Abstract
In prior studies we demonstrated that: 1) CXCL1/KC is essential for NF-κB and MAPK activation, and expression of CXCL2/MIP-2 and CXCL5/LPS-induced CXC chemokine in Klebsiella-infected lungs; and 2) CXCL1 derived from hematopoietic and resident cells contributes to host immunity against Klebsiella. However, the role of CXCL1 in mediating neutrophil Leukotriene B4 (LTB4), reactive oxygen species (ROS), and reactive nitrogen species (RNS) production is unclear, as is the contribution of these factors to host immunity. Here, we investigated: 1) the role of CXCL1 in LTB4, NADPH oxidase, and iNOS expression in lungs and neutrophils; and 2) whether LTB4 after infection reverses innate immune defects in CXCL1-/- mice via regulation of NADPH oxidase and iNOS. Our results demonstrate reduced neutrophil influx, attenuated LTB4 levels, and decreased ROS and iNOS production in the lungs of CXCL1-/- mice following K. pneumoniae infection. Using neutrophil depletion and repletion, we found that neutrophils are the predominant source of pulmonary LTB4 following infection. To treat immune defects in CXCL1-/- mice, we intrapulmonary administered LTB4. Post-infection LTB4 treatment reversed immune defects in CXCL1-/- mice and improved survival, neutrophil recruitment, cytokine/chemokine expression, NF-κB/MAPK activation, and ROS/RNS production. LTB4 also enhanced MPO, H2O2, RNS production, and bacterial killing in K. pneumoniae-infected CXCL1-/- neutrophils. These novel results uncover important roles for CXCL1 in generating ROS and RNS in neutrophils and in regulating host immunity against K. pneumoniae infection. Our findings suggest that LTB4 could be used to correct defects in neutrophil recruitment and function in individuals lacking or expressing malfunctional CXCL1.
INTRODUCTION
Gram-negative bacterial pneumonia continues to be a major cause of morbidity, mortality, and health care costs (1-3). Neutrophils are the first responders to migrate towards the site of infection in order to clear causative bacteria, however their excessive accumulation is associated with devastating pathological consequences including acute lung injury and acute respiratory distress syndrome (4-6). ELR+ CXC chemokines, including CXCL1/KC, CXCL2/MIP-2 and CXCL5/LIX, are potent chemotactic mediators for neutrophils (7-12). To determine the impact of CXCL1 on host immunity in the lung, we previously used a mouse model of Klebsiella pneumoniae infection and found CXCL1 to be important for neutrophil-dependent bacterial clearance in the lung (13). We also demonstrated that CXCL1 regulates the activation of NF-κB and MAPKs, and the expression of other neutrophil chemokines, including CXCL2 and CXCL5 (13).
Bacterial clearance by neutrophils depends on the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (14, 15). Formation of ROS is catalyzed by NADPH oxidase and myeloperoxidase (MPO), whereas nitric oxide synthases (NOSs) catalyze the reaction to form RNS (16, 17). Upon activation, oxygen consumption in neutrophils increases and the oxygen molecule is univalently reduced to superoxide by the membrane-bound NADPH oxidase complex (18, 19). Although the core enzyme consists of five subunits including p67phox, p47phox, p40phox, p22phox and gp91phox, only p67phox, p47phox and p40phox exist in the cytoplasm in an unactivated state (18, 19). Upon cell activation, p67phox, p47phox, and p40phox translocate onto the membrane. This complex is an electron transport chain that produces H2O2 in combination with superoxide dismutase (18, 19) Superoxide is further converted to reactive hypochlorite by myeloperoxidase (18, 19). Furthermore, nitric oxide is produced from guanidino nitrogen during the conversion of L-arginine to L-citrulline by NOSs (20).
Leukotriene B4 (LTB4) has been shown to be a neutrophil chemoattractant derived from membrane phospholipids (21, 22). The role of LTB4 in the context of ROS and RNS production and bacterial killing has largely been explored in macrophages. LTB4 induces NADPH oxidase activation in alveolar macrophages (AMs) in response to Klebsiella infection. LTB4-deficient human AMs exhibit impaired phagocytosis and killing of pneumococci, and these defects can be restored by addition of exogenous LTB4 (23). Genetic deletion of 5-lipoxygenase (5-LO) or pharmacological inhibition of LTB4 biosynthesis in mice results in enhanced mortality and attenuated microbial clearance following pneumococcal infection; this occurs via recruitment of macrophages but not neutrophils (24, 25). One of these reports also demonstrated that LTB4 augmented p47phox expression and bacterial clearance in primary lung macrophages (24). In this regard, LTB4 has been shown to augment killing of K. pneumoniae by murine AMs via ROS but not RNS (26). In human AMs, nitric oxide has been shown to be important in Klebsiella clearance (27). However, more detailed mechanisms underlying LTB4 restoration in the lung or in macrophages have yet to be explored.
