Abstract
Gonorrhea, a sexually transmitted disease caused by Neisseria gonorrhoeae, is an important cause of morbidity worldwide. A safe and effective vaccine against gonorrhea is needed because of emerging resistance of gonococci to almost every class of antibiotic. A gonococcal lipooligosaccharide (LOS) epitope defined by the monoclonal antibody (mAb), 2C7, is being evaluated as a candidate for development of an antibody-based vaccine. Immune antibodies against N. gonorrhoeae need to overcome several subversive mechanisms whereby gonococcus evades complement, including binding to C4b-binding protein (C4BP; classical pathway inhibitor) and factor H (alternative pathway [AP] inhibitor). The role of AP recruitment and in particular properdin in assisting killing of gonococci by specific antibodies is the subject of this study. We show that only those gonococcal strains that bind C4BP require properdin for killing by 2C7, whereas strains that do not bind C4BP are efficiently killed by 2C7 even when AP function is blocked. C3 deposition on bacteria mirrored killing. Recruitment of the AP by mAb 2C7, as measured by factor B binding, occurred in a properdin-dependent manner. These findings were confirmed using isogenic mutant strains that differed in their ability to bind to C4BP. Immune human serum that contained bactericidal antibodies directed against the 2C7 LOS epitope as well as murine anti-gonococcal antiserum, required functional properdin to kill C4BP binding strains, but not C4BP non-binding strains. Collectively, these data point to an important role for properdin in facilitating immune antibody-mediated complement-dependent killing of gonococcal strains that inhibit the classical pathway by recruiting C4BP.
Keywords: Neisseria gonorrhoeae, complement, properdin, C4b-binding protein, serum bactericidal activity
Introduction
Neisseria gonorrhoeae (the gonococcus) is the causative agent of gonorrhea, a sexually transmitted disease that causes considerable morbidity worldwide. In 2009, 301,174 cases of gonorrhea were reported in the United States (http://www.cdc.gov/std/stats09/gonorrhea.htm), although the Centers for Disease Control and Prevention (CDC) estimates that the true incidence may be about twice the number of reported cases (1). Gonorrhea elicits a wide variety of clinical syndromes at local genital sites, ranging from uncomplicated lower genital tract infection (cervicitis and urethritis) to upper tract endometritis and salpingitis in women and occasionally epididymitis in men. In some instances, N. gonorrhoeae disseminate causing tenosynovitis, septic arthritis, and/or papulo-pustular skin lesions (2–5). Over the years, N. gonorrhoeae has demonstrated a remarkable capacity to develop resistance to almost every class of antibiotic used to treat infection (6). Thus, there is an urgent need to develop a safe and effective vaccine against this disease.
Prior efforts to develop vaccines against N. gonorrhoeae have not come to fruition because the antigens selected showed extreme variability across strains as in the case of pilin (Pil) and porin (Por), which may limit protection to a limited repertoire of strains (7–8). Antibodies directed against outer membrane proteins following natural infection, such as opacity protein (Opa) and porin B (PorB) may provide protection against homologous or closely related strains but again, antigenic variability of these proteins preclude eliciting cross-protective antibodies against diverse strains (9–11). In contrast, antibodies elicited against antigenically conserved targets on N. gonorrhoeae such as reduction modifiable protein (Rmp) are often either non-bactericidal or even subversive (‘blocking’ antibodies) (12–16).
Lipooligosaccharide (LOS) is an important gonococcal virulence factor and plays a key role in several aspects of gonococcal pathogenesis including but not limited to resistance to complement, adhesion and entry into cells and recognition by the innate immune system (17–26). Antibodies directed against LOS activate the complement system that result in direct killing of N. gonorrhoeae (27) which has prompted efforts to evaluate LOS as a vaccine candidate. Antibodies against LOS may protect against re-infection with the homologous strain as suggested in the male urethral experimental gonococcal infection model; those males who showed a 4-fold or greater rise in anti-LOS IgG were more resistant to re-infection with the homologous strain than volunteers who did not mount an anti-LOS response (28). Prior work in our laboratory has identified an LOS epitope that is recognized by a bactericidal monoclonal antibody (mAb) called 2C7 (29) as a potential vaccine candidate and have prompted efforts to develop a mimitopes of the 2C7 LOS epitope as an approach to circumvent the potential toxicity of the LOS molecule (30–31).
The complement system forms an important line of defense against Neisserial infections. The complement cascade comprises three major pathways called the classical, lectin and alternative pathways, all of which converge at the level of C3 (32–33). While an intact classical pathway of complement is required for complement-dependent killing of N. gonorrhoeae (34–35), the role of the alternative pathway, in particular properdin, in facilitating killing by specific antibodies remains unclear. Properdin functions as a positive regulator of the alternative pathway by virtue of its ability to stabilize C3 convertase (C3b,Bb) and prolong its half-life from ~1.5 min by 5- to 10-fold (36). The secondary granules of polymorphonuclear neutrophils (PMNs) are the principal reservoirs of properdin (37). Cervical secretions contain an intact complement system (38), the result of local synthesis of alternative pathway proteins by cervical epithelial cells (39) and exudation of serum components into the cervical lumen (40). A requirement for properdin in bacterial killing could suggest an important role for PMNs in determining the efficacy and efficiency of complement activation and killing of gonococci.
In this study, we sought to examine the role of the alternative pathway and properdin in determining the bactericidal efficacy of antibodies directed again the 2C7 epitope, which is present on diverse gonococcal strains. Studies used a murine monoclonal antibody directed against the 2C7 epitope and were also performed using specific immune human serum derived from a gonococcal vaccine trial that contained excess antibody against the 2C7 epitope.
Materials and Methods
Strains
Strains used in this study were 15253 (41–42), 252 (40) and 442089 (43–44), all of which express porin (Por) B.1A molecules and strains FA1090 (45) and 24-1(46) (both express PorB.1B molecules). N. gonorrhoeae strains were chosen based on their ability to i) resist killing (100% survival) in 16.7% pooled non-immune normal human serum (NHS) used in this study and ii) be killed ≥50% when 5 µg/ml of mAb 2C7 was added to NHS. With the exception of strain 24-1, all the wild-type strains fulfilled these criteria. 24-1 is sensitive to the bactericidal action of NHS, but develops resistance to complement-mediated killing when grown in media supplemented with 5′ cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA) (47). Resistance of sialylated 24-1 is related to its ability to bind to human factor H and down-regulate the alternative pathway of complement (48). Note that strain 252 is killed by select normal human sera, but none of the individual sera that were pooled in this study as a source of complement had intrinsic bactericidal activity against this strain. A mutant derivative of strain 15253 (lgtG::ermR) called 15253 ΔlgtG that does not bind mAb 2C7 has been described previously (49).
An isogenic mutant derivative of strain 252 that differed only in its PorB molecule called 252/PorFA19 was created. Strain FA6564, a derivative of C4BP-binding and serum resistant strain FA19 (provided by Drs. P. Frederick Sparling and Christopher Elkins [University of North Carolina, Chapel Hill]) had been created by reintroducing the FA19 porB into FA19 background using plasmid pUNCH62 (50). This mutant strain possesses the chloramphenicol resistance marker ~900 bp 3′ to porB. Chromosomal DNA extracted from FA6564 was used to transform strain 252 and transformants were selected on GC agar supplemented with 2 µg/ml chloramphenicol. porB genes of transformants were sequenced and two clones that: i) contained the entire FA19 porB sequence and ii) also bound to mAb 2C7 were selected for further study.
Sera and complement reagents
Sera obtained from three healthy adult human volunteers (normal human serum; NHS) were pooled, aliquoted and stored at −80° C until used. The use of these sera was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical School. All subjects who donated blood for this study provided written informed consent. None of the sera used individually or pooled, killed any of the gonococcal strains in a serum bactericidal assay that contained 16.7% serum. Further, the addition of 5 µg/ml of mAb 2C7 to 16.7% serum mediated ≥50% killing of test strains as indicated above. Factor B depleted serum, C2 depleted serum, purified factor B and purified C2 were all purchased from Complement Technologies, Inc. (Tyler, TX). In some experiments, complement activity of NHS was destroyed by heating at 56° C for 30 min.
Sera from adult male volunteers immunized with a gonococcal outer membrane preparation enriched for porin (PorB.1B), but also containing 2C7 epitope bearing LOS, taken as part of a 1985 vaccine and challenge trial (51–52) were tested for bactericidal activity against strain15253 and its lgtG deletion mutant (15253 ΔlgtG) that did not express the 2C7 epitope. Four (of seven tested) sera killed the parent but not the mutant strain; one of these sera was selected for use because it displayed the greatest change in antibody titer (from corresponding pre-immunization serum) directed against all the strains used in this study.
In experiments to measure the effects of anti-factor Bb to block alternative pathway activation in zymosan (see below), the classical and lectin pathways of complement were blocked by adding EGTA (10 mM) and MgCl2 (10 mM) to NHS (NHS-Mg/EGTA).
Antibodies
MAb 2C7 (IgG3λ; Refs. (52–53)) is directed against an epitope that comprises at a minimum a lactose extension off HepI and a lactose residue on HepII of LOS. In addition, N-acylation of LOS appears to be required for mAb 2C7 binding (29). mAb 2C7 binding is not affected by HepI glycan extensions beyond the proximal lactose substitution (29).
