benefit from lower, more frequent dosing schemes optimized to sustain and maximize killing by cytotoxic immune effector systems.
Introduction
The B cell-targeting CD20 mAbs, rituximab (RTX) and ofatumumab (OFA), achieve the high levels of cytotoxicity necessary for effective cancer treatment by employing effector mechanisms of the body’s innate immune system (1–11). These mechanisms include complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis. In CDC, mAb-targeted cells activate the classical pathway of complement by which they are covalently tagged with activated complement protein fragments C4b and C3b, and are then lysed due to generation of membrane attack complexes (12–14). However, the increased understanding of immunotherapeutic mAb cytotoxic mechanisms, including that of alemtuzumab (ALM) which also kills targeted cells by CDC (15,16), has not yet led to scientifically formulated fundamental approaches to dosing regimens. Indeed, most modifications of dosing strategies have been empirical, with the unstated presumption that for CD20 mAbs the usual weekly 375 mg/m2 RTX treatment is likely to be close to an optimal dose (17–19). For the CD52 mAb ALM, dosing has been set at 10–30 mg three times weekly.
Because of low CD20 expression on chronic lymphocytic leukemia (CLL) cells together with high tumor burden, the efficiency of OFA-mediated CDC is particularly relevant for CLL treatment (6,8,10,20–22). As part of a phase II trial in CLL (NCT 01145209) combining intravenous OFA infusion with chemotherapy, we investigated the in vivo consequences of OFA treatment on circulating B cells, and evaluated absolute lymphocyte counts (ALC), complement consumption, C3 fragment deposition on cells and levels of B cell-associated CD20 and bound OFA.
At the trial start, patients had high burdens of circulating B cells which were significantly reduced by day 29. In addition, large reductions in complement titers were observed, most notably after the first OFA infusion. Intriguingly, non-depleted cells included B cells with substantial amounts of deposited complement C3 breakdown fragment C3d; these cells could continue circulating for extended time periods. Based on these findings we conducted parallel quantitative in vitro investigations comparing OFA and RTX with respect to their potential to activate and consume complement and to promote CDC upon binding to CD20+ cells.
In vitro studies demonstrated the ability of OFA to induce robust CDC in which only a fraction of available complement components were required to effect cell killing. Using high cell burden conditions, we demonstrated that complement could be severely depleted, leading to inadequate killing of a second target cell challenge. Significantly, we were able to reduce complement consumption and retain killing capability by reducing OFA concentrations. Our studies suggest that standard doses of CD20 mAb, in contrast with current dogma, may be excessive, resulting in wasteful complement consumption which depletes the body’s complement reservoir and cytotoxic capacity. This insight provides a framework for the design of mAb-based immunotherapy regimens that preserve complement as well as other effector functions, thus leading to increased overall tumor cell killing and potentially enhanced efficacy.
Materials and Methods
NIH Clinical trial NCT01145209
Cycle 1: Patients received intravenous OFA (day 1, 300 mg; day 8, 1 g) and either fludarabine (25 mg/m2 days 2–6, FO), or fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2/d (days 2–4, FCO). Cycle 2: day 29, 1 g of OFA plus chemotherapy. Blood samples from patients N1 to N9 (obtained with informed consent) were drawn immediately before starting OFA infusions and several times after infusions started. Absolute lymphocyte counts (ALC) were determined at the NIH clinical laboratory. Analyses of B cells were based on flow cytometry protocols described in previous studies (23,24). B cells were identified by light scattering and development with PE CD19 and PERCP CD45. CD20 was measured by reacting washed whole blood samples with excess OFA and developed with Alexa (Al) 488 mAb HB43 (specific for human IgG Fc). OFA that bound to cells in vivo was measured by reacting washed blood samples with Al488 mAb HB43. Cells were probed with Al488 mAbs 7C12 (C3b/iC3b specific) or 1H8 (C3b/iC3b/C3d specific) to evaluate C3 fragment deposition. Fluorescence intensities were converted to Molecules of Equivalent Soluble Fluorochome (MESF) for quantitation (25,26). To allow quantitative comparisons for all measurements, all assays were conducted with one reagent set, with constant settings on the flow cytometer (BD FACSCalibur).
CLL cells were obtained from blood samples of de-identified patients at the University of Virginia, in accordance with protocols of the UVA institutional review board. All authors had access to primary clinical data, but only investigators at the NIH knew the identities of the patients (N1–N9) in the trial.
Cell lines and Primary Cells
Human Burkitt’s lymphoma cell lines Daudi, Raji (European Collection of Cell Cultures, Porton Down, UK), Z138 mantle cell lymphoma cell line (27) and diffuse large B cell lymphoma cell line SU-DHL-4 (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) were cultured in RPMI 1640 containing 10% heat-inactivated calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 4 mM L-glutamine (Invitrogen, Carlsbad, CA) [REF 31]. Wien-133 cells were provided by Dr. George Hale (Oxford University). Blood samples were obtained from CLL patients at the University of Virginia Hospital (not on the trial, numbers V1–V16) and B cells were isolated using Ficoll-Paque (10,28).
Serum
Pooled normal human AB serum (NHS) was from Sanquin (The Netherlands), or was prepared from pooled sera (25,29). Complement deficient serum and components were from Quidel (San Diego, CA) and C3-depleted (immunodepleted) sera were from Complement Technology Inc. (Tyler, TX).
Monoclonal antibodies
Ofatumumab (OFA, 2F2, Arzerra), and 11B8 are human IgG1, CD20 antibodies generated in HuMab mice (6,8,10,20–22). HuMab-KLH (human IgG1), a human antibody against irrelevant antigen (keyhole limpet hemocyanin), is an isotype control. Rituximab (RTX, Mabthera), a chimeric IgG1, was from Roche (Basel, Switzerland) and the UVA pharmacy. Alemtuzumab (ALM, Campath-1H, anti-CD52) was from Genzyme (Cambridge, MA) and the UVA pharmacy. RTX and OFA were labeled with Al 488 (Invitrogen) to the same fluorochrome/protein ratios of 1–2 following the manufacturer’s instructions; CDC activity of labeled mAbs was equal to that of unlabeled starting material (not shown). MAb 5G9, specific for C3b/iC3b, has been described (25,30).
