Abstract
Background
Heightened stress reactivity is associated with hippocampal atrophy, age-related cognitive deficits, and increased risk for Alzheimer s disease. This temperament predisposition may aggravate age-associated brain pathology or be reflective of it. This association may be mediated through repeated activation of the stress hormone axis over time. Dietary interventions, such as calorie restriction (CR), affect stress biology and may moderate the pathogenic relationship between stress reactivity and brain in limbic and prefrontal regions.
Methods
Rhesus monkeys (Macaca mulatta) aged 19–31 years consumed either a standard diet (N=18) or were maintained on 30% CR relative to baseline intake (N=26) for 13–19 years. Behavior was rated in both normative and aversive contexts. Urinary cortisol was collected. Animals underwent magnetic resonance imaging and diffusion tensor imaging (DTI) to acquire volumetric and tissue microstructure data respectively. Voxel-wise statistics regressed a global stress reactivity factor, cortisol, and their interaction on brain indices across and between dietary groups.
Results
CR significantly reduced stress reactivity during aversive contexts without affecting activity, orientation, or attention behavior. Stress reactivity was associated with less volume and tissue density in areas important for emotional regulation and the endocrine axis including prefrontal cortices, hippocampus, amygdala, and hypothalamus. CR reduced these relationships. A Cortisol by Stress Reactivity voxel-wise interaction indicated that only monkeys with high stress reactivity and high basal cortisol demonstrated lower brain volume and tissue density in prefrontal cortices, hippocampus, and amygdala.
Conclusions
High stress reactivity predicted lower volume and microstructural tissue density in regions involved in emotional processing and modulation. A CR diet reduced stress reactivity and regional associations with neural modalities. High levels of cortisol appear to mediate some of these relationships.
Keywords: aging, stress, prefrontal cortex, hippocampus, calorie restriction, monkey, cortisol
Introduction
Heightened emotional reactivity to psychological stressors has been associated with neural, cognitive, and glucocorticoid dysregulation in aged rats (Dellu et al., 1994, 1996; Sandi & Touyarot, 2006) and humans (Wilson et al., 2005). This affective style is related to a smaller total brain volume (Knutson et al., 2001), less dendritic arborization in medial temporal lobe (Sandi & Touyarot, 2006), and increased risk for developing cognitive impairment and dementia (Wilson et al., 2007a,b; Crowe et al., 2007). Adults with mood and psychotic disorders also exhibit this predisposition of higher psychological reactivity to exogenous stress (Watson et al., 1999; Myin-Germeys et al., 2001; Brown et al., 2005; Rapoport et al., 2005; Carter, 2007), and have been found to have reduced gray and white matter (GM, WM) volumes in the hippocampus and prefrontal cortices (Halliday, 2001; Zou et al., 2010; Abe et al., 2010). Studies in nonhuman primates have shown that these brain areas are important for regulating behavioral and physiological reactivity to aversive psychogenic stimuli (Fox et al., 2008).
A psychological predisposition to react negatively to stressful stimuli may impact neurodegeneration in a recurrent and progressive manner through activation of stress-responsive physiological pathways, including the release of cortisol by the Hypothalamic-Pituitary-Adrenal (HPA) axis (Leverenz et al., 1999). Although evidence for this glucocorticoid cascade hypothesis (Sapolsky et al., 1986) has been inconsistent for hippocampal volume in humans, nonhuman primates, and rodents (Leverenz et al., 1999; O’Brien et al., 2004; Bao et al., 2008; Schubert et al., 2008; Gold et al., 2010), high levels of cortisol alone can mediate circumscribed cellular neuropathology and vulnerability to neurotoxins (Sapolsky et al., 1990) and may affect the tissue microstructure of GM and WM more than volume. Alternatively, given that chronic hypercortisolemia in the absence of stress does not cause hippocampal degeneration in aged nonhuman primates (Leverenz et al., 1999), high cortisol levels may primarily affect limbic and prefrontal regions only when an animal is also chronically stressed or has a highly emotionally reactive temperament. As in humans (Elgh et al., 2006), stress-induced increases in cortisol in nonhuman primates would be expected to mediate neuropathology in areas with a high density of glucocorticoid receptors, including hippocampus, hypothalamus, and prefrontal cortex. Alternatively, higher cortisol could interact with age-induced neuropathology in these areas to heighten psychological reactivity to exogenous stress.
To investigate these relationships in a nonhuman primate model, arousal and fear-related behaviors were evaluated in aged rhesus monkeys during normative and aversive situations. It is known that routine husbandry events, such as relocation to a new cage, handling, brief immobilization, and eye contact with a human, will arouse monkeys and stimulate the HPA axis (Willette et al., 2007; Jahn et al., 2010). Urinary cortisol levels were also determined in a nondisturbed baseline condition to see if day-to-day glucocorticoid output was a potential contributing factor to associations between stress reactivity and brain. Regional brain volume and microstructure were assessed using volumetric T1-weighted magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) respectively.
Further, a subset of the monkeys were maintained on a moderate 30% calorie reduced (CR) diet beginning in middle age. Several beneficial, anti-aging effects of a CR diet have been found in this cohort, including a reduction in oxidative metabolism, improved glucoregulation, and extension of life span (Kemnitz et al., 1993; Ramsey et al., 2000; Cefalu et al., 2000; Colman et al., 2009). However, the effect of a CR diet on temperament and behavior has been mixed. For instance, in rodents, 25% CR for approximately one month lead to more central area field entries in the open field test, which may reflect decreased anxiety (Levay et al., 2007). Lutter and colleagues (2008) found that 10 days of 40% CR increased social interaction after a social defeat paradigm, as well as lowered swim latencies and reduced inactivity in the forced swim test, which may be indicative of less depressive-like behavior. By contrast, a 5 day period of 34% CR followed by replenishment lead to longer forced swim test latencies and increased inactivity (Chandler-Laney et al., 2007). In humans, restriction to 1200 kilocalories per day over a 3-week period increased perceived stress if participants were required to actively monitor intake (Tomiyama et al., 2010). Given these relatively short CR regimens and limited indices of psychogenic stress, it is important to examine this question more systematically in aged monkeys on long-term CR in several environments of varying aversive intensity.
In accord with prior studies, we hypothesized that higher stress reactivity would be related to reduced volumes and microstructural density in brain regions and white matter tracts that involve affect regulation, including threat detection and modulation of stress-related physiological responses (Rodrigues et al., 2009). Specifically, we expected these associations in limbic and cortical areas such as the medial portions of dorsal, orbital, and ventral prefrontal cortex, anterior cingulate cortex, insula, and thalamic nuclei that connect these areas, as well as white matter tracts such as the cingulum and uncinate fasciculus. Given that an emotionally reactive temperament has also been related to accelerated cellular aging (Knapman et al., 2009), and that CR diet can improve cellular homeostasis (Mattson et al., 2001), we predicted that the association of stress reactivity and brain volume or microstructure would be reduced in monkeys consuming a CR diet since middle age, suggesting an ameliorative effect. We also tested whether or not urinary cortisol levels were correlated with stress reactivity, and if basal cortisol was a biological mediator of the association between stress reactivity and either brain volume or microstructure. Given that aging and temperament can have curvilinear effects on physiology and brain (Sowell et al., 2003; Capitanio et al., 2004), both linear and quadratic models were tested.
Methods and Materials
Subjects
Forty-four rhesus monkeys (Macaca mulatta) between 19 and 31 years of age were evaluated from a larger longitudinal project at the Wisconsin National Primate Research Center (WNPRC; Kemnitz et al., 1993;Colman et al., 2009). Eighteen animals were fed an enriched diet ad libitum for ~8 hours/day, while the remaining twenty-six subjects were fed 30% fewer calories relative to their own baseline intake since middle age. Temporary increases in food allotment were made if body fat percentage or amount of food consumed fell below WNPRC veterinarian guidelines (Ramsey et al., 2000). Details of the CR manipulation, housing, and husbandry have been described previously (Kemnitz et al., 1993). Monkeys were housed individually, which was advantageous because social group housing increases the complexity of behavioral observations due to factors such as dominance rank (Vogt et al., 1981; Coe et al., 1982).
