Abstract
We recently identified a protective MHC class Ib-restricted CD8 T cell response to infection with mouse polyomavirus. These CD8 T cells recognize a peptide from aa 139–147 of the VP2 viral capsid protein bound to the nonpolymorphic H-2Q9 molecule, a member of the Qa-2 family of β2m-associated MHC-Ib molecules. Q9:VP2.139-specific CD8 T cells exhibit an unusual inflationary response characterized by a gradual expansion over 3 months followed by a stable maintenance phase. We previously demonstrated that Q9:VP2.139-specific CD8 T cells are dependent on Ag for expansion, but not for the long-term maintenance. Here, we tested the hypothesis that the expansion and maintenance components of the Q9:VP2.139-specific T cell response are differentially dependent on CD4 T cell help and CD28 costimulation. Depletion of CD4+ cells and CD28/CD40L blockade impaired expansion of Q9:VP2.139-specific CD8 T cells, and intrinsic CD28 signaling was sufficient for expansion. In contrast, CD4 T cell-insufficiency, but not CD28/CD40L blockade, resulted in a decline in frequency of Q9:VP2.139-specific CD8 T cells during the maintenance phase. These results indicate that the Q9:VP2.139-specific CD8 T cell response to mouse polyomavirus infection depends on CD4 T cell help and CD28 costimulation for inflationary expansion, but only on CD4 T cell help for maintenance.
Introduction
Nonclassical MHC class Ib molecules are generally distinguished from the classical MHC class Ia molecules in being less polymorphic, having limited tissue distribution, and lower cell surface expression levels. Although some class Ib molecules present non-peptide molecules such as lipids, transferrin, or odorants (1), others structurally resemble class Ia molecules and present oligopeptides to CD8 T cells. Class Ib-restricted CD8 T cell responses to peptides and lipids contribute to both innate and adaptive immunity (2). Most class Ib-restricted T cell responses described to date participate in anti-bacterial host defense. For example, Qa-1b-restricted T cells mediate protection to infection by Listeria monocytogenes (3, 4), and H2-M3 presents N-formyl peptides to T cell responses to several bacterial infections, including Listeria monocytogenes (5) and Mycobacterium tuberculosis (6).
Sequence homology between the Q9 class Ib molecule and class Ia molecules is closer than for other class Ib molecules (7), with the structures between Q9 and H-2Kb showing close overlap. However, unlike class Ia molecules, Q9 lacks a transmembrane domain and is instead bound to cell membranes by a glycosylphosphatidylinositol linkage (8). Q9 is expressed on all somatic cells, although expression levels may be lower than for class Ia molecules (9). The Q9 gene is situated in the murine Qa-2 locus, and has no allelic polymorphisms among mice of inbred strains, although in some strains Q9 exists as a pseudogene (10). Only two dominant residues are critical for anchoring nonameric peptides to Q9 (i.e., histidine at position 7 and hydrophobic residue at position 9), allowing Q9 to bind a diverse peptide repertoire, akin to class Ia molecules (7).
We recently identified a novel population of mouse polyomavirus (MPyV)-specific αβ TCR+ CD8 T cells whose ligand consists of Q9 complexed to a nonamer peptide (aa 139–147) of the VP2 capsid protein (11). Using MHC class Ia-deficient (B6.Kb−/−Db−/−) mice, we demonstrated that this Q9:VP2.139-specific CD8 T cell response controls MPyV infection. These Q9:VP2.139-specific CD8 T cells exhibit response kinetics and Ag dependence that depart dramatically from those of conventional class Ia-restricted anti-MPyV CD8 T cells, being initially detected 8 d after infection and then progressively increasing in magnitude for three months. Thereafter, the population is stably maintained, comprising up to 80% of the CD8 T cell compartment with minimal proliferation or apoptosis (12). However, Q9:VP2.139-specific CD8 T cells exhibit a marked defect in cytokine effector activity, with only 20–50% of these cells capable of producing IFN-γ, a dominant anti-MPyV cytokine (13). We recently reported that the Q9:VP2.139-specific CD8 T cell response depends on Ag for its expansion, but not for its maintenance phase (12); however, it is unclear what additional determinants are critical for maintenance of this population.
In this study, we explored roles of CD4 T cell help and CD28/CD40 ligand costimulation as determinants of expansion and maintenance for the Q9:VP2.139-specific CD8 T cell response. In the absence of CD4 T cell help, conventional class Ia-restricted CD8 T cells show no deficiency in recruitment to acute MPyV infection, but then suffer massive attrition during persistent infection; this response profile cannot be attributed to elevated viral infection levels (14). We further showed that the class Ia-restricted anti-MPyV CD8 T cell response depends on both CD28 and CD40L costimulation for expansion, but is independent of these signals during maintenance (15). We hypothesized that, in parallel with their Ag dependence, the Q9:VP2.139-specific response would be dependent on CD4 T cell help and CD28/CD40L costimulation for expansion, but not for maintenance. However, although inflationary expansion of these cells was found to be dependent on CD28 costimulation and CD4 T cell help, CD4 T cells were also required for maintenance of Q9:VP2.139-specific CD8 T cells.
Materials and Methods
Mice
B6.Kb−/−Db−/− mice (Thy1.2) were purchased from Taconic Farms (Germantown, NY); B6.Kb−/−Db−/− Thy1.1 mice (16) were originally provided by Peter Jensen (University of Utah, Salt Lake City, UT). CD40−/− mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6NCr (B6) mice were purchased from the Frederick Cancer Research and Development Center of the National Cancer Institute (Frederick, MD). B6.129S2-Cd28tm1Mak/J (CD28−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were bred and housed by the Division of Animal Resources at Emory University (Atlanta, GA) in accordance with the guidelines of the Institutional Animal Care and Use Committee of Emory University. Female mice were 6–12-wk old at time of infection.
Viruses
MPyV wild-type virus (strain A2) was prepared as described previously (17). An MPyV mutant with a histidine to alanine substitution at aa 145 in VP2 (A2.H145A) was prepared as described previously (11). Mice were inoculated s.c. with 1 × 106 PFU in the hind footpads.
Costimulation blockade and CD4+ cell depletion
Anti-CD4 (clone GK1.5) was administered i.p. at 250 μg/mouse on d −3, −1, and 1 relative to MPyV infection, and then weekly thereafter. CD4+ cell depletion was verified by staining blood samples with anti-CD4 clone RM4-5 (eBioscience). Anti-CD40 ligand (clone MR-1; BioXCell) and/or CTLA-4-Ig (Bristol-Meyers Squibb) were administered i.p. at 500 μg/mouse on d 0 and 2 of MPyV infection, and then weekly thereafter. After mice were infected for 3 mo, GK1.5, MR-1, and/or CTLA-4 was was administered twice in the first 3 d, and weekly thereafter using 500 ug/mouse of each protein.