Complement-dependent Cytotoxicity assays
CDC assays were conducted using previously described procedures (10,21,25). Cells were incubated at 37°C with mAbs in NHS for periods of between 15 min and one hr, and after washing, viability was determined by staining with TOPRO-3 or propidium iodide (PI). Under these brief incubation conditions there was no killing of cells reacted with mAbs in the absence of NHS, indicating little, if any apoptosis. In assays described in Supplementary Figures 2–3, cells were opsonized with mAbs for 15 min at room temperature, washed twice, and resuspended in RPMI 1640/10% fetal calf serum and pooled NHS or complement-component deficient sera (20% vol/vol) was added. After incubation for 30–60 min at 37°C, cells were harvested and lysis was detected by flow cytometry (FACSCanto II, BD) based on PI or TOPRO-3 uptake. In CDC experiments described in Figures 2–4 and Supplementary Figure 4, cells (107–108/mL) were combined with media or with pooled NHS (10–50%) and either OFA or RTX added. After incubation for 10–30 min at 37°C, cells were washed and CDC was determined based on staining with TOPRO-3 (FACS-calibur, BD). In two-step experiments (Figure 4) similar procedures were followed, except after the first incubation more PKH26-labeled cells (28,31) were added, with additional mAb, for incubation in step 2.
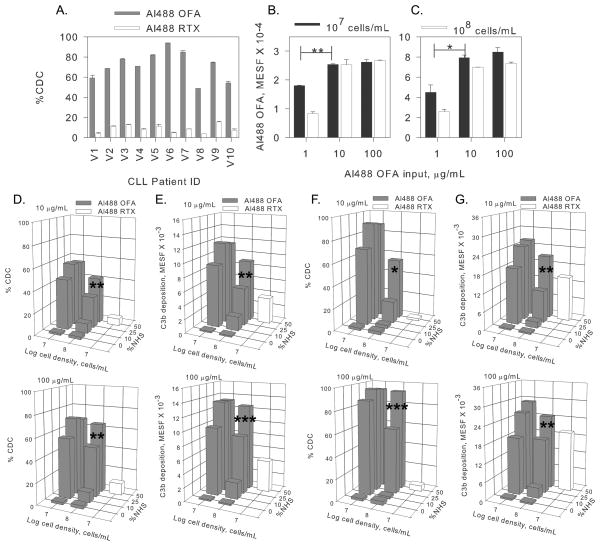
Figure 2. High concentrations of Al488 OFA, but not Al488 RTX, promote substantial CDC of CLL cells.
A. CLL cells from 10 patients (V1 to V10) seen at the University of Virginia (UVA) Hospital were isolated and tested for CDC in the presence of 100 μg/mL mAb, 50% NHS, for 15 min at 37°C. Means and SD (n=2) are displayed. Longer incubation periods did not lead to increased CDC. B–C. Dose-response experiments for OFA binding in media reveal that OFA concentrations as low as 10 μg/mL are sufficient to saturate the cells, even for cell densities of 108 cells/mL. Means and SD (n=2) are displayed for cells from patient V2 (B) and V6 (C). D–G. Dose-response experiments reveal that for high densities of CLL cells (from patients V2 (D,E) and V6 (F,G), optimal OFA-mediated CDC and C3b deposition require adequate complement (50% NHS) and OFA, but under comparable conditions RTX-mediated CDC and C3b deposition is modest. For each condition, final OFA and RTX concentrations of 10 or 100 μg/mL were examined. These results are similar to the findings for all 10 patients. Differences in Al488 OFA binding (B,C) and in CDC for cells reacted in 50% NHS vs 25% NHS were compared for significance, as noted. *, p<0.05; **, p< 0.01; ***, p< 0.001.
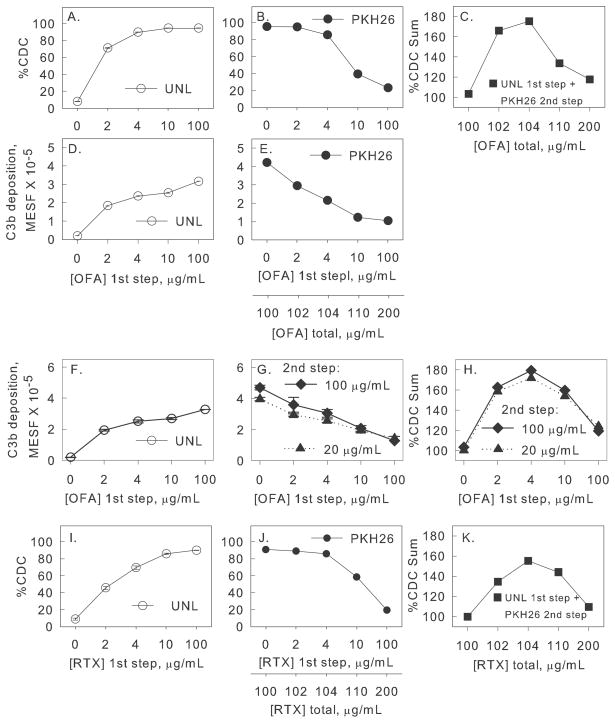
Figure 4. Two-step experiment: Lower mAb concentrations in Step 1 are more effective in promoting overall CDC of the combined cell populations.
A–C. Step 1: Unlabeled Daudi cells (1.8 × 107 cells/mL in 50% NHS), were reacted (one hr at 37°C) with OFA (0 to 100 μg/mL). In step 2 an equal number of PKH26-labeled Daudi cells was added, + 100 μg/mL additional OFA, and after incubation, CDC and C3b deposition was determined. A,B. The %CDC of unlabeled cells after step 1 (open circles), and of PKH26 labeled cells after step 2 (filled circles), is provided. C. The summation of %CDC for unlabeled cells after step 1 plus %CDC for PKH26 cells in step 2 is a bell-shaped curve. The decrease in CDC at high OFA concentrations is not due to final high OFA concentrations (200 μg/mL). In a separate one-step control, varying amounts of OFA were added to Daudi cells (3.6 × 107 cells/mL in 50% NHS); CDC was 85% for OFA concentrations of 100–400 μg/mL. D–E. C3b deposition on unlabeled cells after step 1 or on PKH26-labeled cells after step 2. F–H. Similar experiment, except OFA added in step 2 was 100 μg/mL or 20 μg/mL. Close to maximal C3b deposition and CDC is achieved for OFA concentrations of 4 μg/mL in step 1 and 20 μg/mL in step 2. I–K similar to A–C, except for RTX. Means and SD (n = 2) are displayed; usually SD are smaller than symbols. Each figure represents two or more experiments.
