Summary
Plants and fungi often produce toxic secondary metabolites that limit their consumption [1-4], but herbivores and fungivores that evolve resistance gain access to these resources and can also gain protection against non-resistant predators and parasites [3, 5-8]. Given that larvae of the fruitfly Drosophila melanogaster consume yeasts growing on rotting fruit and have evolved resistance to yeast fermentation products such as ethanol [9, 10], we decided to test whether ethanol protects fruitflies from one of their most common natural parasites, endoparasitoid wasps [11-13]. Here, we show that exposure to ethanol reduces wasp oviposition into fruitfly larvae. Furthermore, if infected, ethanol consumption by fruitfly larvae causes increased death of wasp larvae growing in the hemocoel and increased fly survival without need of the stereotypical anti-wasp immune response. This multi-faceted protection afforded to fly larvae by ethanol is significantly more effective against a generalist wasp than a wasp that specializes on D. melanogaster. Finally, fly larvae seek out ethanol-containing food when infected, indicating they use alcohol as an anti-wasp medicine. Although the high resistance of D. melanogaster may make it uniquely suited to exploit curative properties of alcohol, it is possible that alcohol consumption may have similar protective effects in other organisms.
Results and Discussion
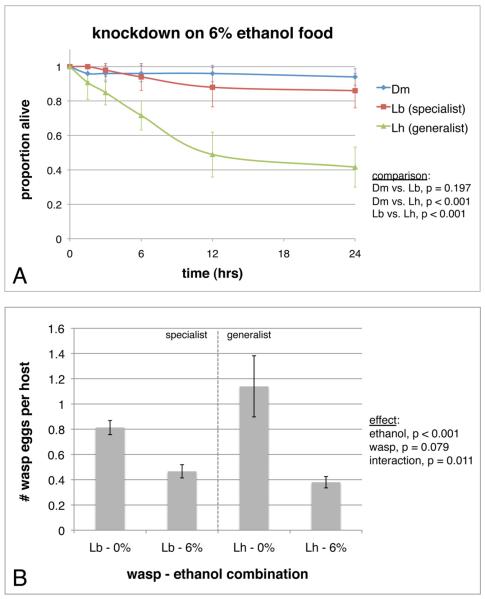
Ethanol levels found in natural D. melanogaster habitats range up to 6% ethanol by volume in rotting fruits, and 11% in wine seepages found at wineries [14, 15]. Fly consumption of food with moderate levels of ethanol (i.e. less than 4% by volume) results in increased fitness [16-18], but consumption of higher ethanol concentrations (i.e. greater than 4%) causes increasing fly mortality [18-20]. Given that secondary metabolites were shown to harm endoparasitoid wasps in other systems [3, 7, 21, 22], and the suggestion that D. melanogaster living in fruits with high ethanol concentrations might experience less wasp parasitism [23], we decided to test whether natural levels of ethanol could act as a protective toxin in fly interactions with two wasp species: Leptopilina boulardi is a specialist parasite of D. melanogaster and its close relatives that was previously shown to have relatively high ethanol knockdown resistance, while L. heterotoma is a generalist parasite that infects a diversity of Drosophila species living in fermenting fruits, decaying plant materials, and sap fluxes [24-26]. Both wasp species are attracted to the odor of fermentation products such as ethanol, presumably as a means to locate hosts [25, 27], and they are each highly infectious in D. melanogaster lab strains [28]. We compared ethanol knockdown resistance of adult female flies and wasps over a 24 hr period using Drosophila food mixed with concentrations of ethanol ranging from 4% to 10% by volume (Figure 1A, S1). At 6% ethanol, D. melanogaster adults and adults of the specialist wasp L. boulardi both showed significantly greater knockdown survival than adults of the generalist wasp L. heterotoma (Figure 1A). Considering all ethanol concentrations used, D. melanogaster is most ethanol resistant, followed by the specialist wasp L. boulardi, followed by the generalist wasp L. heterotoma (Figure S1).
Figure 1.