Flow cytometry
1 × 106 RBC-lysed splenocytes, incubated with BD FcBlock™ for 15 min at 4°C, were stained with a chimeric Q9(Kb)VP2.139 tetramer, generated by swapping the α3 chain of H-2Kb with that of Q9 (NIH Tetramer Core Facility). This tetramer binds Q9:VP2.139-specific cells at a 4-fold higher dilution than wild-type Q9 tetramers (unpublished observations). Cells were also stained with antibodies against the following molecules: CD44, CD62L, CD25, NKG2A/C/E (Clone 20d5), 2B4, 4-1BB and Thy1.1 (BD Pharmingen), CD4, CD127, CD122, CD27, PD-1, CD28, NKG2A (Clone 16a11), ICOS, OX-40, rat IgG2a and rat IgG2b (ebioscience), CD69 (Invitrogen), CD43 (BioLegend), KLRG-1 (SouthernBiotech), and CD8α (BD Pharmingen and BioLegend). Intracellular cytokine staining was performed as described (18). After incubation with and without 1uM VP2.139 peptide for 5–6 h at 37°C in the presence of GolgiPlug (BDBiosciences), cells were stained intracellularly with antibodies to IFN-γ, TNF-α, and IL-2 (BD Pharmingen), and for CD40L (eBioscience) and CD69 (Invitrogen). Samples were acquired on a FACSCalibur (BD Biosciences), and data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Quantitation of MPyV genomes
Tissue samples were taken from spleen, kidney, salivary gland and heart and snap-frozen. DNA isolation and Taqman-based quantitative real-time PCR were performed as previously described (18). The limit of detection of this assay is 10 copies of genomic viral DNA unless specified elsewhere.
Generation of Q9:VP2.139-specific TCR retrogenic mice
A Q9:VP2.139-specific CD8 T cell hybridoma (C3K) was derived by fusing the C3-8 clone (described in ref (11)) with a CD8-transfected BW5147 fusion partner (described in ref (19)). Generation of replication-defective retroviral producer cell lines was performed as described in ref (20). TCRα and TCRβ chains from C3K were cloned by PCR into pGEM using the following primer sequences: α chain (vα10) forward: 5′ GCGCCAGAATTCAGATCTACCATGAAGAGGCTGCTGTGCTCTCTGCTGG 3′; β chain (vβ4) forward: 5′ CTGTTAAAGCAAGCAGGAGACGTGGAAGAAAACCCCGGTCCCATGGGCTCCATTTT CCTCAGTTGCCT 3′; The products were then subcloned into the GFP-encoding murine stem cell virus-based retroviral vector pMIG at the BamHI site using the following reverse primers to generate a 2A-linked multicistronic construct: P2A-Cα reverse: 5′ CTTCCACGTCTCCTGCTTGCTTTAACAGAGAGAAGTTCGTGGCTCCGGAGCCGGACCACAGCCTCAGCGTCATGAG 3′ P2A-Cβ reverse: 5′ GCGTCGCTCGAGGGATCCTCAGGAATTTTTTYTCTTGACCATRGC 3′. 293T cells were transiently transfected with three plasmids, separately encoding packaging genes, envelope genes, and the C3K TCR retroviral vector. Retroviruses produced by these 293T cells were used to infect GP+E86 cells to generate replication-deficient retroviruses. Retrovirus-mediated stem cell gene transfer was carried out as described in ref (20) using B6 or CD28−/− bone marrow and B6 recipients to generate retrogenic donor mice. Briefly, bone marrow isolated from B6 or CD28−/− mice was infected by culture with irradiated GP+E86 producer cells, then transferred i.v. into B6 recipients. After 5 wk, retrogenic donor mice were checked for reconstitution by staining with anti-Vβ4 and Q9:VP2.139 tetramer, and by GFP expression. In our hands, 80–90% of retrogenic donor mice’s splenocytes express GFP. Of the CD8+ T cells in this population 20–30% stain with Vβ4. Splenocytes were FACS-sorted for GFP+ cells (FACSAria, BD Biosciences) and either 1 × 105 or 1 × 106 cells were transferred i.v. Mice received wild-type MPyV or A2.H145A 1 d post-transfer.
IL-2 treatment
Recombinant human IL-2 (Amgen) was administered i.p. at 15,000 units/mouse in PBS and 0.1% B6.Kb−/−Db−/− mouse serum every 12 h for 16 d.
Statistics
Statistical analyses were performed using Prism software (GraphPad, La Jolla, CA). For blood kinetic studies, data were analyzed using 2-way ANOVA with Bonferroni post tests. For phenotyping and quantitative PCR (qPCR) studies, two-tailed Mann-Whitney t-tests were used, with the exception of qPCR data for singly administered anti-CD40L or CTLA-4Ig, for which a Kruskal-Wallis Test was used, with Dunn’s Multiple Comparison Test.
Results
Expansion of Q9:VP2.139-specific cells is dependent on CD4 T cell help
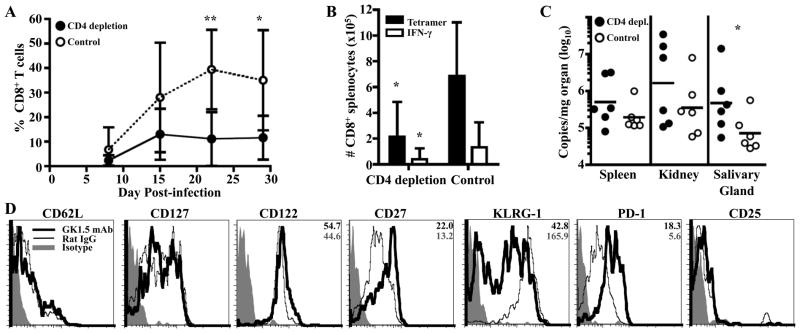
To investigate whether CD4 T cell help is required for expansion of Q9:VP2.139-specific T cells, MHC class Ia-deficient B6.Kb−/−Db−/− mice were depleted of CD4+ cells by administering anti-CD4 (clone GK1.5). Although CD4 T cell insufficiency in B6.Kb−/−Db−/− mice has been shown to foster homeostatic expansion of the CD8 T cell compartment (16), we did not observe an increase in numbers of CD8+ cells in the spleens of GK1.5 mAb-treated, MPyV-infected B6.Kb−/−Db−/− mice at 1 mo p.i. (unpublished observations). As shown in Fig. 1A & B, CD4-insufficiency was associated with severely blunted expansion of Q9:VP2.139-specific T cells, as detected by both tetramer binding and VP2.139 peptide-stimulated intracellular IFN-γ production. Depletion of CD4 cells was associated with a modest loss in viral control that differed between organs examined. As shown in Fig. 1C, MPyV load in the salivary gland was approximately 6-fold higher in CD4+ cell-depleted mice, although the MPyV genome copy numbers in kidneys and salivary glands were not significantly different from those in rat IgG-treated control mice. In this connection, it merits noting that MPyV infection elicits a T cell-independent virus-neutralizing IgG response response that controls viral burden (21). Compared to rat IgG-treated infected mice, at 1 mo p.i. Q9:VP2.139-specific CD8 T cells in GK1.5-treated mice expressed higher cell surface levels of CD27, PD-1, and slightly higher levels of CD122, with lower levels of KLRG-1 (Fig. 1D). This phenotypic profile resembles the effector/effector-memory phenotype seen during chronic infection with LCMV with CD4 depletion (22), suggesting that the unhelped Q9:VP2.139-specific cells may be similarly fated for functional exhaustion.
