Abstract
Background
Two understudied risk factors that have been linked to emotional difficulties in adolescence are chronotype and sleep deprivation. This study extended past research by using an experimental design to investigate the role of sleep deprivation and chronotype on emotion in adolescents. It was hypothesized that sleep deprivation and an evening chronotype would be associated with decreased positive affect (PA), increased negative affect (NA), and lower positivity ratios.
Methods
Forty-seven healthy adolescents (aged 10–15 for girls, 11–16 for boys) participated in a sleep deprivation and a rested condition. A subsample of 24 adolescents was selected on the basis of extreme morningness or eveningness scores (based on outer quartiles of scores on the Children’s Morningness-Eveningness Preferences Scale). PA and NA was measured using the Positive and Negative Affect Schedule for Children, and positivity ratios were calculated by dividing PA by NA.
Results
Participants reported less positive affect and lower positivity ratios when sleep deprived, relative to when rested. Evening chronotypes reported less positive affect and lower positivity ratios than morning chronotypes in both rested and sleep deprivation conditions.
Conclusions
These findings extend previous research by suggesting that adolescents are adversely impacted by sleep deprivation and that an evening chronotype might serve as a useful marker of emotional vulnerability. Early intervention and prevention strategies can focus on improving sleep and on using chronotherapy principles to reduce eveningness.
Keywords: adolescence, circadian rhythm, emotion, sleep
Adolescence is one of the most important and vulnerable stages of the lifespan and a time of great vulnerability for developing emotional problems, with rates of psychopathology as high as 20% (Satcher, 1999). This paper will focus on two understudied contributors to emotional difficulties in adolescence; namely, sleep deprivation and chronotype.
Sleep Deprivation and Affect
Sleep deprivation adversely impacts emotion in adults. For example, Franzen, Siegle, and Buysse (2008) reported increased negative emotion and decreased positive emotion in participants after a night of sleep deprivation. The literature on sleep deprivation in adolescents is small but growing. Based on correlational research conducted with healthy adolescents, sleep deprivation is associated with more anxious and depressive symptoms (National Sleep Foundation, 2006). Furthermore, sleep disturbances are common among adolescents with depression (Dahl et al., 1996) and anxiety (Alfano, Ginsburg, & Kingery, 2007), and a recent longitudinal study showed that reduced sleep in adolescents predicts depressive symptoms (Roberts, Ramsay Roberts, & Duong, 2009). We have reported elsewhere that sleep deprivation reduces positive affect and increases anxiety in adults and adolescents (McGlinchey et al., 2011; Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010).
Although previous studies on the effects of sleep deprivation have examined positive and negative emotion independently, there is an intriguing and burgeoning literature focusing on the relative balance between positive and negative emotion. For example, Diener (2000) proposed that a high ratio of positive to negative emotion (also known as a positivity ratio) predicts subjective well-being, and Fredrickson and Losada (2005) found that normally functioning adults have a positivity ratio of 2.1–2.3 while flourishing adults have ratios higher than 2.9. These findings are framed by Fredrickson’s broaden-and-build model of positive emotion, which suggests that positive emotions enhance psychological well-being by broadening individuals’ thought-action repertoire and building their personal resources (Fredrickson, 2001). Because negative emotions have greater weight than positive emotions, a high positivity ratio is needed to offset the adverse effects of negative emotions (Rozin & Royman, 2001). Positivity ratios have not yet been examined in the context of sleep deprivation or in adolescents.
Chronotype and Affect
Chronotype refers to an individual’s preferred sleep-wake schedule (Roenneberg, Wirz-Justice, & Merrow, 2003). There are two primary chronotypes: evening and morning. Evening chronotypes tend to follow a delayed-phase sleep schedule, being more active later in the day and both going to sleep and getting up later, compared to morning chronotypes (Horne & Ostberg, 1976). There is evidence that the onset of puberty triggers a general preference for eveningness (Carskadon, Vieira, & Acebo, 1993), although even among adolescents there is much variation in chronotype, possibly with biological underpinnings (Carskadon, 2002).