The effect of ethanol on wasp knockdown and oviposition. Survival curves were generated for adult insects living in petri dishes with 6% ethanol food (A). Error bars indicate 95% confidence intervals. The numbers of wasp eggs laid per host (B) were counted by dissecting fly larvae grown on food containing 0 or 6% ethanol and exposed to wasps for two hours. Error bars indicate standard deviation. Dm = D. melanogaster, Lb = L. boulardi, Lh = L. heterotoma. There were five dish replicates for all treatments. See also Figure S1.
Given wasps suffer knockdown by natural levels of environmental ethanol, we tested whether wasps also show a reduction in oviposition when presented with host fly larvae grown in 6% ethanol food (Figure 1B). There was a significant effect of ethanol in reducing oviposition in both wasp species. A significant ethanol-by-wasp interaction effect also indicated that ethanol had a stronger effect in reducing oviposition by the generalist L. heterotoma than the specialist L. boulardi. This difference is not explained by a difference in wasp mortality, as there was no wasp death over the course of the two-hour trial. Wasps may lay fewer eggs because they are sickened by ethanol fumes and attack less, but it is also possible that they insert their ovipositors into fly larvae growing on ethanol food at a normal level and limit oviposition because they detect a hostile environment for their offspring. Given that wasp oviposition was not reduced in fly larvae briefly removed from ethanol (data not shown), we favor the former hypothesis. Thus, ethanol can provide protection to fly larvae from being attacked by endoparasitoid wasps.
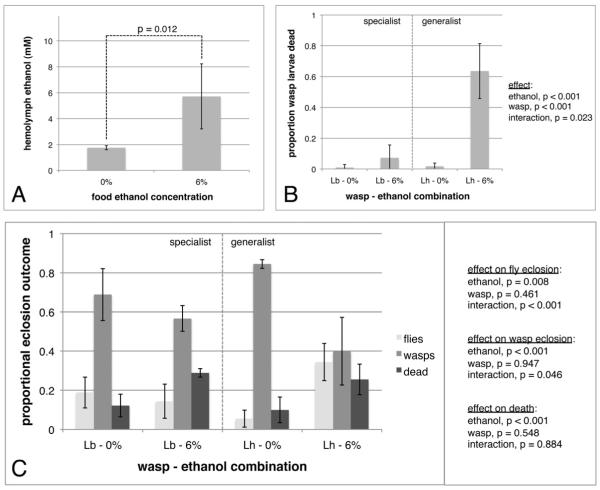
We next considered whether ethanol can help flies kill wasp parasites in the hemocoel once flies are infected. First, we measured the hemolymph ethanol concentration of D. melanogaster larvae grown in 6% ethanol food and found that fly hemolymph ethanol concentration was significantly higher in flies grown on food containing ethanol, with concentrations reaching approximately 6 mM (0.02% hemolymph ethanol content by volume) (Figure 2A). This ethanol concentration is low relative to those found in adult flies and honeybees [29-32], suggesting D. melanogaster larvae may be particularly resistant to passage of ethanol across the gut wall or cuticle into the hemolymph, and/or may have very efficient ethanol detoxification mechanisms. Fly hemolymph ethanol returned to baseline level within 24 hrs of being removed from ethanol food, and wasp infection did not result in increased fly hemolymph ethanol concentration or prolong the presence of ethanol in the hemolymph (Figure S2A, S2B). Altogether, these data show that wasp eggs and larvae living in fly hemolymph are exposed to a moderate level of ethanol (and presumably to ethanol breakdown products such as acetaldehyde) when flies live in or consume ethanol. Any protective effect ethanol might have for infected flies is likely passive, as infected flies do not appear to purposefully increase hemolymph ethanol levels, for example by down-regulating ethanol breakdown enzymes.
Figure 2.
Increased hemolymph ethanol is associated with wasp death and fly survival. Hemolymph ethanol concentration was compared between 72 hrs old fly larvae grown on food with or without 6% ethanol (A). Error bars indicate standard deviation across five dish replicates. Infected fly larvae grown on control or ethanol food were dissected to determine the viability of wasp larvae growing within them (B). Error bars indicate 95% confidence intervals across five dish replicates. The proportion of infected fly larvae resulting in each of the three infection outcomes (fly eclosion, wasp eclosion, and death of both fly and wasp) was compared across ethanol and wasp treatments (C). Error bars represent 95% confidence intervals across three dish replicates. See also Figure S2.