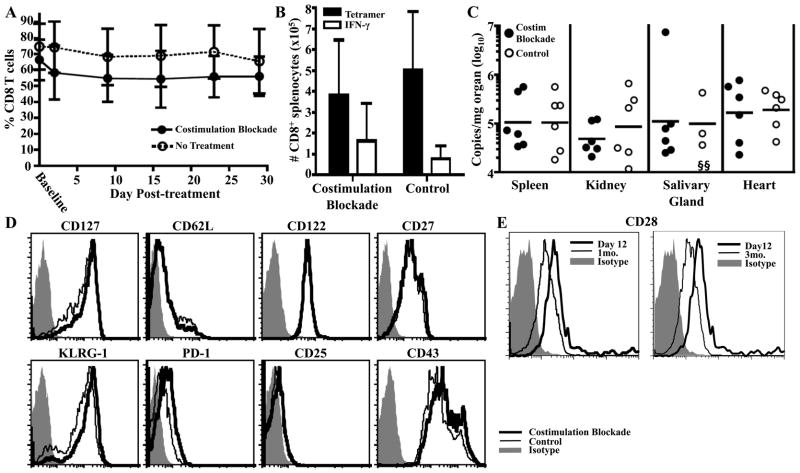
Figure 1.
Q9:VP2.139-specific cells depend on CD4 T cell help for expansion. GK1.5 mAb or rat IgG were administered to B6.Kb−/−Db−/− mice coincident with MPyV inoculation and then once weekly for 1 mo. A. Frequency (± SD) of Q9:VP2.139-specific CD8 T cells in blood over time, determined by cell surface Q9:VP2.139 tetramer staining. B. Number (± SD) of Q9:VP2.139-specific CD8 T cells in spleen detected by cell surface Q9:VP2.139 tetramer binding and VP2.139 peptide stimulated intracellular IFN-γ production at d 29 p.i. C. Numbers of MPyV genome copies as determined by qPCR in indicated organs at d 29 p.i. Each point corresponds to an individual mouse, and horizontal lines indicate geometric mean. D. Expression of indicated surface molecules by splenic Q9:VP2-139-specific CD8 T cells on d 29 p.i. Average gMFI valuess are indicated in the top right corner of molecules for which a significant difference was observed between experimental and control groups. Data are representative of six mice per group from two independent experiments. *p < 0.05, **p < 0.01.
Based on evidence that increased CD27 signaling may suppress effector differentiation (23), we asked whether unhelped Q9:VP2.139-specific cells have less effector cytokine function than helped cells. However, the same frequency (~19%) of Q9:VP2.139-specific CD8 T cells elaborated IFN-γ production after ex vivo VP2.139 peptide stimulation as those in rat IgG-treated infected mice (Fig. 1B). Together, these results indicate that CD4 T cell help is important for normal expansion of Q9:VP2.139-specific CD8 T cells during MPyV infection.
Expansion of Q9:VP2.139-specific cells is dependent on CD28 costimulation
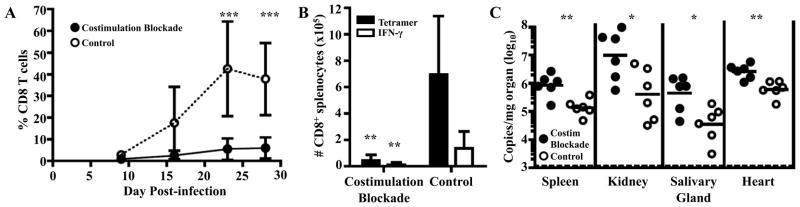
CD4 T cell help is thought to act through CD40:CD40L-mediated activation of dendritic cells, leading to CD28:CD80/86 interactions between dendritic cells and CD8 T cells (24). Because we saw a detrimental effect on Q9:VP2.139-specific expansion due to CD4 depletion, we next asked whether expansion of Q9:VP2.139-specific CD8 T cells requires costimulation by CD28 and CD40L. We applied an established combined costimulation blockade regimen using anti-CD40L (clone MR1) and CTLA-4-Ig, which were administered together to MPyV-infected B6.Kb−/−Db−/− mice for the first month p.i. As shown in Fig. 2A & B, CD28-CD40L costimulation blockade profoundly crippled the expansion of Q9:VP2.139-specific CD8 T cells. By 1 mo p.i., Q9:VP2.139 tetramer+ CD8 T cells had expanded to more than 9% of the CD8 population in only one-third of treated mice. In contrast, Q9:VP2.139 tetramer+ CD8 T cells in all untreated mice ranged from 18–62% of total CD8 T cells. Q9:VP2.139-specific CD8 T cells in the costimulation blockade responders phenotypically resembled those in untreated mice, except for having fewer KLRG-1hi cells and more PD-1hi cells (unpublished observations); interestingly, this shift in KLRG-1 and PD-1 expression is similar to that of Q9:VP2.139-specific CD8 T cells in CD4+ cell-depleted mice (Fig. 1D). Mirroring its deleterious effect on the magnitude of the Q9:VP2.139-specific CD8 T cell response, CD28-CD40L costimulation blockade also resulted in a greater loss of viral control than in CD4+ cell-depleted mice (Fig. 2C).
Figure 2.