Interestingly, an association has been found between an evening chronotype and emotional difficulties during adolescence. Giannotti, Cortesi, Sebastiani, and Ottaviano (2002) found that evening-type adolescents exhibit more anxious and depressed symptoms compared to morning-type adolescents. Moreover, Gau, Soong, and Merikangas (2004) reported that evening-type adolescents exhibit more negative emotion relative to their morning counterparts. Later studies confirmed and extended these findings, noting that evening-type adolescents are also at higher risk for suicidality, have a higher incidence of aggressive and delinquent behavior, and are more likely to have problems in the clinical/borderline range, compared to morning-type adolescents (Gau et al., 2007; Goldstein, Hahn, Hashar, Wiprzycka, & Zelazo, 2007). Taken together, this evidence suggests that eveningness poses a vulnerability to emotional difficulties during adolescence.
Aims and Hypotheses
The present study is predicated on the possibility that evening-type adolescents are doubly vulnerable to emotional difficulties, both because of the correlation between eveningness and emotional problems and because they accrue a higher sleep debt across the week, going to bed later but getting up for school at the same time as morning chronotypes (Giannotti et al., 2002). Given that the relevant studies involving adolescents are correlational in design, they preclude causal inferences. The present study sought to extend previous research by employing an experimental design to index affect in adolescents under both a sleep deprived and a rested condition.
The first aim was to examine the consequences of sleep deprivation on affect in adolescents. Two hypotheses were tested. First, given that studies have shown that sleep deprivation is associated with decreased positive emotion (e.g., Franzen et al., 2008) and increased negative emotion (e.g., National Sleep Foundation, 2006), we hypothesized that in the sleep deprivation condition participants would report less positive affect (PA) and more negative affect (NA), relative to the rested condition. Second, considering that high positivity ratios are related to optimal functioning (Fredrickson & Losada, 2005) and sleep deprivation is related to suboptimal emotional functioning (e.g., Roberts et al., 2009), we hypothesized that participants would exhibit lower positivity ratios when sleep deprived than when rested.
The second aim was to examine the role of chronotype in the effects of sleep deprivation on adolescent affect. Two hypotheses were tested. First, given that an evening chronotype is associated with emotional difficulties and suboptimal functioning (e.g., Gau et al., 2007), we hypothesized that evening chronotypes would exhibit less PA, more NA, and lower positivity ratios relative to morning chronotypes, both when rested and when sleep deprived. Second, given the added adverse effect of sleep deprivation on emotion (e.g., Roberts et al., 2009), we also predicted that both evening and morning chronotypes would report less PA, more NA, and lower positivity ratios when sleep deprived relative to when rested, but that this effect would be more pronounced in evening chronotypes relative to morning chronotypes.
Methods
Participants
Participants were recruited from fliers posted in the community and from online advertisements. They were included if they (a) did not meet Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994) criteria for any past or current Axis I disorder, (b) did not meet criteria for any past or current sleep disorder, (c) had no medical conditions, and (d) had no history of head trauma.
A total of 47 healthy adolescents (22 girls ages 10–15, 25 boys ages 11–16, Mage = 13.06) completed this study. The age inclusion for girls was set at one year earlier on the basis that girls begin puberty one to two years earlier than boys (Gordon & Laufer, 2005). Participants took part in a larger study on the effects of sleep deprivation on emotional functioning in adolescents and adults. We note that two other papers report findings from this study (McGlinchey et al., 2011; Talbot et al., 2010). The unique contribution of the present paper is the focus on chronotype and the outcome measures, which were conceptually and practically difficult to incoporate into the other papers.
The analyses for Aim 1 were based on all 47 participants. To examine the role of chronotype (Aim 2), 24 adolescents were classified as evening-types (n = 13) or morning-types (n = 11) based on the two outer quartiles of the Children’s Morningness-Eveningness Preferences Scale (CMEP; Carskadon et al., 1993). We chose to use an outer quartile split in following the precedent set by previous studies of chronotype in adolescents (e.g., Gau et al., 2004). Participant characteristics of the full adolescent sample as well as the chronotype subsamples are presented in Table 1.
Table 1.