Hormone assays
Urine was collected in the home cage proximal to the behavioral paradigms. Free cortisol was derived through methylene chloride extraction using iodinated radioimmunoassay (DiaSorin, Saluggia, Italy) with an assay sensitivity of 2 ng. Creatinine levels were determined by enzyme immunoassay (Sigma-Aldrich, Madison, WI) with an assay sensitivity of 0.01 mg and used to correct urinary cortisol concentrations for partial voids.
Behavioral Observations
Two observational paradigms were employed to assess emotional reactivity in the home cage and during aversive conditions: 1) the Rhesus Aging and Temperament Examination (RATE); and 2) the Rhesus Reactivity to Environmental Anxiogenic Contexts Test (RREACT). A detailed description of the format, reliability, and validity of these measures is provided in Supplementary Text 1. The RATE used Likert scales to assess the emotional valence and magnitude of hostile and anxious behaviors in the home cage, including displays, vocalizations, and movement. Complementary behavior, such as apathy, lethargy, and inattentiveness, were also analyzed. After the RATE data were collected, the RREACT was conducted to grade each monkey s response to three distinctive contexts of differing aversive intensity using frequency scores: 1) following routine enrichment feeding in the home cage, 2) after relocation alone into an uncompressed restraint cage normally used for blood sample collection, and 3) following acute housing in a novel test cage alone. The scored indices included motor activity, hostility, resistance, and fear behaviors that have been defined previously in other studies (Kalin & Shelton, 1989; Friedman et al., 1996). To reduce type 1 error concerns, RATE and RREACT variables were z-scored and separately averaged into one RATE factor and one RREACT factor using a unit-weighted linear composite strategy (Cascio et al., 1980; Grice et al., 2001). Additional details about this statistical method are available in Supplementary Text 1.
MRI Data Acquisition
MR scan sessions and image acquisition parameters have been described previously (McLaren et al., 2009; Willette et al., 2010). Briefly, animals were anesthetized while images were acquired using a General Electric 3.0 T scanner (GE Medical Systems, Milwaukee, WI, USA). T1-weighted scan parameters were: repetition time (TR)=8.772 ms; echo time (TE)=1.876 ms; inversion time (TI)=600 ms; flip angle=10°; number of excitations (NEX)=2; matrix=256×224; field of view (FOV)=160 mm. 124 coronal slices were acquired with a thickness of 0.7 mm and no gap resulting in near isotropic 0.625×0.625×0.7 mm voxels. DTI was acquired with a diffusion-weighted echo-planar imaging sequence with 12 non-collinear direction gradients and one non-diffusion weighted direction (i.e. the b = 0) using the following parameters: TR/TE=10,000/77.2 ms, b=816s/mm2, NEX=6, field of view=140 mm, 128 × 128 in-plane matrix (interpolated to 256×256 on the scanner), slice thickness=2.5 mm, no gap, and 35 slice locations, with 0.547 × 0.547 × 2.500 voxels. DTI field mapping did not appreciably improve warping artifact. Spatial distortion was corrected by nonlinear normalization of a subject s B0 image to their T2 image, followed by normalization to a T2-weighted template in the same space as the T1 template (McLaren et al., 2009). Voxels for both scans were resampled to 0.5×0.5×0.5 mm. T2-weighted scans were acquired for global volume estimation and are described elsewhere (McLaren et al., 2009).
Imaging artifacts
All images were inspected in order to locate scan artifacts that might influence volumetric or microstructural results. One T1-weighted and three DTI scans had motion, respiratory, phase, or other artifacts that rendered data unusable for regional brain analyses and were excluded. Six DTI scans (Controls=2, CR=4) showed one or more punctate (~1–2 mm) abnormalities located in the white matter of the dorsal convexity. These scans were excluded to avoid spurious results in the voxel-wise analysis.
MRI Pre-processing
Pre-processing steps have been described previously (McLaren et al., 2009; Willette et al., 2010). Briefly, T1-weighted images were segmented, normalized to 112RM-SL macaque atlas space using DARTEL in SPM5 (Ashburner, 2007), and smoothed with a 4 mm kernel. GM and WM volumes were masked using binary transforms of their respective prior probability maps. Partial volume effects were minimized by using absolute thresholding of 0.30 for GM and 0.25 for WM. DTIFIT in FSL was used to compute fractional anisotropy (FA), mean diffusivity (MD), and both axial and radial diffusivity to examine if stress reactivity was primarily associated with differences in myelin or axons (Song et al., 2002). DTI maps were aligned to the macaque atlas and smoothed with a 4 mm Gaussian kernel.
MRI Analyses
Multiple regression voxel-wise analyses were conducted in SPM5 using a general linear model framework (Ashburner & Friston, 2000). DTI, GM, or WM tissue maps were used as dependent variables. Voxel-wise analyses using RREACT or RATE factors produced comparable imaging result maps (data not shown). Given these results and to avoid type 1 error inflation using multiple comparisons, we therefore averaged these factors to create a single global stress reactivity factor. Age, gender, and dietary condition were entered as covariates. Total brain volume (TBV) was also added for volumetric analyses to control for global effects; TBV was calculated by adding whole brain GM and WM. In separate models, a Stress Reactivity by Dietary Condition interaction term was added to evaluate if CR monkeys showed more volume and microstructural tissue integrity relative to controls per unit increase in stress reactivity, suggesting a protective effect. Based on the effect of the CR diet on behavior and previous reports of age-related health benefits (Colman et al., 2009; Willette et al., 2010), the interactions were restricted to a one-tailed test evaluating a hypothesized protective effect of CR. In order to determine if cortisol was a biological mediator for predicted changes in stress reactivity, the main effect of urinary cortisol and its interaction with stress reactivity were also tested. Linear and non-linear models were used, because aging and temperament can have curvilinear relationships to brain volume and physiological indices (Sowell et al., 2003; Capitanio et al., 2004). The voxel and cluster level thresholds were p < .005 (uncorrected) and p < .05 (corrected) respectively. Type 1 error was minimized by using Monte Carlo simulations in AFNI to determine the number of contiguous voxels needed for a given cluster to have not occurred by chance (Forman et al., 1995; Willette et al., 2010). Although violation of nonstationarity is a concern for permutational cluster correction of structural imaging data, smoothing with a 4 mm kernel does not appreciably bias the true alpha when using this method (Hayasaka et al., 2004). A priori cluster thresholds were similarly derived for amygdala, hippocampus, and thalamus. To gain a better understanding of whether myelin or axon changes predominantly drove FA results (Song et al., 2002), radial and axial diffusivity were examined using a binary mask of the FA results at a p < .05 (uncorrected) voxel threshold. Whole brain cluster coordinates correspond to the Saleem-Logothetis atlas (Saleem et al., 2002) and are displayed on the 112RM-SL underlays (McLaren et al., 2009). Standard monkey atlases were used to identify WM fibers (Schmahmann & Pandya, 2006) and subcortical structures (Paxinos et al., 2000).
Non-MRI Analyses
Behavioral data were analyzed using a mixed model multivariate repeated measures analysis of covariance (MANCOVA). The independent variable was Dietary Condition (CR vs. control diet). The dependent variables were the stress reactivity factor from the RATE and the stress reactivity factor from the RREACT. The cortisol-to-creatinine ratio was analyzed using ANCOVA. Covariates were age and gender. ANOVA was used to test for post hoc main effects and interactions for the stress reactivity factors, demographics, and activity behaviors during testing. For cortisol, a one-tailed Pearson s correlation was used to examine if higher basal hormone secretion was related to greater stress reactivity. Results were computed with or without outlier data, as data transformations were not entirely effective in normalizing high values. All tests were conducted with SPSS 16.0 at an alpha of .05 (SPSS, Chicago).