Expansion of Q9:VP2.139-specific CD8 T cells depends on CD28 and CD40L costimulation. A cocktail of MR-1 (α-CD40L) and CTLA-4-Ig were administered to B6.Kb−/−Db−/− mice coincident with MPyV inoculation and then once weekly for 1 mo. Control mice received no Ab. A. Frequency (± SD) of CD8+ Q9:VP2.139-specific cells in blood over time, determined by cell surface Q9:VP2.139 tetramer staining. B. Number (± SD) of splenic Q9:VP2.139-specific CD8 T cells at d 28 p.i. was determined by tetramer staining and VP2.139 peptide-stimulated intracellular IFN-γ production. C. Numbers of MPyV genome copies were determined by qPCR in indicated organs at d 28 p.i. Each point corresponds to an individual mouse, and horizontal lines indicate geometric mean. Data are representative of six mice per group pooled from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
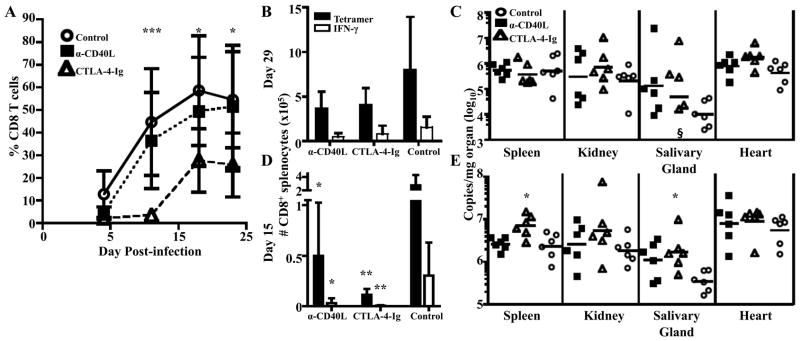
To determine whether both CD28- and CD40L- mediated costimulation were necessary for expansion of Q9:VP2.139-specific CD8 T cells, MPyV-infected B6.Kb−/−Db−/− mice were treated separately with α-CD40L or CTLA-4-Ig. In the first two weeks p.i., CTLA-4-Ig-treated mice showed only modest expansion of Q9:VP2.139-specific CD8 T cells; in contrast, Q9:VP2.139-specific CD8 T cell expansion progressed unimpaired in α-CD40L-treated mice (Fig. 3A). However, after two wk the Q9:VP2.139-specific CD8 T cell population in CTLA-4-Ig-treated mice became detectable and reached magnitudes that were not significantly different than in α-CD40L-treated and untreated control mice (Fig. 3A & B) and virus loads were equivalent (Fig. 3C). Breakthrough T cell responses during costimulation blockade has been observed in mouse skin allograft transplantation, with anti-donor T cell responses overcoming blockade by 21 d after transplantation (25). To examine the Q9:VP2.139-specific CD8 T cell response before breakthrough occurs, mice were sacrificed at two wks p.i. At this timepoint, CD28 blockade was observed to clearly dampen the response (Fig. 3D), and was associated with significantly higher virus levels in the spleen and salivary glands (Fig. 3E). As shown in Fig. 3D, α-CD40L-treated mice also had a diminished number and frequency (unpublished observations) of Q9:VP2.139-specific CD8 T cells in the spleen, but there was no significant difference in viral titers compared to the untreated control group. The frequency of Q9:VP2.139-specific CD8 T cells in the blood of α-CD40L-treated mice at d 15 was equivalent to that in untreated mice (unpublished observations). Impaired cellular trafficking in the presence of CD40L blockade (26, 27) may explain the discord between the frequency of Q9:VP2.139-specific CD8 T cells in the blood and spleen. These results demonstrate that expansion of Q9:VP2.139-specific CD8 T cells is dependent upon CD28 costimulation.
Figure 3.
Expansion of Q9:VP2.139-specific CD8 T cells depends on CD28, costimulation. MR-1 (α-CD40L ) or CTLA-4-Ig were administered to B6.Kb−/−Db−/− mice before and weekly during MPyV infection. Control mice received no Ab. A. Frequency (± SD) of CD8+ Q9:VP2.139-specific cells in the blood over time. Significance indicated is the result of comparing CTLA-4-Ig-treated mice to controls. There was no significant difference between α-CD40L-treated mice and controls. B. Number (± SD) of Q9:VP2.139-specific cells in spleen were determined by tetramer staining and intracellular anti-IFN-γ staining at d 29 p.i. C. Number (± SD) of MPyV genome copies were determined by qPCR in indicated organs at d 29 p.i. D. Number (± SD) of Q9:VP2.139-specific cells in spleen were determined by tetramer surface and intracellular anti-IFN-γ staining at d 15 p.i. Significance indicated is the result of comparing tetramer+ or IFN-γ+ cell numbers of experimental to control mice. E. Number of MPyV genome copies were determined by qPCR in indicated organs at d 15 p.i. For C & E, each point corresponds to an individual mouse, and horizontal lines indicate geometric mean. Data are representative of six mice per group pooled from two independent experiments. §, sample is below the limit of detection. *p < 0.05, **p < 0.01, ***p < 0.001.
Q9:VP2.139-specific CD8 T cells require intrinsic CD28 signaling for expansion
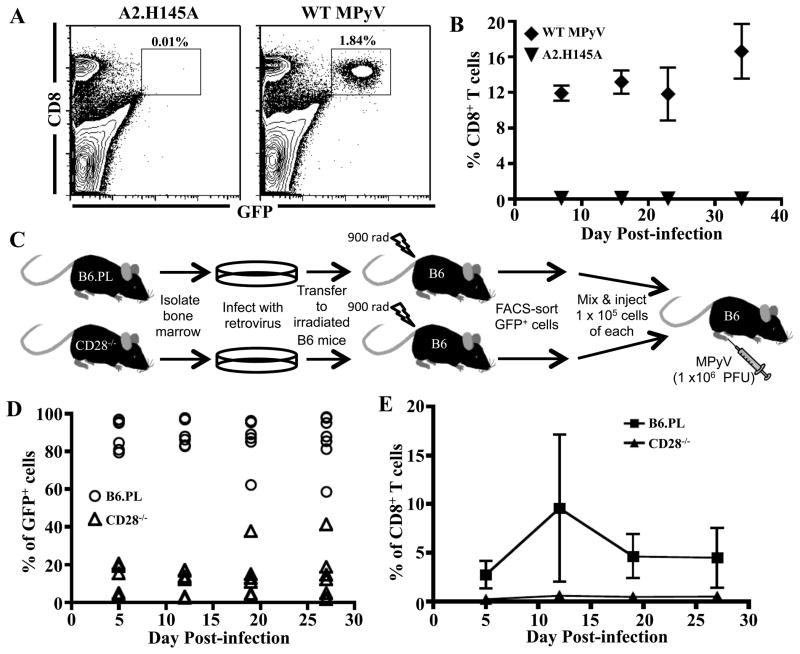
To directly determine whether the dependence on CD28-mediated costimulation for expansion of Q9:VP2.139-specific CD8 T cells is autochthonous, we used retrogenic TCR technology to create mice with monoclonal Q9:VP2.139-specific CD8 T cells (20). Briefly, lethally irradiated B6 mice were engrafted using bone marrow infected by a recombinant, replication-deficient retrovirus bicistronic for genes for GFP and a TCR from a cloned line of Q9:VP2.139-specific CD8 T cells. To demonstrate Ag specificity, GFP+ CD8+ cells were FACS-sorted from spleens of these TCR retrogenic mice, and transferred to naïve B6 recipients, which were then infected with either wild-type MPyV or a mutant MPyV in which the codon for the dominant histidine anchor residue in VP2.139 epitope was replaced with one for alanine (A2.H145A). As shown in Fig. 4A, GFP+ (i.e., donor TCR retrogenic) CD8 T cells were recruited in mice infected by wild-type MPyV but not A2.H145A. It is important to note that expansion of the immunodominant class Ia-restricted CD8 T cell response to MPyV was not affected in recipients of the retrogenic T cells (unpublished observations). To investigate if CD28 expression by Ag-specific CD8 T cells per se was essential for their expansion in MPyV-infected mice, Q9:VP2.139 TCR retrogenic mice were generated using bone marrow from Thy1.1+ wild-type B6 mice (B6.PL) and Thy1.2+ CD28−/− B6 mice. Equal numbers (1 × 105) of FACS-sorted GFP+ CD8+ cells from B6.PL and CD28−/− bone marrow TCR retrogenic mice were co-transferred into naïve Thy 1.2+ B6 recipients (Fig. 4C), then infected with parental MPyV. As shown in Fig. 4D, CD28-sufficient GFP+ retrogenic cells efficiently expanded in mice infected by wild-type MPyV. In contrast, Thy1.1+ GFP+ (i.e., CD28−/−) cells only expanded in one of three infected recipients, did so with delayed kinetics and reached substantially lower frequencies than those of CD28-sufficient donor T cells (Fig. 4E). Importantly, wild-type Q9:VP2.139 TCR retrogenic cells transferred into CD40L−/− mice expanded equivalently to that of wild-type retrogenic cells transferred into wild-type mice after infection by MPyV (unpublished observations). These data demonstrate that CD28 signaling is intrinsically required by Q9:VP2.139-specific CD8 T cells for expansion in MPyV-infected mice.