Sleep Conditions Manipulation Checks
| All Adolescents | Morning Chronotypes (n = 11) | Evening Chronotypes (n = 13) | |
|---|---|---|---|
| Habitual sleep | |||
| TST | 8.23 (1.16) | 8.21 (1.16) | 7.85 (1.22) |
| Sleep Deprivation Condition | |||
| First Night TST | 6.28 (1.52) | 6.57 (1.34) | 6.31 (2.04) |
| Second Night Maximum TST | 2.00 | 2.00 | 2.00 |
| TST over First and Second Nights | 8.28 | 8.57 | 8.31 |
| Habitual TST x 2 Nights | 16.46 | 16.42 | 15.70 |
| Sleep Debt Accrued during Sleep Deprived Condition | 8.18 | 7.85 | 7.39 |
| Rested Condition | |||
| Average TST of First and Second Nights | 7.96 (0.96) | 8.32 (0.93) | 7.89 (1.08) |
| TST over First and Second Nights | 15.92 (1.92) | 16.64 (1.86) | 15.77 (2.16) |
Note. Mean values presented with standard deviations in parentheses. TST = total sleep time. All times are quoted in hours.
Measures
Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (KSADS-PL)
The KSADS-PL (Kaufman et al., 1997), a semi-structured diagnostic interview, was used to screen out potential adolescent participants who met criteria for psychiatric illness. Diagnoses on the KSADS-PL have been shown to yield reliable and valid psychiatric diagnoses in children (Kaufman et al., 1997).
Duke Structured Interview for Sleep Disorder (DSISD)
The DSISD is a semi-structured interview that assesses research diagnostic criteria for sleep disorders (Edinger et al., 2004). This measure was used to assess sleep disorders in all participants. The DSISD is the only structured interview for sleep disorders that has established reliability and validity (Edinger et al., 2004).
Sleep diary
The sleep diary is a self-report measure completed upon waking in which participants estimate a number of sleep variables, including total sleep time (TST). The sleep diary is considered the gold standard subjective measure of sleep (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006).
Actigraphy
Actigraphs (Mini Mitter Actiwatch, model AWLP) are wristwatch-like devices that provide an estimate of an individual’s sleep/wake cycle via movement using a sensor, a processor, and memory. Actigraphy has been shown to have good reliability with sleep diaries and has been shown to provide a good estimate of TST in healthy adolescents (Acebo et al., 1998).
Self-Rating Scale for Pubertal Development
The Self-Rating Scale for Pubertal Development is a self-report measure of pubertal status adapted by Carskadon and Acebo (1993) from the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988). The scale includes items rating physical development that are combined to classify the adolescents’ pubertal development. The measure has established validity and reliability (Carskadon & Acebo, 1993).
Children’s Morningness-Eveningness Preferences (CMEP) Scale
The CMEP (Carskadon et al., 1993) is a 10-item scale adapted from the Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976). The total score ranges from 10 to 43. Cutoff scores based on the two outer quartiles of the CMEP were 27 or lower for evening type and 32 or higher for morning type. The CMEP has been shown to have good reliability and validity (Kim, Dueker, Hasher, & Goldstein, 2002).
Positive and Negative Affect Schedule for Children (PANAS-C)
The PANAS-C (Laurent et al., 1999) is a 30-item scale with 12 items measuring positive affect (PA) and 15 items measuring negative affect (NA). At each administration, participants rated how strongly they currently felt each item on a 5-point Likert scale, and PA and NA scores were obtained by a sum of ratings for the corresponding items. The PANAS-C has been shown to have good convergent and discriminant validity (Laurent et al., 1999).
Procedure
Study procedures were approved by the Institutional Review Board of the University of California, Berkeley. All parents provided consent and adolescents provided assent, and all participants were compensated for their time and effort. During the first visit, doctoral student interviewers with previous experience conducting structured clinical interviews administered the K-SADS-PL and DSISD. When clinical parent/child reports were discrepant, any Axis I psychopathology or sleep disorder endorsed by either parent or child rendered a participant ineligible for further participation. Adolescents also completed the Self-Rating Scale for Pubertal Development and the CMEP.
Following the first visit, eligible participants kept sleep diaries throughout the study and a portion (66%) also wore actigraphs. There was moderate correlation between actigraphy and sleep diaries (r = .52, p < .01). Data analysis was based on actigraphy data when available, and when actigraphy data was not available sleep diary data was used on the basis that actigraphy and sleep diary data are interchangeable when determining sleep start and end and TST (Werner, Molinari, Guyer, & Jenni, 2008). Habitual sleep was obtained from an average of five weekdays of data from actigraphy or sleep diary. After this habitual sleep period, participants completed either the sleep deprived or the rested condition. The other condition was completed approximately one week later. The order of these two conditions was counterbalanced across participants.