Cellular deposition of C3b and C4b
Deposition of C3b/iC3b fragments on OFA- or RTX-opsonized cells was determined according to published procedures (23,29). To assess cellular C4b deposition, saturating concentrations of mAbs were reacted with B cells for 15 min at room temperature. C5-deficient serum was added (final concentration of 20%) followed by incubation at 37°C for 45 min. After washing, fluorescein isothiocyanate (FITC)-conjugated polyclonal antibodies against C4c (F0169) (DakoCytomation, Glostrup, Denmark) were added (1:100) and samples incubated for 30 min at 4°C and analyzed by flow cytometry, with readouts being mean fluorescent intensities.
To distinguish between C4c deposited on anti-B-cell mAbs, or on B-cell membranes, an acidic wash (RPMI 1640 + 0.3% BSA, pH 2.5) was implemented.(31,32) MAb binding was determined based on staining with FITC-F(ab′)2 fragments of goat anti-human IgG, Fab-specific.
CH50 assays
Complement hemolytic assays (CH50) were performed according to standard procedures (33,34). Briefly, washed sheep E (Lampire) were reacted with anti-sheep E stroma (hemolysin, Sigma), washed, and reconstituted to a hematocrit of 2% EA in gelatin veronal buffer supplemented with Ca2+ and Mg2+ (GVB++). Test and standard sera were first diluted 10-fold in GVB++ and then further diluted sequentially by factors of 2 across 6 wells of a conical 96 well plate. The hemolytic reaction was initiated by addition of 25 μl of 2% EA to 25 μl of diluted sera. Assay plates were incubated for 60 min at 37°C. The reaction was stopped by addition of 200 μl ice cold GVB++ to each well. The lysed EA were pelleted by centrifugation at 1800 × g for 3 min, the supernatant removed to a flat bottom plate and the optical densities measured at 405 nm. CH50 titers were reported relative to a NHS pool. Assays were performed in triplicate.
Statistics
All experiments were conducted independently two or more times, and representative results are presented. Where replicates measurements were performed, means and SD are provided. Unpaired 2-tailed t tests (Sigmastat) were used to compare selected results in Figure 2.
Results
OFA infusion in CLL patients rapidly exhausts complement and induces loss of CD20 from the tumor cells
A first group of 9 CLL patients (N1–N9, NCT01145209) was treated with OFA (300 mg for the first dose (day 1) and 1 g for all doses thereafter (days 8 and 29)) in combination with chemotherapy (Table I). Blood samples were collected before, during and after OFA infusions on days 1, 8 and 29. Measurements included absolute lymphocyte counts (ALC), deposition of C3 fragments on target cells, complement titers and binding of OFA to circulating cells.
Table I.
Patient Characteristics.
| Pat ID | Age/Sex | Rai Stage | Cytogenetics | TreatMent | Spleen Sizea (cm) | Preb ALC (1000x/μL) | Preb CD20 (MESF) ave ± SD | Max Boundc OFA (MESF) ave ± SD | % Saturation | Preb Rel CH50d ave ± SD | Max Bound C3de (MESF) ave ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 26/ M | II | 11q | FCOf | 11 | 131 | 44,600±600 | 17,100±20 | 38 | 4±1 | 10,300±100 |
| N2 | 49/ F | III | 13q | FO | 9.5 | 306 | 13,100±100 | 12,600±200 | 96 | 68±16 | 15,400±100 |
| N3 | 61/ F | II | 17p, 11q, 13q | FCO | 16 | 115 | 13,300±20 | 1,700±100 | 13 | 87±9 | 7,700±200 |
| N4 | 56/ F | III | T12 | FO | 16 | 142 | 33,600±900 | 10,900±400 | 32 | 51±12 | 17,500±400 |
| N5 | 47/ M | III | 17p, T12, 13q | FCO | 24.5 | 158 | 18,800±200 | 12,200±100 | 65 | 94±22 | 20,800±600 |
| N6 | 50/ M | II | 11q, 13q | FCO | 16.5 | 273 | 5,500±200 | 3,200±40 | 58 | 69±8 | 5,200±30 |
| N7 | 57/ F | IV | 13q | FO | 22.5 | 168 | 9,700±200 | 11,700±100 | 121 | 49±9 | 7,400±200 |
| N8 | 66/ F | II | 17p, 13q | FCO | 11.5 | 253 | 4,600±100 | 2,400±40 | 52 | 109±12 | 4,900±20 |
| N9 | 78/ M | III | Normal | FO | 16 | 90 | 29,200±1600 | 25,300±800 | 87 | 84±4 | 25,000±600 |
No patient had bulky disease (> 5 cm).
Craniocardal diameter on CT.
The “pre” values are based on measurements on blood samples taken just before the first OFA infusion; n =2 for CD20 determinations.
Max OFA bound was observed in blood samples taken 2 hrs after the first infusion for all patients except pat 3; in this case maximum OFA bound was observed at 6 hrs (n=2). %Sat = 100 X (Max Bound OFA/Pre CD20).
The relative CH50 values are based on a pool of sera taken from normal volunteers with the CH50 titer set to 100 (n=3).
Max C3d bound was observed in the 2 hr samples (day 1) for patients 2, 4–6, and 8–9. For patients 3 and 7, C3d binding peaked at the 6 hr point. Apparently due to low initial CH50 titer in patient 1, C3d binding did not peak until day 29 (n=2).
FCO: OFA and fludarabine/cyclophosphamide; FO: OFA and fludarabine.