The function of the alternative pathway in serum was blocked by the addition of an anti-factor Bb mAb (Quidel Corporation; catalog no. A227) used at a final concentration of 125 µg/ml. Properdin function was blocked with an anti-properdin mAb (anti-properdin mAb #1; catalog no. A233) used at a final concentration of 50 µg/ml (54). C7 function in NHS was blocked by adding anti-C7 mAb (Quidel; catalog no. A221) at a concentration of 100 µg/ml. Blockade of C7 function was confirmed by loss of hemolytic activity of NHS as measured using the Total Haemolytic Complement Kit (The Binding Site, Inc., Birmingham, U.K). Azide (0.1%), present in the commercial anti-properdin, anti-factor Bb and anti-C7 mAb preparations, was removed by spin-concentration and dialyzed against PBS/0.1% BSA using a 30kDa cut-off Amicon Ultra-15 centrifugal filter device (Millipore). Polyclonal goat anti-human C3 antiserum was from Complement Technologies and goat polyclonal anti-human factor B was from Quidel Corporation. An antibody preparation directed against a membrane preparation derived from strain FA1090 was kindly provided by Dr. Ann E. Jerse (Uniformed Services University of the Health Sciences, Bethesda, MD).
Flow cytometry
Flow cytometry was used to determine the concentration of anti-Bb mAb required to block C3 deposition on zymosan, using anti-human C3c conjugated to FITC (Biodesign / Meridian Life Science, Saco, ME). C3 deposition on zymosan either in the absence or in the presence of increasing concentrations of anti-factor Bb mAb, was measured using previously described methods (54). Briefly, NHS-Mg/EGTA (final concentration of 15% (v/v)) containing concentrations of anti-factor Bb mAb ranging from 0 to 500 µg/ml was added to 5 × 107 zymosan particles suspended in HBSS containing 10 mM MgCl2 and 10 mM EGTA for 30 min at 37 °C. The final volume of the reaction mixture was 100 µl. Total C3 deposited on zymosan was detected using anti-C3c FITC (reacts with C3b and iC3b). Data were collected on a FACSCalibur instrument (Becton Dickinson, Franklin Lakes, NJ) and analyzed using the FlowJo analysis software program (Version 7.2.4, TreeStar, Ashland, MA).
Serum bactericidal assays
Serum bactericidal assays were performed as described previously (55), with minor modifications. Bacteria that had been harvested from an overnight culture on chocolate agar plates were re-passaged onto fresh chocolate agar and allowed to grow for 6 h at 37 °C in an atmosphere containing 5% CO2. Bacteria were then suspended in HBSS containing 1 mM MgCl2 and 0.15 mM CaCl2 (HBSS++) for use in serum bactericidal assays. Strain 24-1 was sialylated by growth to the exponential phase in gonococcal liquid media supplemented with CMP-NANA (2 µg/ml) as described previously (47). Approximately 2000 CFU of gonococci were incubated with serum (concentration specified for each experiment) that had all complement pathways intact, or where factor B or properdin function had been blocked, either in the presence or absence of mAb 2C7. The final reaction volumes were maintained at 150 µl, with the exception of experiments to measure the rate of bacterial killing where the initial reaction volume was 450 µl. Aliquots of 25 µl of reaction mixtures were plated onto chocolate agar in duplicate at the beginning of the assay (t0) and again after incubation at 37 °C for 30 min (t30). Survival was calculated as the number of viable colonies at t30 relative to t0.
Western blotting
Gonococcal lysates in NuPAGE® LDS Sample Buffer (4×) (Invitrogen) were separated on NuPAGE® 12% Bis-Tris (Invitrogen) gels using Novex® MES running buffer (Invitrogen) followed by transfer to an Immobilon PVDF membrane (Millipore) (56). Membranes were blocked with PBS/1% milk and probed with tissue culture supernatants that contained mAb 2C7. mAb 2C7-reactive LOS bands were disclosed with anti-mouse IgG conjugated to alkaline phosphatase followed by the addition of BCIP®/NBT-Purple Liquid Substrate (Sigma). The specificity of IgG in the immune serum of mice immunized with FA1090 membrane preparations was determined by incubating western blotted membrane preparations and bacterial lysates of FA1090 and 252 with a 1:1000 dilution of the immune serum, followed by disclosing the mouse IgG reactive bands with anti-mouse IgG alkaline phosphatase.
Whole cell ELISA
C3 deposition on, and factor B binding to bacteria were measured by whole cell ELISA as described previously (40, 57–58). Briefly, 2 × 108 organisms in HBSS++ were incubated with NHS (final concentration 16.7%) that contained either no anti-complement components mAbs, or contained anti-C7 alone, or anti-C7 plus either anti-factor Bb or anti-properdin (same concentrations used for bactericidal assays) in a reaction volume of 100 µl and then incubated for 10 min at 37°C. This time point was chosen because maximal killing was observed at 10 min here (see Results) and based on complement deposition on gonococci in previously published data (40). Reactions were stopped after 10 min by washing three times with ice-cold HBSS containing 5 mM phenylmethylsulfonyl fluoride at 4 °C. Organisms were resuspended in 200 µl of the same buffer, and 50 µl of each sample applied per well of a 96-well U-bottomed polystyrene microtiter plate (Dynatech Laboratories, Inc., Chantilly, VA) and incubated for 3 h at 37°C. Plates were washed with PBS containing 0.05% Tween 20. Primary antibodies (goat anti-human C3 and goat anti-human factor B) were diluted in PBS, and secondary antibodies diluted in PBS-0.05% Tween 20 prior to use. To ensure similar capture of bacteria incubated under different conditions, we measured the amount of gonococcal H.8 antigen (59–60) expression using MAb 2-8C-4-1 (58), followed by antimouse IgG-alkaline phosphatase conjugate.
Results
Characterization of mAb 2C7 binding to the selected serum resistant N. gonorrhoeae strains
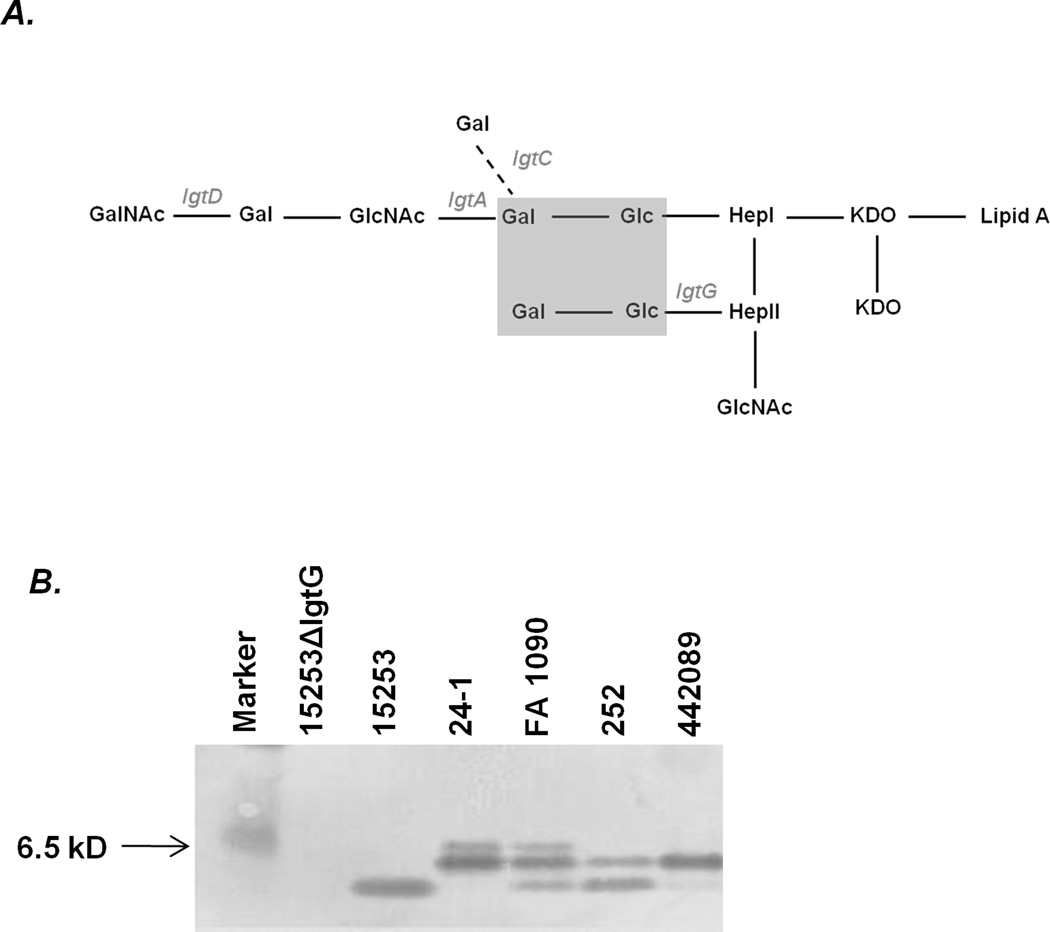
A simplified schematic of the organization of LOS glycans on gonococcal LOS is provided in Fig. 1A. The phase-variable genes involved in gonococcal LOS biosynthesis are indicated in grey italic font. Expression of lactose on both HepI and HepII (indicated by the grey shaded box) is required for mAb 2C7 reactivity with gonococcal LOS (Fig. 1A). Glycan extensions beyond the lactose residue on HepI do not affect 2C7 binding (29). Thus, while expression of LgtG is required for mAb 2C7 binding, phase variation of the lgtA, lgtC and lgtD do not affect 2C7 reactivity. mAb 2C7 reactivity with the LOS of the strains used in this study is shown by western blot in Fig. 1B and as expected, LOS phase variation resulted in variations in the pattern of mAb 2C7-reactive bands across strains. The lgtG deletion mutant of strain 15253 (15253 ΔlgtG) that does not bind to mAb 2C7 was used as a negative control.
