Abstract
Epstein-Barr virus-induced gene 3 (EBI3) encoded protein can form heterodimers with IL-27P28 and IL-12P35 to form IL-27 and IL-35. IL-27 and IL-35 may influence autoimmunity through inhibiting Th17 differentiation, and facilitating the inhibitory roles of Foxp3+ Treg cells, respectively. In this study we have evaluated the development of experimental autoimmune encephalomyelitis (EAE) in EBI3-deficient mice that lack both IL-27 and IL-35. We found that MOG peptide immunization resulted in marginally enhanced EAE development in EBI3-deficient C57BL6 and 2D2 TCR transgenic mice. EBI3-deficiency resulted in significantly increased Th17 and Th1 responses in the central nervous system (CNS) and increased T cell production of IL-2 and IL-17 in the peripheral lymphoid organs. EBI3-deficient and -sufficient 2D2 T cells had equal ability in inducing EAE in Rag1−/− mice, however more severe disease was induced in EBI3−/−Rag1−/− mice than in Rag1−/− mice by 2D2 T cells. EBI3-deficient mice had increased numbers of CD4+FoxP3+ Treg cells in peripheral lymphoid organs. More strikingly, EBI3-deficient Treg cells had more potent suppressive functions in vitro and in vivo. Thus, our data support an inhibitory role for EBI3 in Th17, Th1, IL-2 and Treg responses. While these observations are consistent with the known functions of IL-27, the IL-35 contribution to the suppressive functions of Treg cells is not evident in this model. Enhanced Treg responses in EBI3−/− mice may explain why the EAE development is only modestly enhanced compared to WT mice.
Introduction
Epstein-Barr virus-induced gene 3 (EBI3) encoded protein can form heterodimmers with IL-27P28 (1) or IL-12P35 (2) to form two recently discovered cytokines, namely IL-27 (1) and IL-35 (3–4). IL-27 is mainly produced by antigen presenting cells (APCs) and signals through a heterodimeric receptor (IL-27R) consisting of the WSX-1 and the gp130 subunits (5). A variety of cell types including T cells express IL-27R (5–7) and ligation of IL-27R induces activation of cell type-specific signaling pathways involving Janus kinases and STAT transcription factors (8–10). Even though IL-27 was first described as a cytokine promoting Th1 responses (11), recent evidence suggest that IL-27 is a negative regulator of immune response capable of inhibiting Th1, Th2 and Th17 responses (12–14). IL-27 is also a potent inducer of IL-10 production by T cells (15–17). Studies using IL-27Rα−/− (WSX-1−/−) mice have revealed that IL-27 inhibits activation and proliferation of T cells and plays a critical role in inhibiting differentiation of Th17 cells (12, 18–19).
EBI3 has also been reported to be associated with the IL-12 p35 to form a heterodimmeric hematopoietin (2), which was later named IL-35 (3–4). Although the expression pattern of IL-35 may differ in human (20), it is constitutively expressed in FoxP3+CD4+CD25+ Treg cells in mice and contributes to their suppressive activity (4). IL-35-deficient (either deficient for EBI3 or P35) Treg cells have significantly reduced regulatory activity in vitro and fail to control homeostatic proliferation or cure inflammatory bowel disease (IBD) in vivo (4). Recombinant IL-35 suppresses T cell proliferation (3–4), Th17 differentiation and experimental arthritis (3). Moreover, exogenous IL-35 or Treg-produced IL-35 has been shown to either expand Foxp3+ Treg cells (3) or to induce a population of Foxp3-negative “iTR35” cells that mediates suppression via IL-35 but not the inhibitory cytokines IL-10 or TGF-β (21). Adoptively transferred iTR35 cells inhibited inflammatory bowel disease (IBD) and experimental autoimmune encephalomyelitis (EAE) in mice (21). Thus, IL-35 is a novel cytokine with therapeutic effects against autoimmune diseases.
EBI3−/− mice are deficient for both IL-27 and IL-35, thus the deficiency of EBI3 could have additive effects on the development of autoimmune inflammation. However, no overt autoimmunity or inflammatory disease has been reported in EBI3−/− mice (22). The role of IL-27 in promoting EAE development has been mainly demonstrated in IL-27Rα-deficient mice (12) and by IL-27 systemic injection (17). Recently, the EBI3 partner, p28-deficient mice have been shown to develop enhanced autoimmune inflammation in the CNS (23). Given the often distinct expression kinetics and the reported differences of the phenotypes between EBI3−/− and IL-27Rα−/− mice (24–25), evaluation of autoimmune responses in EBI3−/− may offer a new opportunity to identify novel pathways that regulate autoimmunity. In this study we evaluated the EAE development in EBI3-deficient mice. We found that MOG peptide immunization resulted in enhanced EAE development in EBI3-deficient C57BL6 mice and EBI3-deficient 2D2 TCR transgenic mice. Enhanced EAE development in EBI3-deficient mice was associated with increased IL-2, Th17, Th1 and Treg responses. Increased numbers and enhanced suppressive function of Tregs in EBI3−/− mice may explain why the EAE development is only modestly enhanced compared to WT mice.
Materials and methods
Mice
C57BL6 mice were purchased from the Jackson Laboratories. 2D2 TCR transgenic mice (26) were described previously (27). EBI3-deficient mice in the C57BL6 background have been described (22). 2D2 mice deficient for EBI3 (2D2+EBI3−/−) mice were generated through breeding of 2D2 mice with EBI3−/− mice for two generations. PCR was used for identification of mice genotypes. The primers used were: EBI3 (forward): 5′-CTG ATG GGT CAC TAA CTC GGA TCC-3′ and EBI3 (reverse): 5′-ACG ACA TCA GGG TCT GAT ATC AAG-3′; 2D2 (forward): 5′-CCC GGG CAA GGC TCA GCC ATG CTC CTG-3′ and 2D2 (reverse): 5′-GCG GCC GCA ATT CCC AGA GAC ATC CCT CC-3′. EBI3−/−Rag1−/− mice were generated through breeding EBI3−/− mice with Rag1−/− mice for two generations. Rag1-deficiency was identified by analyzing peripheral blood cells for lack of B220+ cells. All mice were maintained in the animal facilities of The Ohio State University. The animal facilities are fully accredited by American Association for Accreditation of Laboratory Animal Care.