Sleep deprived condition
This condition took place over two nights. On the first night, participants spent the night at home and were asked to aim for a maximum sleep time of 6.5 hours. Compliance was checked using actigraphy or participants’ sleep diary. On the second night, participants reported to the laboratory at 22:00, where they completed a baseline PANAS-C. They were continuously monitored by trained laboratory staff and were permitted to choose from a range of activities (e.g., read, watch movies) to help them stay awake. Snacks were available, but caffeine or other stimulants were not allowed. Exposure to light was not controlled, to mimic real world conditions. At 03:00 participants were given a two-hour sleep opportunity until 05:00. Participants each had their own bed in their own room and were monitored by laboratory staff to ensure they were sleeping. All participants slept during the two-hour sleep opportunity. Throughout the night participants completed the PANAS-C again at 00:00, 02:00, 03:00, 05:00 and 08:00.
The rationale for the two-night partial sleep deprivation protocol, as opposed to a night of total sleep deprivation, was threefold. First, because to the best of our knowledge this is the first experimental study of sleep deprivation in adolescents, the consequences of a night of total sleep deprivation in this population were unclear. Second, we were concerned that adolescents would be unable to stay awake to complete the morning PANAS-C after a night of total sleep deprivation; hence, adolescents were given a two-hour sleep opportunity on the second night. Finally, partial sleep deprivation may more closely mimic real-world conditions experienced by adolescents.
Rested condition
The rationale for the rested protocol was based on findings showing that, although the need for sleep during adolescence is about 9 hours (Carskadon & Acebo, 2002), the average adolescent only gets 7.5 hours of sleep per night (National Sleep Foundation, 2006). Participants were asked to extend their habitual sleep time to 8.5 hours for two consecutive nights at home. Compliance was checked using actigraphy and sleep diary data. At 08:00 or 10:00 the morning following the second night, participants came into the laboratory and completed the PANAS-C. A later testing time was necessary to ensure that all participants had an adequate amount of time to spend the required 8.5 hours of time in bed.
Manipulation Checks
Sleep deprivation manipulation check
Participants were asked to aim for a maximum sleep time of 6.5 hours on the first night of the sleep deprivation condition (at home). Mean values of total sleep time (TST) on the first night showed that adolescents in the full sample complied with this instruction (see Table 1). Furthermore, an independent samples t-test indicated that there was no difference between morning and evening chronotypes on this night, t (22) = 0.23, p = 0.819, η2 = 0.00 (see Table 1). Given that the maximum TST on the second night was 2 hours, over the two nights participants accrued substantial sleep debt relative to habitual TST. Moreover, there were no differences between morning and evening chronotypes in the sleep debt accrued, t (22) = 0.03, p = 0.980, η2 = 0.00 (see Table 1). In sum, these data suggest that the sleep deprivation manipulation was delivered successfully.
Rested manipulation check
Participants in the rested condition were asked to extend their habitual sleep time to 8.5 hours for two nights. An inspection of the mean values in Table 1 reveals that participants’ TST was below 8.5 hours An independent samples t-test on average TST of the first and second nights indicated there was no significant difference between morning and evening chronotypes, t (22) = −0.47, p = 0.645, η2 = 0.00. Furthermore, paired t-tests examining the difference between average TST on the two rested nights and habitual TST yielded no significant results in the full sample, t (45) = 1.81, p = 0.079, η2 = 0.09, in the morning chronotype group, t (22) = 0.42, p = 0.686, η2 = 0.0, or on the evening chronotype group, t (22) = 0.36, p = 0.725, η2 = 0.01. These results suggest that participants were unsuccessful in extending their sleep beyond their habitual sleep, perhaps due to noncompliance or difficulty altering prior habitual sleep patterns (e.g., earlier sleep times may be unachievable for evening types without specific prior planning or intervention).