Results
Demographics and Stress Reactivity
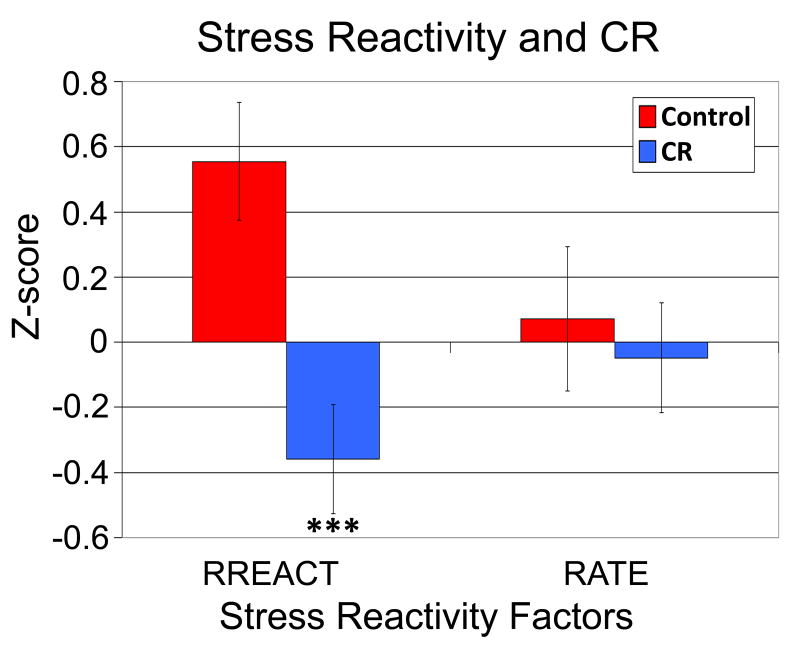
The age and gender composition of animals in the two dietary conditions did not differ (control: mean age±SD, 24.0±2.8 years, 10 females, 8 males; CR: mean age±SD, 24.3±2.8 years; 15 females, 11 males). For stress reactivity, a repeated measures MANCOVA showed that animals on the CR diet exhibited lower reactivity across the RATE and RREACT temperament assessment tests [F(1,40)=5.56, p=.023]. A Stress Reactivity by Dietary Condition interaction [F(1,40)=7.59, p=.009] modified this main effect. CR monkeys were less reactive versus controls during the more aversive RREACT [F(1,40)=12.02, p ≤.001], but similar during the RATE assessment in the home cage [F(1,40)=0.27, p=.606] (Figure 1). The removal of one highly reactive control animal (z=2.58) did not change these RREACT results [F(1,39)=6.23, p=.017]. Groups did not significantly differ for any orientation, attention, or motor behavior variable collected during the RATE and RREACT, nor for an averaged z-score factor of these indices [F(1,40)=0.63, p=.432].
Figure 1.
The effect of a calorie restriction diet on stress reactivity assessed during the RATE and RREACT behavioral assessment paradigms. *** = p-value of .001. Please refer to the internet version of this article for interpretations of color in this figure.
Cortisol
Cortisol values were statistically similar for the control (mean±SD=468±371 ng/mg) and CR monkeys (mean±SD=401±217 ng/mg) [F(1,40)= 0.63, p=.432] with or without one control and two CR monkeys that had cortisol values higher than two standard deviations above the mean (1812, 1180, and 823 ng/mg, respectively). Higher stress reactivity was not related to increased basal cortisol when all animals were included in the analysis. However, this relationship did attain significance [r =.29, p=.034] after removing the outliers. Further removal of a very stress reactive control monkey did not further influence the relationship evident between temperament and cortisol [r=.29, p=.036].
Regional MD
Stress Reactivity Association
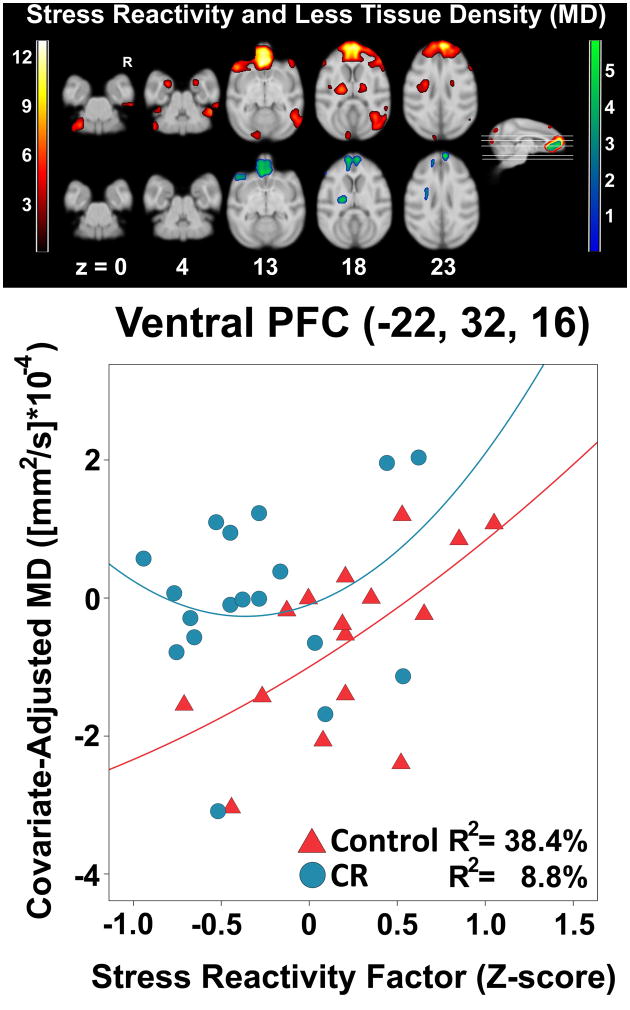
Using a linear fit, the global stress reactivity factor was regressed onto every voxel in the brain to gauge where there were significant associations with reduced tissue density (i.e., increased MD). Several clusters within a priori regions were found, with a maximally significant voxel in right dorsomedial prefrontal cortex (Supplementary Figure 1, Supplementary Table 1). A quadratic term was then added to the existing model to see if a curvilinear fit improved the voxel-wise regression analysis, because aging (Sowell et al., 2003) and psychological stress (Lupien et al., 2007) can exert non-linear effects on the brain. The power of the MD analysis was substantially increased and expanded existing clusters; new clusters were also found incorporating left granular insula and right amygdala (Figure 2; Table 1). The findings for these regions were not driven by edge, partial volume, or artifact effects. The main cluster in medial temporal lobe extended bilaterally along the axial plane to include: gyrus rectus; agranular insula; frontal pole; caudate; precentral operculum; ventral, orbital, and dorsal prefrontal cortices; anterior cingulate cortex; mediodorsal thalamus; and amygdala. An unexpected region in brainstem encapsulated the dorsal column medial lemniscus tract and the raphe nuclei. WM tracts of interest included the anterior corpus callosum, uncinate fasciculus, and the cingulum bundle.
Figure 2.
Statistical parametric map of the quadratic fit of higher stress reactivity predicting less microstructural tissue density (i.e., higher MD). The linear fit produced similar but smaller clusters. A representative voxel illustrates the protective effect of CR for the interaction. The ‘yellow-red’ color map represents regions where there was a significant association between stress reactivity and MD for both CR and control animals. The green-blue color map represents areas where there was a significant Dietary Condition × Stress Reactivity interaction. Color bars represent t values. Slice numbers below images correspond to the distance (mm) from the origin located at ear bar zero. Axial cross-sections correspond to slices seen on the sagittal representation of the brain. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.
Table 1.