Figure 1.
Specificity of mAb 2C7 and 2C7 reactivity against gonococcal strains used in this study. A. A simplified schematic of glycan organization of gonococcal LOS. Lipooligosaccharide glycosyl transferase (lgt) genes that are involved in LOS biosynthesis and are subject to phase variation are indicated in grey italic font. The grey shaded box represents the minimum glycan extensions expressed by naturally occurring gonococcal strains that are required for mAb 2C7 binding. B. Reactivity of mAb 2C7 with the LOS of gonococcal strains used in this study. Bacterial lysates were subjected to denaturing electrophoresis and Western blotting and membranes probed with tissue culture supernatants that contained mAb 2C7. Strain 24-1 was grown in media supplemented with CMP-NANA (2 µg/ml).
mAb 2C7-dependent killing of select serum-resistant strains of N. gonorrhoeae requires properdin
We next asked whether the bactericidal activity of mAb 2C7 required participation of the alternative pathway of complement. In order to maintain activity of the classical (and lectin) pathways, but block activity of the alternative pathway, the function of factor B was blocked with an anti-factor Bb mAb. To determine the concentration of this mAb needed to fully block alternative pathway activation, we measured C3 deposition on zymosan, an alternative pathway activating particle (61–62), in the presence of Mg/EGTA-NHS that contained anti-factor Bb mAb at concentrations ranging from 62 µg/ml to 500 µg/ml. Deposition of C3 was blocked (no increase in fluorescence over baseline) even at anti-factor Bb concentrations as low as 62.5 µg/ml (data not shown). Thus, an anti-factor Bb concentration of 125 µg/ml was chosen to ensure maximal alternative pathway blockade in subsequent experiments. The ability of the anti-properdin mAb (50 µg/ml) to limit C3 deposition on zymosan and on the surface of N. gonorrhoeae has been demonstrated previously (54).
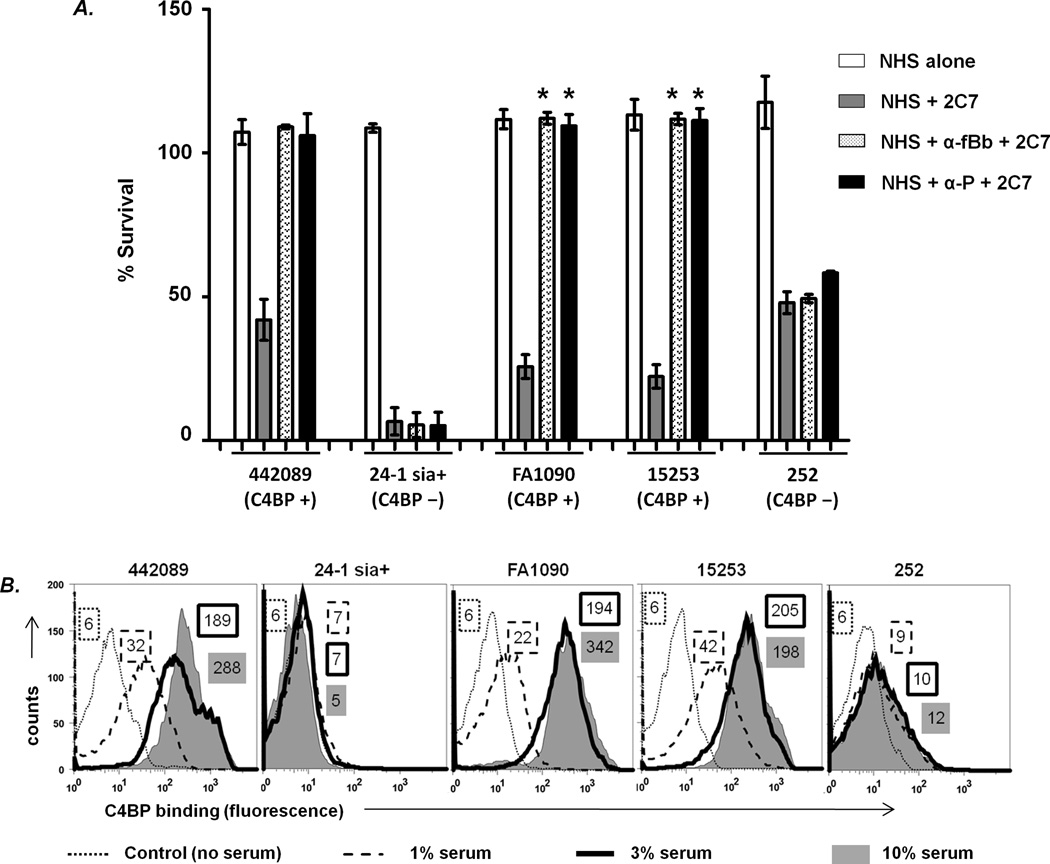
As shown in Fig. 2A, all strains resisted killing by normal human serum (NHS) alone (>100% survival at 30 min), but were killed >50% in the presence of 5 µg/ml of added 2C7. Blocking the alternative pathway with anti-factor Bb resulted in complete (>100%) survival of strains 442089, FA1090 and 15253, the C4BP binding strains, but had little effect on the survival of strains 24-1 sia+ and 252 that did not bind C4BP. These results showed that antibody-mediated killing of some, but not all, serum-resistant strains of N. gonorrhoeae required an intact alternative pathway. Blocking properdin function with anti-properdin (P) yielded similar results as with anti-factor Bb mAb, suggesting that the positive regulatory function of properdin on the alternative pathway was required for antibody-dependent killing of select strains of N. gonorrhoeae (Fig. 2A). The negative control for this experiment included organisms incubated with mAb 2C7 and heat-inactivated NHS (no complement activity), which showed no killing (data not shown). In addition, the mutant strain 15253 ΔlgtG (does not bind to mAb 2C7) was not killed in a bactericidal assay that contained NHS (complement intact) and mAb 2C7 (data not shown).
Figure 2.
The alternative pathway of complement and properdin is required for killing of C4BP-binding strains of N. gonorrhoeae by mAb 2C7. A. Survival of gonococcal strains was measured in serum bactericidal assays in the presence of NHS alone, NHS plus mAb 2C7, or NHS plus mAb 2C7 where either factor B or properdin function was blocked with anti-factor Bb (α-Bb) or anti-properdin (α-P), respectively. Each data point represents the mean (±SD) of at least 2 independently repeated experiments. *, p<0.001 (2-tailed Student’s t-test) compared to the corresponding survival seen with mAb 2C7+NHS. B. C4BP binding to N. gonorrhoeae strains. N. gonorrhoeae strains 442089, 24-1 (LOS sialylated by growth in media containing CMP-NANA; labeled 24-1 sia+), FA1090, 15253, and 252 were incubated with 1%, 3% or 10% NHS and C4BP bound to the bacteria surface was measured by flow cytometry. One representative experiment of 3 independently performed experiments is shown. Numbers represent the average of the median fluorescence of each of the three experiments.
We used factor B depleted serum to confirm the results obtained with anti-factor Bb blocking mAb (Table). Note that the factor B depleted serum alone (without added mAb 2C7) killed strain 252, so this strain could not be tested in this assay. Factor B depleted serum supported significantly greater killing of 24-1 sia+ than the C4BP binding strains (FA1090, 15253 and 442089) by mAb 2C7. Almost identical results were shown using properdin depleted serum instead of factor B depleted serum (24-1 sia+ showed only 9% survival, while C4BP binders FA1090, 15253 and 442089 survived 62%, 69% and 107%, respectively).
Table.
Factor B in killing of N. gonorrhoeae by mAb 2C7
| Strain | % survival [mean (range)] | ||
|---|---|---|---|
| fB-dep/fBA | fB-dep + 2C7 | fB-dep/fB + 2C7 | |
| 24-1 sia+ (C4BP−) | 117 (4) | 6 (0) | 7.3 (2) |
| FA1090 (C4BP+) | 110 (2) | 70 (2) | 18 (2) |
| 15253 (C4BP+) | 105 (2) | 65.5 (3) | 16.8 (6) |
| 442089 (C4BP+) | 107.5 (3) | 107 (6) | 50.5 (3) |
factor B-depleted serum (fB-dep) reconstituted with purified human factor B (200 µg/ml)
An intact classical pathway of complement is required for mAb 2C7-mediated killing
A requirement of the alternative pathway for manifestation of bactericidal activity of mAb 2C7 against select gonococcal strains raised the possibility that antibody-dependent alternative pathway activation (63) may have been fully responsible for killing of bacteria. To address this possibility, strains 24-1 sia+ and FA1090 were incubated with mAb 2C7 in the presence of C2 depleted human serum (classical and lectin pathways blocked, alternative pathway intact). No killing of either strain (>100% survival) was seen. Reconstitution of C2 depleted serum with physiological concentrations of C2 restored killing by mAb 2C7 (92.5% and 83% killing of strains 24-1 sia+ and FA1090, respectively). Controls included C2 depleted serum alone and depleted serum reconstituted with C2; neither control showed killing (>100% survival). To underscore the importance of an intact classical pathway in mediating killing by specific antibody, we tested wild-type strain 24-1(unsialylated) that is extremely sensitive to killing by normal human serum (46–47)). Incubating this strain with mAb 2C7 and C2 depleted serum together also resulted in >100% survival. As expected, killing was restored (0% survival) when purified C2 was added to the reaction mixture. These data suggest that an intact classical pathway is required for 2C7-dependent bactericidal activity; recruitment of the alternative pathway alone is insufficient to support gonococcal killing under these conditions.