Induction and assessment of EAE
Myelin oligodendrocyte glycoprotein (MOG) peptide35-55 (MEVGWYRSPFSRVVHLYRNGK), purchased from Genemed Synthesis, Inc (San Antonio, TX), was used as the immunogen. Mice of 8 to 12 weeks of age were immunized subcutaneously with 200 μg MOG peptidein CFA (containing 400 μg of Mycobacterium tuberculosis) in a total volume of 100 μL. Mice also received 150 ng of pertussistoxin (List Biological, Campbell, CA) in 200 μLPBS via the tail vein immediately after the immunization and again 48 hours later. In some experiments, Rag1−/− or EBI3−/−Rag1−/− mice received 1 × 106 CD4+ T cells from 2D2 mice or 5 × 106 CD4+ T cells from C57BL6 mice. The recipient mice were then immunized for the induction of EAE, as described above. The mice were observed every day and were scored on a scale of 0 to 5 with gradations of 0.5 for intermediate scores: 0, no clinical signs; 1, loss of tail tone;2, wobbly gait; 3, hind limb paralysis; 4, moribund; and 5, death.
Histology
Mice were sacrificed by inhaling CO2. Lumbar spinal cords were removed and fixed in 10% formalin/PBS. Paraffin sections were prepared and stained with hematoxylin and eosin (H&E) in the histology core facilities of the Department of Pathology (Ohio State University). Neurologic lesions were graded on each of the 10 cross sections per spinal cord, according the following criteria: 0, no infiltrate; 1, three or less focal meningeal infiltrates; 2, more than three focal meningeal infiltrates; 3, up to five perivascular infiltrate foci in the parenchyma with involvement of less than 5% of the white matter; 4, five to ten perivascular foci in the parenchyma or invasions involving 5–25% the white matter; 5, more than ten perivascular foci or diffuse infiltration involving more than 25% of the white matter.
Isolation of Total RNA from Spleen, Spinal Cord and qRT-PCR
Total RNA was isolated from spleens and spinal cords by using the Trizol method (Invitrogen). The first strand cDNA of each sample was synthesized using a reverse transcription kit (Invitrogen). Quantitative real time PCR was performed using an ABI Prism 7900-HT sequence system (PE Applied Biosystems) with the QuantiTect SYBR Green PCR kit (Qiagen) in accordance with the manufacturer’s instructions. The following primers were used: IL-2.F: 5′-TCC TGA GCA GGA TGG AGA AT-3′; IL-2.R: 5′-CGC AGA GGT CCA AGT TCA T-3′; IL10.F: 5′-ACA GCC GGG AAG ACA ATA AC-3′; IL10.R: 5′-CAG CTG GTC CTT TGT TTG AA-3′; IL17.F: 5′-CCT CCA GAA TGT GAA GGT CA-3′; IL17.R: 5′-CTA TCA GGG TCT TCA TTG CG-3′; IFNγ.F: 5′-AGC TCT TCC TCA TGG CTG TT-3′; IFNγ.R: 5′-TTT GCC AGT TCC TCC AGA TA-3′; TNFα.F: 5′-ATG AGA AGT TCC CAA ATG GC-3′; EBI3.F: 5′-ATG TCC AAG CTG CTC TTC CT-3′; EBI3.R: 5′-AGA GGA GTC CAG GAG CAG TC-3′; TGF-β1.F: 5′-ACA ATT CCT GGC GTT ACC TT-3′; TGF-β1.R: 5′-GAA AGC CCT GTA TTC CGT CT-3′; HPRT.R: 5′-TTA CTA GGC AGA TGG CCA CA-3′. The hypoxanthine-guanine phosphoribosyl transferase (HPRT) gene was amplified and served as endogenous control. PCR was performed using an optimal condition. One μl of first strand cDNA product was amplified with platinum Taq polymerase (Invitrogen) and gene-specific primer pairs. Each sample was assayed in triplicate and experiments were repeated twice. The relative expression was calculated by plotting the Ct (cycle number), and average relative expression was determined by the comparative method (2−ΔΔCt).
Cytokine ELISA
ELISA kits for the detection of IL-2, IL-10, IL-17 and IFN-γ were purchased from eBiosciences. Standard procedures were followed to detect release of cytokines in culture supernatants in a variety of settings (detailed in figure legends to each experiment).
Preparation of mononuclear cells from the CNS, intracellular cytokine staining and flow cytometry
On days 17 to 20 after EAE induction, mice were sacrificed and perfused with PBS through the left heart ventricle to eliminate contaminating blood cells in the CNS. The CNS mononuclear cells were then prepared, as we have described (28). For intracellular cytokine staining, the CNS infiltrating cells from mice were stimulated in vitro with phorbal 12-myristate 13-acetate (PMA) (50 ng/ml) and ionomycin (1 μM) for 5 h. GolgiStop (BD Biosciences) was added (1:1500) during the last 2 h of incubation. The cells were stained for the cell surface marker CD4, followed by a standard intracellular cytokine staining for IFN-γ, IL-10 and IL-17. The following antibodies were used: anti-CD4 FITC (GK1.4, BD Biosciences), anti-IL-10 PE (JES5-16E3, eBioscience), anti-IFN-γ APC (XMG1.2, BD Biosciences), anti-IL-17 PE (TC11-18H10, BD Biosciences), rat IgG1 isotype control PE or APC (BD Biosciences). Cells were analyzed on a FACScalibur flow cytometer and respective isotype controls were obtained from BD Pharmingen. Cells were analyzed on a FACScalibur flow cytometer.
Purification of CD4+CD25+ and CD4+CD25− T cells from 2D2 TCR Transgenic mice or C57BL6 mice
CD4+ T cells were purified from 2D2 TCR transgenic mice or C57BL6 mice by negative selection. Briefly, spleen and lymph node cells from donor mice were incubated with a cocktail of mAbs (anti-CD8 mAb TIB210, anti-FcR mAb 2.4G2 and anti-CD11c mAb N418). After removing the unbound antibodies, the cells were incubated with anti-rat IgG coated magnetic beads (Dynal Biotech). A magnet was used to remove the Ab-bound cells. The remaining cells were CD4+ T cells. To isolate CD4+CD25+ and CD4+CD25− T cells, the purified CD4+ T cells were further stained with anti-CD25-PE antibody (7D4, BD Biosciences) followed by separation of CD4+CD25+ and CD4+CD25− cells using anti-PE MACS bead technology. In some experiments, the purified CD4+ T cells were stained with anti-CD4-FITC and anti-CD25-PE, CD4+CD25+ and CD4+CD25− cells were subsequently separated by high speed sorting using a flow cytometer.