Figure 4.
Q9:VP2.139-specific cells depend on intrinsic CD28 costimulation for expansion. 1 × 106 Q9:VP2.139 TCR retrogenic GFP+ cells were transferred i.v. into B6 mice, which were subsequently infected with wild-type (WT) MPyV or A2.H145A. A. Representative dot plots of splenocytes in recipient mice at d 40 p.i., gated on viable lymphocytes. B. Frequency (± SD) of GFP+ Q9:VP2.139-specific CD8 T cells in blood over time. n = 3 mice. C. Experimental design: Q9:VP2.139 TCR retrogenic CD8 T cells derived from Thy1.1 B6.PL and B6.CD28−/− bone marrow were mixed 1:1, transferred to B6 mice, which were inoculated with WT MPyV. D. Frequency of Q9:VP2.139-specific CD8 T cells from B6.PL and CD28−/− bone marrow donors in blood over time. Dots represent individual mice. n = 3 mice. E. Frequency of Q9:VP2.139-specific cells from B6.PL bone marrow donors compared to cells from CD28−/− bone marrow donors in the blood over time following a co-transfer (± SD). n = 3 mice.
Maintenance of Q9:VP2.139-specific cells does not require CD28 and CD40L costimulation
We previously demonstrated that CD28 and CD40L costimulation is dispensible for maintenance of class Ia-restricted antiviral CD8 T cells during persistent MPyV infection (15). Based on this and our prior data showing that maintenance of Q9:VP2.139-specific CD8 T cells in persistently infected mice is Ag-independent (12), we hypothesized that CD28 and CD40L costimulation blockade would not impact long-term maintenance of Q9:VP2.139-specific CD8 T cells. To test this possibility, CD28 and CD40L costimulation blockade was administered to B6.Kb−/−Db−/− mice for 1 mo beginning at 3 mo p.i. As shown in Fig. 5A, the frequency of circulating Q9:VP2.139-specific CD8 T cells in mice given anti-CD40L and CTLA-4-Ig was maintained at the same levels as in untreated, infected B6.Kb−/−Db−/− mice. Moreover, after one mo p.i., direct ex vivo analyses showed minimal differences in frequency, magnitude, cytokine effector function (Fig. 5B), and surface phenotype (Fig. 5D) of Q9:VP2.139-specific CD8 T cells in untreated mice and mice receiving costimulation blockade. Consistent with these findings, MPyV loads were similar in the kidney, spleen, salivary glands and heart in both groups (Fig. 5C). Interestingly, CD28 expression by Q9:VP2.139-specific CD8 T cells in B6.Kb−/−Db−/− mice declined between d 12 and 1 mo p.i. (Fig. 5E) and remained low long-term (unpublished observations), a finding that may correlate with the differential impact of CD28 blockade during expansion and maintenance phases of this T cell response.
Figure 5.
Q9:VP2.139-specific cells do not depend on costimulation for maintenance. B6.Kb−/−Db−/− mice were infected with MPyV and 3 mo p.i. given a cocktail of MR-1 (α-CD40L) and CTLA-4-Ig weekly for 1 mo. Control infected mice received no Ab. A. Frequency (± SD) of Q9:VP2.139-specific CD8 T cells in blood over time. Baseline indicates frequency before start of treatment. B. Number (± SD) of splenic Q9:VP2.139-specific CD8 T cells was determined by tetramer staining and VP2.139 peptide-stimulated intracellular anti-IFN-γ staining after 1 mo of treatment. C. Number of MPyV genome copies was determined by qPCR in indicated organs after 1 mo of treatment. Each point corresponds to an individual mouse, and horizontal lines indicate geometric mean. D. Expression of indicated surface molecule by CD8+ Q9:VP2-139-specific splenocytes after 1 mo of treatment. E. CD28 expression on Q9:VP2.139-specific CD8 T cells in MPyV-infected B6.Kb−/−Db−/− mice at the indicated time post-infection. Data are representative of six mice per group pooled from two independent experiments. The limit of detection of this assay is 20 copies of genomic viral DNA. §, sample is below the limit of detection.
Maintenance of Q9:VP2.139-specific CD8 T cells is dependent on CD4 T cell help
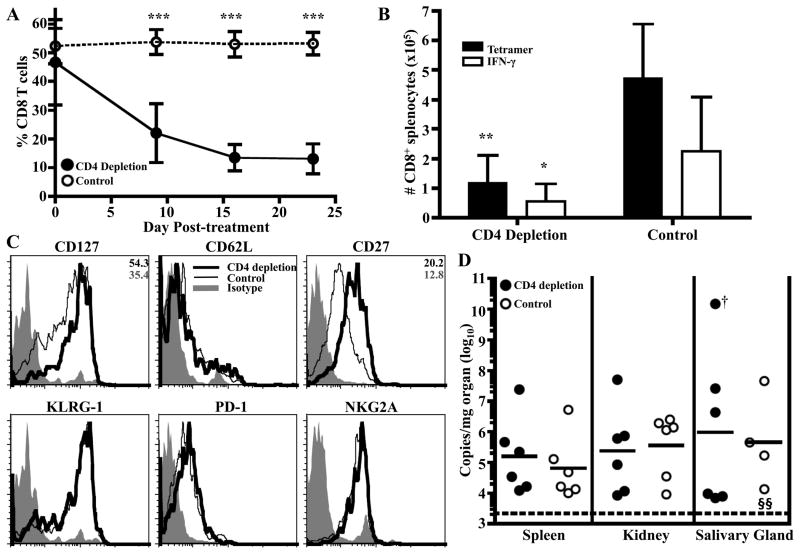
Because the Q9:VP2.139-specific CD8 T cell population was independent of Ag and CD28/CD40L costimulation for its maintenance, we hypothesized that CD4 T cell help during this phase would similarly be dispensable. To test this possibility, GK1.5 mAb-mediated CD4+ cell depletion was administered to B6.Kb−/−Db−/− mice 3 mo after infection. Unexpectedly, CD4 depletion resulted in a steady attrition of circulating Q9:VP2.139-specific CD8 T cells, while rat IgG-treated mice maintained their Q9:VP2.139-specific population (Fig. 6A). The rapid attrition of the Q9:VP2.139 population during CD4+ cell deficiency may be explained by the loss of IL-2 secretion by CD4+ cells. However, administering recombinant human IL-2 to B6.Kb−/−Db−/− mice depleted of CD4+ cells at 3 mo p.i. failed to prevent loss of the Q9:VP2.139-specific CD8 T cell population (Supplemental Fig 1). By 1 mo p.i., GK1.5 mAb treated mice had 5-fold fewer Q9:VP2.139-specific CD8 T cells in the spleen than rat IgG-treated controls (Fig. 6B). Q9:VP2.139 tetramer+ CD8 T cells from GK1.5 mAb-treated mice, while still CD62Llo, displayed higher expression of CD127 and CD27 (Fig. 6C), which may indicate that those CD8 T cells with more central memory-like characteristics (28) are less dependent on CD4 help for survival. CD4 cell depletion-mediated attrition of Q9:VP2.139-specific cells did not lead to a change in viral loads after 1 mo (Fig. 6D). We demonstrated above that CD4 depletion during expansion had only a modest effect on viral loads, suggesting 10% of the CD8 T cell compartment, comprised of Q9:VP2.139-specific cells, can control viral loads. Control would be enhanced during the maintenance phase, as virus levels are lower at this time. Taken together, these data show that the Q9:VP2.139-specific CD8 T cell response depends on CD4 T cell help for both expansion and maintenance, while CD28 costimulation is only required for expansion of this MHC class Ib-restricted T cell response.