The relationship of higher stress reactivity and increased mean diffusivity across and between groups
| Description | x, y, z | t-value | Cluster Size (voxels) |
|---|---|---|---|
| Stress Reactivity Association | |||
| L mPFC | −2, 34, 13 | 13.12 | 41,473 |
| R dmPFC | 1, 46, 20 | 12.77 | |
| L oPFC/vlPFC | −22, 31, 10 | 8.11 | |
| R Ventral Visual Areas (temporal lobe) | 26, −6, 7 | 7.82 | 13,592 |
| R Extrastriate Cortex | 18, −10, 16 | 7.12 | |
| R Dorsal Inferotemporal Cortex | 25, 4, 0 | 5.97 | |
| L MD of Thalamus (parvicellular) | −8, 12, 18 | 7.27 | 4,042 |
| L Granular Insula | −16, 14, 21 | 3.85 | |
| L Lateral Posterior Cerebellum | −14, −10, −2 | 6.91 | 2,162 |
| L Lateral Posterior Cerebellum | −22, −14, 3 | 5.59 | |
| R Superior Temporal Gyrus | 30, 14, 12 | 6.12 | 607 |
| L Amygdala | −11, 18, 4 | 5.94 | 706 |
| R MD/VPL of Thalamus | 8, 14, 18 | 5.37 | 887 |
| R Amygdala | 10, 19, 4 | 5.05 | 460 |
| L Primary Visual Cortex | −5, −23, 15 | 4.68 | 2,909 |
| L Primary Visual Cortex | −5, −20, 28 | 4.61 | |
| L Ventral inferotemporal cortex | −18, −2, 5 | 4.54 | 1,183 |
| CR*Stress Reactivity Interaction | |||
| R dmPFC | 2, 44, 22 | 6.26 | 8,765 |
| L mPFC | −4, 44, 16 | 5.58 | |
| L mPFC | −3, 36, 12 | 5.44 | |
| L VPL of Thalamus | −9, 12, 17 | 5.12 | 1,252 |
| L StB | −12, 18, 24 | 3.19 | |
| L oPFC/vlPFC | −21, 31, 10 | 4.36 | 1,550 |
Local maxima are included underneath the maximum of larger clusters. L = Left hemisphere, R = Right hemisphere, where non-designated areas follow the medial longitudinal fissure and are bilateral. CB = Cingulum Bundle; CR = Calorie Restriction; dmPFC = Dorsomedial PFC; MD = Mediodorsal Nucleus; mPFC = Medial PFC; oPFC = Orbital PFC; PFC = Prefrontal Cortex; StB = Striatum Bundle; vlPFC = Ventrolateral PFC; VPL = Ventral Posterior Lateral Nucleus. A priori cluster estimates of the MD nucleus of the thalamus and amygdala were used beyond typical cluster correction. This quadratic model increased statistical power and expanded existing clusters from the linear model, but also produced new clusters in left insula and right amygdala. Brains are oriented in neurological space.
Stress Reactivity by Dietary Condition Interaction
The next analysis tested for a slope difference between CR and control monkeys in the relationship between stress reactivity and MD. Specifically, we expected stress reactivity and increasing MD to be less related in CR animals versus controls, suggesting a protective effect. The linear fit resulted in significant clusters primarily in prefrontal regions (Supplementary Figure 1). The quadratic fit yielded a result map that was similar but with expanded clusters, which was due to a better sub-fit line for controls (Figure 2; Table 1). Clusters of interest for WM included prefrontal corticocortical connections, cingulum bundle, and uncinate fasciculus. In GM, a bilateral region encompassed the gyrus rectus; ventral, orbital, and dorsal prefrontal cortices; frontal pole; and anterior cingulate cortex. A representative voxel in medial prefrontal cortex indicated that even after removing the highly reactive control monkey, there was a strong correlation between higher stress reactivity and MD [r=.64, p=.019]. By contrast, the association in the CR monkeys was non-significant [r=.22, p=.405], suggesting that stress reactivity and microstructural tissue density in these regions did not influence one another for CR animals.
Regional FA
Stress Reactivity Association
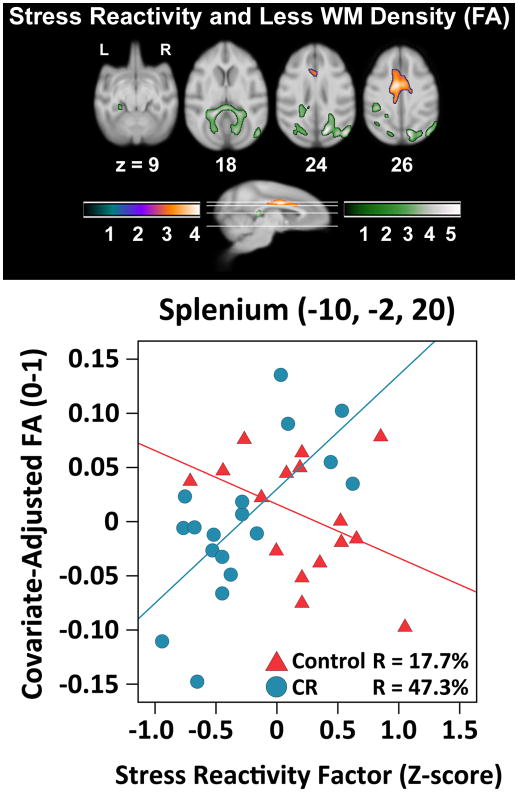
Higher stress reactivity was related to lower FA values along the cingulum bundle suprajacent to cingulate gyrus for 24mm (Figure 3, Table 2). Axial diffusivity results overlapped with this result map, suggesting that stress reactivity may primarily be related to axonal integrity in cingulum bundle. No significant quadratic relationships were found for FA.
Figure 3.
The linear relationship between greater stress reactivity and less tract integrity (i.e., lower FA). The quadratic fit produced similar results. Out of concern for partial volume effects, follow-up analyses using stringent FA thresholding (.30) excluded voxels along the midline, but preserved regions of association bilaterally in separate clusters. A representative voxel illustrates the protective effect of CR. Slice numbers below images correspond to the distance (mm) from the origin located at ear bar zero. Slices correspond to cross-sections along the sagittal representation of the brain. The purple-orange and green color schemes respectively represent analyses examining the association within groups and interaction between groups. Color bars represent t-values. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.
Table 2.
The relationship of higher stress reactivity and lower fractional anisotropy of white matter fibers across and between groups
| Description | t-value | x, y, z | Cluster Size (voxels) |
|---|---|---|---|
| Stress Reactivity Association | |||
| L Middle CB | 4.11 | −7, 16, 27 | 4,021 |
| R Middle CB | 3.28 | 8, 13, 27 | |
| L Middle CB | 3.26 | −4, 8, 25 | |
| CR*Stress Reactivity | |||
| R dOB | 5.52 | 8, −14, 22 | 14,385 |
| Splenium of CC | 4.63 | −10, −2, 20 | |
| R ILF | 4.49 | 22, −16, 21 | |
| L ILF | 4.43 | −16, −10, 28 | 2,084 |
Local maxima are included underneath the maximum of larger clusters. L = left hemisphere, R = right hemisphere, where non-designated areas follow the medial longitudinal fissure and are bilateral. CB = Cingulum Bundle; CC = Corpus Callosum; CR = Calorie Restriction; dOB = Dorsal Occipital Bundle; ILF = Inferior Longitudinal Fasciculus. Brains are oriented in neurological space.
Stress Reactivity by Dietary Condition Interaction
In line with our hypothesis of a protective effect of CR, we tested the extent to which CR monkeys showed greater tract density per unit increase in stress reactivity as compared to controls. Bilateral clusters were seen throughout the splenium of the corpus callosum, extending down to WM within extrastriate dorsal occipital bundle and the inferior longitudinal fasciculus. As shown in Figure 3, a plot from a representative voxel in the splenium indicates the interaction. Stress reactivity predicted lower FA [r=−.56, p=.009] but was reduced to marginal significance after removing the one outlier [r=−.42,p=.052]. CR monkeys showed the opposite pattern [r=.69, p=.001] (Figure 3; Table 2). The overlap with results for radial diffusivity suggests that the CR diet might have had protective effects on axonal myelin for these tracts.