Lymphocyte proliferation and Treg-mediated suppression assay
For lymphocyte proliferation assay, splenocytes from EBI3−/− or WT mice with EAE, or draining lymph node cells from EBI3−/− or WT mice immunized with MOG35-55/CFA, or splenocytes from 2D2+EBI3−/− or 2D2+EBI3+/+ mice were cultured in Click’s EHAA medium in the presence of graded concentrations of MOG35-55 for 60 h, 1 μCi/well 3H-Tritium (ICN Pharmaceuticals) was then pulsed into the cultures, and incorporation of 3H-Tritium was measured in a liquid scintillation β plate counter 12 h later.
For Treg-mediated suppression assay, 1 × 106/ml purified CD4+CD25− T cells from EBI3−/− mice were co-cultured with graded numbers of CD4+CD25+ Treg cells from WT or EBI3−/− mice in the presence of irradiated splenocytes (2 × 106/ml) from EBI3−/− Rag1−/− mice and 0.1 μg/ml of anti-CD3 mAb (2C11). After 48 h, 1 μCi/well 3H-Tritium was pulsed into the cultures and incorporation of 3H-Tritium was measured in a liquid scintillation β plate counter 12 h later.
Statistics
Mann-Whitney U test was used for comparison of EAE clinical scores and histology scores at a given time point. We used Wilcoxon signed-rank test to compare EAE scores in the time course experiments. Student’s t-test was used for all other comparisons. In all cases, the alpha level was set at P < 0.05.
Results
1. EAE development is marginally enhanced in EBI3−/− mice
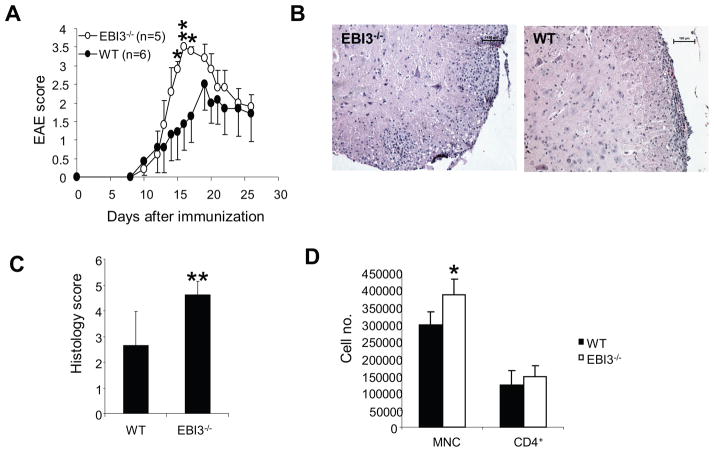
To understand the role of EBI3 formed cytokines in EAE development, we induced EAE in EBI3−/− and wild type mice using MOG 35-55 emulsified in CFA with two doses of pertussis toxin. As shown in Figure 1A, EBI3−/− mice were susceptible to EAE induction and had similar disease onset compared to WT mice. However, in wild type mice, disease peaked at about day 19 and persisted thereafter; EBI3−/− mice reached to peak disease earlier (at about day 17) and had significantly higher disease scores than wild type mice between days 14–16. However, after day 20, EAE scores were similar between both groups of mice. In five independent experiments, we consistently observed increased mean maximal scores (Table 1) and mean accumulating scores (Table 1) in EBI3−/− mice compared with WT mice. However, mean day of onset and mean incidences of EAE did not differ between the two groups of mice. Examination of histology in spinal cords revealed more inflammatory lesions in the white matter of EBI3−/− mice than in WT mice on day 17 (Figure 1B and Figure 1C). In addition, more CNS-infiltrating mononuclear cells were harvested from the brains and spinal cords of EBI3−/− mice with EAE on day 17 than in WT mice (Figure 1D). However, the number of CNS-infiltrating CD4+ T cells did not differ significantly between the two groups on day 17 p.i.
Figure 1. EAE development in EBI3−/− and wild type mice.
A. EAE development in EBI3−/− and wild type mice. To induce EAE, C57BL6 and EBI3−/− mice were immunized with MOG35-55 as described in materials and methods. A representative experiment out of five experiments is shown. *: p<0.05; **: p<0.01 by Mann-Whitney U test.
B. Representative histology of spinal cords from a EBI3−/− and a WT mouse. Bars represent 100 μm.
C. Inflammation scores of the spinal cords from mice with EAE. 17 days after EAE inductions, groups of EBI3−/− (n=5) and WT mice (n=5) were sacrificed and spinal cords were processed for H&E staining. Each spinal cord was evaluated and scored as described in materials and methods. **P<0.01 by Mann-Whitney U test.
D. CNS infiltrating cells in mice with EAE. 17 days after EAE inductions, groups of EBI3−/− (n=5) and WT mice (n=5) were sacrificed and spinal cords were removed. Mononuclear cells (MNC) were isolated, counted and stained for CD4 as described in materials and methods. * P<0.05 by student’s t test.
Table 1.
Summary of EAE development in five independent experiments
| Experiment | Mice | Incidence | Mean accumulating score | Mean maximal score | Mean day of onset |
|---|---|---|---|---|---|
| 1 | WT | 7/10 | 22.1 ± 11 | 1.85 ± 1.42 | 11.3 ± 0.8 |
| EBI3−/− | 10/10 | 26.1 ± 7 | 2.45 ± 0.87 | 12.43 ± 1.7 | |
| 2 | WT | 4/4 | 22.2 ± 6.1 | 2.1 ± 0.93 | 13.75 ± 0.37 |
| EBI3−/− | 5/5 | 27.7 ± 9.4 | 3.1 ± 0.88* | 13.6 ± 1.36 | |
| 3 | WT | 5/5 | 17.2 + 2.2 | 1.6 ± 0.32 | 14.6 ± 0.6 |
| EBI3−/− | 6/6 | 17 ± 3.3 | 2 ± 0.5 | 14.8 ± 0.8 | |
| 4 | WT | 6/7 | 19.6 ± 10.6 | 2.92 ± 0.9 | 16.1 ± 4.16 |
| EBI3−/− | 5/5 | 29.1 ± 4.6 | 3.6± 0.16* | 13 ± 1.2 | |
| 5 | WT | 5/5 | 20.6 ± 7.2 | 1.4 ± 0.36 | 13.2 ± 0.32 |
| EBI3−/− | 4/4 | 29.2 ± 11.8 | 1.6 ± 0.6 | 12 ± 0 |
Mean maximal score=the average of maximal score of each mouse in each experiment. Mean accumulating score=the average of accumulating scores of each mouse. The daily EAE scores of each mouse were added together for a period of 25–40 days to obtain the accumulating score for each mouse. Day of EAE onset was considered as when the first sign of EAE appears in each mouse. Data shown are Mean ± Standard deviations. In experiment#3 and #5, reduced pertussis toxin (100 ng/mouse) was used for the EAE induction.
p<0.05 by Mann-Whitney U test.