Figure 6.
Q9:VP2.139-specific cells depend on CD4 help for maintenance. B6.Kb−/−Db−/− mice were infected with MPyV for 3 mo before the start of GK1.5 or rat IgG treatment. A. Frequency (± SD) of Q9:VP2.139 tetramer+ CD8 T cells in blood over time. B. After 1 mo of treatment, the number (± SD) of splenic Q9:VP2.139-specific CD8 T cells (same mice as in A) was determined by tetramer staining and intracellular anti-IFN-γ staining white bars) after ex vivo VP2.139 peptide stimulation. C. Expression of indicated surface molecules by Q9:VP2-139 tetramer+ CD8+ splenocytes after 1 mo of treatment. Average gMFI values are indicated in the top right corner of molecules for which a significant difference was observed between experimental and control groups. Data are representative of six mice per group pooled from two independent experiments. D. Number of MPyV genome copies was determined by qPCR in indicated organs after 1 mo of treatment. Each point corresponds to an individual mouse, and horizontal lines indicate geometric mean. The limit of detection of this assay is 20 copies of genomic viral DNA. †, mouse with salivary gland tumor. §, sample is below the limit of detection. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In this study, we provide evidence that the inflationary kinetic profile for an antiviral MHC class Ib-restricted CD8 T cell response varies over time in its dependence on CD28 costimulation, but requires continuous CD4 T cell help. Following a prolonged period of gradual expansion that requires CD28 costimulation and CD4 T cell help, the Q9:VP2.139-specific CD8 T cell response to MPyV infection plateaus to a CD4 T cell-dependent maintenance phase that is independent of CD28 or CD40L costimulation. The dependence on CD28 signaling is an intrinsic property of the Q9:VP2.139-specific CD8 T cells. Although this expansion-maintenance phase difference in CD28 costimulation for Q9:VP2.139-specific CD8 T cells parallels the dependence of this response on Ag (12), the dispensability for viral Ag during maintenance is at odds with the ongoing dependence on CD4 T cell help.
The role of CD4 T cell help for the primary expansion of CD8 T cells has been investigated in numerous models, with varied results. Early experiments with non-inflammatory cell-based immunization (29) or HSV infection (30) indicated a clear role for CD4 T cell help in generating CD8 T cell responses. In contrast, CD4 T cell-independent primary expansion of pathogen-specific class Ia-restricted CD8 T cells has been documented in several experimental infection systems, including MPyV (14, 31–36). Unlike for these virus-specific, class Ia-restricted CD8 T cell responses, the primary expansion of Q9:VP2.139-specific CD8 T cells is CD4 T cell dependent as shown by its apparently abbreviated peak magnitude response. However, a similar pattern has been seen for unhelped primary CD8 T cell responses to Plasmodium yoelii (37, 38), Listeria monocytogenes, and vaccinia virus (36). This apparent CD4 T cell independence for naïve CD8 T cell priming, but dependence on CD4 T cells for sustained proliferation, was predicted for pathogens triggering intermediate levels of danger signals (39). The CD4 T cell-independence of the MPyV-specific, class Ia-restricted CD8 T cell response to acute infection, however, argues against inflammation level as a major determinant of expansion by these unhelped MHC class Ib-restricted CD8 T cells.
Irrespective of the availability of CD4 T cell help, there is a similar dissociation between tetramer-binding and IFN-γ production by Q9:VP2.139-specific CD8 T cells. We previously demonstrated that IFN-γ has direct anti-MPyV activity and is important for controlling MPyV infection (13). Although CD4 T cell depletion reduced expansion of the Q9:VP2.139-specific CD8 T cell response, viral load was kept in check in most of the organs examined. It is interesting to speculate that the high magnitude Q9:VP2.139-specific CD8 T cell response in Kb−/−Db−/− mice may compensate for their depressed effector functionality and enable them to exert effective control of MPyV infection.
Secretion of IL-2 by CD4 T cells constitutes a well-recognized mechanism of T cell help (40). D’Souza and Lefrancois reported that IL-2 receptor signaling is unnecessary for the initiation of CD8 T cell cycling, but required for sustained expansion. CD4 T cells may similarly be involved in expansion of activated naïve Q9:VP2.139-specific CD8 T cells, possibly resulting from upregulation of high-affinity IL-2 receptors through CD28:CD80/86 signaling (36). Furthermore, CD25 expression on CD8 T cells has been shown to be independent of CD40 signaling (36), which also holds for expansion of Q9:VP2.139-specific CD8 T cells. This is in contrast to the class Ia-restricted CD8 T cell responses to MPyV infection, which display dependence on both CD28 and CD40L signals for expansion (15). Thus, CD28 signaling may be primarily involved in recruitment of naïve Q9:VP2.139-specific CD8 T cells, with CD4 T cell help promoting upregulation of high-affinity IL-2 receptors to enable expansion by IL-2. However, we did not observed decreased CD25 expression on Q9:VP2.139-specific cells CD4-depleted B6.Kb−/−Db−/− mice on d 10 p.i. (unpublished observations).