Results
Participant Characteristics
Characteristics of the full sample as well as the chronotype subsamples are presented in Table 2. Independent samples t-tests confirmed that there were no significant differences between morning and evening chronotypes in age, t (22) = 1.07, p = 0.297, η2 = 0.05 (see Table 2), or habitual TST, t (22) = 0.08, p = 0.936, η2 = 0.00 (see Table 1). Chi-square tests verified that morning and evening chronotypes did not differ in gender, χ2 (1) = 2.48, p = 0.116, ϕ = 0.32, pubertal status, χ2 (2) = 0.39, p = 0.824, V = 0.44, or race/ethnicity, χ2 (4) = 5.40, p = 0.249, V = 1.16 (see Table 2).
Table 2.
Participant Characteristics
| Demographic Variable | All Adolescents (N = 47) | Morning Chronotypes (n = 11) | Evening Chronotypes (n = 13) |
|---|---|---|---|
| Mean Age (SD) | 13.06 (1.59) | 12.55 (1.57) | 13.38 (1.80) |
| Gender | |||
| Female | 22 | 6 | 5 |
| Male | 25 | 5 | 8 |
| Pubertal status | |||
| Pre/early | 5 | 2 | 2 |
| Mid/late/post | 37 | 8 | 10 |
| Missing | 5 | 1 | 1 |
| Race/Ethnicity | |||
| African American | 4 | 2 | 1 |
| Asian-American/ | 11 | ||
| Pacific Islander | 2 | 3 | |
| Caucasian | 26 | 5 | 7 |
| Hispanic | 3 | 2 | 0 |
| Other | 3 | 0 | 2 |
Aim 1: Sleep Deprivation and Affect
These analyses were based on the full sample of 47 participants. Participants’ PA and NA scores from the PANAS-C completed in the rested and sleep deprived conditions were compared using repeated-measures t-tests. Given that affect appears to vary throughout the day (Hasler, Mehl, Bootzin, & Vazire, 2008), we used scores from the PANAS-C completed at 08:00 from the sleep deprived condition to match the 08:00 or 10:00 time from the rested condition. Positivity ratios were computed by dividing the total PA score by the total NA score. Mean values are presented in Table 3.
Table 3.
Comparison of Mean Emotion Variables by Sleep Condition
| Variable | Rested
|
Sleep Deprived
|
t(45) | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Positive affect | 30.45 | 10.26 | 23.28 | 8.58 | 6.45* |
| Negative affect | 16.56 | 2.07 | 16.26 | 2.40 | 0.67 |
| Positivity ratio | 1.84 | 0.61 | 1.46 | 0.57 | 5.17* |
Note.
p < 0.05
There was a significant difference in PA, t (45) = 6.45, p = 0.001, η2 = 0.47, such that participants reported more PA when rested relative to when sleep deprived (see Table 3). There was also a significant difference in positivity ratios when participants were rested relative to when they were sleep deprived, t (45) = 5.17, p = 0.001, η2 = 0.30. here was no difference in adolescents’ NA when sleep deprived relative to when rested, t (45) = 0.67, p = 0.508, η2 = 0.01.
Aim 2: Sleep Deprivation, Chronotype, and Affect
These analyses were based on participants classified as extreme morning types or evening types. Two-way repeated-measures ANOVAs were conducted, one for each of the emotion variables (PA, NA, positivity ratio). Chronotype (Morning, Evening) was the between-subjects factor and Sleep Condition (Rested, Sleep Deprived) was the within-subjects factor. The PANAS-C completed at 08:00 formed the basis for these analyses for the sleep deprived condition. Mean values are presented in Table 4.
Table 4.