2. Enhanced Th17 responses in EBI3−/− mice
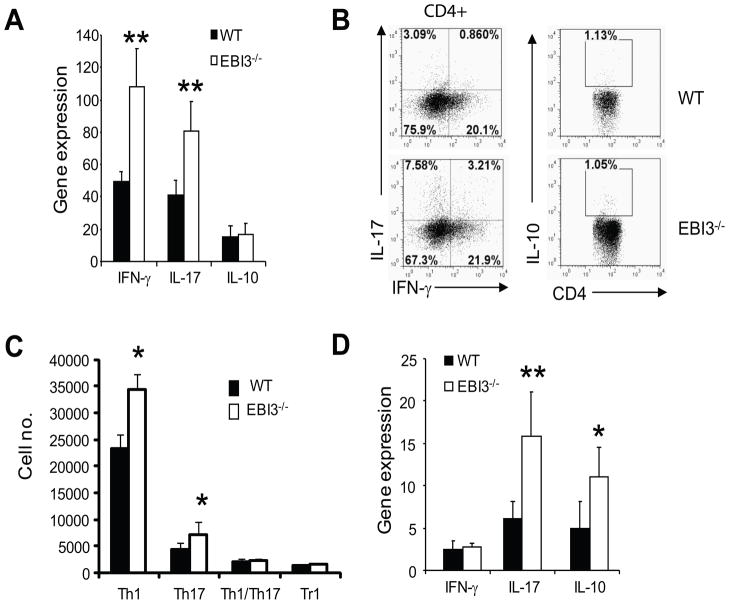
To understand the phenotype of EAE in EBI3−/− mice, we investigated the immune responses in the CNS of mice with EAE. Increased expression of IFN-γ and IL-17 genes (Figure 2A) and increased numbers of IFN-γ and IL-17 producing cells (Figure 2B and Figure 2C) were detected in the spinal cords of EBI3−/− mice at day 17 post immunization (p.i.). Expression of IL-10 gene and number of IL-10 producing CD4+ T cells (Tr1) were low in the spinal cords of mice with EAE on day 17 p.i.; no significant differences were detected between EBI3−/− and WT mice (Figure 2A–C). The expression of cytokine genes including IFN-γ and IL-17 in spinal cords diminished during EAE recovery (day 29), however, IL-10 gene expression was still higher in spinal cords of EBI3−/− mice (Figure 2D). We also detected increased IL-10 gene expression in the spinal cords of EBI3−/− mice (Figure 2D).
Figure 2. T cell responses in the CNS of WT and EBI3−/− mice.
A. qPCR was used to detect the expression of cytokine genes in spinal cords of mice with EAE (day 17 after EAE induction). Expression of cytokine genes in a normal spinal cord of a WT mouse was set as 1. Average and standard deviation of five mice per group are shown. Data shown represents two experiments with similar results. **: p<0.01 by student’s t test.
B. Production of cytokines by CNS-infiltrating CD4 T cells. MNCs were prepared from spinal cords of mice with EAE (day 17 after EAE induction). Cells were first stained for CD4 followed by intracellular cytokine staining as described in materials and methods. Data shown are representative of three experiments with similar results.
C. Quantification of CD4 T cell subsets in the spinal cords of mice with EAE. MNCs were prepared from each spinal cord of mouse with EAE (day 17 after EAE induction). Cells were counted and stained for CD4 followed by intracellular cytokine staining. Numbers of IL-17 producing CD4 T cells (Th17), IFN-γ producing CD4 T cells (Th1) and IL-10 producing CD4 T cells (Tr1) were calculated and data is expressed as mean + SD (n=5 for each group). * P<0.05 by student’s t test.
D. Cytokine gene expression in the CNS of mice with EAE (day 29 after EAE induction). Total RNA was prepared from the spinal cords of mice (n=3 in each group). *: p<0.05; **: p<0.01 by student’s t test. Data represents two experiments with similar results.
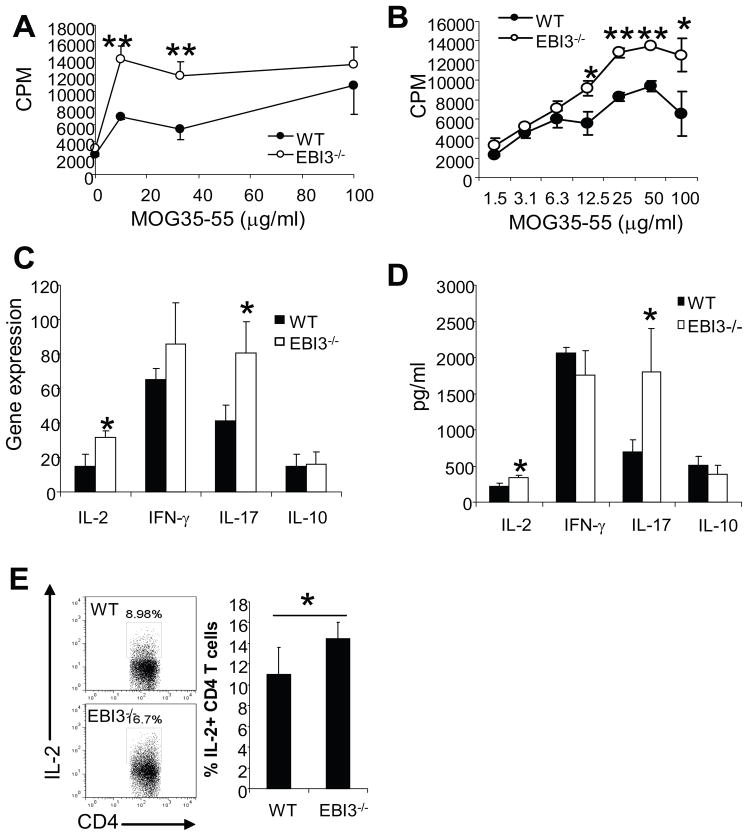
To determine the impact of EBI3-deficiency on the priming and differentiation of myelin antigen specific T cells in the peripheral lymphoid organs, we immunized EBI3−/− and WT mice with MOG35-55/CFA. Ten days later, draining lymph node cells were evaluated by lymphocyte proliferation assay. As shown in Figure 3A, lymph node cells from EBI3−/− mice had a stronger proliferation in response to MOG35-55 compared with WT lymph node cells. Similarly, when examining recall proliferative responses of splenocytes from mice with EAE (day 29 p.i.), we found that splenocytes from EBI3−/− mice underwent significantly higher proliferation (Figure 3B). We examined the expression of cytokine genes in MOG-peptide re-stimulated lymph node cells and found that expression of IFN-γ, IL-17 and IL-2 genes were significantly upregulated in cells from EBI3−/− mice (Figure 3C). We measured cytokines released in the culture supernatants and found that MOG 35-55-stimulated lymph node cells from EBI3−/− mice produced more IL-17 and IL-2 than lymph node cells from wild type mice. The production of IL-10 and IFN-γ did not differ significantly (Figure 3D). Since IL-2 production was low in the culture supernatant after four days of cell culture, we directly measured IL-2 production in CD4+ T cells from un-stimulated lymph nodes by intracellular cytokine staining. As shown in Figure 3E, higher numbers of CD4+IL-2+ T cells were detected in the lymph nodes from EBI3−/− mice than in WT mice.