Despite the critical role of neutrophil recruitment and responses during pulmonary K. pneumoniae clearance, little is known about the role of CXCL1, LTB4, NADPH oxidase, or iNOS in neutrophils during K. pneumoniae infection. We illustrate that CXCL1 controls neutrophil immunity by regulating LTB4, ROS, and RNS production following Klebsiella infection. Compared to WT controls, exogenous LTB4 corrected host immunity in CXCL1-/- mice by restoring neutrophil influx, bacterial clearance, cytokine/chemokine production, activation of NF-κB and MAPKs, as well as expression of ROS and RNS. Moreover, LTB4 restored ROS and RNS generation and bacterial killing capacity in K. pneumoniae-stimulated CXCL1-/- neutrophils. These findings identify novel molecular and cellular mechanisms underlying the enhancement of neutrophil immunity in CXCL1-/- mice by a single dose of intrapulmonary LTB4 administration.
MATERIALS AND METHODS
Animals
Eight to ten-week old CXCL1 gene-deficient (CXCL1-/-) female mice were backcrossed 10 times with C57Bl/6 mice for this study; therefore C57Bl/6 (WT) mice were used as age- and gender-matched controls (28). Animal experiments were conducted as per Louisiana State University Animal Welfare Committee's approved protocol.
Bacterial preparation and infection
The K. pneumoniae strain (ATCC 43816) was grown in tryptic soy broth overnight to mid-logarithmic phase at 37°C while shaking at 200 rpm. Following PBS washings, bacteria were resuspended in isotonic saline at a concentration of 103 CFU/50 μl/mouse. For infection, a ketamine/xylazine mixture was used to anesthetize mice and the trachea was exposed for inoculation with 103 CFU/mouse (13, 29). A 10-fold serially diluted suspension of initial inoculum was plated onto Tryptic Soy Agar (TSA) plates and MacConkey plates for validation of the inoculum.
LTB4 administration
LTB4 (Cayman Chemicals, Ann Arbor, MI) was prepared in PBS containing 0.1% BSA to a final concentration of 2 μg/ml, and 50 μl/mouse (100 ng/mouse) was administered i.t. at 1 h post-Klebsiella challenge as described (23). Following 48 h post-infection, bronchoalveolar lavage fluid (BALF) or lungs were collected for LTB4 determination as described in our previous publications (24).
BALF isolation and lung harvesting
BALF was collected as described previously (13, 29-31). In brief, trachea were cannulated with a 20-gauge catheter, then a total of 0.9 ml BAL buffer was introduced, flushed 4 times, and retrieved. A total of 3.0 ml BALF was retrieved from each mouse. Cytospin slides prepared with 0.5 ml of BALF were stained by Diff-Quick reagents (Fisher, Chicago, IL) to enumerate leukocyte subtypes based on their cellular and nuclear morphological properties. Lungs were excised at designated time-points post-K. pneumoniae challenge and were immediately snap frozen followed by storage at -80°C for later use.
Total WBC, PMN, cytokine/chemokine, and LTB4 determination
We used BALF and lungs that were obtained from animals following K. pneumoniae infection, K. pneumoniae+LTB4 administration or K. pneumoniae +BSA (vehicle) instillation. ELISA kits for TNF-α and IL-6 were obtained from eBioscience San Diego, CA) whereas kits for CXCL1, MIP-2, LIX and LTB4 were obtained from R&D Systems (Minneapolis, MN). The minimum detection limit was 8 pg/ml of cytokine/chemokine protein, whereas the detection limit for LTB4 was 13.7 pg/ml (13, 29-32). For cellular recruitment, cytospin samples were subsequently prepared from BALF cells and stained with Diff-Quick. Total leukocytes in BALF were determined using a hemocytometer whereas leukocyte subsets were examined by direct counting of stained slides based on cell and nuclear morphology (13, 29-32).