Comparison of Mean Emotion Variables by Chronotype and Sleep Condition
| Variable | Morning
|
Evening
|
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Positive affect | ||||
| Rested | 25.55 | 10.39 | 26.17 | 6.85 |
| Sleep deprived | 29.45 | 9.46 | 20.25 | 6.50 |
| Negative affect | ||||
| Rested | 15.72 | 0.79 | 16.62 | 2.50 |
| Sleep deprived | 16.55 | 3.05 | 16.15 | 3.00 |
| Positivity ratio | ||||
| Rested | 2.26 | 0.63 | 1.57 | 0.42 |
| Sleep deprived | 1.87 | 0.71 | 1.29 | 0.46 |
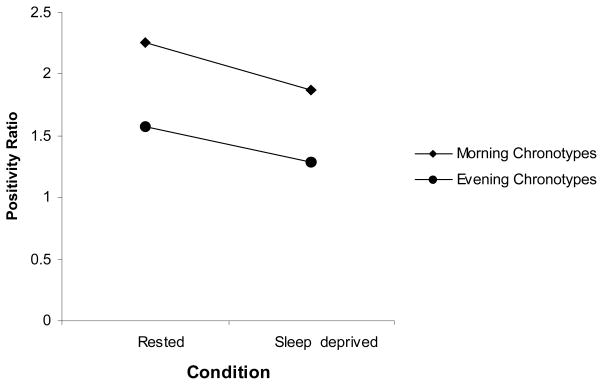
There was a main effect of chronotype for PA, F (1, 23) = 8.07, MSE = 122.83, p = 0.010, η2 = 0.28, and positivity ratios, F (1, 23) = 8.33, MSE = 0.55, p = 0.009, η2 = 0.28, but not for NA, F (1, 23) = 0.10, MSE = 7.51, p = 0.757, η2 = 0.00.
There was a main effect of sleep condition for PA, F (1, 23) = 23.24, MSE = 0.17.81, p = 0.000, η2 = 0.53, and positivity ratios, F (1, 23) = 14.33, MSE = 0.90, p = 0.001, η2 = 0.41 (see Figure 1), but not for NA. There was no significant Sleep Condition x Chronotype interaction for any of the affect variables.
Figure 1.
Positivity ratios across sleep conditions.
Discussion
The goal of this study was to examine the effects of sleep deprivation and chronotype—two understudied risk factors—on positive and negative affect in adolescence.
As hypothesized, participants reported less PA when sleep deprived compared to when rested. This extends the findings with adults (e.g., Franzen et al., 2008) by providing evidence that adolescents also exhibit decreased positive affect after sleep deprivation. Furthermore, as hypothesized, adolescents exhibited lower positivity ratios when sleep deprived relative to when rested. This is an important finding given that the balance between positive and negative emotion may be more indicative of individuals’ well-being than independent scores of positive or negative emotion (e.g., Diener, 2000).
Contrary to the hypothesis, there was no change in NA. This is surprising given that previous research has shown an association between sleep deprivation and negative emotion (e.g., Franzen et al., 2008). It is possible that negative emotion may be amplified after sleep deprivation only in certain contexts. For example, Zohar and colleagues (2005) found that sleep loss in adults was associated with a change in negative emotion only when they were in a context that included a goal-disruptive event. In the present study, participants were aware that the purpose of their visit to the laboratory was to remain awake during the night, and they were given freedom to choose their preferred activity for achieving this goal. Hence, it is unlikely that participants in this study experienced events that were goal-disruptive, but an important direction for future research is to control the context in which the sleep deprivation takes place. In addition, note that the variability of participants’ NA scores was very small compared to the variability of their PA scores (see Tables 3 and 4). The overall low scores in NA may be indicative of a floor effect, and it is possible that the PANAS-C is not a sensitive measure of subjective negative emotion in adolescents. Finally, given that our sample excluded participants with any past or current psychiatric disorders, it is possible that the lack of change in NA between conditions reflects the above-average psychological health of our participants.
We also hypothesized that evening chronotypes would report less PA, more NA, and lower positivity ratios than morning chronotypes, both when sleep deprived and when rested. Consistently, evening chronotypes reported less PA and lower positivity ratios than morning chronotypes in both the rested and the sleep deprivation condition. This is a noteworthy finding that extends research on the emotional difficulties associated with evening-type adolescents by including positive emotion as well as the balance between positive and negative emotion, which may be a better indicator of healthy emotional functioning than independent levels of positive or negative emotion (e.g., Diener, 2000). One interpretation of these findings is that the emotional difficulties experienced by evening chronotypes may not solely be a consequence of insufficient sleep, because even in the rested condition evening chronotypes had significantly lower PA and positivity ratios, but may instead reflect differences inherent to chronotype. For instance, finding an association between an evening chronotype and antisocial behavior in pre-pubertal adolescents, Susman and colleagues (2007) suggested that eveningness may represent a dimension of temperament that places individuals with this chronotype at risk for emotional and behavioral problems. Another possibility is that the difficulties evening chronotypes experience may in part stem from conflict between their circadian preference and family and school expectations. However, further research needs to be conducted to examine this possibility.