Figure 3. T cell responses in the peripheral lymphoid organs of EBI3−/− and WT mice.
A. Proliferative responses of draining lymph node cells in MOG-peptide immunized EBI3−/− and WT mice. Mice were immunized s.c. with MOG 35-55/CFA, 10 days after immunization, mice were sacrificed and draining lymph nodes were collected and lymph node cells were prepared. 2 × 106/ml of draining lymph node cells were stimulated with different concentrations of MOG 35-55 for three days. 3H-Tritium incorporation assay was performed as described in materials and methods. Five mice per group were used for this experiment, and data represents three experiments with similar results. **: p<0.01 by student’s t test.
B. Proliferative responses of splenocytes from mice with EAE (day 29 after EAE induction). 2 × 106/ml splenocytes were stimulated with different concentrations of MOG 35-55 for three days. Three mice per group was used in this experiment, data represents three experiments with similar results. *: p<0.05; **: p<0.01 by student’s t test.
C. Cytokine gene expression in draining lymph node cells of EBI3−/− and WT mice. 2 × 106/ml of draining lymph node cells described in A were stimulated with 50 μg/ml of MOG 35-55 for three days. RNA was prepared from the cultured cells and expression of cytokine genes was quantified by qPCR. Expression of cytokine genes in the lymph node cells of a naïve WT mouse was set as 1. Average and standard deviation of five mice per group are shown. * P<0.05 by student’s t test.
D. 2 × 106/ml of draining lymph node cells described in A were stimulated with 50 μg/ml of MOG 35-55 for four days. Production of cytokines in the culture supernatants were detected by ELISA. Data shown are average + SD (n=5 for each group). *: p<0.05 by student’s t test.
E. IL-2 producing CD4+ T cells in WT and EBI3−/− mice. Draining lymph node cells were stimulated with PMA (100 ng/ml) + Ionomycin (100 ng/ml) for 4 h in the presence of Gorgistop. Intracellular IL-2 staining (mAb: JES6-5H4) was performed and analyzed by flow cytometry. Data shown are average + SD (n=5 for each group). *: p<0.05 by student’s t test.
3. Enhanced Th17 response and EAE development in EBI3-deficient 2D2 mice
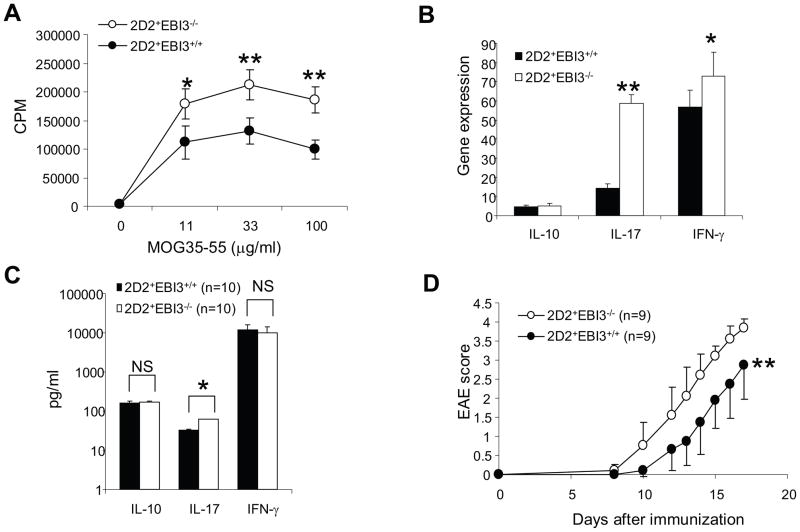
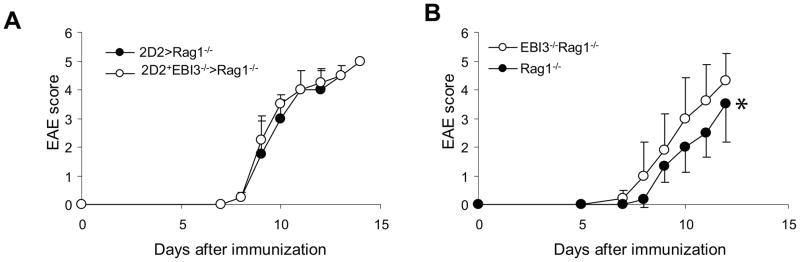
To further validate the observations in EBI3−/− mice, we bred 2D2 TCR transgenic mice with EBI3−/− mice and generated EBI3-deficient 2D2 mice (2D2+EBI3−/− mice). Although the frequencies of 2D2 T cells (Vα3.2+Vβ11+) in spleens of 2D2+EBI3−/− mice were similar to that of 2D2+EBI3+/+ mice (not shown), the splenocytes of 2D2+EBI3−/− mice had stronger proliferative response to MOG peptide stimulation (Figure 4A). After 5 d stimulation with MOG 35-55, qPCR analysis of expression for cytokine genes revealed that the most dramatic alteration was IL-17 (Figure 4B). In the culture supernatants, we detected about one fold difference in IL-17 production between splenocytes from 2D2+EBI3−/− and 2D2+EBI3+/+ mice, while production of IL-10 and IFN-γ did not significantly differ (Figure 4C). Finally, although 2D2+EBI3−/− mice rarely develop spontaneous EAE, similar to their wild type counterparts, they were more sensitive to induced EAE (Figure 4D). Upon induction, 2D2+EBI3−/− mice had earlier disease onset and rapidly reached peak disease compared with 2D2+EBI3+/+ mice. Thus, EBI3-deficiency enhances both Th17 responses and EAE development in 2D2 TCR transgenic mice. To test if EBI3-deficient T cells have superior capacity to cause EAE compared to WT T cells, we purified CD4+CD25− T cells from 2D2+EBI3−/− and 2D2+EBI3+/+ mice, and injected 1 × 106 purified T cells into each Rag1−/− mice i.v. followed by induction of EAE. As shown in Figure 5A, mice receiving T cells from 2D2+EBI3−/− mice developed equal disease compared with recipients of 2D2+EBI3+/+ T cells. Thus, the disease enhancing effect caused by EBI3-deficiency is not T cell intrinsic. In contrast, when we adoptively transferred CD4+CD25− T cells from 2D2+EBI3−/− mice into EBI3−/−Rag1−/− or Rag1−/− mice, we found that EBI3−/−Rag1−/− mice developed more severe EAE compared with Rag1−/− mice (Figure 5B). Thus, EBI3-deficiency in the non-T cell compartment enhances EAE development.