To determine whether host ethanol consumption affects wasp larval development, D. melanogaster larvae raised in food containing 6% ethanol were briefly removed from the food for attack by wasps before being returned to the food. There was a significant effect of host ethanol consumption on wasp larval mortality (Figure 2B). There was also a significant effect of wasp species and a significant interaction between ethanol treatment and wasp species, indicating that the increase in wasp larval mortality due to host consumption of ethanol was significantly greater for the generalist L. heterotoma than the specialist L. boulardi. To determine if wasp larval mortality was an effect of ethanol experienced by the host fly larvae before or after attack, a similar infection experiment was performed in which food treatments were switched after the fly larvae were attacked (Figure S2C). Although there was no overall effect of different ethanol treatments on wasp larval mortality, in a regression analysis stratified by wasp type there was a significant increase in death of L. boulardi larvae in hosts grown on ethanol food post-infection compared to pre-infection (p = 0.003), whereas L. heterotoma larvae suffered high mortality regardless of ethanol consumption timing (p = 0.623). Larval wasp death resulted in a decreased proportion of wasps surviving through eclosion and a significant increase in the proportion of flies that eclosed, despite an overall increase in ethanol-mediated mortality (Figure 2C). There were significant ethanol-by-wasp interaction effects on the proportion of flies and wasps eclosed, again indicating that ethanol has a stronger protective effect in flies infected by the generalist L. heterotoma. Altogether, these results indicate that ethanol consumption enhances fitness of wasp-infected flies, and that flies can receive maximal therapeutic benefit by consuming ethanol post-infection.
Wasp larvae dissected from singly infected control hosts invariably had defined internal organs and moved vigorously (Figure S2D). However, wasp larvae dissected from fly larvae grown on 6% ethanol food often did not move, showed amorphous internal organ structure, and had everted tissues, in many cases in close proximity to their anuses (Figure S2E), suggesting ethanol causes defects in wasp organ development or maintenance. Normally, flies attempt to kill wasps in a process termed encapsulation, and the increased mortality of wasps growing in ethanol-fed host flies might be the result of a heightened fly encapsulation response. Encapsulation involves constitutively produced plasmatocytes recognizing a wasp egg or larva as foreign and signaling to induce differentiation of lamellocytes, which spread over the wasp in a multi-layered capsule, leading to wasp death [33]. The wasp strains used here are highly virulent in D. melanogaster hosts and normally completely suppress the encapsulation response, but no wasp eggs or larvae dissected from ethanol-consuming fly larvae were found to be encapsulated by host hemocytes either. Although ethanol consumption was associated with a significant increase in fly plasmatocyte numbers, ethanol consumption was associated with a significant decrease in the number of lamellocytes, the hemocyte type specifically induced to mount the encapsulation response (Figure S2F, S2G). Lack of induction and/or death of host lamellocytes could be the result of ethanol toxicity, but it may be adaptive for hosts to purposefully suppress induction of an immune response that is un-needed in the presence of an anti-parasite toxin, given the presumed energetic cost of mounting an immune response [34].