Subcellular fractionation
To obtain cytosolic and membrane fractions, harvested lung tissues were fractionated using a cell fractionation kit (Biovision, City, State) following the manufacturer's recommendations (33). Membrane and cytoplasm fractions were obtained and equal amounts of protein for each fraction were subjected to SDS-PAGE. Western blots were probed with anti-p67phox and p47phox antibodies. The same membranes were immunoblotted with GAPDH and Pan cadherin to indicate the cytoplasm and membrane fractions, and to demonstrate equal loading on gels.
Neutrophil purification
Neutrophils were purified from bone marrow derived cells by negative magnetic selection (StemCell Technologies, Vancouver, Canada). Bone marrow cells were flushed from tibias and femurs using PBS. The cell suspension was passed through a 0.70 μ filter then resuspended in RoboSep buffer (PBS without Ca2+/Mg2+, 2% FBS, 1 mM EDTA). Purified neutrophils were obtained from bone marrow cells by using a custom mixture containing Abs to CD5, CD4, CD45R/B220, TER119, F4/80, CD11c, c-KIT (Cat No# 19709). Briefly, bone marrow cells were incubated at 4° C in RoboSep buffer containing 5% normal rat serum along with the custom antibody cocktail, biotin selection cocktail, and magnetic colloid according to manufacturer's instructions. Samples were then placed in the EasySep magnet. Following three minutes of incubation, samples highly enriched with neutrophils were poured off into the new tube (34). Purified cells were washed and resuspended in RPMI-1640 containing 10% FBS. Cells were then counted using a hemocytometer and used for neutrophil-mediated bacterial killing, MPO activity, and release of H2O2, nitric oxide (NO) and to determine the expression levels of the components of NADPH oxidase system after K. pneumoniae infection. Neutrophil purity neared 91%, as assessed by cytospin slides and flow cytometry (data not shown).
Neutrophil depletion
The neutrophil depletion protocol (GR1/Ly6G) used here has been described earlier (13, 31). A total of 50 μg of anti-mouse Ly6G mAb (clone IA8; BD Pharmingen, City, State) per mouse was administrated i.p. in a 50 μl at 12 and 2 h prior to bacterial infection. As a control, 50 μg of isotype-matched control mAb in an equal volume was administrated prior to infection. To validate the efficiency of anti-Gr1/Ly6G mAb in neutrophil depletion, we determined blood neutrophil counts every 12 h up to 3 days and observed that after depletion only 2-3% of neutrophils remained in circulation during this period (data not shown).
Neutrophil repletion
To examine the role of neutrophils in contributing to LTB4, PMN repletion was performed i.t. in neutropenic mice with PMNs (107 cells/mouse) isolated from WT or CXCL1-/- mice 30 min prior to K. pneumoniae infection.
Bone marrow transplantation
Generation of CXCL1 chimeras has been described in our earlier publications (13, 35). Briefly, donor and recipient animals (between 6–8 wk old) were used to make chimeras. Recipients were γ-irradiated in two 525-rad doses delivered over 3 h. Isolated bone marrow cells (8 × 106/mouse) were injected into the tail vein of recipients and animals were kept on 0.2% antibiotic (neomycin sulfate) for the first 3 wks. Transplanted mice were used 6-8 wk after bone marrow transfer. We found that greater than 84% of blood leukocytes were derived from donor mice at the time of experiments.
Immunoblotting
At the designated times, harvested lungs were homogenized in 1 ml of buffer containing 0.1% Triton X-100 in PBS, with complete protease and phosphatase inhibitor cocktail (Roche Co, Indianapolis, IN) for 30 s and centrifuged at maximum speed in a microcentrifuge at 4°C. The resulting supernatant fluids were used for immunoblotting. To ensure that equal amounts of protein were loaded onto the gel, a Bradford protein assay was used to measure protein levels in the sample (Bio-Rad, Hercules, CA). The whole lung homogenates were resolved on 10% SDS-PAGE and the resolved proteins were transferred to PVDF membrane using standard protocols (13, 29). Antibodies to p67phox, p47phox, and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); whereas antibodies to iNOS, ICAM-1, VCAM-1, phospho-NF-κB (Ser536), NF-κB, phospho-Iκ-Bα (Ser 32/36),Iκ-Bα, phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), phospho-p38 MAPK (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), total p38 MAPK, and pan-cadherin were obtained from Cell Signaling Technology (Danvers, MA). Primary antibodies were added at a 1:1000 dilution whereas monoclonal Ab to mouse GAPDH was added at 1:5000 dilution. Immunostaining was performed using appropriate secondary Ab at a dilution of 1: 5000, and developed with ECL plus Western blot detection system (ThermoFisher, Piscataway, NJ). The intensity of immunoreactive bands was determined using gel Digitizing Software (UN-SCAN-IT gel™ from Silk Scientific, Inc., City, Utah) for densitometric analysis.