Figure 4. T cell responses and EAE development in EBI3-deficient 2D2 mice (2D2+EBI3−/−).
A. 3H-Tritium assay was used to measure proliferative responses of splenocytes to MOG35-55. Error bars representing mice groups of three (2D2+EBI3+/+) and four (2D2+EBI3−/−). *: p<0.05; **: p<0.01 by student’s t test. Data shown represents 5 experiments with similar results.
B. Assessment of cytokine gene expression in MOG35-55 activated splenocytes by real-time PCR. Splenocytes from 2D2+EBI3−/− and 2D2+EBI3+/+ mice were cultured with MOG35-55 (50 μg/ml) for 5 days, the resulting effector cells were then analyzed for expression of different cytokine genes using qPCR. Data shown represents two experiments with similar results.
C. The day 5 culture supernatants from the above culture were measured for different cytokines by ELISA. Data shown are average ± standard error (n=10 for 2D2+EBI3−/− and 2D2+EBI3+/+ mice). *: p<0.05 by student’s t test.
D. EAE development in 2D2+EBI3−/− and 2D2+EBI3+/+ mice. Each mouse was immunized with 100 μg of MOG35-55 in CFA subcutaneously. Each mouse also received 100 ng of pertussis toxin at immunization and 48 h later. **: P=0.0078 by Wilcoxon signed-rank test.
Figure 5. EBI3-deficient Rag1−/− mice are more susceptible to EAE.
A. 1 × 106 CD4+CD25− T cells from 2D2+EBI3−/− or 2D2+EBI3+/+ mice were injected into each Rag1−/− mouse i.v. The recipient mice were then immunized with MOG35-55/CFA/p.t. Data shown represents two experiments with similar results.
B. 1 × 106 CD4+CD25− T cells from 2D2+EBI3−/− mice were injected into each EBI3−/−Rag1−/− or Rag1−/− mouse i.v. The recipient mice were then immunized with MOG35-55/CFA/p.t. *: P=0.0313 by Wilcoxon signed-rank test. Data represents two experiments with similar results.
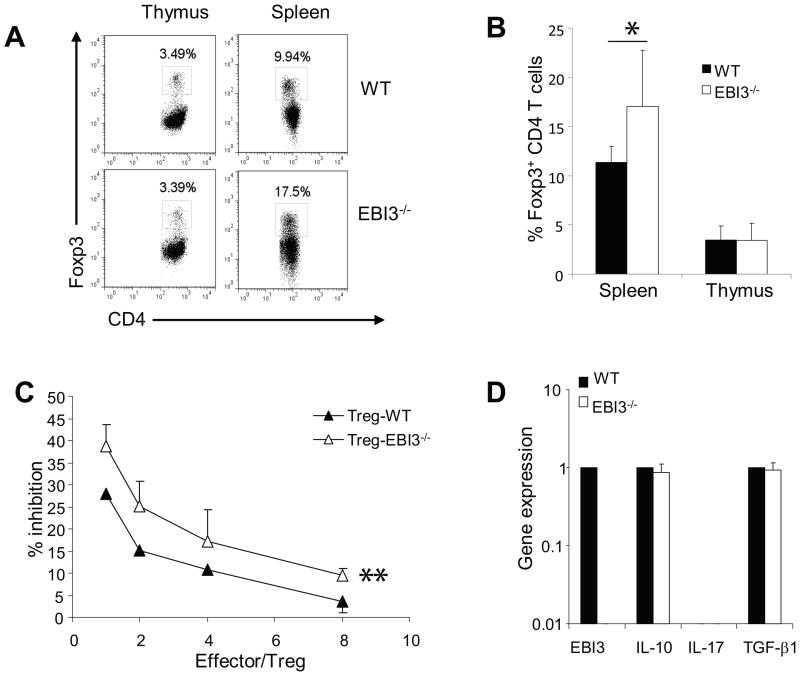
4. Enhanced suppressive functions of CD4+CD25+ Treg cells in EBI3−/− mice
EBI3 was also demonstrated to co-express with IL-12p35 (2) to form IL-35 in FoxP3+ Treg cells and contributed to the suppressive activity of Treg cells (4). To understand if the enhanced EAE development is due to reduced Treg functions, we investigated the impact of EBI3-deficiency on Treg numbers and functions in the EAE model. As shown in Figure 6A and 6B, similar numbers of CD4+FoxP3+ Treg cells were found in the thymi of EBI3−/− and WT mice. Interestingly, significantly increased numbers of Treg cells were found in the spleens of EBI3−/− mice. To test if EBI3-deficiency affects the functions Treg cells, we sorted CD4+CD25+ Treg cells from EBI3 and WT mice, and compared their suppressive activities to the proliferation of EBI3-deficient CD4+CD25− T cells in response to low dose anti-CD3. The experiement was done in the presence of irradiated splenocytes from EBI3−/−Rag1−/− mice. As shown in Figure 6C, EBI3-deficient Treg cells had more potent inhibitory effects compared with WT Tregs. We compared the expression of an array of cytokine genes between EBI3-deficient and WT Tregs, and found similar expression of inhibitory cytokine genes such as IL-10 and TGF-β1 (Figure 6D).
Figure 6. Increased numbers and suppressive functions of CD4+Foxp3+ cells in EBI3−/− mice.
A. Flow cytometry analysis of Treg cells in EBI3−/− and WT mice. Thymocytes and splenocytes from sex and age matched EBI3−/− or WT mice were stained for CD4 and FoxP3 followed by flow cytometry analysis. Data from a representative mouse from each group are shown.