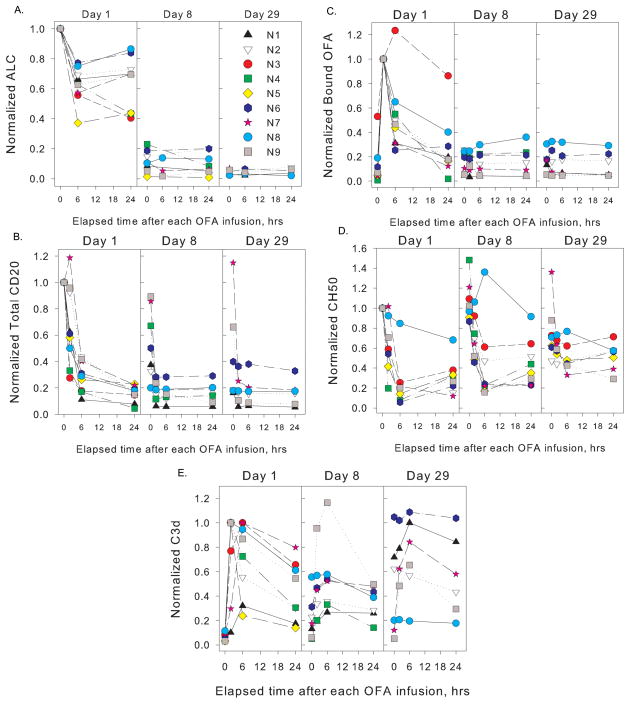
Significant B cell depletion in peripheral blood was achieved with the first cycle (Figure 1A). However, six of 8 patients had ALC in excess of 2,000 cells/μL on day 29 (one patient withdrew on day 10). The results of our measurements on blood samples taken over the first 29 days reveal several trends. The effect of OFA infusion on ALC was modest within the first 24 hours, achieving a median reduction of 30% (range 15–60%). Cell counts were greatly reduced on day 8 with a median reduction of 90% (range 80–95%) and on day 28 with a median reduction of 95% (range 93–98%). Based on the trial design that reflects current standard of care it is not possible to determine the relative contribution of OFA and chemotherapy to cytotoxicity. OFA infusions promoted substantial decreases in CD20 levels on circulating cells of all patients within 24 hrs (Figure 1B), likely as a result of trogocytosis as described for RTX (27,29,31); however, in many cases CD20 was partially restored on cells before the next infusion (day 8 and day 29). Two hours after starting the first infusion (day 1), substantial levels of OFA were demonstrable on circulating cells, in 6 cases corresponding to saturation of >50% of available CD20 sites (Figure 1C and Table I). This is all the more remarkable as by this timepoint only about 10% of the total dose, i.e. 30 mg of OFA, had been infused. These results indicate that during the first treatment, even when ALC are in the range of 100,000–200,000 cells/μL, relatively low doses of OFA (in the range of 20 mg/m2) (23,24) are adequate to achieve rapid and effective targeting of circulating CLL cells. Binding of OFA to cells decreased by 24 hrs (Figure 1C), likely due to CDC, clearance of OFA-opsonized cells by the mononuclear phagocytic system and trogocytosis of the OFA-CD20 complexes by effector cells expressing FcγR (23,29,31,35,36); indeed CD20 on circulating cells was reduced by 90% ± 5% (mean ± SD), 24 hrs after the start of the first infusion. In cases in which CD20 was partially restored between infusions, modest binding of OFA to circulating cells was demonstrable for subsequent infusions (Figures 1B,C). Notably, in most patients complement levels were reduced considerably due to OFA infusion (Figure 1D), and this was most evident during the first infusion when CD20 levels were initially high (Figure 1B).
Figure 1. Correlative studies on patients for the first 30 days of the trial.
OFA was infused on days 1 (300 mg), 8 (1 g) and 29 (1 g). Blood samples were obtained immediately before, and 2, 6 and 24 hrs after starting OFA infusions. Results are normalized to pre-treatment values for absolute lymphocyte counts (ALC), CD20, and CH50. Bound OFA is normalized to the 2 hr mark (first infusion), usually the maximum amount bound; values for bound C3d are normalized to maximum amount bound, observed at 2 or 6 hrs. Absolute values for these parameters are provided in Table I, along with representative uncertainties (SD). A., ALC; B., B cell surface levels of CD20; C., cell-bound OFA; D., complement titers (CH50 determinations); E., C3d deposition on B cells. The complement titer of patient N1 was low throughout the study (1–4 day 1; 7–8 day 29) and is not plotted in panel D. Results in B, C and E are based on duplicate determinations; CH50 titers were determined in triplicate.
OFA promoted deposition of C3d fragments on CLL B cells (Figure 1E). C3d, a degradation product derived from active C3b during complement activation, remains covalently attached to circulating cells that are targeted by complement-fixing antibodies but are not lysed (23,29). In most cases, circulating cells with deposited C3d fragments were demonstrable 24 hrs after OFA infusion. We also found that neither C3b nor iC3b were demonstrable on circulating cells (not shown), indicating these fragments are converted to C3d. A particularly interesting finding centers on the increased levels of C3d deposition on circulating cells of patients 1, 2 and 6 observed between day 9 (24 hrs after the second OFA infusion, when CD20 levels were quite low) and day 29, before the third OFA infusion. The most likely explanation is that after day 9, as CD20 was re-expressed by circulating CLL B cells, the cells were opsonized by circulating OFA, leading to C3b deposition followed by its degradation to C3d. CD20 levels were modestly restored on B cells of patient 6 by day 29 (Figure 1B.). It is also noteworthy that after the start of the OFA infusion on day 29 there was substantial C3d deposition on cells of several of the patients, reflecting binding of OFA and subsequent complement activation, due to partially restored levels of CD20.
In vitro studies
To investigate the basis of our in vivo findings, we studied the interplay of complement with OFA-opsonized cells using various in vitro experimental designs. As demonstrated in Supplementary Figures 1–3, cell lines opsonized with OFA demonstrate substantial deposition of C4b directly on the cell surface and are effectively lysed by CDC in the presence of relatively low concentrations of complement components C2 or C3. Indeed maximal OFA-induced CDC required only 10–100 μg/mL C3 which represents less than 10% of the amount present in normal plasma. Nevertheless, killing of high tumor cell loads required high serum concentrations (i.e. 50% NHS) (Supplementary Figure 4). OFA induced substantially more CDC of Daudi and Z138 cells than did RTX.
Dose Response Tests: CLL Cells
It has been reported that OFA promotes high levels of CDC and kills CLL B cells more effectively than RTX (6,8,10,20–22). To evaluate this phenomenon quantitatively, we labeled OFA and RTX with fluorophore Al488 (which does not affect CDC potency (10,21)) and evaluated CDC, cell binding and C3b deposition on CLL cells from 10 patients (seen at the University of Virginia (UVA) Hospital) not in the trial (V1–V10, Figure 2). In order to model physiologic conditions, we used 50% NHS and varying cell densities typical for CLL (i.e. 107 and 108 cells/mL). The results, using B cells from two representative CLL patients, reveal the following: saturation of OFA binding to cells in media is achieved at 10 μg/mL OFA (Figures 2B,C) and substantial CDC is observed at this concentration in 50% NHS for both cell burdens. At cell inputs of 108 cells/mL, OFA concentrations of 100 μg/mL and 50% NHS maximize CDC and C3b deposition (Figures 2D–G); less OFA-mediated CDC occurs if less complement (lower %NHS) is available. Finally, even at RTX concentrations of 100 μg/mL, there was little CDC, but complement activation was evident, as demonstrated by C3b deposition; thus RTX would appear to be less efficient in making use of complement to kill cells.