Nitric oxide release by neutrophils
The NO assay was performed as described in previous reports (36, 37). Neutrophils isolated from bone marrow were infected with 1 MOI of K. pneumoniae for different time intervals. Media were collected at designated time-points for the detection of NO2 and NO3 anions using a colorimetric assay kit (Cayman Chemical Company, MI). A standard curve was plotted by diluting standards with incubation media (36, 37)
Hydrogen peroxide release by neutrophils
Hydrogen peroxide levels were measured to evaluate reactive oxygen species production by using the Fluorescent H2O2/Peroxidase Detection Kit (Cell Technology, Inc, Mountain View, CA). The Fluoro H2O2 detection kit utilizes a non-fluorescent detection reagent to detect H2O2. H2O2 oxidizes the detection reagent in a 1:1 stoichiometry to produce a fluorescent product, resorufin, which is catalyzed by peroxidase in a homogeneous no wash assay system. After incubation, fluorescence was measured at an excitation of 540 nm and an emission of 595 nm (38).
MPO activity in neutrophils and lungs
MPO release by the neutrophils was measured as previously described with minor modifications (13, 29). Briefly, culture media obtained at different time intervals was stored at -80 °C until evaluation. The reaction mixture consisted of 100 μl of culture media and 50 μl of 1.0% hexadecyltrimethylammoniumbromide (HTAB) (Sigma Chemical, St. Louis, MO) in 100 mmol/l phosphate buffer (pH 6.0) and 50 μl of 50 mmol/l potassium phosphate buffer (pH 6.0) containing o-dianisidine hydrochloride (0.167 mg/ml; Sigma Chemical, St. Louis, MO) and 0.0005% hydrogen peroxide. Absorbance change at 460 nm was recorded with a spectrophotometer (U-2001, Hitachi, Tokyo, Japan) every 5 min at room temperature. For MPO activity in the lung and neutrophils, tissues were homogenized or cells were lysed in HTAB-phosphate buffer containing o-dianisidine hydrochloride and hydrogen peroxide, and MPO activity was measured in cells as described in our earlier publication (13, 29-31).
Survival studies
WT and CXCL1-/- mice were inoculated i.t. with 103 CFU of K. pneumoniae in 50 μl of 0.9% saline and subsequently divided into two groups. One group received LTB4 in PBS containing 0.1% BSA to a final concentration of 2 μg/ml; 50μl/mouse was administered i.t. at 1 h post- Klebsiella challenge. The control animals received 50 μl/mouse PBS containing 0.1% BSA and animal survival was examined over 15 days (13, 29).
Lung bacterial CFUs
The lungs of mice were weighed and homogenized in 1 ml of 0.9% saline using a tissue homogenizer. Supernatants were serially diluted and 20 μl aliquots of each sample were plated on MacConkey agar and TSA plates. The number of colonies was enumerated after incubation at 37°C overnight (13, 29).
Phagocytosis assay
Klebsiella expressing green fluorescent protein (GFP) were opsonized in RPMI containing 10% FBS for 30 mins at 37°C with constant agitation. Purified neutrophils and bacteria were mixed at a 1:1 ratio in polystyrene tubes (1 MOI) with agitation at 37°C in 100 μl of RPMI containing 10% FBS. After incubation at designated time-points, tubes were placed on ice to stop phagocytosis and gentamicin was added to tubes at a final concentration of 100 μg/ml. The rationale for using 100 μg/ml gentamicin is that this dose was sufficient to kill 100% of extracellular bacteria, as confirmed by others (39, 40). After 15 min of incubation on ice, the suspension was washed twice and resuspended in PBS for flow cytometric analysis. As a control, one tube containing neutrophils only (no bacteria) was carried through the procedure to evaluate the background fluorescence of PMNs alone (41, 42). Antibactericidal activity of gentamicin was determined by plating the samples at each time point on MacConkey agar. At the 100 μg/ml concentration, gentamicin was able to kill 100% of extracellular bacteria (data not shown). Similar findings have been reported in earlier studies (39, 40).