Regional WM
Stress Reactivity Association
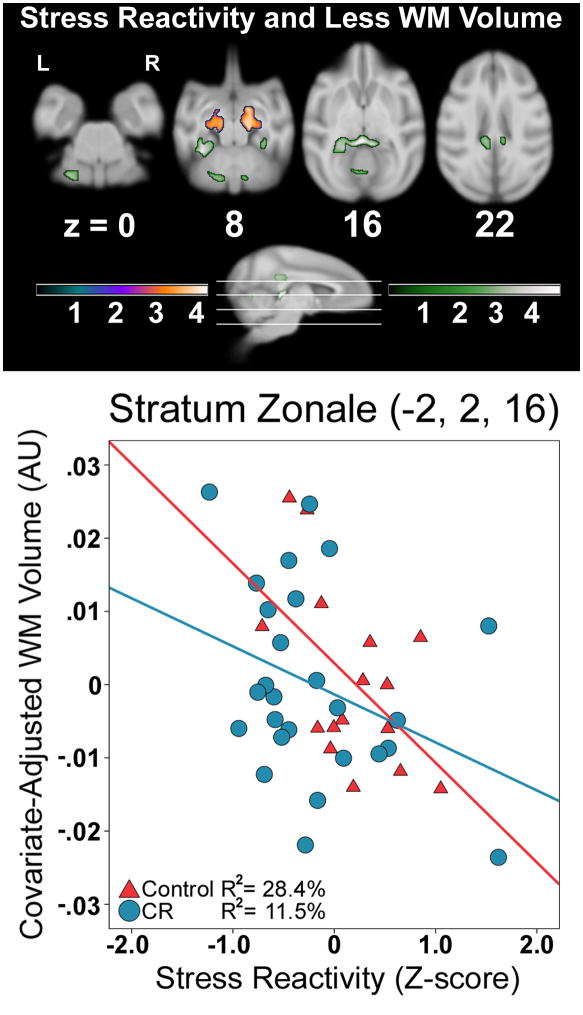
Higher stress reactivity scores were maximally associated with less WM within the right pontine bundle (Figure 4, Table 3), extending to much of the thalamic and striatum bundles. A comparable cluster was found in the left hemisphere. No significant quadratic relationships were found between stress reactivity and regional WM volume.
Figure 4.
The linear relationship between more stress reactivity and less WM volume. The quadratic fit produced similar results. A representative voxel illustrates the protective effect of CR. Slice numbers below images correspond to the distance (mm) from the origin located at ear bar zero. Slices correspond to cross-sections along the sagittal representation of the brain. The purple-orange and green color schemes respectively represent analyses examining the association within groups and interaction between groups. Color bars represent t-values. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.
Table 3.
The relationship of higher stress reactivity and lower regional white matter volume across and between groups
| Description | t-value | x, y, z | Cluster Size (voxels) |
|---|---|---|---|
| Stress Reactivity Association | |||
| R PB | 4.03 | 7, 12, 10 | 2085 |
| L PB | 3.88 | −8, 14, 11 | 1770 |
| CR*Stress Reactivity | |||
| L SZ | 4.79 | −2, 2, 16 | 4504 |
| L CB; ILF | 4.26 | −16, −2, 8 | |
| L Fx | 3.56 | −10, 0, 15 | |
| L Posterior fCbl | 3.29 | −7, −16, 5 | 866 |
| L Anterior fCbl | 3.00 | −4, −13, 14 | |
Local maxima are included underneath the maximum of larger clusters. L = left hemisphere, R = right hemisphere, where non-designated areas follow the medial longitudinal fissure and are bilateral. CB = Cingulum Bundle; CR = Calorie Restriction; fCbl = Fibers of the Cerebellum; Fx = Fornix; ILF = Inferior Longitudinal Fasciculus; PB = Pontine Bundle; SZ = Stratum Zonale. Brains are oriented in neurological space.
Stress Reactivity by Dietary Condition Interaction
The next analysis tested if a unit increase in stress reactivity was associated with less of a predicted impact on WM in CR monkeys versus controls, indicating a protective effect. This pattern of results was found in lamina 1 and intermediate lamina 5 of left and right superior colliculus. The cluster included the fornix, cingulum bundle, and the splenium. As shown in Figure 4, a plot of a representative voxel in lamina 1 illustrated this relationship among the control animals [r=−.48, p=.049]; removing one highly reactive control animal did not change the result map and slightly strengthened the correlation. The regression line for the CR monkeys had a shallower slope that was a statistical trend in the same direction [r=−.34, p=.089] (Figure 4, Table 3).
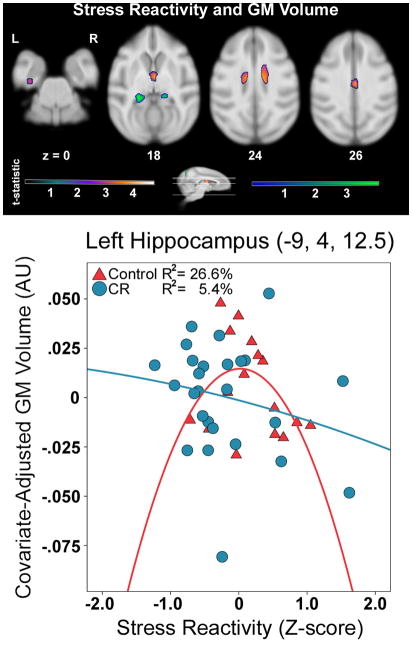
Regional GM
Stress Reactivity Association
Stress reactivity was maximally related to less GM in the mediodorsal nucleus of the thalamus (Figure 5, Table 4). The paraventricular nucleus of the hypothalamus, caudate head, mid cingulate cortex and left anterior hippocampus were also included.
Figure 5.
The relationship between higher stress reactivity and lower GM volume. A representative voxel illustrates the protective effect of CR for the interaction effect. The purple-orange and green color schemes respectively represent analyses examining the association within groups and interaction between groups. Color bars represent t-values. Slice numbers below images correspond to the distance (mm) from the origin located at ear bar zero. Slices correspond to cross-sections along the sagittal representation of the brain. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.
Table 4.
The relationship of higher stress reactivity and lower regional gray matter volume across and between groups
| Description | t-value | x, y, z | Cluster Size (voxels) |
|---|---|---|---|
| Stress Reactivity Association | |||
| R MD of Thalamus | 4.65 | 2, 16, 20 | 2712 |
| R Caudate | 3.38 | 3, 28, 22 | |
| L Hippocampus | 3.07 | −16, 9, −2 | 184 |
| CR*Stress Reactivity Interaction | |||
| L Hippocampus | 4.00 | −9, 4, 12 | 2096 |
| R Hippocampus | 3.56 | 9, 5, 12 | 945 |
Local maxima are included underneath the maximum of larger clusters. L = left hemisphere, R = right hemisphere. A priori cluster estimation of the hippocampus was used beyond typical cluster correction. CR = Calorie Restriction; MD = Mediodorsal Nucleus. The quadratic model for the interaction produced the two hippocampus clusters, whereas the linear model did not. Brains are oriented in neurological space.
Stress Reactivity*Dietary Condition Interaction
This contrast examined if CR versus control monkeys showed less GM atrophy per unit increase in stress reactivity, which would be suggestive of a graded, protective effect. A linear fit yielded clusters in primary (−20,−16,10; t=3.33; cluster size=375 voxels) and extrastriate (−6,−20,13; t=3.33; cluster size=89 voxels) visual cortices. A quadratic term was then added to test for non-linear relationships. Two clusters appeared in left and right hippocampus including parasubiculum and presubiculum, dentate gyrus, and CA3 and CA4 (Figure 5, Table 4). The right cluster also overlapped with the pulvinar of the thalamus. A representative voxel in left hippocampus showed that stress reactivity predicted less GM for controls [r=−.70, p=.005]. Removal of one control outlier lessened but did not alter this result [r=−.52, p=.041]. CR animals did not show this association [r=−.22, p=.271], suggesting that stress reactivity on the health-promoting diet did not influence hippocampus volume or vice-versa.