Complement Depletion
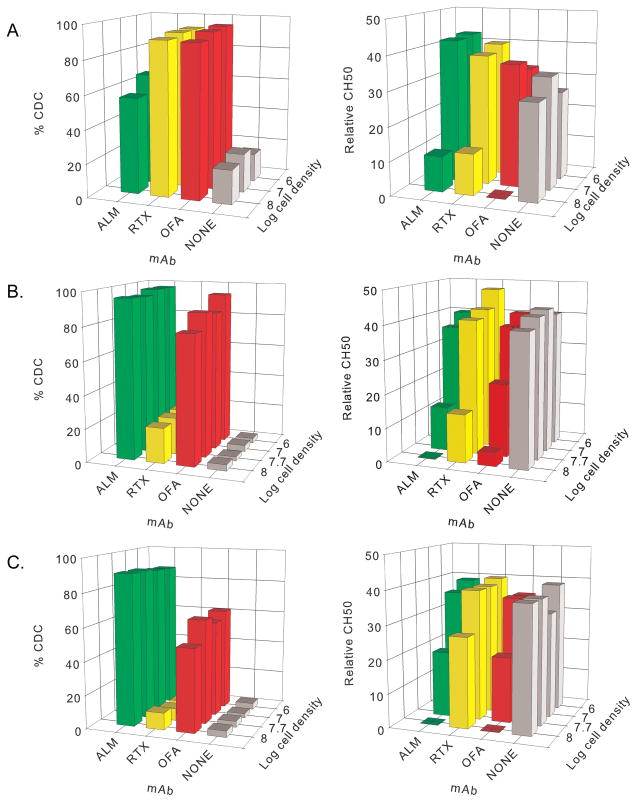
We reported that CD20+ B cell lines opsonized with RTX (100 μg/mL) at high cell burdens (108 cells/mL) activate and deplete complement in vitro (29). The present correlative results for CLL patients treated with OFA demonstrate complement consumption in vivo (Figure 1D). We extended our in vitro measurements to include alemtuzumab (ALM), with Wien cells as targets because these cells can be killed in NHS via CDC induced with either RTX, OFA or ALM. We varied target cell burdens with mAb concentrations held at 100 μg/mL, and measured CDC and complement depletion. In intact 50% NHS, all three mAbs promote CDC, but complement was depleted at high cell burdens (108 cells/mL) (Figure 3A). Similar results for CDC and complement depletion were obtained when OFA and RTX were tested with Daudi cells or Raji cells (not shown).
Figure 3. At cell densities as high as 108 cells/mL, CDC mediated by ALM, RTX or OFA at 100 μg/mL can be quite effective but at the highest cell densities complement activity is substantially depleted.
A. Results for Wien cells. In these experiments CDC was maximized at only ~70% in the presence of ALM, because a fraction of the Wien cells did not express CD52. B,C. Results for CLL cells of patients V11 (B) and V12 (C) (representative of results for cells from UVA patients V11–V16). Moderate to large reductions in complement titers occur when high densities of CLL cells are subjected to CDC mediated by ALM, RTX, or OFA, but only ALM and OFA promoted high levels of CDC. Cetuximab (CET) which does not bind to or induce CDC in B cells, was used as a second negative control and no complement was consumed (not shown).
We next used varying concentrations of CLL cells from six additional UVA CLL patients (V11–V16) and evaluated CDC and complement consumption mediated by OFA, RTX and ALM, in 50% NHS. Representative results from 2 patients are shown in Figures 3B,C. OFA and ALM induced CDC at high cell burdens (108/mL), and complement was consumed. RTX promoted modest CDC of the B cells of patient V11 (Figure 3B), and some complement was consumed. However, RTX-opsonized cells of patient V12 were not killed by CDC, and less complement was consumed for this RTX-treated sample (Figure 3C).
Alternative mAb dosing strategies better preserve complement activity for more effective CDC
Our findings raise fundamental questions with respect to mAb dosing paradigms in cancer immunotherapy. If a mAb depends on host innate effector systems (CDC, ADCC or ADCP) to mediate cytotoxicity of malignant cells, it would seem important to determine the most effective immunotherapeutic doses, and their optimal timing, rather than to base treatment on maximum tolerated doses. Circulating cell burdens in CLL, readily accessible to infused mAbs, can be quite high (23,24,37,38). The infused mAb may thus promote clearance/destruction of cells at the cost of available effector mechanisms. Therefore, several hours after mAb infusion, as more cells re-equilibrate to the bloodstream from other compartments, effector functions such as complement may be saturated or exhausted for periods of days or weeks. Under these conditions clearance/killing of the next wave of malignant cells may be severely compromised (11,23,39). To examine this potential problem, we established a two-step model to approximate likely scenarios with respect to infusion of mAbs for CLL treatment, based on treatment paradigms currently used for RTX and OFA.
In step 1, varying amounts of OFA were added to multiple identical aliquots of Daudi cells in 50% NHS; after a one hr incubation at 37°C cell death and C3b deposition were determined. In step 2, an equal number of naive PKH26-labeled Daudi cells were added along with additional mAb. These PKH26-labeled cells represent CLL cells re-equilibrating into the bloodstream. The mixtures were incubated for an additional one hour at 37°C and CDC and C3b deposition on the PKH26 cell population was measured. The results for OFA and RTX are shown in Figures 4A–H and 4I–K respectively.