Bacterial killing activity of neutrophils
A neutrophil killing assay was performed as described earlier with slight modifications (43). Briefly, 1×106 neutrophils were suspended in RPMI-1640 with 10% v/v FBS, and 1×106 opsonized bacteria were added to polypropylene tubes (1 MOI). The tubes were incubated in a shaking water bath at 37°C for 180 min with continuous agitation, then samples were harvested at 30, 60, or 180 min and a portion of the sample was spun at 100 × g for 10 min to collect the viable bacteria in media. Gentamicin (100 μg/ml) was added to the neutrophil pellet for 15 min to kill extracellular bacteria (39, 40). The neutrophil pellet was resuspended in 1 ml of PBS with 0.05% (w/v) saponin and the debris was broken up using a ground glass homogenizer to evaluate engulfed bacteria. Colony counting of viable bacteria was conducted by plating 20 μl aliquots of each sample on MacConkey agar and TSA plates. The number of colonies was enumerated after incubation at 37°C overnight. .
Statistical analysis
Data were analyzed by Student's t-test for two-way comparisons, for comparisons among three or more groups analysis was carried out by two-way ANOVA followed by Bonferroni post-hoc test. Survival curves were compared by Wilcoxon signed-rank test. All statistical calculations were performed using InStat software and GraphPad Prism 4.0 (San Diego, CA). Significance was defined as p<0.05.
Results
CXCL1 controls neutrophil recruitment and LTB4 production in the lungs following K. pneumoniae infection
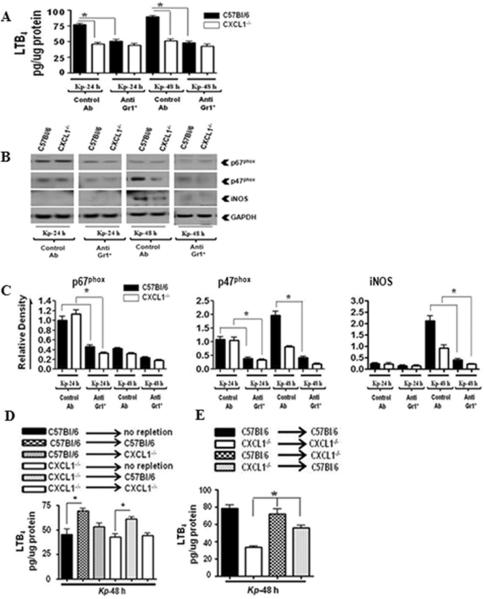
Leukocyte recruitment to the lungs is a critical step in host immunity to clear bacteria. In our previous report, we showed that pulmonary Klebsiella challenge leads to substantially reduced total WBC and neutrophil counts in the BALF of CXCL1-/- mice as compared to their littermate controls (13). In the current study, we extended these observations by specifically examining neutrophil-specific responses that might be regulated by CXCL1 deficiency. We began by examining MPO, and found it to be significantly decreased in the lungs of CXCL1-/- mice at 24 and 48 h post-infection (Fig. 1A). Since LTB4 is a neutrophil chemotactic lipid, we also examined LTB4 levels in the lungs of CXCL1-/- mice following K. pneumoniae administration. We found significantly reduced levels of LTB4 in CXCL1-/- mice at 24 and 48 h post-infection as compared to littermate controls (Fig. 1B). These findings indicate that CXCL1 is an essential regulator of neutrophil influx and LTB4 production in the lungs during K. pneumoniae infection.
Figure 1. MPO activity and LTB4 production in the lungs is dependent on CXCL1 during K. pneumoniae infection.