Strains that require properdin for mAb 2C7-dependent killing bind the classical pathway inhibitor, C4BP
Direct complement-dependent killing of N. gonorrhoeae requires an intact classical pathway (34–35). Active inhibition of the classical pathway, as occurs on strains that bind to the classical pathway inhibitor, C4b-binding protein (C4BP) (43), may reduce the ability of the classical pathway alone to support bactericidal activity although small amounts of active C3b deposited on organisms are sufficient to amplify the alternative pathway. We reasoned, therefore, that killing of C4BP-binding strains may require participation of the alternative pathway. Properdin is critical for stabilization of the positive feedback loop of the alternative pathway and therefore is likely to be critical for killing. On the other hand, antibody-dependent killing, via the classical pathway, of strains that do not bind to C4BP should be unimpeded. We speculated that strains that bound C4BP were killed by mAb 2C7 via the alternative pathway, which required properdin. C4BP binding to strains 15253, 442089 and FA1090 in 1%, 3% and 10% human serum (Fig. 2B and Ref (64)) correlated with a properdin requirement for 2C7-mediated killing of these strains (Fig. 2A), while strains 252 and 24-1 sia+ that bound C4BP minimally or not at all were killed in the absence of properdin (Fig. 2A)
Properdin does not alter the rate of killing by mAb 2C7
Although 2C7-mediated killing of strains 252 and 24-1 sia+ at 30 min in the presence or absence of properdin was similar, we asked whether properdin influenced the rate of bacterial killing as may be expected if the alternative pathway was amplifying antibody-dependent Neisserial killing to a significant extent (65). Blocking properdin function did not alter the kinetics of killing of strain 252 by mAb 2C7. Both in the presence and absence of properdin, bactericidal activity was almost complete within 10 min, suggesting that properdin did not augment the rate of killing of a strain that bound minimal levels of C4BP (data not shown) and that bacterial killing of these strains was less influenced by the alternative pathway.
Maximal C3 deposition on C4BP-binding gonococci requires an intact alternative pathway
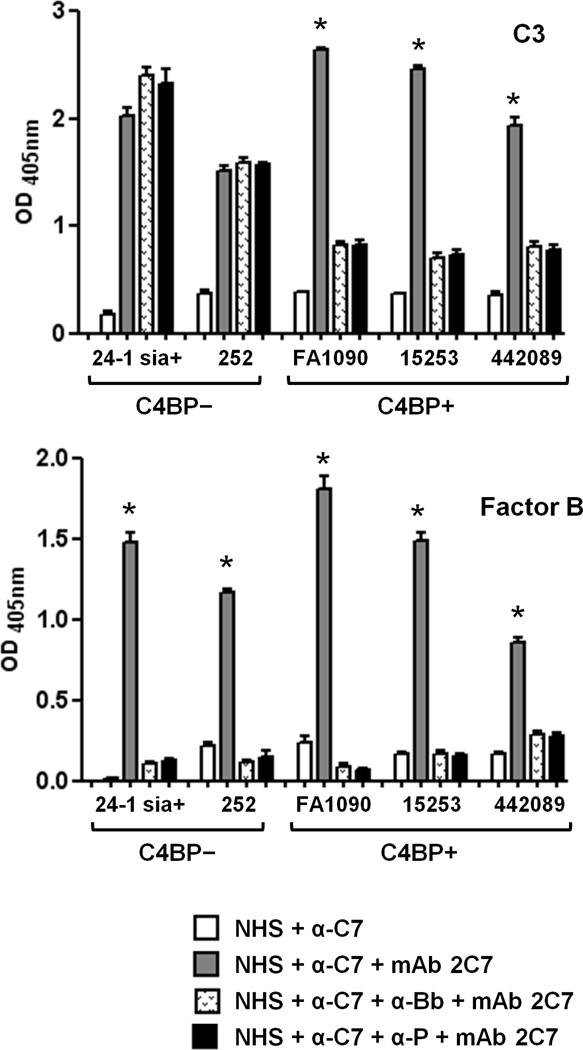
Having shown that killing of C4BP-binding N. gonorrhoeae by mAb 2C7 required properdin and an otherwise intact alternative pathway, we next determined the roles for factor B and properdin in C3 fragment deposition on, and factor Bb binding to gonococci by whole cell ELISA. A 10 min incubation period was chosen based on the killing kinetics presented above and our previous studies of complement activation on N. gonorrhoeae (40). In these experiments, a function-blocking anti-C7 mAb was added to NHS to avoid a possible effect from bacterial killing and membrane lysis. When compared with NHS, the addition of anti-C7 to NHS did not affect C3 or factor Bb binding to bacteria; therefore only data with NHS plus anti-C7 are shown for simplicity. Shown in Fig. 3 (upper graph) and in accordance with the results of bactericidal assays presented in Fig. 2A, blocking factor B or properdin function did not affect mAb 2C7-mediated C3 deposition on the C4BP non-binding strains 24-1 sia+ and 252, but significantly reduced mAb 2C7-dependent C3 deposition on the C4BP binders (FA1090, 15253 and 442089). Controls where bacteria were incubated with heat-inactivated NHS or with buffer alone showed OD405nm readings <0.1 (data not shown).
Figure 3.
C3 deposition on, and factor B binding to N. gonorrhoeae. Bacterial strains were incubated with NHS that contained anti-C7 to prevent bacterial lysis, either in the presence or absence of mAb 2C7. Anti-factor Bb or anti-properdin mAbs to block function of these components was added to some reactions as indicated. C3 deposition on (upper graph) and factor B binding to (lower graph) bacteria was measured by whole cell ELISA. * indicates p≤0.001 compared to all other data points for the corresponding strain (2-tailed Student’s t test). C3 and factor B binding when bacteria were incubated with NHS alone were similar to results obtained when organisms were incubated with NHS + anti-C7 mAb (data not shown). Gonococcal H.8 antigen measured using mAb 2-8C-4-1 was similar across wells and ensured equal bacterial capture (data not shown). Controls where bacteria were incubated with heat-inactivated NHS showed OD405nm readings <0.1 and have been omitted for simplicity.
Addition of mAb 2C7 to NHS resulted in a marked increase in factor Bb binding to all strains (Fig. 3, lower graph), suggesting that this antibody recruited the alternative pathway on all strains even though only C4BP binding strains required the alternative pathway for killing (Fig. 2A.). Of note, blocking properdin function resulted in almost complete loss of detectable factor Bb binding to all strains to levels comparable those seen when factor B function was blocked, suggesting a key role for properdin in alternative pathway recruitment by mAb 2C7.
Collectively, these data suggest that unimpeded classical pathway activation on C4BP non-binders was sufficient for maximal C3 deposition and killing of these strains even in the absence of alternative pathway activation (factor B function blocked) or when the alternative pathway lacked function of its positive regulator, properdin. In contrast, C4BP binding gonococcal isolates required an intact alternative pathway for mAb 2C7-mediated killing. Further, properdin played a critical role in mAb 2C7-mediated recruitment of the alternative pathway and assembly of C3 convertases on gonococci.