This two-step experiment demonstrates that use of moderate to high concentrations of OFA in step 1 (4 to 100 μg/mL) promotes high levels of CDC of unlabelled Daudi cells (95% CDC) in 50% NHS (Figure 4A, open circles; UNL). However, PKH26-labeled cells added in step 2 (along with an additional 100 μg/mL OFA) are effectively lysed only in samples which contained relatively low concentrations of OFA in step 1 (≤4 μg/mL, Figure 4B, filled circles, PKH). That is, although “excess” additional OFA (100 μg/mL) was added in step 2, only a fraction of PKH26-labeled Daudi cells were killed when added to samples exposed to higher OFA concentrations in step 1 (39% and 23% CDC, for samples reacted with 10 and 100 μg/mL OFA, respectively). The bell shaped curve in Figure 4C represents the sum of CDC achieved for both populations in this two-step experiment (% CDC of unlabeled cells in step 1 plus % CDC of PKH26-labeled cells in step 2). Thus, most effective killing is achieved with an OFA concentration (4 μg/mL) just sufficient to achieve maximal killing in step 1 (Figure 4A). Presumably complement activity is preserved under these conditions and the second wave of B cells is effectively killed in step 2, whereas higher OFA concentrations in step 1 do not markedly increase killing of the first wave of cells but promote exaggerated complement activation and consumption. This interpretation is supported by examining C3b deposition in our two-step paradigm (Figures 4D,E). C3b deposition for unlabeled cells modestly increases as the OFA input increases from 10 to 100 μg/mL in step 1 (Figure 4D), indicating increasing complement activation and deposition even though maximal cell killing is already achieved. In contrast, C3b opsonization of the PKH26-labeled cells (after addition of OFA at 100 μg/mL) decreases continuously for samples in which higher OFA concentrations were used in step 1 (Figure 4E), indicating that complement was consumed in step 1, thus limiting C3b opsonization of the PKH26 cells in step 2.
We next performed a more comprehensive experiment in which OFA doses in step 1 and step 2 were varied (Figures 4F–H), and the findings are consistent with the observations above. Close to the maximum sum of CDC for the two populations was achieved at OFA inputs of only 4 μg/mL in step 1 and 20 μg/mL in step 2 (Figure 4H). Similar patterns were evident based on evaluation of C3b deposition (Figures 4F–G); opsonization with 4 μg/mL OFA in step 1 and 20 μg/mL OFA in step 2 gave more C3b deposition on PKH26-labeled cells than when 100 ug/mL OFA was used in both steps. Finally, we replicated these findings for RTX in similar experiments (Figures 4I–K).
Discussion
The most important finding in the present study is that the potential of the complement cascade to support mAb-mediated CDC of tumor cells is finite and saturable, and the degree of CDC is strongly influenced by the nature of the opsonizing mAb and its concentration. Our findings in the CLL clinical study demonstrate that at high initial cell burdens, infusion of OFA activates and consumes complement, and this was also made evident by the appearance of C3d-tagged circulating cells (Figures 1D,E). Apparently, after an initial wave of clearance/killing, some complement activation may continue, but complement as well as the mononuclear phagocytic system and NK cell-mediated ADCC are insufficient to deplete the cells (Figure 1A). The increase in net C3d found on cells of three patients by day 29, relative to day 9, suggests that as CD20 is re-expressed by cells, the infused OFA may promote chronic low level complement activation as it targets CD20 on cells.
Our findings of the rapid loss of CD20 due to infusion of OFA are most likely mediated by trogocytosis, but direct internalization of OFA by the CLL B cells may also play a role in loss of CD20 (40,41). However, we have directly compared the relative rates and extent of loss of CD20 for trogocytosis versus internalization, and these experiments demonstrate that trogocytosis reduces CD20 expression more strongly and in a shorter time frame (42).
We compared ALM, OFA and RTX with respect to their potential to utilize and activate individual components of the classical pathway of complement activation. OFA is more effective in promoting C4b deposition on opsonized Wien cells than RTX or ALM (Supplementary Fig. 1). The results are not due to higher densities of cell-bound OFA; binding of RTX and OFA to Wien cells was comparable and OFA binding was lower than ALM binding. Less cell-associated C4b was released from OFA-opsonized cells via acid wash than from RTX- or ALM-opsonized cells, indicating more deposited C4b was covalently bound to the cell membrane; this finding may be because OFA binds close to the cell membrane (6,8,10,20–22). CDC studies in sera depleted of C2 or C3 provide additional support for the concept that cell-bound OFA can use limited amounts of complement to lyse cells and that complete serum therefore initially contains a relatively large excess of complement components required for cell killing (Supplementary Figures 2 and 3).
Indeed, due to downstream amplification in the complement cascade, small amounts of early components are adequate to allow for deposition of large amounts of C3 activation fragments on cells (12–14). However, complement component C2, at lowest concentration in the plasma, is often the limiting factor that is first depleted during classical pathway activation. Based on our previous observations of C2 consumption in CLL patients who received RTX therapy (25,29), it is reasonable to predict that C2 was also first depleted in the present studies. These considerations, taken in context with the work of Ziccardi (43) may also explain the sharp drop in CDC observed for serum concentrations of 25% at the higher cell densities (Supplemental Figure 4). Under these conditions the potential of C1 inhibitor to control C1 activation may be abrogated, thus leading to uncontrolled activation of C1 followed by complete consumption of C2.
In patients with CLL, circulating B cells in excess of 108/mL are commonly observed (23,24,37–39), and we reported that infusion of the usual RTX dose can deplete complement in CLL patients for a week or longer (29). We extended these findings and show that at these physiologically relevant cell burdens complement activity in 50% NHS can be severely depleted (reduced 20-fold) when cells are reacted with mAbs such as ALM or OFA and are killed by CDC (Figure 3). Indeed, under our standard assay conditions, 50% NHS is required to promote effective CDC at 108 cells/mL, and CDC and C3b deposition are substantially reduced if the NHS concentration is reduced only 2-fold to 25% (Figure 2 and Supplementary Figure 4). Moreover, our two-step experiments described in Figure 4 provide dramatic evidence that at moderate to high cell burdens, large amounts of mAb indeed promote effective CDC of CLL cells, but if more mAb is added than essentially required, this leads to exhaustion of complement activity and therefore additional CDC is severely compromised upon challenge with additional cells, even if adequate mAb concentrations are present. The present results, taken along with our previous findings for CLL patients treated with RTX (29), indicate that after the standard (high) mAb doses, patients’ complement levels can be depleted in vivo for periods of several days to more than one week. It would seem reasonable that mAb dosing strategies that take into account patient complement titers as well as other indices of immune function would help provide guidance for more efficacious, individualized therapies.