Stress Reactivity and Brain: Mediation by Cortisol
In this analysis, we tested if basal cortisol alone or its interaction with stress reactivity predicted less regional brain volume or microstructure across all subjects (i.e. CR and control combined). Cortisol or the interaction between cortisol and stress reactivity were used as predictors of interest in multiple regression voxel-wise analyses. A priori areas of interest included prefrontal cortex, amygdala, and hippocampus. The main effect of cortisol did not produce any significant clusters after correcting for type 1 error. A Cortisol by Stress Reactivity interaction for GM (Figure 6, Table 5) and MD (Supplementary Table 2) produced several clusters in brain areas that were also implicated when looking at the main effect association of stress reactivity to tissue volume and density. These areas included hippocampus, caudate, parventricular nucleus of the hypothalamus, amygdala, and gyrus rectus. No relationships were noted for WM and FA. To validate this interaction for GM and MD, a mean split for stress reactivity was used to create “hi stress” and “low stress” groups. To minimize selection bias for our confirmational analyses (Vul et al., 2009), sub-maximum voxels in hippocampus (Leverenz et al., 1999) and orbital prefrontal cortex (Elgh et al., 2006) were selected a priori. For GM, “hi stress” animals with higher cortisol had significantly less GM volume [r=−.493, p=.020], whereas “low stress” monkeys showed no significant effect [r=.359, p=.110]. For MD, “hi stress” monkeys [r=.483, p=.050], but not “low stress” monkeys [r=−.361, p=.141] with higher cortisol had greater diffusion of water and thus less microstructural tissue density. These results suggest that cortisol can synergize with stress reactivity to affect brain regions involved in emotional regulation that are also relevant for aging and dementia. Alternatively, high levels of cortisol may synergize with existing pathology in these regions, leading to higher stress reactivity.
Figure 6.
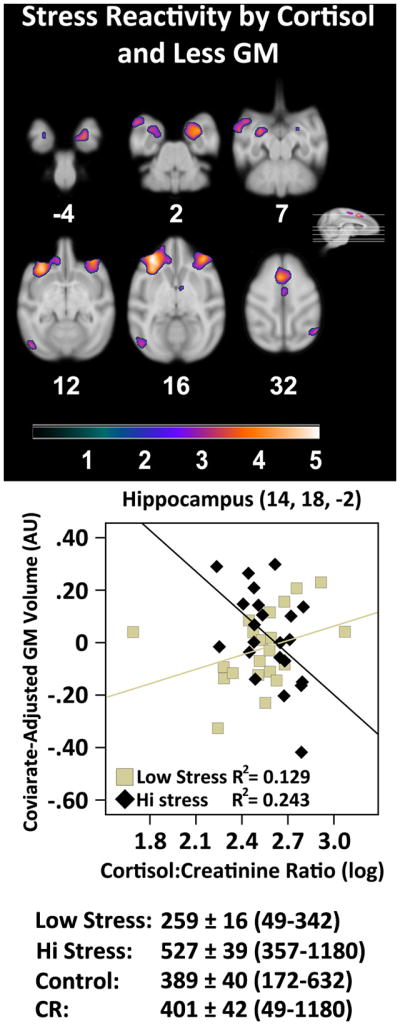
The linear relationship between GM volume and a Cortisol × Stress Reactivity interaction. A quadratic fit produced a similar result. The graph depicts the interaction in a representative voxel in hippocampus. Mean ± SEM and value ranges for cortisol output in ng/mg are provided below the graph. The purple-orange color scheme represents areas with a significant between group interaction. The color bar represents t-values. Slice numbers below images correspond to the distance (mm) from the origin located at ear bar zero. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.
Table 5.
The interaction effect of stress reactivity and cortisol on gray matter volume
| Description | t-value | x, y, z | Cluster Size (voxels) |
|---|---|---|---|
| L oPFC | 5.08 | −14, 36, 16 | 8813 |
| L oPFC | 3.18 | −6, 36, 12 | |
| L Frontal Pole | 3.05 | −8, 44, 16 | |
| R Hippocampus | 4.45 | 14, 18, −2 | 4147 |
| R Striatum | 2.82 | 10, 20, 6 | |
| R vPFC | 4.27 | 16, 37, 15 | 4314 |
| R mPFC | 2.97 | 8, 36, 17 | |
| L SMA | 4.17 | −2, 24, 31 | 2192 |
| L Arcuate Sulcus (lower limb) | 3.94 | −12, 26, 19 | 407 |
| L Anterior Amygdaloid Area | 3.84 | −13, 18, 8 | 540 |
| R Frontal Eye Field | 3.71 | 20, 30, 34 | 500 |
| L STS | 3.69 | −23, 23, 4 | 2077 |
| L Entorhinal Cortex | 3.69 | −14, 19, −2 | 1713 |
| R Extrastriate Cortex | 3.58 | 22, −8, 30 | 435 |
| L Primary Visual Cortex | 3.38 | −21, −16, 12 | 699 |
| R Primary Motor Cortex | 3.31 | 2, 16, 32 | 1723 |
| PVN of the Hypothalamus | 3.27 | 4, 18, 18 | 371 |
Local maxima are included underneath the maximum of larger clusters. L = left hemisphere, R = right hemisphere, where non-designated areas follow the medial longitudinal fissure and are bilateral. mPFC = Medial Prefrontal Cortex; Paraventricular Nucleus; SMA = Supplementary Motor Area; STS = Superior Temporal Sulcus; oPFC = Orbital Prefrontal Cortex; vPFC = Ventral Prefrontal Cortex. Brains are oriented in neurological space.
Discussion
In this study we found that consuming a CR diet during the lifespan led to a substantial reduction in the stress reactivity of monkeys in old age. This was most evident during the arousing contexts of the RREACT paradigm. By contrast, the RATE was used in a low stimulation, home cage context where monkeys were proximal to familiar conspecifics, which can substantially mitigate psychological stress (Vogt et al., 1981; Coe et al., 1982; Friedman et al., 1996; Willette et al., 2007). CR does not appear to induce striking differences in activity for this cohort (Ramsey et al., 2000), and our results in this study suggest that decreased stress reactivity in CR animals is not likely due to group differences in motor activity levels, inattentiveness, or somnolence. The RATE and RREACT stress reactivity measures were combined because they are complementary constructs, produced similar main effect result maps, and it was important to reduce type 1 error. This global stress reactivity factor was related to less volume and tissue density in limbic and prefrontal tracts and regions important in emotional regulation and age-associated cognitive decline, although relationships with GM volume were modest. Furthermore, CR monkeys showed protective effects in prefrontal cortex and hippocampus, where higher stress reactivity predicted less pathology in contrast to controls--although directionality of these effects cannot be inferred. This ameliorative effect is in concert with our previous work illustrating anti-aging effects in brain due to CR, including reduced iron deposition and related improvements in motor task performance (Kastman et al., 2010), lower inflammation and higher tissue density throughout brain (Willette et al., 2010), and more GM in cingulate and middle temporal cortices as a function of CR alone (Colman et al., 2009).
We also found that the trait of heightened stress reactivity was moderately associated with basal cortisol levels, although it should be acknowledged that this assessment was limited because it was only collected at one time point and that outliers needed to be removed for this association to be significant. Indeed, it was not feasible to collect cortisol immediately before or after stress assessments. Even though the hormone collection was circumscribed, the result suggests that a temperament predisposition of psychological reactivity to stress could chronically potentiate the release of cortisol and affect the brain. Although high cortisol levels per se were not related to variation in brain volume or microstructure, a stress reactivity by cortisol interaction indicated that high stress reactivity and high cortisol may synergize to affect areas like hippocampus, amygdala, and prefrontal cortex. This result is in line with Leverenz and colleagues (1999), who found that chronic hypercortisolemia in the absence of stress was not related to hippocampal volume or neuronal density in aged rhesus monkeys using stereological techniques.