Inconsistent with the hypothesis and echoing the findings from the first aim, there were no differences in NA between morning and evening chronotypes in either condition. This is unexpected given that previous studies have shown an association between an evening chronotype and negative emotions during adolescence (e.g., Gau et al., 2004), but as previously stated there are several interpretations for these findings.
The final hypothesis was that both evening and morning chronotypes would exhibit less PA, more NA, and lower positivity ratios when sleep deprived relative to when rested, and this effect would be more marked in evening chronotypes relative to morning chronotypes. As with the larger adolescent sample, evening and morning chronotypes exhibited less PA and lower positivity ratios when sleep deprived relative to when rested, but there was no difference in the magnitude of this effect between chronotypes. Furthermore, there was no difference in NA across the sleep conditions.
To the best of our knowledge, this is the first experimental sleep deprivation study with adolescents. We learned a great deal. Hence, we wish to offer some recommendations based on issues and limitations that arose over the course of this study. First, due to our small sample size it is possible that the non-significant differences (e.g., no interaction between sleep condition and chronotype) reflect the small sample size and inadequate power rather than ‘no difference.
Second, participants’ sleep in the rested condition was below the 9 hours recommended for adolescents (Carskadon & Acebo, 2002), so it is possible that our results underestimated the emotional consequences of sleep deprivation in adolescents. Future studies should consider conducting the sleep manipulations in a more controlled laboratory setting to ensure adolescents get sufficient sleep. In addition, future studies should include nights of recovery sleep prior to the experimental conditions to dispel any potential effects of accumulated sleep deprivation and to ensure that the emotion ratings reflect the experimental conditions rather than acquired habits. Third, it is possible that self-report measures of emotion may be indexing habits of feeling rather than current feelings. Hence, the inclusion of emotion processing and regulation tasks (e.g., Franzen et al., 2009) would be valuable. Fourth, the fact that some participants did not wear actigraphs introduces an additional measure of variability between sleep diary and actigraphy data in our data analyses. However, we note that the correlation between both measures was good. Finally, data collection for this study took place in the morning, which may have disadvantaged evening chronotypes (Goldstein et al., 2007). Nevertheless, the suboptimal emotional functioning of evening chronotypes is important because they suggest that evening chronotypes may have impaired mood and functioning at a time when they are expected to be in school.
These findings have important clinical and public health implications. It is vital that the challenges of adolescence are not aggravated by a lack of sleep. Also, it is also possible that an evening chronotype per se (even with sufficient sleep) is a marker of vulnerability. Prevention and intervention efforts can target either sleep or chronotype (most typically both would be targeted: Dahl & Harvey, 2007; Harvey, 2009). If focused on sleep, the goal would be to promote sleep-enhancing behavior (e.g., teach savoring or imagery rather than worrying and rumination between lights out and sleep onset) and reduce sleep-diminishing behavior (e.g., remove technology from the bedroom). If focused on eveningness, careful and slow planned shifts toward earlier bed and rise times with dim light conditions prior to bedtime and bright sunlight on waking (via developmentally-adapted chronotherapy principles, e.g., Crowley, Acebo, & Carskadon, 2007) may minimize the adverse emotional consequences of sleep deprivation in adolescents. There are also have practical implications for school policies. For example, a longitudinal study reported that later high school start times were associated with better academic functioning and lower depressive symptoms (Wahlstrom, 2002). Given these findings, later school start times will encourage healthy sleep habits, allow for the slight tip toward eveningness during puberty (Carskadon et al., 1993), and help curtail the harmful effects of sleep deprivation on emotional functioning.
Key Points.
Correlational research has suggested that reduced sleep adversely affects emotion in adolescents and that adolescents with an evening chronotype experience more emotional difficulties that adolescents with a morning chronotype.
Recent literature has suggested that the balance between positive and negative emotion (i.e., positivity ratio) is more relevant for well-being than independent levels of positive and negative emotion.
This experimental study demonstrates that adolescents report lower positive emotion and lower positivity ratios when sleep deprived relative to when rested.