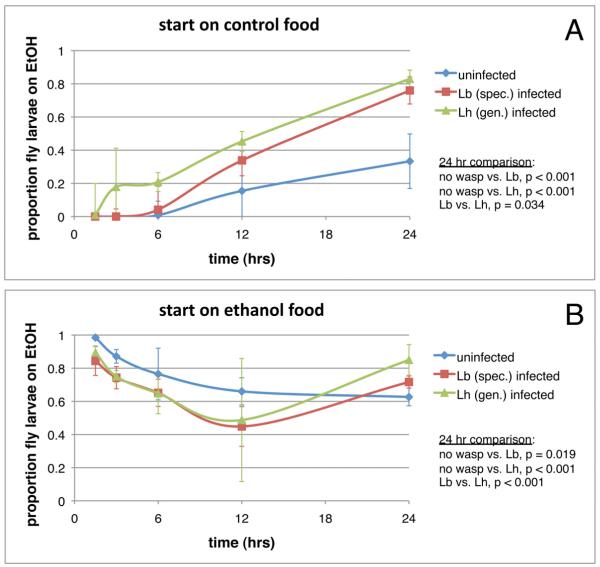
Use of toxic secondary metabolites in defense against enemies is usually preventative, i.e. organisms consume a toxic food source as part of their normal diet and the presence of toxin in their bodies results in internal host conditions that limit subsequent predation and infection. However, parasitized organisms can also therapeutically self-medicate, whereby they actively seek out compounds that help cure pre-existing infections [35]. The fact that fly consumption of ethanol post-infection has strong protective effects (Figure S2C) led us to consider the possibility that D. melanogaster might self-medicate. To test this idea, infected and uninfected fly larvae were placed in bisected petri dishes containing half control food and half 6% ethanol food, and the proportions of fly larvae that moved to (Figure 3A) or remained on (Figure 3B) the ethanol food side of the dish were measured over time. Fly larvae initially placed on control food showed a significant effect of wasp treatment at 24 hrs, with fly larvae infected by each wasp species significantly more likely to have moved to the ethanol food side of the dishes (Figure 3A). Infected fly larvae initially placed on ethanol food moved off the ethanol food faster than uninfected fly larvae, but returned to the ethanol food in significantly greater numbers than uninfected fly larvae by 24 hrs (Figure 3B).
Figure 3.
Choice of ethanol food by wasp-infected fly larvae. Preference for food containing 6% ethanol was compared between infected and uninfected flies over time using bisected petri dishes, with fly larvae initially placed on the control food side (A) or ethanol food side (B) of the dish. Error bars indicate 95% confidence intervals across three dish replicates. EtOH = ethanol. See also Figure S3.
These results are not caused by an increased sensitivity to ethanol sedation in infected fly larvae, which might cause the ethanol half of the dishes to act as an “absorbing state” for these flies, because infected larvae were highly mobile and vigorously masticated the food once they were settled on the ethanol side of the dishes. Instead, these results show that infected flies self-medicate by actively sampling their environment for a food source containing levels of ethanol most suitable for fighting off wasp infection, despite the otherwise toxic effects of ethanol consumption on fly developmental rate and survival found by us (Figure S3) and others [18-20]. Interestingly, in both choice experiments, fly larvae infected by the generalist L. heterotoma showed a significantly stronger preference for ethanol food than fly larvae infected by the specialist L. boulardi (Figure 3). These data suggest that fly larvae can distinguish between endoparasitoids with different levels of ethanol resistance, or that L. boulardi can better manipulate the ethanol seeking behavioral immune response of D. melanogaster.
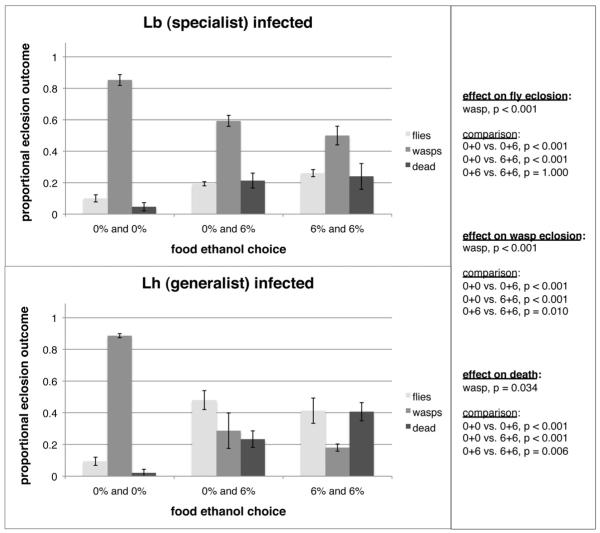
Finally, we tested the eclosion success of infected flies allowed to self-medicate by giving them the option of 0% or 6% ethanol food in bisected petri dishes (Figure 4). Survival of self-medicating flies was significantly greater than that of flies given no ethanol, and equivalent to that of flies grown in dishes where both sides contained ethanol. Death of infected flies given a choice between control and ethanol food was significantly greater than that of flies given no ethanol, indicating the choice of ethanol food results in ethanol-mediated death, but death was significantly lower than for flies grown in dishes where both sides contained ethanol. Altogether, these data show that flies not only choose to consume ethanol as self-medication against wasp infection, but also balance their ethanol intake to limit toxic effects on themselves. Furthermore, there were significant effects of wasp species on infection outcomes, where flies infected by the generalist wasp L. heterotoma achieved a relatively greater increase in eclosion success due to self-medication.