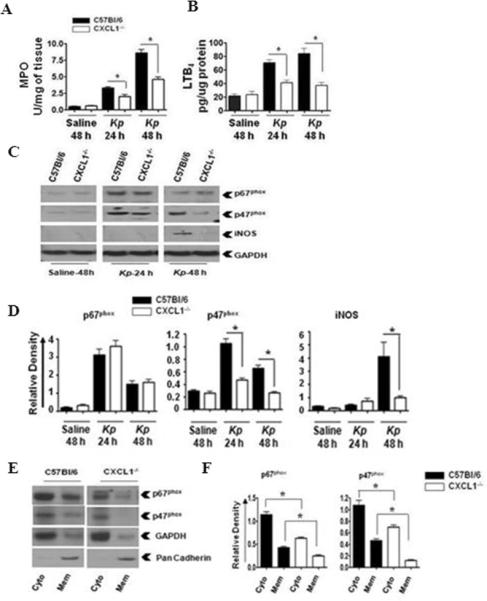
MPO activity (A) and LTB4 levels (B) in homogenized (unlavaged) whole lungs of WT (C57Bl/6) and CXCL1-/- mice infected with K. pneumoniae (103 CFU/mouse) for 24 and 48 h. Data are presented as means ± SEM. n=6-8 mice/group. (* indicates p<0.05 compared with CXCL1-/- mice). The levels of p67phox, p47phox, and iNOS (C) in K. pneumoniae-infected homogenized whole lungs of WT and CXCL1-/- mice at 24 and 48 h post-infection. The blot is representative of three individual blots with identical results. Densitometric analysis of p67phox, p47phox and iNOS expression (D) in homogenized lungs. Densitometry was performed from three separate blots. Data are expressed as means ± SEM. n=6-8 mice/group. (* indicates p<0.05 compared to CXCL1-/- mice). Levels of p67phox and p47phox in cytosolic and membrane fractions of the lung (E) upon K. pneumoniae infection. This is a representative blot of three independent experiments. GAPDH is a cytosolic marker, pan-cadherin is a plasma membrane marker. Densitometric analysis of p67phox and p47phox levels (F) in cytosol and membrane fractions of the lung following K. pneumoniae infection. Protein expression was quantitated from three separate blots. Data are shown as means ± SEM. For experiments E-F, a total of 6-8 mice were used in each group. (* indicates p<0.05 as compared to CXCL1-/- mice.)
CXCL1 regulates the expression of NADPH oxidase and iNOS in the lung following K. pneumoniae infection
The NADPH oxidase complex includes p67phox and p47phox, which regulate the production of H2O2, whereas inducible nitric oxide synthase (iNOS) controls the production of reactive nitrogen species; both mechanisms are critical for bacterial degradation (14-17). Therefore, we next determined whether CXCL1 is important for the expression of NADPH oxidase and iNOS in the lung following K. pneumoniae infection. We found that the expression of p47phox, a key component of the NADPH oxidase, was decreased in CXCL1-/- mice at 24 and 48 h post-infection, whereas the expression of iNOS was decreased at 48 h post-infection, the time point at which it was first detected in WT (Figs. 1C-D). Furthermore, the expression of p67phox was not reduced in CXCL1-/- mice compared to WT at either 24 or 48 h post-infection (Figs. 1C-D). Nevertheless, the expression of both p67phox and p47phox was higher at 24 h, whereas the expression of iNOS was higher at 48 h (Figs. 1C-D). To determine the level of activation of NADPH oxidase, we explored the translocation of p67phox and p47phox from the cytoplasm to the plasma membrane in lung cells 48 h post-K. pneumoniae challenge. Our results show attenuated p67phox and p47phox translocation in the lungs of CXCL1-/- mice compared to their littermate controls (Fig. 1E-F). These data suggest that CXCL1 is essential for the regulation of ROS and RNS generation in the lungs.
Neutrophils are the major source of LTB4, NADPH oxidase, and iNOS expression in the lung following Klebsiella infection
Neutrophils are known to be critical in controlling K. pneumoniae infection in the lung (13, 31). To determine whether neutrophils are important for NADPH oxidase and iNOS expression in the lung during K. pneumoniae infection, we depleted granulocytes in WT and CXCL1-/- mice using anti-Gr-1/Ly6G antibody, then determined the expression of LTB4, p67phox, p47phox, and iNOS in lungs after infection. To our surprise, we found substantial reduction in LTB4, p67phox, p47phox and iNOS in the lungs of granulocyte-depleted mice following K. pneumoniae challenge (Figs 2A-C). Furthermore, intratracheal neutrophil repletion in neutropenic mice supported the predominant role of neutrophils in LTB4 production (Fig. 2D), as bone marrow chimera experiments demonstrated that bone marrow cells were a major source of LTB4 (Fig. 2E). These observations suggest that neutrophils are the predominant contributors of NADPH oxidase and iNOS expression in the lungs of K. pneumoniae-infected mice.