These findings extend previous research by suggesting that adolescents are adversely impacted by sleep deprivation and that an evening chronotype might serve as a useful marker of emotional vulnerability. Early intervention and prevention strategies can focus on improving sleep and on using chronotherapy principles to reduce eveningness
Acknowledgments
This project was supported by National Institute of Mental Health Grant R24 MH067346 awarded to RED and by National Institute of Child Health and Human Development Grant F31 HD058411 awarded to ELM, by NRSA Institutional Research Training Grant 5T32 MH020006 awarded to LST, and by an NSF Graduate Research Fellowship award to KAK.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, Carskadon MA. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep. 1998;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:224–232. doi: 10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: Author; 1994. [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Factors influencing sleep patterns of adolescents. In: Carskadon MA, editor. Adolescent sleep patterns: Biological, social, and psychological influences. New York: Cambridge University Press; 2002. [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–195. doi: 10.1016/1054-139X(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. Regulation of sleepiness in adolescence: Update, insights, and speculation. Sleep. 2002;25:606–616. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Harvey AG. Sleep in children and adolescents with behavioral and emotional disorders. Sleep Medicine Clinics. 2007;2:501–511. [Google Scholar]
- Dahl RE, Ryan ND, Matty MK, Birmaher B, al-Shabbout M, Williamson DE, Kupfer DJ. Sleep onset abnormalities in depressed adolescents. Biological Psychiatry. 1996;39:400–410. doi: 10.1016/0006-3223(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Diener E. Subjective well-being: The science of happiness and a proposal for a national index. American Psychologist. 2000;55:34–43. doi: 10.1037/0003-066X.55.1.34. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychiatry. 2009;80:300–305. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist. 2001;56:218–226. doi: 10.1037/0003-066X.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. American Psychologist. 2005;60:678–686. doi: 10.1080/17439760500510981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Gau SS, Soong WT, Merikangas KR. Correlates of sleep-wake patterns among children and young adolescents in Taiwan. Sleep. 2004;27:512–519. [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: Is there a synchrony effect? Personality and Individual Differences. 2007;42:431–440. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CM, Laufer MR. The physiology of puberty. In: Emans SJ, Laufer MR, Goldstein DP, editors. Pediatric and Adolescent Gynecology. 5. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Harvey AG. The adverse consequences of sleep disturbance in pediatric bipolar disorder: Implications for intervention. Child and Adolescent Psychiatric Clinics of North America. 2009;18:321–338. doi: 10.1016/j.chc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. Journal of Research in Personality. 2008;42:1537–1546. doi: 10.1016/j.jrp.2008.07.012. [DOI] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim S, Dueker GL, Hasher L, Goldstein D. Children’s time of day preference: Age, gender and ethnic differences. Personality and Individual Differences. 2002;33:1083–1090. doi: 10.1016/S0191-8869(01)00214-8. [DOI] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner JTE, Rudolph KD, Potter KI, Lambert S, Gathright T. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. doi: 10.1037/1040-3590.11.3.326. [DOI] [Google Scholar]
- McGlinchey EL, Talbot LS, Chang K, Kaplan KA, Dahl RE, Canny JF, Harvey AG. Effect of sleep deprivation on vocal expression of emotion in adolescents. Sleep. 2011;34:1233–1241. doi: 10.5665/SLEEP.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. 2006 Sleep in America Poll: Summary of Findings. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. Measuring pubertal status: Reliability and validity of a self-report measure. Journal of Youth and Adolescence. 1988;7:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Ramsay Roberts C, Duong HT. Sleepless in adolescence: Prospective data on sleep deprivation, health and functioning. Journal of Adolescence. 2009;32:1045–1057. doi: 10.1016/j.adolescence.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5:296–320. doi: 10.1207/S15327957PSPR0504_2. [DOI] [Google Scholar]
- Satcher D. Mental health: A report of the surgeon general. Rockville, MD: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health; 1999. Children and mental health. [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21:7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Development and Psychopathology. 2007;43:811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: Changes in affect. Emotion. 2010;10:831–841. doi: 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom K. Changing times: Findings from the first longitudinal studyd of later high school start times. NASP Bulletin. 2002;86(633):3–21. doi: 10.1177/019263650208663302. [DOI] [Google Scholar]
- Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Archives of Pediatrics & Adolescent Medicine. 2008;162:350–358. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: A cognitive-energy model. Sleep. 2005:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]