CD28 signaling by Q9-restricted CD8 T cells may be essential for amplifying weak TCR signaling. Data from our lab and others support the concept that Q9:VP2.139-specific cells bind weakly with their pMHC ligands. Preliminary surface plasmon resonance data indicates that the Q9:VP2.139 TCR binds to its cognate ligand with lower affinity than that typical for class Ia-restricted TCR (A. Brooks and L. Sullivan, unpublished observations). Furthermore, the orientation of the CD8-binding loop in the α3 domain of Q9 suggests that it weakly engages CD8 coreceptors (7). This possibility is supported by our data showing that Q9:VP2.139 tetramers constructed using a chimeric Q9 molecule with a Kbα3 domain, which strongly binds CD8α (41), stains Ag-specific CD8 T cells with higher MFI than Q9 tetramers with native α3 domains (unpublished observations). The tissue distribution and expression levels of Q9 are similar to that of MHC class Ia molecules (9); however, Q9 is upregulated by IFN-γ (42) and the defect in IFN-γ effector function by Q9:VP2.139-specific CD8 T cells may impact Q9 expression levels. Suboptimal TCR signaling may also explain the slow inflationary expansion of the Q9:VP2.139-specific cells and their effector function defect. By corollary, high epitope density and/or strong CD28 signaling may compensate for inefficient CD8 coreceptor binding and enable full activation of Q9-restricted CD8 T cells. Because the inflationary kinetics of the Q9:VP2.139-specific response depend on persistent infection (12), it is possible that continuous TCR stimulation together with CD28 costimulation is needed for Q9:VP2.139-specific CD8 T cells to maximally expand.
As seen here for memory MHC class Ib-restricted CD8 T cells to MPyV infection, CD4 T cell help has been shown to maintain memory CD8 T cells in the setting of other persistent infections (14, 31, 32, 43). The nature of the CD4 T cell help for maintaining the MHC class Ib-restricted CD8 T cell population is unknown. CD4 T cells likely provide other forms of help aside from IL-2, as exogenous addition of IL-2 during the maintenance phase did not prevent decay of the Q9:VP2.139 population following CD4+ cell depletion. We have recently demonstrated that MPyV-specific CD4 T cells, in addition to producing IL-2, produce IFN-γ, TNF-α, IL-10 and IL-21 (44). IL-21 has been demonstrated to sustain CD8 T cells during chronic infection (45–47), and it is tempting to speculate that IL-21 signaling may also support the sustained expansion and maintenance of Q9:VP2.139-specific CD8 T cells. In contrast to CD4 help, CD28 and CD40L costimulation are not required during the maintenance phase. This is consistent with Ag independence of the Q9:VP2.139-specific CD8 T cell population at this time (12), as lack of TCR signaling precludes a requirement for costimulation (48).
Costimulatory molecules other than CD28 and CD40L may contribute to the expansion and maintenance of Q9:VP2.139-specific CD8 T cells. The kinetics of CD27 expression by Q9:VP2.139-specific CD8 T cells parallel those of CD28 expression (Fig. 5E and Supplemental Figure 2). The decreased expression of CD27 over the course of MPyV infection would imply declining dependence on CD27-mediated signals. 2B4 is expressed at a steady but low level throughout the response (Supplemental Figure 2). This receptor can be activating or inhibitory, depending on the level of 2B4 expression, the extent of 2B4 cross-linking, and the relative abundance of signaling lymphocyte activation molecule-associated protein (49). OX-40, 4-1BB, and ICOS are expressed at only low levels by Q9:VP.139-specific CD8 T cells (Supplemental Figure 2). In contrast, CD94/NKG2A expression increases after d 12 p.i. and is maintained at high expression levels (Fig. 6D and Supplemental Figure 2). We previously showed that CD94/NKG2A is expressed by MHC class Ia-restricted, MPyV-specific CD8 T cells, where it inhibits cytotoxicity while increasing IL-2 production and proliferative potential (50, 51). However, the impact of CD94/NKG2A expression varies depending on infection model (52–54). Whether CD27, 2B4 or CD94/NKG2A expression on Q9:VP2.139-specific cells regulate IFN-γ functionality of these cells remains to be determined.
In this study, we explored CD4 T cell and CD28/CD40L costimulation as determinants for the inflationary kinetic profile of an MHC class Ib-restricted antiviral CD8 T cell population. We demonstrated that CD4 T cell help and intrinsic CD28 costimulation are necessary for expansion of Q9-restricted, MPyV-specific CD8 T cells and that CD4 T cell help is important for survival of these cells during their Ag-independent maintenance phase. Together with our previous study on the role of Ag in expansion and maintenance of Q9:VP2.139-specific CD8 T cells (12), we propose the following model: Q9:VP2.139-specific CD8 T cells are primed in a CD4 T cell-independent fashion, expand in response to combined signals provided by cognate Ag, CD4 T cell help and intrinsic CD28 signaling, and are then maintained independent of Ag and CD28/CD40L but dependent on CD4 T cell help. This sustained dependence on CD4 T cell help, which does not involve a CD40L-dependent mechanism or IL-2, suggests a novel mechanism of help for maintaining anti-MPyV MHC class Ib-restricted CD8 T cell response.
Supplementary Material
Acknowledgments
We thank the NIH Tetramer Core for developing a chimeric Q9:VP2.139 tetramer with the H2-Kb α3 domain, Linda Stempora for expertise with FACS-sorting, Kendall Smith for providing recombinant human IL-2, Erin West for protocols for administering IL-2 to mice, and Eugene Lin for helpful discussions and critical review of this manuscript.
Abbreviations used
- B6
C57BL/6
- MFI
mean fluorescence intensity
- gMFI
geometric MFI
- MPyV
mouse polyomavirus
- p.i
postinfection
- qPCR
quantitative PCR
Footnotes
This work was supported from NIH grants RO1 CA139220 (AEL) and R01 AI073707 (MLF).