Isogenic mutant strains confirm the association between C4BP binding and the requirement of properdin for antibody-dependent killing
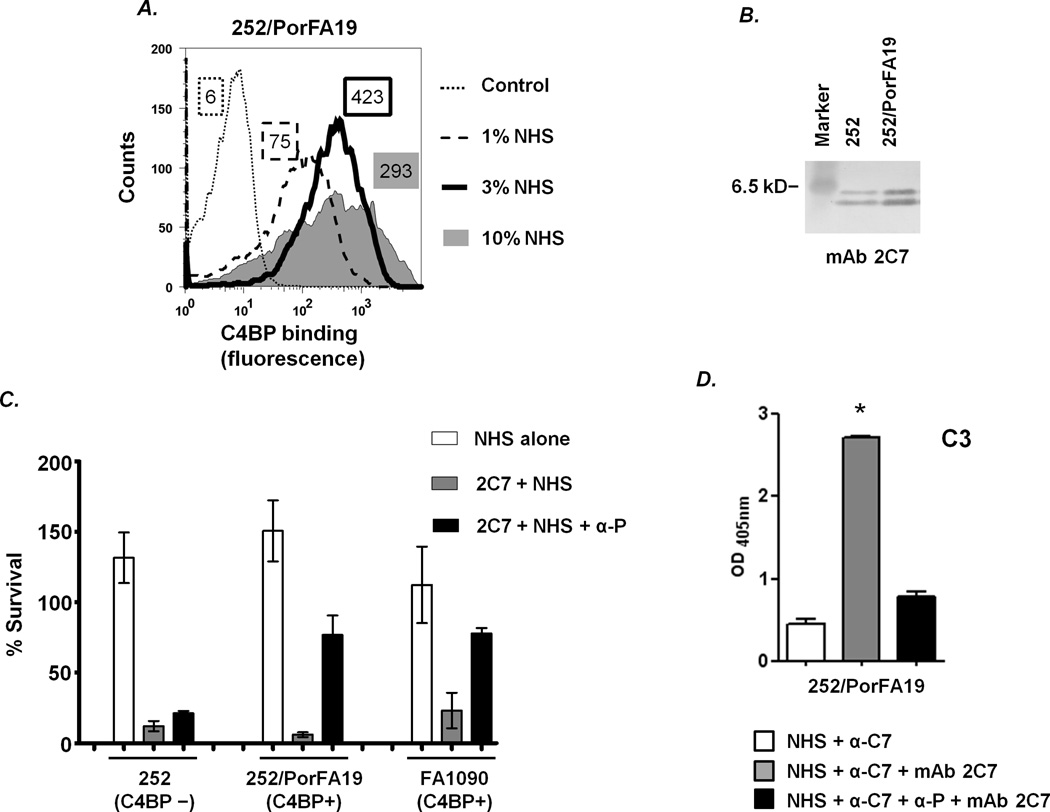
In addition to their ability to bind to complement inhibitors, additional factors such as LOS structure can modulate serum resistance of N. gonorrhoeae (22–23, 66). To provide further evidence linking C4BP binding to the requirement for properdin for antibody-mediated killing, we created isogenic mutants that differed in their ability to bind C4BP by replacing the PorB.1A molecule in strain 252 (binds C4BP minimally (Fig. 2B)), with the PorB.1A molecule of strain FA19 that binds C4BP strongly (43). The resulting mutant, called 252/PorFA19 showed no change in the 2C7 binding profile compared to the parent strain, 252 (Fig. 4B). As expected, the 252/PorFA19 mutant strain also bound C4BP (Fig. 4A). Shown in Fig. 4C, C4BP binding to strain 252/PorFA19 resulted in resistance to killing by mAb 2C7 when properdin function was blocked. A second 252/PorFA19 clone yielded similar results (data not shown). Similar to results seen with the anti-properdin mAb, blocking factor B function with the anti-Bb mAb yielded a mean bacterial survival of 66% of strain 252/PorFA19 (results omitted from the graph for simplicity) thereby demonstrating the absolute need for properdin for recruitment of the alternative pathway and killing of C4BP-binding gonococci. Similar to strain 252/PorFA19, the C4BP binding strain FA1090, used as a control, showed restored survival when properdin function was blocked, while mAb 2C7-mediated killing of the C4BP non-binding parent strain 252, was unaffected by loss of properdin function (Fig. 4C). The effects of blocking factor B on survival of control strains 252 and FA1090 under similar conditions are shown in Fig. 2A. C3 deposition on strain 252/PorFA19, measured by whole cell ELISA, showed that blocking properdin function resulted in a significant decrease in C3 deposition on the organism (Fig. 4D). This stood in contrast to the parent strain 252, where the total amount of C3 deposition was not affected by blockade of the alternative pathway. Blocking factor B function reduced C3 deposition to levels similar to those seen when properdin function was blocked (mean (±SD) OD405nm 0.8 (±0.05)).
Figure 4.
Restoring C4BP binding to N. gonorrhoeae is associated with a properdin requirement for killing by mAb 2C7. The PorB.1A molecule of strain 252 (binds C4BP minimally) was replaced with the PorB.1A from strain FA19 (strong C4BP binder; Ref. (43)) to yield mutant strain 252/PorFA19. A. Binding of C4BP to 252/PorFA19. Bacteria were incubated with increasing concentrations of NHS as indicated and bound C4BP was measured by flow cytometry. Deficient C4BP binding to parent strain 252 incubated with 10% NHS is shown as the Control. Numbers adjacent to each histogram represent the average of the median fluorescence of at least two independently performed experiments. B. 2C7 epitope expression by the LOS of mutant strain 252/PorFA19. Western blotting was performed as described in Fig 1A. Parent strain 252 was used as a control. C. Functional properdin is required for 2C7-dependent killing of C4BP-binding isogenic mutant strain 252/PorFA19. Bacteria were incubated with NHS alone, 2C7 + NHS or 2C7 + NHS + α-P (anti-properdin mAb). Bacterial survival following incubation of the reaction mixture for 30 min at 37 °C was measured in a serum bactericidal assay. Controls included parent strain 252 (binds C4BP minimally) and strain FA1090 (strong C4BP binder). D. C3 deposition on 252/PorFA19. Bacteria were incubated with NHS containing anti-C7, either in the presence or absence of mAb 2C7. Where indicated, properdin function was blocked with an anti-properdin mAb. *, p<0.001 (2-tailed Student’s t test).
Taken together, these results strongly suggest that killing of N. gonorrhoeae strains that bind to C4BP by specific anti-LOS antibody requires participation of the alternative pathway. Further, properdin plays a key role in facilitating alternative pathway recruitment to mediate bactericidal activity.
Requirement for properdin for killing by human antibody elicited by vaccination with a gonococcal vaccine preparation
The studies described above were performed with a murine bactericidal mAb against LOS. To address the applicability of the results to human immune antibody against LOS, we asked whether properdin was required for killing by immune serum obtained from a human volunteer, administered a gonococcal outer membrane-derived vaccine candidate. The vaccine candidate used was enriched with PorB.1B, derived from a gonococcal strain called 2399, but the candidate also contained other components of the outer membrane that were immunogenic including LOS (51–52). The PorB.1B antibody response was restricted to that of the homologous strain (2399) used to prepare the vaccine candidate; no cross-reacting PorB.1B antibodies to any of the PorB antigens present in the strains used in this study were detected by Western blotting (data not shown). However, in several vaccinees, the resultant bactericidal antibody response was directed principally against the LOS derived 2C7 epitope.
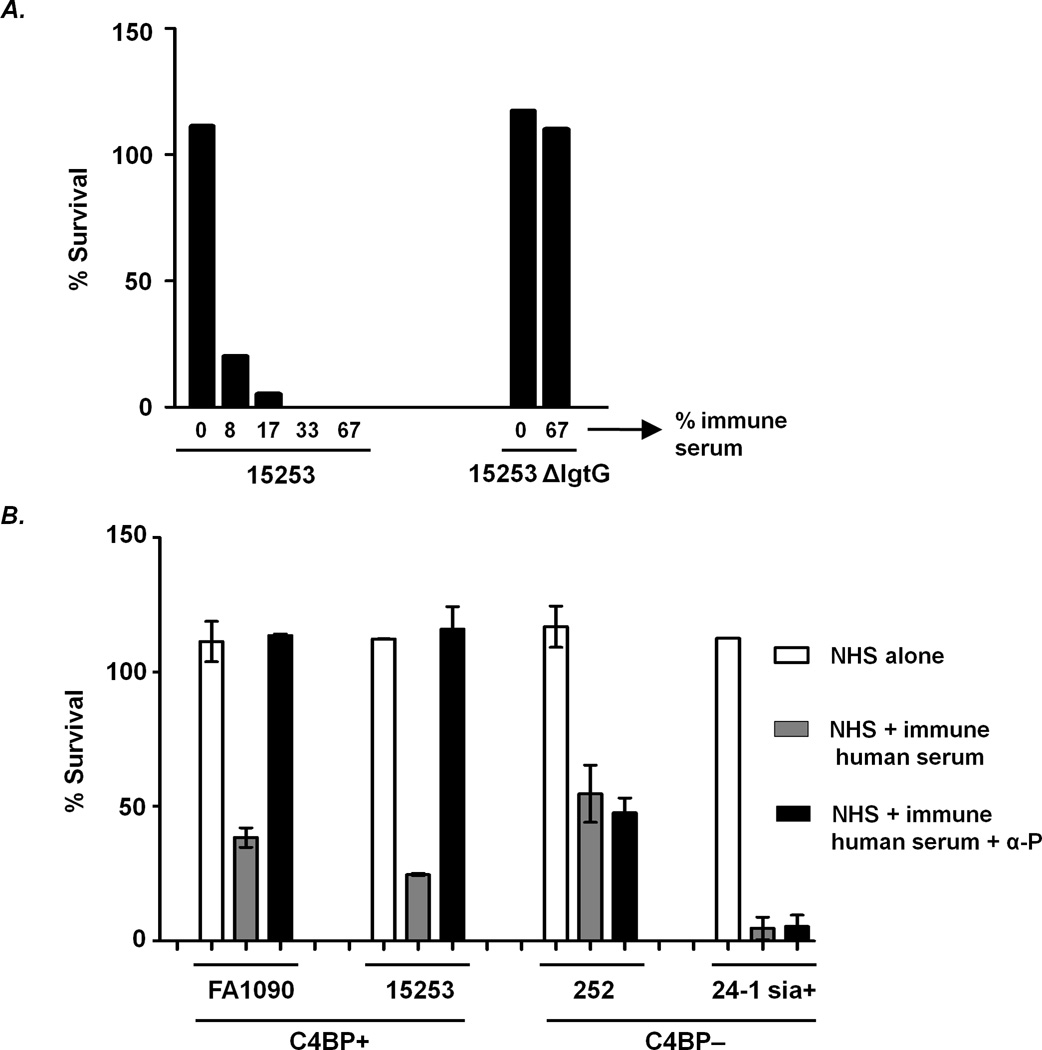
We used such a vaccinee serum here (2C7 epitope-specific antibody was 3.61 µg/ml, which was 91% of the total LOS antibody (3.95 µg/ml); Ref. (52)), where bactericidal activity depended solely on LgtG expression of the target organism as evidenced by complete killing (no survival) of 15253, but no killing (≥100% survival) of 15253 ΔlgtG (shown in Fig. 5A).