Prior research on the glucocorticoid cascade hypothesis for the hippocampus has yielded mixed results. On the one hand, cognitive deficits and smaller hippocampal volume were present in aged humans who showed high, but not moderate, cortisol output at baseline and a progressive increase in secretion with age (Lupien et al., 2007). Yet, chronic administration of glucocorticoids in rodents, nonhuman primates, or humans treated with synthetic agents does not necessarily produce significant atrophy or neuronal loss (Leverenz et al., 1999; Bao et al., 2008; Schubert et al., 2008). In addition, aging in rodents does not appear to produce progressive deficits in corticosterone secretion during the recovery period after restraint stress (Sabatino et al., 1991), suggesting that the HPA axis is not necessarily sensitized to stress in a feed-forward manner as the organism ages. Results for basal cortisol are also contradictory even in the presence of a chronically stressful state like major depression. Gold and colleagues found that higher salivary cortisol levels correlated with lower hippocampal volume in the dentate gyrus and cornu ammonis fields 2 and 3 in depressed compared to non-depressed multiple sclerosis patients (Gold et al., 2010). By contrast, longitudinally assessed elders with major depression had a 6% decrease in right hippocampal volume, but this effect was not correlated with basal salivary cortisol (O’Brien et al., 2004).
Our monkey cohort may provide a useful intermediate animal model, because we have examined the interplay between a temperament-derived measure of stress reactivity collected under several exogenous stress contexts, basal cortisol, and brain. Akin to the work of Dal-Pan and colleagues (2011) in mouse lemurs, we found no significant effect of CR on basal cortisol output. Some CR regimens actually induce mild pre-prandial increases in cortisol that may benefit the brain (Sabatino et al., 1991), possibly via anti-inflammatory effects. Our findings suggest that elevation of glucocorticoids may exacerbate existing neuropathology to affect psychological reactivity to stress, or that chronic stress across contexts and higher cortisol may synergize to induce lower GM and tissue density.
For WM, heightened stress reactivity was associated with less volume or microstructural integrity throughout a subcortical-thalamo-insular-prefrontal neuroaxis that has been related to emotional and neuroendocrine regulation. Several such brain areas along this axis include the amygdala and hippocampus, prefrontal cortex, mediodorsal thalamus, the paraventricular nucleus of the hypothalamus, anterior cingulate cortex, and other regions (Diorio et al., 1993; Sullivan & Gratton, 1999; Li et al., 2004; McEwen, 2007). For WM, differences in axial diffusion presumably related to axonal alterations were seen in cingulum and uncinate fasciculus, which connect temporal and prefrontal areas. These areas have been found to be adversely affected by chronic psychological stress associated with mood disorders (Houenou et al., 2007; Frodl et al., 2008).
Similar to GM results, there was a protective effect of CR on WM when examining the Dietary Condition by Stress Reactivity interaction. Specifically, for CR animals, higher stress reactivity was not significantly related to lower WM volume or tract density in tracts extending from extrastriate cortex to superior colliculus and the cingulum. This circuit is related to the orientation and defensive body posturing that accompanies cardiovascular changes in stressful and threatening situations (Dean et al., 1989; Brandao et al., 2003; Sung et al., 2009). Biological mediators of this CR effect may include lower stress-induced cortisol release (Sorrells et al., 2009), or alterations in energy and oxidative metabolism that occurs in part by increasing orexin signaling and decreasing markers of cellular aging (Inoue et al., 2004; Lutter et al., 2008). For instance, in peripheral Schwann cells from aged rats, 40% CR starting from 16 weeks of age showed markedly more myelin protein expression in concert with improved expression and binding activity of heat shock proteins, improved autophagy related to non-functional proteins, and reduced redistribution of ion channels, all of which suggest less glial dysfunction that may reflect similar changes in brain (Rangaraju et al., 2009). Alternatively or in concert, CR may ameliorate WM via repression of reactive oxygen species and inflammation. In myelinated peripheral nerves, 40% CR in aged rats significantly decreased accumulation of oxidatively damaged intracellular proteins and induction of inflammatory cascade pathways (Opalach et al., 2010).
There are several limitations of this cross-sectional analysis that should be acknowledged. Despite several previous reports that characterize behavioral phenotypes in nonhuman primates (Fox et al., 2008), there is no standardized and universally accepted method for measuring anxious temperaments in monkeys. We sought to capture the core temperament of each animal through ratings generated by multiple observers, repeated over many trials, in both the undisturbed home environment and a range of aversive environmental contexts over several months. In addition, we employed previously validated scoring methodologies when classifying similar traits in monkeys (Capitanio et al., 2004; Segerstrom et al., 2006). Furthermore, the monkeys assigned to the CR diet were randomly allocated to that dietary group, eliminating any bias in assignment to group based on temperament. Due to the nature of our cross-sectional design, it not possible to causally determine if individual differences in temperament were temporally antecedent to neural alterations. Longitudinal data on stress reactivity or brain volume and microstructure would be necessary to infer directionality and causality. Due to the dramatic change in body composition caused by CR, observers may have been influenced in ratings even though they were blind to condition. Because animals were in the CR condition for over a decade before the behavioral and neural assessments, it was not possible to counterbalance this aspect of the study design. Finally, it should be acknowledged that the neuroendocrine assessment was limited to only a single basal determination instead of analyzed over the course of the data or near the time of stress induction. Nevertheless, basal cortisol did interact with stress reactivity to mediate lower brain volume and microstructure in areas with a dense array of glucocorticoid receptors, such as hippocampus and prefrontal cortex, suggesting a physiologically plausible effect.
In conclusion, higher stress reactivity in aged rhesus monkeys was related to less regional volume and tissue density in some brain areas that are important for stress-related emotion and neuroendocrine regulation. High cortisol levels appear to synergize with brain pathology or stress reactivity to mediate some of these relationships. Overall, consumption of a CR diet reduced anxious responsiveness, which was most evident in the moderate to highly arousing contexts commonly encountered by monkeys in laboratory settings. Furthermore, an interaction between dietary condition and temperament indicated an ameliorative effect for CR monkeys. Specifically, per unit increase in stress reactivity, CR monkeys would have more volume and tissue density relative to controls in important regions and tracts in prefrontal cortex and hippocampus. Cortisol appeared to mediate effects in GM volume and microstructure, but not in WM. It must again be emphasized that directionality between stress reactivity, cortisol, and brain indices cannot be inferred. Pharmacological mimetics that induce the same physiological and cellular changes as CR may be able to confer some of these benefits without the challenges of sustaining a prolonged commitment and adherence to a CR diet in humans. For example, adult grey mouse lemurs given the polyphenol resveratrol had significant improvements in cognition that were comparable to conspecifics on chronic 30% CR (Dal-Pan et al., 2011).
Supplementary Material
The linear relationship between more stress reactivity and less microstructural tissue density (i.e., higher MD). A representative voxel illustrates the protective effect of CR. Numbers below images correspond to the distance (mm) from the origin located at ear bar zero. The ‘purple-orange’ and green color schemes respectively represent areas that showed significant association and interaction relationships. Color bars represent t-values. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.