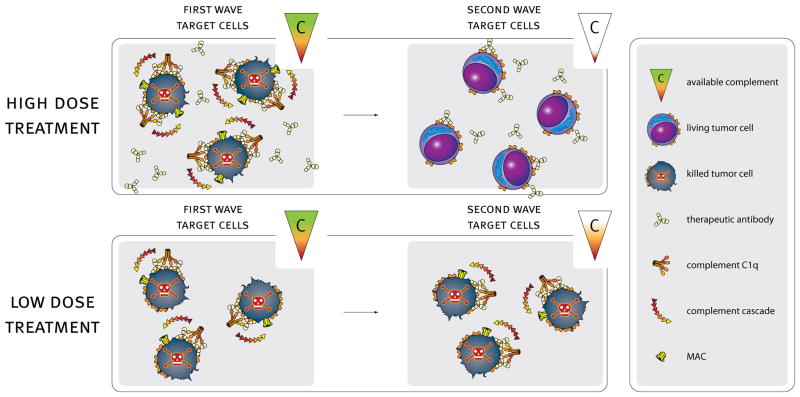
On the basis of accumulated evidence, high tumor burdens combined with high antibody concentrations may exhaust effector functions of the body’s innate immune system, including mAb-mediated CDC (Figure 5). Our results specifically relate to prominent therapeutic mAbs which are highly dependent on Fc-mediated effector functions for target cell cytotoxicity, such as type I CD20 mAbs. Provocative results in uncontrolled trials with RTX suggest that infusion of fresh frozen plasma to increase or restore complement activity may increase clinical efficacy of single agent treatment in CLL, in support of our findings (29,44–46). Our results support this concept with respect to maximizing the potential CDC that can be achieved by normal complement levels in the bloodstream. It is possible, based on findings in several reports, that other effector functions important in mAb-based immunotherapy of cancer, such as ADCC, are similarly subject to exhaustion, likely as a consequence of down-modulation of CD16 and depletion of NK cell-associated perforin and granzymes (47–51). Moreover, the substantial and protracted loss of CD20 mediated by infusions of large doses of mAb, coupled with the loss of potency of cytotoxic effector systems, would generate a “perfect storm” that can allow malignant cells to continue to proliferate for extended time periods, even after infusion of gram quantities of mAb.
Figure 5. Generalization of the findings in Fig. 4 to a likely in vivo scenario.
High dose mAb treatment may result in excess complement consumption (depletion of available complement). Complement depletion will lead to inadequate killing of a second target cell challenge even in the presence of additional excess mAb. Tailoring mAb doses to optimal levels (e.g. alternative dose or frequency of treatment) should be aimed at preserving complement-mediated killing activity and maintaining adequate complement levels. This paradigm may allow design of future mAb-based immunotherapy regimens in which effector function is retained over time to achieve increased overall killing and potentially increase efficacy.
We hypothesize that our results not only apply to OFA or RTX treatment, but impact all therapeutic mAbs which use Fc- or complement-mediated effector functions as part of their cytotoxic mechanisms. Re-formulation of mAb dosing schedules based on more frequent treatments (several times per week) with lower doses may allow for recovery and/or preservation of effector functions between doses (23,24) and should be investigated. Our study emphasizes that the time-honored approach to use drugs at maximum tolerated doses as derived from the chemotherapy field may not necessarily be optimal for mAb-based immunotherapy of cancer.
Supplementary Material
Acknowledgments
We thank Marleen Voorhorst for technical assistance and Joost Bakker for graphic design. We thank the patients for their participation in the trial.
Abbreviations used
- ADCC
antibody-dependent cell-mediated cytotoxicity
- Al
alexa
- ALC
absolute lymphocyte counts
- ALM
alemtuzumab
- Al
Alexa
- CDC
complement-dependent cytotoxicity
- CLL
chronic lymphocytic leukemia
- MESF
molecules of equivalent soluble fluorophore
- PI
propidium iodide
- OFA
ofatumumab
- RTX
rituximab
Footnotes
Glaxo Smith Kline funded the correlative studies and provided Ofatumumab for the trial. The clinical trial was funded by NHLBI, NIH. Genmab supported the in vitro studies.
The online version of this article contains supplemental material.
Reference List
- 1.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 2.Parren PWHI, van de Winkel JGJ. An integrated science-based approach to drug development. Cur Opin Immunol. 2008;20:426–430. doi: 10.1016/j.coi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, Golay J. Mechanism of action of Type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol. 2011;186:3762–3769. doi: 10.4049/jimmunol.1000303. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Cartron G, Watier H, Golay J, Solai-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 6.Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie MJ. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin’s lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of Rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother. 2006;29:388–397. doi: 10.1097/01.cji.0000203081.43235.d7. [DOI] [PubMed] [Google Scholar]
- 8.Teeling JL, Mackus WJM, Wiegman LJJM, van den Brakel JHN, Bees SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, Parren PWHI, Glennie MJ, van de Winkel JGJ. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 9.Glennie MJ, French R, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 10.Beum PV, Lindorfer MA, Beurskens F, Stukenberg PT, Lokhorst HM, Pawluczkowycz AW, Parren PW, van de Winkel JG, Taylor RP. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol. 2008;20:444–449. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walport MJ. Complement. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 13.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 14.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zent CS, Secreto CR, LaPlant BR, Bone ND, Call TG, Shanafelt TD, Jelinek DF, Tschumper RC, Kay NE. Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res. 2008;32:1849–1856. doi: 10.1016/j.leukres.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zent CS, Chen JB, Kurten RC, Kaushal GP, Lacy HM, Schichman SA. Alemtuzumab (CAMPATH 1H) does not kill chronic lymphocytic leukemia cells in serum free medium. Leuk Res. 2004;28:495–507. doi: 10.1016/j.leukres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles T, Dallaire BK, Wey K, Royston I, Davis T, Levy R. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 18.Byrd J, Murphy T, Howard R, Lucas M, Goodrich A, Park K, Pearson M, Waselenko J, Ling G, Grever M, Grillo-Lopez A, Rosenberg J, Kunkel L, Flinn I. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien S, Kantarjian H, Thomas D, Giles F, Freireich E, Cortes J, Lerner S, Keating M. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 20.Bleeker WK, Munk ME, Mackus WJM, van den Brakel JHN, Pluyter M, Glennie MJ, van de Winkel JGJ, Parren PWHI. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol. 2008;140:303–312. doi: 10.1111/j.1365-2141.2007.06916.x. [DOI] [PubMed] [Google Scholar]