Figure 5.
Properdin is required for killing of C4BP-binding strains of N. gonorrhoeae by immune human antibody directed against the 2C7 LOS epitope. A. Bactericidal activity of gonococcal vaccine-elicited human immune serum (directed against the 2C7 LOS epitope) requires expression of lactose on HepII (Fig. 1A). Strain 15253 and its isogenic lgtG deletion mutant (15253 ΔlgtG) were incubated with NHS alone (indicated as 0 on the graph) as a constant source of complement and with increasing concentrations of heat-inactivated vaccine-elicited human immune serum (complement inactivated, but antibodies intact); bacterial survival following a 30 min incubation period was measured. B. Properdin-dependent killing of C4BP-binding N. gonorrhoeae by vaccine-elicited human immune serum. C4BP-binding strains FA1090 and 15253, and minimal C4BP-binding strains 252 and sialylated 24-1 (24-1 sia+), were incubated with NHS alone, NHS + immune human serum (final concentration 6.7%) or NHS + immune human serum + α -P (anti-properdin mAb). Bacterial survival was measured following incubation of reaction mixtures for 30 min at 37 °C. Each data point represents the mean (±SD) of 2 separate experiments.
Consistent with the presence of 2C7 epitope-specific bactericidal antibodies, the vaccinee serum also killed strains FA1090, 252 and sialylated 24-1 (sia+), all displaying the 2C7 epitope (Figs. 1B and 5B). In accordance with results using mAb 2C7, the immune human serum also required functional properdin to kill strains FA1090 and 15253 and (both bound C4BP), but not 252 or 24-1 sia+ (bound C4BP minimally or not at all (Fig. 5B)). Survival of 252 and 24-1 sia+ in the presence of the human immune serum when factor B function was blocked (no alternative pathway activity) were similar to results seen when properdin was blocked (mean survival of 252 and 24-1 sia+ in 2 independent experiments were 48.5% and 1.6%, respectively, demonstrating that killing of the C4BP non-binders by specific human anti-LOS antibody did not require any input from the alternative pathway. Survival of the C4BP binding strains ≥100% when only the positive regulatory function of the alternative pathway was blocked with anti-properdin obviated the need for testing survival of these strains when the entire pathway was rendered non-functional with anti-Bb.
Properdin is required for maximal bactericidal activity against C4BP-binding gonococci of a polyclonal murine anti-OMV antibody
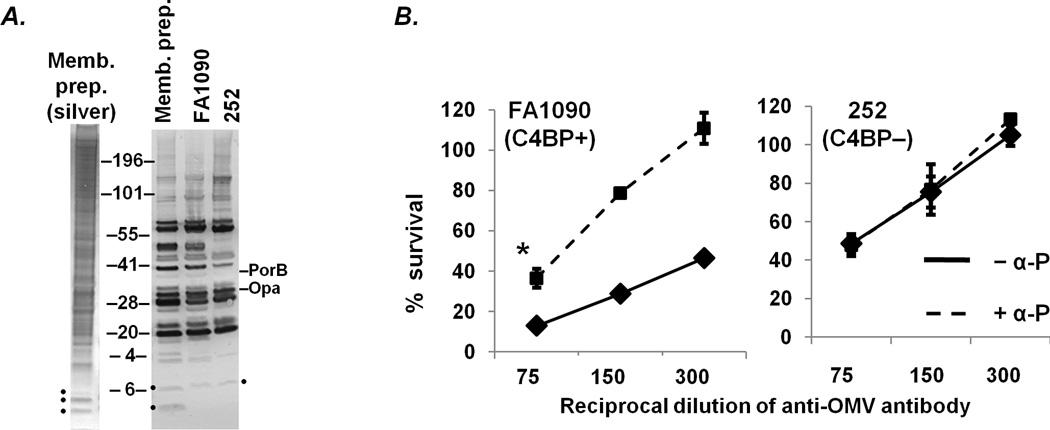
The data thus far have focused on the requirement of properdin for the bactericidal activity of anti-LOS antibodies, in particular antibodies directed against the 2C7 epitope. To determine whether properdin was required for killing activity of antibodies directed at potential bactericidal antibody-reactive targets other than LOS, we tested complement-dependent killing mediated by a murine polyclonal antibody raised against a membrane preparation of FA1090 (Fig. 6A shows a silver stain of the immunogen). The broad cross-reactivity of the elicited murine antibodies against the membrane preparation as well as whole cell lysates of FA1090 and 252 was determined by Western blot, shown in Fig. 6A. The antiserum reacted strongly with a variety of proteins, but only weakly against LOS. In the presence of an intact complement cascade, the polyclonal anti-membrane antibody was bactericidal against strain FA1090 (the homologous strain) and to a similar extent against heterologous strain 252 (solid lines, Fig. 5). Akin to observations with anti-LOS antibodies, the addition of the anti-properdin mAb to block properdin function resulted in increased survival of C4BP binding strain FA1090 (left-hand panel, Fig. 5B), but had no effect on survival of 252 (right-hand panel, Fig. 5B). Thus, the critical role for properdin in maximizing antibody-mediated bactericidal activity against C4BP-binding gonococcal strains likely extends to antigens other than LOS.
Figure 6.
Killing of C4BP-binding gonococcal strain FA1090 by anti-OMV antibody requires functional properdin. A. Broad cross-reactivity of murine antiserum raised against a FA1090 membrane preparation (“Memb. prep.”). The membrane preparation that was used as the immunogen (a silver stain of the same is also shown) and whole cell lysates of FA1090 and 252 were separated on a 4–12% Bis-Tris gel, transferred to a PVDF membrane that was then overlaid with a 1:1000 dilution of anti-FA1090 OMV in PBS/0.05% Tween 20. Mouse IgG bound to bacterial antigens was disclosed with anti-mouse IgG alkaline phosphatase and substrate. Localization of IgG- reactive PorB.1B (faintly visualized only in the membrane preparation and FA1090 lanes) and Opacity protein (Opa) bands are indicated. LOS and LOS-reactive IgG bands are indicated with a solid black dots. B. Murine anti-OMV serum antibody (dilutions ranging from 1/75 to 1/300) mediates complement-dependent killing of strains FA1090 (left panel) and 252 (right panel) in the presence (dashed lines) or absence (solid lines) of α-P (anti-properdin mAb) that blocks function of properdin; 16.7% NHS was used as a source of human complement. The 1/75 dilution experiment represents the mean (±SD) of 3 separately performed experiments; the 1/150 and 1/300 dilution experiments, the mean (±SD) of 2 experiments. *, p<0.005 (2-tailed Student’s t-test).
Discussion
In addition to its pivotal role in protection against Neisserial infections (67), complement also plays an important role in innate immune defenses against several other bacterial pathogens. Over 50 years ago, Roantree and Rantz reported that gram-negative bacteria isolated from the bloodstream were almost always resistant to the killing activity of complement (68). Defects in antibody and/or early components of the classical pathway that impair opsonophagocytosis predispose to recurrent respiratory infections and sepsis caused by bacteria such as Haemophilus influenzae and Streptococcus pneumoniae (69–70). The link between deficiencies of the alternative and terminal pathways of complement and recurrent, disseminated meningococcal disease is well established (69–70).
Previous studies have shown that the classical pathway of complement is essential to mediate complement-dependent killing of N. gonorrhoeae (35, 63). Gonococcal strains that are otherwise sensitive to killing by non-immune normal human serum (all pathways of complement intact) survive when the classical (and lectin) pathway is blocked with Mg/EGTA-NHS (to selectively chelate Ca2+) or with serum that lacks C1q (35), while permitting activation of the alternative pathway (34, 63). However, such strains can be killed when the alternative pathway alone is selectively blocked, as with factor B-depleted serum (35). N. gonorrhoeae have evolved several mechanisms to resist killing by human complement. Effective strategies used by N. gonorrhoeae to escape killing by complement include binding to human complement inhibitors such as factor H and C4BP through their PorB molecule (43–44). Sialylation of gonococcal LOS that expresses the lacto-N-neotetraose structure also enhances factor H binding to bacteria.
The rapid and widespread development of antibiotic resistance by N. gonorrhoeae (6, 71) has severely limited therapeutic options and there is an urgent need to develop safe and effective vaccines that prevent gonococcal carriage and disease. Antibody-based vaccines against N. gonorrhoeae could depend on efficient complement activation to either mediate direct killing through membrane attack complex insertion or promote opsonophagocytosis in order to protect the host. To effectively activate complement on the bacterial surface, vaccine induced antibodies need to overcome the complement inhibitory effects of molecules such as factor H and C4BP that bind to the gonococcal surface. mAb 2C7 kills gonococci that inherently resist killing by complement (Fig. 2A and Ref. (52)) via any of several mechanisms (31, 52) and is therefore an attractive vaccine candidate. Antibodies elicited by immunization of mice with a peptide that mimics the 2C7 epitope, bind the 2C7 epitope on gonococcal LOS and kill gonococci whose LOS displays the epitope (31). Binding of mAb 2C7 to gonococcal LOS requires expression of LgtG, which is encoded by lgtG that contains a poly-C tract and may therefore be subject to phase variation (49). Nevertheless, over 90% of minimally passaged clinical isolates of N. gonorrhoeae bind to 2C7 (72) suggesting an important, but undefined role for lactose substitution of the HepII chain (a requirement for 2C7 epitope expression (29)) of gonococcal LOS.