Figure 4.
The option of ethanol food enhances fitness of wasp-infected flies. Larvae were placed in bisected petri dishes with either 0% or 6% ethanol food on each side of the dish. The proportion of wasp-infected fly larvae resulting in each of the three infection outcomes (fly eclosion, wasp eclosion, and death of both fly and wasp) was compared across wasp and ethanol choice treatments. Error bars represent 95% confidence intervals across three dish replicates.
It is not surprising that D. melanogaster are highly attuned to ethanol concentration [36-38] given the previously characterized fitness benefits and costs of different levels of ethanol [16-20], along with the variation in ethanol content across rotting fruits, within rotting fruits, and temporally during the fruit rotting process. We have shown here that ethanol provides novel benefits to flies by reducing wasp infection (Figure 1B), by increasing infection survival (Figure 2B, 2C), and by allowing for a behavioral immune response against wasps based on consumption of it in toxic amounts (Figure 3, 4). To our knowledge, these data are the first to show that alcohol consumption can have a protective effect against infectious disease, and in particular against blood-borne parasites. Given that alcohols are relatively ubiquitous compounds consumed by a number of organisms, protective effects of alcohol consumption may extend beyond fruitflies. Although many studies in humans have documented decreased immune function in chronic consumers of alcohol [39-41], little attempt has been made to assay any beneficial effect of acute or moderate alcohol use on parasite mortality or overall host fitness following infection.
Experimental Procedures
Insect rearing
D. melanogaster strain Oregon R was used for all experiments. L. boulardi strain Lb17 and L. heterotoma strain Lh14 originated from single females collected in Winters, California in 2002 [28] and have been continuously maintained in the lab on D. melanogaster strain Canton S. Instant Drosophila medium (Formula 4-24, Carolina Biological Supply) in 0.25 g aliquots per 35 mm diameter petri dish was used for most experiments, supplemented with approximately 20 granules of active baker’s yeast and specific concentrations of ethanol. For standard experimental infections, Oregon R flies were allowed to lay eggs overnight; 48 hrs after egg lay, second-instar larvae were moved into petri dishes containing the experimental medium in groups of forty per dish. 72 hrs after egg lay, early third-instar fly larvae were moved into new, non-ethanol food dishes to be attacked by groups of ten female wasps for two hrs, after which they were returned to the experimental food conditions. Insects were kept in a 25 degrees C incubator with 12 hr light-dark cycle for all experiments. Further detailed experimental procedures are described in the Supplemental Information.
Supplementary Material
Highlights.