B. Increased numbers of CD4+Foxp3+ cells in EBI3−/− mice. Thymocytes and splenocytes from sex and age matched EBI3−/− or WT mice were stained for CD4 and FoxP3 followed by flow cytometry analysis. Data shown are plotted from groups of five mice. *: P< 0.05 by student’s t test.
C. Treg suppression assay. CD4+CD25+ Treg cells were purified from spleen and lymph node cells of 2D2+EBI3−/− or 2D2+EBI3+/+ mice by MACS bead-based sorting. Purified Treg cells were then mixed with responder cells (CD4+CD25− T cells sorted from EBI3−/− mice) at different ratios. 1 × 106 irradiated EBI3−/−Rag1−/− splenocytes were pulsed with anti-CD3 (2C11, 0.1 μg/ml) and were used as APC. Cells were co-cultured for 36 h and 1 μCi 3H-Tritium was added into each well. 12 h later cells were harvested and 3H-Tritium incorporation was measured in a scintillation counter. % of suppression was calculated by the formula: (Maximal counts-actual counts)/Maximal counts. *: P< 0.01 by paired student’s t test. Data shown represents five experiments with similar results.
D. qPCR was used to detect expression of cytokine genes in sorted Treg cells from WT and EBI3−/− mice. Data shown are representative of two experiments with similar results.
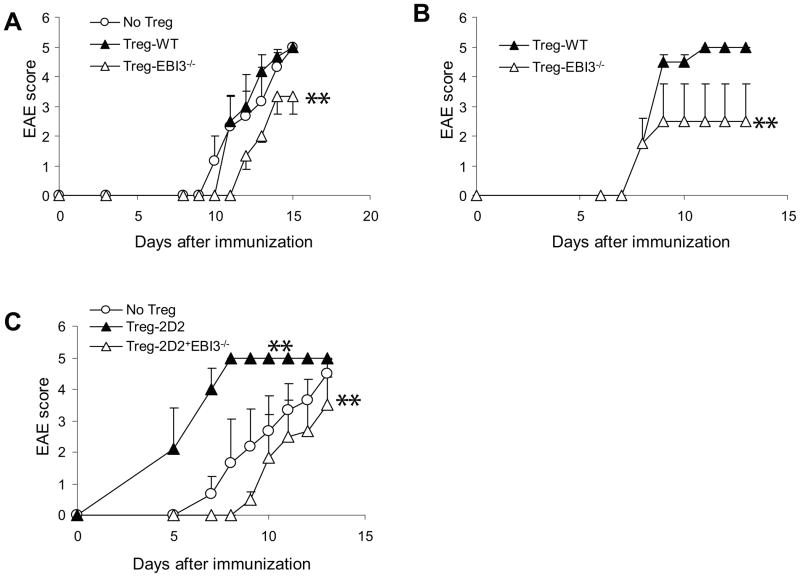
To determine if EBI3-deficiency affects Treg function during EAE development, we performed a series of adoptive transfer experiments. First, we isolated Treg cells from EBI3−/− and WT mice and adoptively transferred them into EBI3−/−Rag1−/− mice. CD4+CD25− EBI3−/− T cells were used as effectors. The recipient mice were then immunized for EAE development. We found that mice receiving WT Treg cells developed similar disease as mice receiving no Treg. In mice receiving EBI3−/− Treg, diminished EAE development was observed (Figure 7A). In a second series of experiments, we injected CD4+CD25− 2D2 T cells from 2D2+EBI3−/− mice into EBI3−/−Rag1−/− mice. The recipient mice also received Treg cells from EBI3−/− or WT mice followed by EAE induction. We also found that mice receiving EBI3-deficient Tregs developed less severe EAE (Figure 7B). Finally, we compared Tregs from 2D2+EBI3−/− mice to Tregs from 2D2+EBI3+/+ mice for their ability to inhibit EAE development caused by EBI3-deficient CD4+CD25− 2D2 T cells. Surprisingly, CD4+CD25+ T cells from 2D2+EBI3+/+ mice did not inhibit, but rather dramatically enhanced EAE development (Figure 7C). In contrast, CD4+CD25+ T cells from 2D2+EBI3−/− mice significantly inhibited EAE development (Figure 7C). Thus, EBI3-deficient Tregs had more potent capacity to inhibit EAE development in vivo.
Figure 7. Increased suppressive functions of EBI3-deficient Treg cells.
A. 5 × 106 CD4+CD25− T cells from EBI3−/− mice were injected into each EBI3−/−Rag1−/− mouse i.v. Each recipient mouse also received 0.7 × 106 Treg-WT cells (n=4), Treg-EBI3−/− (n=4) or no Treg (n=4). The recipient mice were then immunized with MOG35-55/CFA/p.t. Data shown represents two experiments with similar results. **: P<0.01 by Wilcoxon signed-rank test in comparison to no Treg group.
B. 1 × 106 CD4+CD25− T cells from 2D2+EBI3−/− mice were injected into each EBI3−/−Rag1−/− mouse i.v. Each recipient mouse also received 0.5 × 106 Treg-WT cells (n=3) or Treg-EBI3−/− (n=3). The recipient mice were then immunized with MOG35-55/CFA/p.t. **: P<0.01 by Wilcoxon signed-rank test in comparison to Treg-WT group.
C. 1 × 106 CD4+CD25− T cells from 2D2+EBI3−/− mice were injected into each EBI3−/−Rag1−/− mouse i.v. Each recipient mouse also received 0.5 × 106 Tregs from 2D2 mice (n=3) or Tregs from 2D2+EBI3−/− (n=3). The recipient mice were then immunized with MOG35-55/CFA/p.t. Data shown represents two experiments with similar results. **: P<0.01 by Wilcoxon signed-rank test in comparison to no Treg group.
Discussion
Studies using IL-27Rα−/− mice (12) and IL-27 systemic injection (17, 29) have revealed an inhibitory role of IL-27 in EAE development. Recently, IL-27p28−/− mice has also been shown to have enhanced EAE (23). However, the role of the other component of IL-27, EBI3 subunit, in EAE development has not been studied. To investigate the contribution of this cytokine subunit, we compared the immune responses and susceptibility of EBI3−/− versus WT mice, and EBI3-deficient versus EBI3-sufficient 2D2 TCR transgenic mice to MOG peptide induced EAE. We found that EBI3−/− and 2D2+EBI3−/− mice had marginally enhanced EAE development compared to their WT counterparts. The enhanced EAE development in EBI3−/− mice was associated with enhanced T cell IL-2 production, enhanced T cell proliferation and Th17 and Th1 differentiation. In addition, we have also found that in the peripheral lymphoid organs of EBI3−/− mice, numbers of CD4+FoxP3+ Treg cells are increased. More strikingly, the suppressive function of CD4+FoxP3+ Treg cells from EBI3−/− mice is more potent than Tregs in WT mice. Enhanced Treg responses in EBI3−/− mice may explain why the EAE development is only modestly enhanced compared with WT mice, despite the potent Th17 response induction.