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 2.Hofstetter AR, Sullivan LC, Lukacher AE, Brooks AG. Diverse roles of non-diverse molecules: MHC class Ib molecules in host defense and control of autoimmunity. Curr Opin Immunol. 2011;23:104–110. doi: 10.1016/j.coi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ib-restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J Immunol. 1997;159:2795–2801. [PubMed] [Google Scholar]
- 4.Seaman MS, Perarnau B, Lindahl KF, Lemonnier FA, Forman J. Response to Listeria monocytogenes in mice lacking MHC class Ia molecules. J Immunol. 1999;162:5429–5436. [PubMed] [Google Scholar]
- 5.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi T, Yamada H, Yajima T, Wajjwalku W, Hara T, Yoshikai Y. H2-M3-restricted CD8+ T cells induced by peptide-pulsed dendritic cells confer protection against Mycobacterium tuberculosis. J Immunol. 2007;178:3806–3813. doi: 10.4049/jimmunol.178.6.3806. [DOI] [PubMed] [Google Scholar]
- 7.He X, Tabaczewski P, Ho J, Stroynowski I, Garcia KC. Promiscuous antigen presentation by the nonclassical MHC Ib Qa-2 is enabled by a shallow, hydrophobic groove and self-stabilized peptide conformation. Structure. 2001;9:1213–1224. doi: 10.1016/s0969-2126(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 8.Stroynowski I, Soloski M, Low MG, Hood L. A single gene encodes soluble and membrane-bound forms of the major histocompatibility Qa-2 antigen: anchoring of the product by a phospholipid tail. Cell. 1987;50:759–768. doi: 10.1016/0092-8674(87)90334-5. [DOI] [PubMed] [Google Scholar]
- 9.Ungchusri T, Chiang EY, Brown G, Chen M, Tabaczewski P, Timares L, Stroynowski I. Widespread expression of the nonclassical class I Qa-2 antigens in hemopoietic and nonhemopoietic cells. Immunogenetics. 2001;53:455–467. doi: 10.1007/s002510100347. [DOI] [PubMed] [Google Scholar]
- 10.Stroynowski I, Tabaczewski P. Multiple products of class Ib Qa-2 genes which ones are functional? Res Immunol. 1996;147:290–301. doi: 10.1016/0923-2494(96)89642-8. [DOI] [PubMed] [Google Scholar]
- 11.Swanson PA, 2nd, Pack CD, Hadley A, Wang CR, Stroynowski I, Jensen PE, Lukacher AE. An MHC class Ib-restricted CD8 T cell response confers antiviral immunity. J Exp Med. 2008;205:1647–1657. doi: 10.1084/jem.20080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson PA, 2nd, Hofstetter AR, Wilson JJ, Lukacher AE. Cutting edge: shift in antigen dependence by an antiviral MHC class Ib-restricted CD8 T cell response during persistent viral infection. J Immunol. 2009;182:5198–5202. doi: 10.4049/jimmunol.0900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson JJ, Lin E, Pack CD, Frost EL, Hadley A, Swimm AI, Wang J, Dong Y, Breeden CP, Kalman D, Newell KA, Lukacher AE. Gamma interferon controls mouse polyomavirus infection in vivo. J Virol. 2011;85:10126–10134. doi: 10.1128/JVI.00761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemball CC, Pack CD, Guay HM, Li ZN, Steinhauer DA, Szomolanyi-Tsuda E, Lukacher AE. The antiviral CD8+ T cell response is differentially dependent on CD4+ T cell help over the course of persistent infection. J Immunol. 2007;179:1113–1121. doi: 10.4049/jimmunol.179.2.1113. [DOI] [PubMed] [Google Scholar]
- 15.Kemball CC, Lee ED, Szomolanyi-Tsuda E, Pearson TC, Larsen CP, Lukacher AE. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J Immunol. 2006;176:1814–1824. doi: 10.4049/jimmunol.176.3.1814. [DOI] [PubMed] [Google Scholar]
- 16.Jay DC, Reed-Loisel LM, Jensen PE. Polyclonal MHC Ib-restricted CD8+ T cells undergo homeostatic expansion in the absence of conventional MHC-restricted T cells. J Immunol. 2008;180:2805–2814. doi: 10.4049/jimmunol.180.5.2805. [DOI] [PubMed] [Google Scholar]
- 17.Andrews NP, Pack CD, Vezys V, Barber GN, Lukacher AE. Early virus-associated bystander events affect the fitness of the CD8 T cell response to persistent virus infection. J Immunol. 2007;178:7267–7275. doi: 10.4049/jimmunol.178.11.7267. [DOI] [PubMed] [Google Scholar]
- 18.Kemball CC, Lee ED, Vezys V, Pearson TC, Larsen CP, Lukacher AE. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J Immunol. 2005;174:7950–7960. doi: 10.4049/jimmunol.174.12.7950. [DOI] [PubMed] [Google Scholar]
- 19.Gu JJ, Gottlieb PD. Inducible functions in hybrids of a Lyt-2+ BW5147 transfectant and the 2C CTL line. Immunogenetics. 1992;36:283–293. doi: 10.1007/BF00215656. [DOI] [PubMed] [Google Scholar]
- 20.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 21.Szomolanyi-Tsuda E, Welsh RM. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Carr JM, Carrasco MJ, Thaventhiran JE, Bambrough PJ, Kraman M, Edwards AD, Al-Shamkhani A, Fearon DT. CD27 mediates interleukin-2-independent clonal expansion of the CD8+ T cell without effector differentiation. Proc Natl Acad Sci USA. 2006;103:19454–19459. doi: 10.1073/pnas.0609706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 25.Coley SM, Ford ML, Hanna SC, Wagener ME, Kirk AD, Larsen CP. IFN-γ dictates allograft fate via opposing effects on the graft and on recipient CD8 T cell responses. J Immunol. 2009;182:225–233. doi: 10.4049/jimmunol.182.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng L, Wu Z, Wang Y, Lassman C, Busuttil RW, Zhai Y, Kupiec-Weglinski JW. Differential impact of CD154 costimulation blockade on alloreactive effector and regulatory T cells in murine renal transplant recipients. Transplantation. 2008;85:1332–1338. doi: 10.1097/TP.0b013e31816c4f2b. [DOI] [PubMed] [Google Scholar]
- 27.Sitati E, McCandless EE, Klein RS, Diamond MS. CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis. J Virol. 2007;81:9801–9811. doi: 10.1128/JVI.00941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4+ T lymphocytes are required for the generation of the primary but not the secondary CD8+cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 31.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson PG, Belz GT, Altman JD, Doherty PC. Virus-specific CD8+ T cell numbers are maintained during γ-herpesvirus reactivation in CD4-deficient mice. Proc Natl Acad Sci USA. 1998;95:15565–15570. doi: 10.1073/pnas.95.26.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agnellini P, Wiesel M, Schwarz K, Wolint P, Bachmann MF, Oxenius A. Kinetic and mechanistic requirements for helping CD8 T cells. J Immunol. 2008;180:1517–1525. doi: 10.4049/jimmunol.180.3.1517. [DOI] [PubMed] [Google Scholar]
- 36.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overstreet MG, Chen YC, Cockburn IA, Tse SW, Zavala F. CD4+ T cells modulate expansion and survival but not functional properties of effector and memory CD8+ T cells induced by malaria sporozoites. PLoS One. 2011;6:e15948. doi: 10.1371/journal.pone.0015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 39.Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 40.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moody AM, Xiong Y, Chang HC, Reinherz EL. The CD8αβ co-receptor on double-positive thymocytes binds with differing affinities to the products of distinct class I MHC loci. Eur J Immunol. 2001;31:2791–2799. doi: 10.1002/1521-4141(200109)31:9<2791::aid-immu2791>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 42.Wu L, Exley GE, Warner CM. Differential expression of Ped gene candidates in preimplantation mouse embryos. Biol Reprod. 1998;59:941–952. doi: 10.1095/biolreprod59.4.941. [DOI] [PubMed] [Google Scholar]
- 43.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin E, Kemball CC, Hadley A, Wilson JJ, Hofstetter AR, Pack CD, Lukacher AE. Heterogeneity among viral antigen-specific CD4+ T cells and their de novo recruitment during persistent polyomavirus infection. J Immunol. 2010;185:1692–1700. doi: 10.4049/jimmunol.0904210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 47.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 49.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 50.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8+ T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 51.Byers AM, Andrews NP, Lukacher AE. CD94/NKG2A expression is associated with proliferative potential of CD8 T cells during persistent polyoma virus infection. J Immunol. 2006;176:6121–6129. doi: 10.4049/jimmunol.176.10.6121. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Matsuoka M, Cantor H, Homer R, Enelow RI. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 53.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 54.Cush SS, Flano E. KLRG1+NKG2A+ CD8 T cells mediate protection and participate in memory responses during γ-herpesvirus infection. J Immunol. 2011;186:4051–4058. doi: 10.4049/jimmunol.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.