- environmental ethanol protects D. melanogaster from being parasitized by wasps
- consumption of ethanol by D. melanogaster also kills internal wasp parasites
- D. melanogaster choose high ethanol content food when infected by wasps
- protection afforded to flies by ethanol is stronger against a generalist parasite
Acknowledgements
We thank Katherine Ellingson, Thierry Lefevre, Erin Keebaugh, and four anonymous reviewers for a number of helpful comments on the manuscript. This work was supported by NIH grant AI081879 to TAS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berenbaum MR. The chemistry of defense: Theory and practice. Proc. Natl. Acad. Sci. USA. 1995;92:2–8. doi: 10.1073/pnas.92.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraenkel GS. The raison d’etre of secondary plant substances. Science. 1959;129:1466–1470. doi: 10.1126/science.129.3361.1466. [DOI] [PubMed] [Google Scholar]
- 3.Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Ann. Rev. Ecol. Syst. 1980;11:41–65. [Google Scholar]
- 4.Vining LC. Functions of secondary metabolites. Ann. Rev. Microbiol. 1990;44:395–427. doi: 10.1146/annurev.mi.44.100190.002143. [DOI] [PubMed] [Google Scholar]
- 5.Jaenike J. Parasite pressure and the evolution of amanitin tolerance in Drosophila. Evolution. 1985;39:1295–1301. doi: 10.1111/j.1558-5646.1985.tb05695.x. [DOI] [PubMed] [Google Scholar]
- 6.Nishida R. Sequestration of defensive substances from plants by Lepidoptera. Ann. Rev. Entomol. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- 7.Ode PJ. Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Ann. Rev. Entomol. 2006;51:163–185. doi: 10.1146/annurev.ento.51.110104.151110. [DOI] [PubMed] [Google Scholar]
- 8.Rothschild M. Secondary plant substances and warning colouration in insects. In: van Emden HF, editor. Insect/Plant Interactions. Blackwell; Oxford: 1973. pp. 59–83. [Google Scholar]
- 9.David JR, Vanherrewege J. Adaptation to alcoholic fermentation in Drosophila species: Relationship between alcohol tolerance and larval habitat. Comp. Biochem. Physiol. A Physiol. 1983;74:283–288. [Google Scholar]
- 10.Mercot H, Defaye D, Capy P, Pla E, David JR. Alcohol tolerance, Adh activity, and ecological niche of Drosophila species. Evolution. 1994;48:746–757. doi: 10.1111/j.1558-5646.1994.tb01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driessen G, Hemerik L, van Alphen JJM. Drosophila species breeding in the stinkhorn (Phallus impudicus Pers.) and their larval parasitoids. Neth. J. Zool. 1990;40:409–427. [Google Scholar]
- 12.Fleury F, Ris N, Allemand R, Fouillet P, Carton Y, Bouletreau M. Ecological and genetic interactions in Drosophila-parasitoids communities: a case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. Genetica. 2004;120:181–194. doi: 10.1023/b:gene.0000017640.78087.9e. [DOI] [PubMed] [Google Scholar]
- 13.Janssen A, Driessen G, de Haan M, Roodbol N. The impact of parasitoids on natural populations of temperate woodland Drosophila. Neth. J. Zool. 1988;38:61–73. [Google Scholar]
- 14.Gibson JB, May TW, Wilks AV. Genetic variation at the alcohol dehydrogenase locus in Drosophila melanogaster in relation to environmental variation: Ethanol levels in breeding sites and allozyme frequencies. Oecologia. 1981;51:191–198. doi: 10.1007/BF00540600. [DOI] [PubMed] [Google Scholar]
- 15.McKechnie SW, Morgan P. Alcohol dehydrogenase polymorphism of Drosophila melanogaster: Aspects of alcohol and temperature variation in the larval environment. Austral. J. Biol. Sci. 1982;35:85–93. [Google Scholar]
- 16.Chawla SS, Perron JM, Radoucothomas C. Effects of ingested ethanol on adult Drosophila melanogaster (Diptera: Drosophilidae) Can. Entomol. 1981;113:315–323. [Google Scholar]
- 17.Geer BW, Langevin ML, McKechnie SW. Dietary ethanol and lipid synthesis in Drosophila melanogaster. Biochem. Genet. 1985;23:607–622. doi: 10.1007/BF00504295. [DOI] [PubMed] [Google Scholar]
- 18.Parsons PA, Stanley SM, Spence GE. Environmental ethanol at low concentrations: Longevity and development in the sibling species Drosophila melanogaster and D. simulans. Austral. J. Zool. 1979;27:747–754. [Google Scholar]