In the primed peripheral lymphoid organs and in the CNS of EBI3−/− and 2D2+EBI3−/− mice, increased Th17 response was consistently observed. IL-27 has a known suppressive effect on Th17 differentiation, through IL-27-induced activation of STAT1 and STAT3 (12, 18, 30). Thus, in EBI3−/− mice, the enhanced IL-17 response is consistent with enhanced Th17 responses consequent to IL-27 deficiency. We also observed enhanced Th1 response in EBI3−/− mice during EAE development. This effect was evident in the CNS during EAE, however in the peripheral lymphoid organs, the effect was less consistent. The reason could be the smaller difference, or the fact that peripheral lymphoid cells were cultured for a long time. IL-27 induced T cell production of IL-10 has been thought to be an important mechanism in suppressing EAE development (16, 31), however, in EBI3−/− mice with EAE, no decreased IL-10 production was observed. In some cases, especially in the CNS, increased IL-10 expression is observed. Thus, EBI3-deficiency does not seem to globally affect IL-10 production. Since IL-10 has multiple sources in vivo, this observation does not rule out the possibility that IL-10 production of some subsets of T cells are affected in EBI3−/− mice. Another notable observation in this study is that EBI3−/− T cells had enhanced IL-2 production and proliferation. Enhanced IL-2 production by T cells from EBI3−/− mice is consistent with the known anti-IL-2 effect of IL-27 (32–34) and could explain the stronger proliferation of EBI3−/− T cells.
We have also observed increased numbers of CD4+FoxP3+ Tregs cells in the peripheral lymphoid organs in EBI3−/− mice. Since the numbers of Treg cells in the thymi of EBI3−/− and WT mice were similar, it is likely that EBI3-deficiency only affects “converted Tregs”. Consistent with this observation, IL-27 has been shown to antagonize TGF-β driven Treg conversion through restricting the expression of Foxp3, CD25 and CTLA-4(CD152) in vitro (35). A recent study (36) showed that lack of IL-27 signaling (T cell deficient for IL-27Rα−/−) resulted in the rapid conversion of conventional T cells into CD4+FoxP3+ Treg cells, which profoundly blocked colitis development in a T cell adoptive transfer induced colitis model, where Treg cells are critical for controlling disease development. In another study (32), IL-27 transgenic mice were found to develop spontaneous autoimmunity, with greatly reduced CD4+FoxP3+ Treg cells and the effect could be largely attributed to lack of IL-2 production in IL-27 transgenic mice. Indeed, we also found that EBI3−/− T cells produced more IL-2, which likely contributed to the expansion of Tregs in EBI3-deficient mice. Thus, increased numbers of Treg cells in EBI3−/− mice are consistent with the effects of IL-27-deficiency.
Our adoptive transfer experiments suggest that the disease enhancing effect of EBI3-deficiency is not T cell intrinsic, but attributed to EBI3-deficiency in the non-T cell compartment. Given IL-27 is mainly produced in Non-T compartments (5), while IL-35 can be produced by Treg cells in T cell compartment (4), the enhanced EAE in EBI3−/− mice can not be explained by diminished Treg functions due to IL-35-deficiency. In fact, we have found that Treg cells from EBI3−/− mice have more potent inhibitory effect on T cell proliferation and T cell-mediated CNS autoimmunity. Under our experimental condition, we found that Tregs from WT B6 mice or 2D2 mice did not display substantial inhibitory roles for EAE development, but rather enhanced autoimmunity was observed in mice receiving Tregs from 2D2 mice. In contrast, Tregs from EBI3−/− and 2D2+EBI3−/− mice were consistently inhibitory. Since IL-10 and TGF-β1 gene expression are similar in Tregs from EBI3−/− versus WT mice, it will be interesting to determine what other factors contribute to enhanced suppressive functions of EBI3-deficient Tregs.
Taken together, our data suggest that EBI3 inhibits Th17, Th1, IL-2 and Treg responses and suppresses EAE development. While these observations are consistent with the known functions of IL-27, the IL-35 contribution to the suppressive functions of Treg cells is not evident in this model. Enhanced Treg responses in EBI3−/− mice may explain why the EAE development is only modestly enhanced compared to WT mice, despite enhanced Th17 responses are induced.
Acknowledgments
This study is supported in part by a R21 grant (AI078286 to XFB) from the National Institutes of Health.
References
- 1.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 4.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 5.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 6.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 7.Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 8.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 10.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 12.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 13.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 14.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 15.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 18.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 19.Colgan J, Rothman P. All in the family: IL-27 suppression of T(H)-17 cells. Nat Immunol. 2006;7:899–901. doi: 10.1038/ni0906-899. [DOI] [PubMed] [Google Scholar]
- 20.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 21.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci U S A. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 24.Siebler J, Wirtz S, Frenzel C, Schuchmann M, Lohse AW, Galle PR, Neurath MF. Cutting edge: a key pathogenic role of IL-27 in T cell- mediated hepatitis. J Immunol. 2008;180:30–33. doi: 10.4049/jimmunol.180.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka A, Hamano S, Miyazaki Y, Ishii K, Takeda A, Mak TW, Himeno K, Yoshimura A, Yoshida H. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172:3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carl JW, Jr, Liu JQ, Joshi PS, El-Omrani HY, Yin L, Zheng X, Whitacre CC, Liu Y, Bai XF. Autoreactive T cells escape clonal deletion in the thymus by a CD24-dependent pathway. J Immunol. 2008;181:320–328. doi: 10.4049/jimmunol.181.1.320. [DOI] [PubMed] [Google Scholar]
- 28.Bai XF, Li O, Zhou Q, Zhang H, Joshi PS, Zheng X, Liu Y, Wang Y, Zheng P. CD24 controls expansion and persistence of autoreactive T cells in the central nervous system during experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:447–458. doi: 10.1084/jem.20040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 32.Wojno ED, Hosken N, Stumhofer JS, O’Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 34.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 35.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 36.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]