Acknowledgments
The assistance of R. Fisher and the Waisman Center for Brain Imaging is greatly appreciated. This study was supported in part by the National Institutes of Health RR000167, AG011915, AG000213, GM007507, MH085051, and MH062015. The analyses were enabled by the resources and facilities at the W.S. Middleton Memorial Veterans Hospital. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01 from NCRR. CLC receives partial salary support from several NIH awards (AG027343, AG20166, HD057064, AI067518). This paper will be listed as GRECC number: 2010-XX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Inoue H, Takei K, Suga M, Matsuo K, Kato T, Masutani Y, Ohtomo K. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181(1):64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57(2):531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Troncoso AC, de Souza Silva MA, Huston JP. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: empirical and conceptual considerations. Eur J Pharmacol. 2003;463(1–3):225–233. doi: 10.1016/s0014-2999(03)01284-6. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162(4):767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta) Psychoneuroendocrinology. 2004;29(10):1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Carter CJ. eIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull. 2007;33(6):1343–1353. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio WF, Valenzi ER, Silbey V. More on validation and statistical power. J Appl Psych. 1980;65(2):135–138. [Google Scholar]
- Cefalu WT, Terry JG, Thomas MJ, Morgan TM, Edwards IJ, Rudel LL, Kemnitz JW, Weindruch R. In vitro oxidation of low–density lipoprotein in two species of nonhuman primates subjected to caloric restriction. J Gerontol A Biol Sci Med Sci. 2000;55(7):B355–361. doi: 10.1093/gerona/55.7.b355. [DOI] [PubMed] [Google Scholar]
- Chandler-Laney PC, Castaneda E, Pritchett CE, Smith ML, Giddings M, Artiga AI, Boggiano MM. A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol Biochem Behav. 2007;87(1):104–114. doi: 10.1016/j.pbb.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe C, Franklin D, Smith E, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol Behav. 1982;29(6):1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325 (5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Gatz M. Do work-related stress and reactivity to stress predict dementia more than 30 years later? Alzheimer Dis Assoc Disord. 2007;21(3):205–209. doi: 10.1097/WAD.0b013e31811ec10a. [DOI] [PubMed] [Google Scholar]
- Dal-Pan A, Pifferi F, Marchal J, Picq JL, Aujard F RESTRIKAL Consortium. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6(1):e16581. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12(4):137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Le Moal M, Simon H. Reactivity to novelty during youth as a predictive factor of cognitive impairment in the elderly: a longitudinal study in rats. Brain Res. 1994;653(1–2):51–56. doi: 10.1016/0006-8993(94)90371-9. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Maccari S, Piazza PV, Le Moal M, Simon H. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly--a life-span study in rats. Psychoneuroendocrinology. 1996;21(5):441–453. doi: 10.1016/0306-4530(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgh E, Lindqvist Astot A, Fagerlund M, Eriksson S, Olsson T, Nasman B. Cognitive dysfunction, hippocampal atrophy and glucocorticoid feedback in Alzheimer’s disease. Biol Psychiatry. 2006;59(2):155–161. doi: 10.1016/j.biopsych.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PloS One. 2008;3(7):e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Reyes TM, Coe CL. Context-dependent behavioral effects of interleukin-1 in the rhesus monkey (Macaca mulatta) Psychoneuroendocrinology. 1996;21(5):455–468. doi: 10.1016/0306-4530(96)00010-8. [DOI] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Reiser M, Möller HJ, Meisenzahl EM. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65(10):1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Gold SM, Kern KC, O’Connor MF, Montag MJ, Kim A, Yoo YS, Giesser BS, Sicotte NL. Smaller cornu ammonis 2–3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms. Biol Psychiatry. 2010;68(6):553–559. doi: 10.1016/j.biopsych.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice JW. A comparison of factor scores under conditions of factor obliquity. Psychol Methods. 2001;6(1):67–83. doi: 10.1037/1082-989x.6.1.67. [DOI] [PubMed] [Google Scholar]
- Halliday GM. A review of the neuropathology of schizophrenia. Clin Exp Pharmacol Physiol. 2001;28(1–2):64–65. doi: 10.1046/j.1440-1681.2001.03398.x. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, Poupon C, Martinot JL, Paillere-Martinot ML. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- Inoue K, Zorrilla EP, Tabarin A, Valdez GR, Iwasaki S, Kiriike N, Koob GF. Reduction of anxiety after restricted feeding in the rat: implication for eating disorders. Biol Psychiatry. 2004;55(11):1075–1081. doi: 10.1016/j.biopsych.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychiatry. 2010;67(2):175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243(4899):1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Xu G, Canu E, Field AS, Alexander AL, Voytko ML, Beasley TM, Colman RJ, Weindruch RH, Johnson SC. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2010;30(23):7940–7. doi: 10.1523/JNEUROSCI.0835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48(1):B17–26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Knapman A, Heinzmann JM, Hellweg R, Holsboer F, Landgraf R, Touma C. Increased stress reactivity is associated with cognitive deficits and decreased hippocampal brain-derived neurotrophic factor in a mouse model of affective disorders. J Psychiatr Res. 2009;44(9):566–75. doi: 10.1016/j.jpsychires.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Knutson B, Momenan R, Rawlings RR, Fong GW, Hommer D. Negative association of neuroticism with brain volume ratio in healthy humans. Biol Psychiatry. 2001;50(9):685–690. doi: 10.1016/s0006-3223(01)01220-3. [DOI] [PubMed] [Google Scholar]
- Levay EA, Govic A, Penman J, Paolini AG, Kent S. Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol Behav. 2007;92(5):889–896. doi: 10.1016/j.physbeh.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19(6):2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XB, Inoue T, Nakagawa S, Koyama T. Effect of mediodorsal thalamic nucleus lesion on contextual fear conditioning in rats. Brain Res. 2004;1008(2):261–272. doi: 10.1016/j.brainres.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech Ageing Dev. 2001;122(7):757–78. doi: 10.1016/s0047-6374(01)00226-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. NeuroImage. 2009;45(1):52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58(12):1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161(11):2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 2010;13(1):65–74. doi: 10.1089/rej.2009.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga A. The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; Orlando, FL: 2000. [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35(9–10):1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Rangaraju S, Hankins D, Madorsky I, Madorsky E, Lee WH, Carter CS, Leeuwenburgh C, Notterpek L. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 2009;8(2):178–91. doi: 10.1111/j.1474-9726.2009.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Pauls JM, Augath M, Trinath T, Prause BA, Hashikawa T, Logothetis NK. Magnetic resonance imaging of neuronal connections in the macaque monkey. Neuron. 2002;34(5):685–700. doi: 10.1016/s0896-6273(02)00718-3. [DOI] [PubMed] [Google Scholar]
- Sandi C, Touyarot K. Mid-life stress and cognitive deficits during early aging in rats: individual differences and hippocampal correlates. Neurobiol Aging. 2006;27(1):128–140. doi: 10.1016/j.neurobiolaging.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10(9):2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J, Pandya J. Fiber pathways of the brain. Oxford UP; New York: 2006. [Google Scholar]
- Schubert MI, Kalisch R, Sotiropoulos I, Catania C, Sousa N, Almeida OF, Auer DP. Effects of altered corticosteroid milieu on rat hippocampal neurochemistry and structure--an in vivo magnetic resonance spectroscopy and imaging study. J Psychiatr Res. 2008;42(11):902–912. doi: 10.1016/j.jpsychires.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Lubach GR, Coe CL. Identifying immune traits and biobehavioral correlates: generalizability and reliability of immune responses in rhesus macaques. Brain Behav Immun. 2006;20(4):349–358. doi: 10.1016/j.bbi.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19(7):2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KK, Jang DP, Lee S, Kim M, Lee SY, Kim YB, Park CW, Cho ZH. Neural responses in rat brain during acute immobilization stress: a [F-18]FDG micro PET imaging study. NeuroImage. 2009;44(3):1074–1080. doi: 10.1016/j.neuroimage.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Mann T, Vinas D, Hunger JM, Dejager J, Taylor SE. Low calorie dieting increases cortisol. Psychosom Med. 2010;72(4):357–64. doi: 10.1097/PSY.0b013e3181d9523c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, Coe C, Levine S. Behavioral and adrenocorticoid responsiveness of squirrel monkeys to a live snake: Is flight necessarily stressful? Behav Neural Biol. 1981;32(4):391–405. doi: 10.1016/s0163-1047(81)90826-8. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Persp Psych Sci. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11(3):457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behav Immun. 2007;21(6):807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, McLaren DG, Canu E, Kastman EK, Kosmatka KJ, Xu G, Field AS, Alexander AL, Colman RJ, Weindruch RH, Coe CL, Johnson SC. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. NeuroImage. 2010;51(3):987–94. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005;30(1):11–17. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med. 2007a;69(1):47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007b;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Zou K, Deng W, Li T, Zhang B, Jiang L, Huang C, Sun X, Sun X. Changes of brain morphometry in first-episode, drug-naive, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol Psychiatry. 2010;67(2):186–188. doi: 10.1016/j.biopsych.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The linear relationship between more stress reactivity and less microstructural tissue density (i.e., higher MD). A representative voxel illustrates the protective effect of CR. Numbers below images correspond to the distance (mm) from the origin located at ear bar zero. The ‘purple-orange’ and green color schemes respectively represent areas that showed significant association and interaction relationships. Color bars represent t-values. Brains are oriented in neurological space. Please refer to the internet version of this article for interpretations of color in this figure.