A novel finding in this study was the requirement of the alternative pathway and in particular properdin for specific antibody-mediated complement-dependent killing of N. gonorrhoeae strains that bind C4BP. Strains that bind C4BP and limit classical pathway activation require recruitment of the alternative pathway to assemble sufficient C3/C5 convertases for subsequent membrane attack complex (C5b-9) and bacterial killing. In the absence of the alternative pathway or when properdin is blocked, mAb 2C7 deposits only a small amount of C3 on strains such as FA1090, 442089 and 15253 because these strains actively regulate the classical pathway through recruitment of C4BP to their surfaces. However, an intact alternative pathway appears to effectively amplify the C3b that ‘breaks through’ the barrier posed by C4BP and effect bacterial killing. The stabilizing effects of properdin on the alternative pathway C3 convertases are crucial to amplify C3 deposition and mediate bacterial killing. On the other hand, strains that resist complement by mechanisms independent of C4BP binding and do not actively cleave C4b or dissociate the classical pathway convertase (C4b,2b according to a newly proposed nomenclature (32)), do not require properdin for specific antibody-mediated bactericidal activity.
Importantly, the role of properdin in facilitating antibody-dependent killing was not restricted to murine mAb 2C7 but also extended to specific immune human serum that contained antibodies directed against the 2C7-specific LOS epitope. These findings have implications for antibody-based vaccine development because PMNs are a major reservoir and source of properdin (37). In addition, properdin is synthesized by primary cervical epithelial cells (39) and would also be exuded into the cervical lumen from serum. The concomitant presence of neutrophils is likely to ensure high levels of properdin that may enhance complement activation on gonococci. Enhanced complement activation in the vicinity of PMNs may also facilitate opsonophagocytic disposition of organisms. As shown by Braconier et al (73), phagocytic killing was reduced in Streptococcus pneumoniae serotype 23F due to defective opsonization in the presence of P-deficient sera. Addition of native properdin to the P-deficient sera restored opsonization of S. pneumoniae serotype 23F by human granulocytes (73) showing the significance of properdin in phagocytosis. Indeed, a recent report showed that PMNs stimulated by cytokines such as TNF-α activate the alternative pathway of complement and result in C3 fragment deposition on these cells (74). Properdin secreted by PMNs was detected on cell surfaces and it was proposed that the cell-bound properdin could serve as a focus for additional activation of the alternative pathway. Complement activation in turn resulted in further activation of PMNs, which was accompanied by an increases in CD11b expression and the oxidative burst (74). The presence of classical pathway activation by specific antibodies, coupled with this “positive feedback loop” involving PMNs and the alternative pathway could prove important in curtailing gonococcal infection by vaccine-elicited antibody.
In conclusion, we have demonstrated that both human and murine antibodies that are directed against an LOS epitope that is currently being investigated as a potential vaccine candidate (31), as well as specific anti-OMV antibodies more generally, require a functional alternative pathway and in particular properdin, to kill serum-resistant gonococcal strains that bind to the complement inhibitor C4BP. Strains that do not bind to C4BP are efficiently killed by specific antibodies via the classical pathway alone and do not require properdin. These findings provide insights into how complement is activated on N. gonorrhoeae by specific antibody and also shed light on the requirements for effective complement-dependent bactericidal activity by potential vaccine candidates.
Acknowledgments
We greatly appreciate Dr. Anna M. Blom (Lund University, Malmo, Sweden) for the gift of anti-C4BP mAb 104; Drs. P. Frederick Sparling and Christopher Elkins (University of North Carolina, Chapel Hill) for strain FA6564; Dr. Ann E. Jerse and Dr. Abdul Khan (Uniformed Services University of the Health Sciences, Bethesda, MD) for FA1090 membrane preparation and murine antibodies directed against the membrane preparation; Edward W. Hook III (University of Alabama, Birmingham, AL) and Peter K. Kohl (Vivantes-Klinikum Neukölln, Berlin, Germany) for providing vaccinee serum. We would also like to thank Nancy Nowak for excellent technical assistance.
Abbreviations used in this paper
- AP
alternative pathway
- NHS
normal human serum
- LOS
lipooligosaccharide
- lgt
lipooligosaccharide glycosyl transferase
- Hep
heptose
- Glc
glucose
- CMP-NANA
5′-cytidinemonophospho-N-acetylneuraminic acid
- HBSS
Hank’s Balanced Salt Solution
Footnotes
This work was supported by National Institutes of Health grants AI054544 (S.R.), AI32725 and AI084048 (P.A.R),
References
- 1.Gonorrhea - CDC Fact Sheet. Centers for Disease Control and Prevention. Atlanta, GA: [Google Scholar]
- 2.Holmes KK, Counts GW, Beaty HN. Disseminated gonococcal infection. Ann Intern Med. 1971;74:979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- 3.Knapp JS, Holmes KK. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975;132:204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien JP, Goldenberg DL, Rice PA. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine. 1983;62:395–406. [PubMed] [Google Scholar]
- 5.Rice PA, Goldenberg DL. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981;95:175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- 6.Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009;7:821–834. doi: 10.1586/eri.09.63. [DOI] [PubMed] [Google Scholar]
- 7.Blake MS, Wetzler LM. Vaccines for gonorrhea: where are we on the curve? Trends Microbiol. 1995;3:469–474. doi: 10.1016/s0966-842x(00)89012-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SC, Chung RC, Deal CD, Boslego JW, Sadoff JC, Wood SW, Brinton CC, Jr, Tramont EC. Human immunization with Pgh 3-2 gonococcal pilus results in cross-reactive antibody to the cyanogen bromide fragment-2 of pilin. J Infect Dis. 1991;163:128–134. doi: 10.1093/infdis/163.1.128. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TM, Eschenbach DA, Knapp JS, Holmes KK. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980;138:978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- 10.Plummer FA, et al. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989;83:1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, Nagelkerke NJ, Maclean I, Ndinya-Achola JO, Waiyaki P, Brunham RC. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest. 1994;93:1748–1755. doi: 10.1172/JCI117159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joiner KA, Scales R, Warren KA, Frank MM, Rice PA. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985;76:1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice PA, Vayo H, Tam M, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virji M, Heckels JE. Nonbactericidal antibodies against Neisseria gonorrhoeae: Evaluation of their blocking effect on bactericidal antibodies directed against outer membrane antigens. J Gen Microbiol. 1988;134:2703–2711. doi: 10.1099/00221287-134-10-2703. [DOI] [PubMed] [Google Scholar]
- 15.Virji M, Heckels JE. Location of a blocking epitope on outer-membrane protein III of Neisseria gonorrhoeae by synthetic peptide analysis. J Gen Microbiol. 1989;135:1895–1899. doi: 10.1099/00221287-135-7-1895. [DOI] [PubMed] [Google Scholar]
- 16.Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, Maclean I, Ndinya-Achola JO, Waiyaki P, Brunham RC. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J Clin Invest. 1993;91:339–343. doi: 10.1172/JCI116190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards JL, Apicella MA. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol. 2002;4:585–598. doi: 10.1046/j.1462-5822.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 18.Gorby GL, Ehrhardt AF, Apicella MA, Elkins C. Invasion of human fallopian tube epithelium by Escherichia coli expressing combinations of a gonococcal porin, opacity-associated protein, and chimeric lipo-oligosaccharide. J Infect Dis. 2001;184:460–472. doi: 10.1086/322784. [DOI] [PubMed] [Google Scholar]
- 19.Harvey HA, Porat N, Campbell CA, Jennings M, Gibson BW, Phillips NJ, Apicella MA, Blake MS. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol Microbiol. 2000;36:1059–1070. doi: 10.1046/j.1365-2958.2000.01938.x. [DOI] [PubMed] [Google Scholar]
- 20.Lucas CE, Hagman KE, Levin JC, Stein DC, Shafer WM. Importance of lipooligosaccharide structure in determining gonococcal resistance to hydrophobic antimicrobial agents resulting from the mtr efflux system. Mol Microbiol. 1995;16:1001–1009. doi: 10.1111/j.1365-2958.1995.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 21.Quan DN, Cooper MD, Potter JL, Roberts MH, Cheng H, Jarvis GA. TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells. Mucosal Immunol. 2008;1:229–238. doi: 10.1038/mi.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafer WM, Datta A, Kolli VS, Rahman MM, Balthazar JT, Martin LE, Veal WL, Stephens DS, Carlson R. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J Endotoxin Res. 2002;8:47–58. [PubMed] [Google Scholar]