- 21.Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JGJ, Parren PWHI, Taylor RP. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–758. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncology. 2010;28:3525–3530. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 23.Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, Hamil SH, Eggleton JC, Taylor RP. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–7443. doi: 10.4049/jimmunol.177.10.7435. [DOI] [PubMed] [Google Scholar]
- 24.Aue G, Lindorfer MA, Beum PV, Pawluczkowycz AW, Vire B, Hughes T, Taylor RP, Wiestner A. Fractionated subcutaneous rituximab is well tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica. 2009;95:329–332. doi: 10.3324/haematol.2009.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, Foley PL, Taylor RP. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by Rituximab. Blood. 2003;101:1071–1079. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 26.Beum PV, Lindorfer MA, Hall BE, George TC, Frost K, Morrissey PJ, Taylor RP. Quantitative analysis of protein co-localization on B cells opsonized with rituximab and complement using the ImageStream multispectral imaging flow cytometer. J Immunol Meth. 2006;317:90–99. doi: 10.1016/j.jim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: Rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol. 2006;176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 28.Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol. 2008;181:2916–2924. doi: 10.4049/jimmunol.181.4.2916. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–3288. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 30.Lindorfer MA, Pawluczkowycz AW, Peek EM, Hickman K, Taylor RP, Parker CJ. A novel approach to preventing the hemolysis of paroxysmal nocturnal hemoglobinuria: both complement-mediated cytolysis and C3 deposition are blocked by a monoclonal antibody specific for the alternative pathway of complement. Blood. 2010;115:2283–2291. doi: 10.1182/blood-2009-09-244285. [DOI] [PubMed] [Google Scholar]
- 31.Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol. 2008;181:8120–8132. doi: 10.4049/jimmunol.181.11.8120. [DOI] [PubMed] [Google Scholar]
- 32.Beekman JM, van der Poel CE, Van Der Linden JA, van den Berg DL, van den Berghe PV, van de Winkel JG, Leusen JH. Filamin A stabilizes FcγRI surface expression and prevents its lysosomal routing. J Immunol. 2008;180:3938–3945. doi: 10.4049/jimmunol.180.6.3938. [DOI] [PubMed] [Google Scholar]
- 33.Whaley K, North J. Haemolytic assays for whole complement activity and individual components. In: Dodds AW, Sim RB, editors. Complement. A Practical Approach. IRL at Oxford University Press; Oxford: 1997. pp. 19–48. [Google Scholar]
- 34.DiLillo DJ, Pawluczkowycz AW, Peng W, Kennedy AD, Beum PV, Lindorfer MA, Taylor RP. Selective and efficient inhibition of the alternative pathway of complement by a mAb that recognizes C3b/iC3b. Mol Immunol. 2006;43:1010–1019. doi: 10.1016/j.molimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber AD, Frank MM. Role of antibody and complement in the immune clearance and destruction of erythrocytes: in vivo effects of IgG and IgM complement fixing sites. J Clin Invest. 1972;51:575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 37.Rawstron AC, Bennett FL, O’Connor SJM, Kwok M, Fenton JAL, Plummer M, de Tute R, Owen RG, Richards SJ, Jack AS, Hillmen P. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 38.Shanafelt TD, Kay NE, Jenkins G, Call TG, Zent CS, Jelinek DF, Morice WG, Boysen J, Zakko L, Schwager S, Slager SL, Hanson CA. B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome. Blood. 2009;113:4188–4196. doi: 10.1182/blood-2008-09-176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper IA, Ding JC, Adams PB, Quinn MA, Brettell M. Intensive leukapheresis in the management of cytopeias in patients with chronic lymphocytic leukaemia (CLL) and lymphocytic lymphoma. Amer J Hematol. 1979;6:387–398. doi: 10.1002/ajh.2830060411. [DOI] [PubMed] [Google Scholar]
- 40.Beers SA, French RR, Chan CHT, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS, Dixon SV, Kim HJ, Cox KL, Kerr JP, Johnston DA, Johnson PWM, Verbeek S, Glennie MJ, Cragg MS. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 41.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 42.Beum PV, Peek EM, Lindorfer MA, Beurskens FJ, Engelberts PJ, Parren WHI, van de Winkel JGJ, Taylor RP. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. J Immunol. 2011;187:3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 43.Ziccardi RJ. A new role for C1 inhibitor in homeostasis: control of activation of the first component of human complement. J Immunol. 1982;126:2505–2508. [PubMed] [Google Scholar]
- 44.Klepfish A, Schattner A, Ghoti H, Rachmilewitz EA. Addition of fresh frozen plasma as a source of complement to rituximab in advanced chronic lymphocytic leukemia. Lancet Oncol. 2007;8:361–362. doi: 10.1016/S1470-2045(07)70106-7. [DOI] [PubMed] [Google Scholar]
- 45.Taylor R. Fresh frozen plasma as a complement source. Lancet Oncol. 2007;8:370–371. doi: 10.1016/S1470-2045(07)70114-6. [DOI] [PubMed] [Google Scholar]
- 46.Xu W, Miao KR, Zhu DX, Fang C, Zhu HY, Dong HJ, Wang DM, Wu YJ, Qiao C, Li JY. Enhancing the action of rituximab by adding fresh frozen plasma for the treatment of fludarabine refractory chronic lymphocytic leukemia. Intl J Canc. 2011;128:2192–2201. doi: 10.1002/ijc.25560. [DOI] [PubMed] [Google Scholar]
- 47.Bowles JA, Weiner GJ. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Methods. 2005;304:88–99. doi: 10.1016/j.jim.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Bowles JA, Wang SY, Link BK, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, Breitmeyer JB, Weiner GJ. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berdeja JG, Hess A, Lucas DM, O’Donnell P, Ambinder RF, Diehl LF, Carter-Brookins D, Newton S, Flinn IW. Systemic interleukin-2 and adoptive transfer of lymphokine-activated killer cells improves antibody-dependent cellular cytotoxicity in patients with relapsed B-cell lymphoma treated with rituximab. Clin Cancer Res. 2007;13:2392–2399. doi: 10.1158/1078-0432.CCR-06-1860. [DOI] [PubMed] [Google Scholar]
- 50.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells -enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veermani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.