- 19.McKechnie SW, Geer BW. Regulation of alcohol dehydrogenase in Drosophila melanogaster by dietary alcohol and carbohydrate. Insect Biochem. 1984;14:231–242. [Google Scholar]
- 20.McKenzie JA, Parsons PA. Alcohol tolerance: An ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa P. Natural enemies and herbivore-plant interactions: Influence of plant allelochemicals and host specificity. In: Barbosa P, LeTourneau DK, editors. Novel Aspects of Insect-Plant Interactions. Wiley; New York: 1988. pp. 201–229. [Google Scholar]
- 22.Flanders SE. Abortive development in parasitic hymenonoptera, induced by the food-plant of the insect hosts. J. Econ. Entomol. 1942;35:834–835. [Google Scholar]
- 23.Owen RE. Utilization and tolerance of ethanol, acetic acid and acetaldehyde vapor by Asobara persimilis, a parasitoid of Drosophila. Entom. Exper. Appl. 1985;39:143–147. [Google Scholar]
- 24.Bouletreau M, David JR. Sexually dimorphic response to host habitat toxicity in Drosophila parasitic wasps. Evolution. 1981;35:395–399. doi: 10.1111/j.1558-5646.1981.tb04898.x. [DOI] [PubMed] [Google Scholar]
- 25.Carton Y. Attraction de Cothonaspis sp. (Hymenoptera: Cynipidae) par le milieu trophique de son hote: Drosophila melanogaster. Actes Coll. Intern. Tours. 1977;265:285–303. [Google Scholar]
- 26.Fleury F, Gibert P, Ris N, Allemand R. Ecology and life history evolution of frugivorous Drosophila parasitoids. Adv. Parasitol. 2009;70:3–44. doi: 10.1016/S0065-308X(09)70001-6. [DOI] [PubMed] [Google Scholar]
- 27.Dicke M, Vanlenteren JC, Boskamp GJF, Vandongenvanleeuwen E. Chemical stimuli in host-habitat location by Leptopilina heterotoma (Thomson) (Hymenoptera: Eucoilidae), a parasite of Drosophila. J. Chem. Ecol. 1984;10:695–712. doi: 10.1007/BF00988537. [DOI] [PubMed] [Google Scholar]
- 28.Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Path. 2007;3:1486–1501. doi: 10.1371/journal.ppat.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcoholism: Clin. Exp. Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- 30.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 31.Bozic J, DiCesare J, Wells H, Abramson CI. Ethanol levels in honeybee hemolymph resulting from alcohol ingestion. Alcohol. 2007;41:281–284. doi: 10.1016/j.alcohol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Maze IS, Wright GA, Mustard JA. Acute ethanol ingestion produces dose-dependent effects on motor behavior in the honey bee (Apis mellifera) J. Insect Physiol. 2006;52:1243–1253. doi: 10.1016/j.jinsphys.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carton Y, Poirie M, Nappi AJ. Insect immune resistance to parasitoids. Insect Sci. 2008;15:67–87. [Google Scholar]
- 34.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- 35.Clayton DH, Wolfe ND. The adaptive significance of self-medication. Trends Ecol. Evol. 1993;8:60–63. doi: 10.1016/0169-5347(93)90160-Q. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie JA, McKechnie SW. A comparative study of resource utilization in natural populations of Drosophila melanogaster and D. simulans. Oecologia. 1979;40:299–309. doi: 10.1007/BF00345326. [DOI] [PubMed] [Google Scholar]
- 37.Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: Variation within and among populations. Behav. Genet. 1980;10:183–190. doi: 10.1007/BF01066268. [DOI] [PubMed] [Google Scholar]
- 38.Richmond RC, Gerking JL. Oviposition site preference in Drosophila. Behav. Genet. 1979;9:233–241. doi: 10.1007/BF01071304. [DOI] [PubMed] [Google Scholar]
- 39.Brayton RG, Stokes PE, Schwartz MS, Louria DB. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N. Eng. J. Med. 1970;282:123–128. doi: 10.1056/NEJM197001152820303. [DOI] [PubMed] [Google Scholar]
- 40.Szabo G. Consequences of alcohol consumption on host defence. Alcohol and Alcoholism. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- 41.Nelson S, Kolls JK. Alcohol, host defence and society. Nat. Rev. Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.