- 23.Shafer WM, Joiner K, Guymon LF, Cohen MS, Sparling PF. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984;149:175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- 24.Song W, Ma L, Chen R, Stein DC. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med. 2000;191:949–960. doi: 10.1084/jem.191.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Schwartz O, Pantelic M, Li G, Knazze Q, Nobile C, Radovich M, He J, Hong SC, Klena J, Chen T. DC-SIGN (CD209) recognition of Neisseria gonorrhoeae is circumvented by lipooligosaccharide variation. J Leukoc Biol. 2006;79:731–738. doi: 10.1189/jlb.0405184. [DOI] [PubMed] [Google Scholar]
- 26.Gregg CR, Johnson AP, Taylor-Robinson D, Melly MA, McGee ZA. Host species-specific damage to oviduct mucosa by Neisseria gonorrhoeae lipopolysaccharide. Infect Immun. 1981;34:1056–1058. doi: 10.1128/iai.34.3.1056-1058.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Densen P, Zollinger WD, Gulati S, Rice PA. Antibodies against Neisseria gonorrhoeae lipooligosaccharide antigens stimulate neutrophil chemotaxis. In: Poolman JT, Zanen HC, Mayer TF, Heckels JE, Makela PRH, Smith H, Beuvery EC, editors. Gonococci and meningococci. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 511–518. [Google Scholar]
- 28.Schmidt KA, Schneider H, Lindstrom JA, Boslego JW, Warren RA, Van de Verg L, Deal CD, McClain JB, Griffiss JM. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis. 2001;28:555–564. doi: 10.1097/00007435-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki R, Koshino H, Kurono S, Nishinaka Y, McQuillen DP, Kume A, Gulati S, Rice PA. Structural and immunochemical characterization of a Neisseria gonorrhoeae epitope defined by a monoclonal antibody 2C7; the antibody recognizes a conserved epitope on specific lipo-oligosaccharides in spite of the presence of human carbohydrate epitopes. J Biol Chem. 1999;274:36550–36558. doi: 10.1074/jbc.274.51.36550. [DOI] [PubMed] [Google Scholar]
- 30.Gulati S, Ngampasutadol J, Yamasaki R, McQuillen DP, Rice PA. Strategies for mimicking Neisserial saccharide epitopes as vaccines. Int Rev Immunol. 2001;20:229–250. doi: 10.3109/08830180109043036. [DOI] [PubMed] [Google Scholar]
- 31.Ngampasutadol J, Rice PA, Walsh MT, Gulati S. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine. 2006;24:157–170. doi: 10.1016/j.vaccine.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 32.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 34.Ingwer I, Petersen BH, Brooks G. Serum bactericidal action and activation of the classic and alternate complement pathways by Neisseria gonorrhoeae. J Lab Clin Med. 1978;92:211–220. [PubMed] [Google Scholar]
- 35.Lewis LA, Choudhury B, Balthazar JT, Martin LE, Ram S, Rice PA, Stephens DS, Carlson R, Shafer WM. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect Immun. 2009;77:1112–1120. doi: 10.1128/IAI.01280-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fearon DT, Austen KF. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975;142:856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirthmueller U, Dewald B, Thelen M, Schafer MK, Stover C, Whaley K, North J, Eggleton P, Reid KB, Schwaeble WJ. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158:4444–4451. [PubMed] [Google Scholar]
- 38.Price RJ, Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm- immobilizing antibodies. Fertil Steril. 1979;32:61–66. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- 39.Edwards JL, Brown EJ, Uk-Nham S, Cannon JG, Blake MS, Apicella MA. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 2002;4:571–584. doi: 10.1046/j.1462-5822.2002.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 40.McQuillen DP, Gulati S, Ram S, Turner AK, Jani DB, Heeren TC, Rice PA. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J Infect Dis. 1999;179:124–135. doi: 10.1086/314545. [DOI] [PubMed] [Google Scholar]
- 41.Mandrell R, Apicella A, Boslego J, Chung R, Rice P, Griffiss JM. Human immune response to monoclonal antibody - defined epitopes of Neisseria gonorrhoeae lipooligosaccharides. In. In: Poolman JT, Zanen H, Mayer T, Heckels J, Makela PH, Smith H, Beuvery C, editors. Gonococci and meningococci. Dordrecht, Netherlands: Kluwer Academic Publishers; 1988. pp. 569–574. [Google Scholar]
- 42.Yamasaki R, Kerwood DE, Schneider H, Quinn KP, Griffiss JM, Mandrell RE. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of gonococcal lipooligosaccharide. J Biol Chem. 1994;269:30345–30351. [PubMed] [Google Scholar]
- 43.Ram S, Cullinane M, Blom A, Gulati S, McQuillen D, Monks B, O'Connell C, Boden R, Elkins C, Pangburn M, Dahlback B, Rice PA. Binding of C4b-binding Protein to Porin: A molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–296. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- 46.Densen P, Gulati S, Rice PA. Specificity of antibodies against Neisseria gonorrhoeae that stimulate neutrophil chemotaxis. J Clin Invest. 1987;80:78–87. doi: 10.1172/JCI113067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banerjee A, Wang R, Uljon SN, Rice PA, Gotschlich EC, Stein DC. Identification of the gene (lgtG) encoding the lipooligosaccharide beta chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1998;95:10872–10877. doi: 10.1073/pnas.95.18.10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carbonetti N, Simnad V, Elkins C, Sparling PF. Construction of isogenic gonococci with variable porin structure: effects on susceptibility to human serum and antibiotics. Mol Microbiol. 1990;4:1009–1018. doi: 10.1111/j.1365-2958.1990.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 51.Rice PA, Hook EW, Blake MS, Kaslow RS, Gulati S, Kohl PK, VanRadden M, Buchanan TM. A possible influence of vaccine induced Por, LOS, and Rmp antibodies on the outcome of intraurethral challenge with Neisseria gonorrhoeae. In: Evans JS, Yost SE, Maiden MC, Feavers IM, editors. Proceedings of the Ninth International Pathogenic Neisseria Conference; London: Winchester; 1994. pp. 483–484. [Google Scholar]
- 52.Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis. 1996;174:1223–1237. doi: 10.1093/infdis/174.6.1223. [DOI] [PubMed] [Google Scholar]
- 53.Mandrell R, Schneider H, Apicella M, Zollinger W, Rice PA, Griffiss JM. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986;54:63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal S, Ferreira VP, Cortes C, Pangburn MK, Rice PA, Ram S. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J Immunol. 2010;185:507–516. doi: 10.4049/jimmunol.0903598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McQuillen DP, Gulati S, Rice PA. Complement-mediated bacterial killing assays. Methods Enzymol. 1994;236:137–147. doi: 10.1016/0076-6879(94)36013-8. [DOI] [PubMed] [Google Scholar]
- 56.Lewis LA, Ram S, Prasad A, Gulati S, Getzlaff S, Blom AM, Vogel U, Rice PA. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect Immun. 2008;76:339–350. doi: 10.1128/IAI.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulati S, Sastry K, Jensenius JC, Rice PA, Ram S. Regulation of the mannan-binding lectin pathway of complement on Neisseria gonorrhoeae by C1-inhibitor and alpha 2-macroglobulin. J Immunol. 2002;168:4078–4086. doi: 10.4049/jimmunol.168.8.4078. [DOI] [PubMed] [Google Scholar]
- 58.Ram S, Ngampasutadol J, Cox AD, Blom AM, Lewis LA, St Michael F, Stupak J, Gulati S, Rice PA. Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect Immun. 2007;75:4071–4081. doi: 10.1128/IAI.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannon JG. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin Microbiol Rev. 1989;2 Suppl:S1–S4. doi: 10.1128/cmr.2.suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cannon JG, Black WJ, Nachamkin I, Stewart PW. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984;43:994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fearon DT, Austen KF. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977;74:1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120:279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 63.Ingwer I, Petersen BH, Brooks G. Serum bactericidal action and activation of the classic and alternate complement pathways by Neisseria gonorrhoeae. J Lab Clin Med. 1978;92:211–220. [PubMed] [Google Scholar]
- 64.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O'Connell C, Boden R, Elkins C, Pangburn MK, Dahlback B, Rice PA. Binding of C4b-binding protein to porin: A molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–295. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giuntini S, Reason DC, Granoff DM. The Combined Roles of Human IgG Subclass, Alternative Complement Pathway Activation, and Epitope Density on Bactericidal Activity of Antibodies to Meningococcal Factor H Binding Protein. Infect Immun. 2011 doi: 10.1128/IAI.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider H, Griffiss JM, Mandrell RE, Jarvis GA. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985;50:672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 68.Roantree RJ, Rantz LA. A Study of the Relationship of the Normal Bactericidal Activity of Human Serum to Bacterial Infection. J Clin Invest. 1960;39:72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tapsall JW. Neisseria gonorrhoeae and emerging resistance to extended spectrum cephalosporins. Curr Opin Infect Dis. 2009;22:87–91. doi: 10.1097/QCO.0b013e328320a836. [DOI] [PubMed] [Google Scholar]
- 72.Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis. 1996;174:1223–1237. doi: 10.1093/infdis/174.6.1223. [published erratum appears in J Infect Dis 1997 Apr;175(4):1027] [DOI] [PubMed] [Google Scholar]
- 73.Braconier JH, Odeberg H, Sjoholm AG. Granulocyte phagocytosis of Streptococcus pneumoniae in properdin-deficient serum. Infect Immun. 1983;40:219–224. doi: 10.1128/iai.40.1.219-224.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camous L, Roumenina L, Bigot S, Brachemi S, Fremeaux-Bacchi V, Lesavre P, Halbwachs-Mecarelli L. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117:1340–1349. doi: 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]