Figure 2. Neutrophil depletion impairs LTB4, iNOS, and NADPH oxidase component expression in lung tissues during K. pneumoniae infection.
LTB4 levels (A) in the lungs of neutrophil-depleted WT and CXCL1-/- mice at 24 and 48 h following K. pneumoniae infection (103 CFUs/mouse). WT and CXCL1-/- mice were i.p. injected with anti-Gr-1/Ly6G or control Ab at 12 and 2 h before i.t. infection with K. pneumoniae. Data are presented as means ± SEM. (* indicates p < 0.05 compared with control Ab-administered mice.) Protein levels of p67phox, p47phox, and iNOS in whole lung homogenates (B) of neutrophil-depleted WT and CXCL1-/- mice after K. pneumoniae infection (103 CFUs/mouse). This blot is representative of three separate blots. Densitometric analysis (C) of p67phox, p47phox, and iNOS levels in the lungs of neutrophil-depleted WT and CXCL1-/- mice following K. pneumoniae (103 CFUs/mouse) infection. Western blots from three independent blots experiments were used to quantify protein levels compared to GAPDH. Data are expressed as mean ± SE. For experiments A-C, n=6-9 mice/group. (* indicates p<0.05 as compared to control Ab-administered mice.) Role of hematopoietic and resident cells in LTB4 production. Levels of LTB4 (D) in the lungs of i.t. neutrophil repleted (107 cells/mouse) neutropenic WT and CXCL1-/- mice at 48 h following K. pneumoniae infection. Data are presented as means ± SEM. (* indicates p<0.05 compared to depleted (non-repleted) mice.) (E) Bone marrow chimeras were generated and LTB4 levels in lungs were measured at 48 h post K. pneumoniae challenge. A total of 5-7 mice/group were used. (* indicates p < 0.05 compared with CXCL1->CXCL1 mice.)
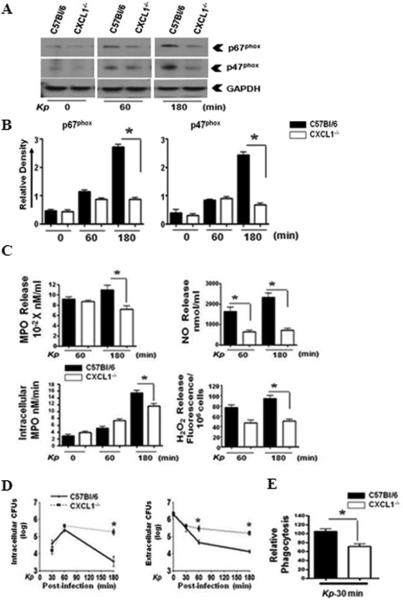
CXCL1 is essential for expression of NADPH oxidase and formation of oxygen free radicals and nitric oxide in neutrophils following K. pneumoniae infection in vitro
Since neutrophil depletion reduced the expression/activation of NADPH oxidase components and iNOS, we specifically examined whether CXCL1 plays an essential role in the expression of NADPH oxidase components and iNOS in purified neutrophils. We found reduced expression of p67phox and p47phox in neutrophils isolated from CXCL1-/- mice at 180 min after K. pneumoniae stimulation (Fig 3A-B). We also observed attenuated MPO, NO, and H2O2 production from neutrophils of CXCL1-/- mice at this time-point whereas NO production was reduced in neutrophils even at an earlier time-point (Fig. 3C). Furthermore, bacterial killing and phagocytosis by neutrophils were impaired in CXCL1-/- neutrophils (Fig. 3D-E).
Figure 3. CXCL1 is essential for Klebsiella-induced expression of p67phox, p47phox, and ROS/RNS production by neutrophils.
Levels of p67phox and p47phox (A) in bone marrow-derived neutrophils from WT and CXCL1-/- mice after infection with K. pneumoniae (MOI of 1). This is a representative blot from three separate experiments. Densitometric analysis from three separate blots (B) shows the expression of p67phox and p47phox in K. pneumoniae stimulated-neutrophils that were normalized against GAPDH. (* indicates p<0.05 as compared to CXCL1-/- neutrophils). MPO activity and nitrite and H2O2 release (C) in WT and CXCL1-/- neutrophils stimulated with K. pneumoniae. The levels of MPO, nitrite, and H2O2 were measured in infected neutrophils at 30, 60, and 180 min post-infection. Experiments were performed in triplicates. Bacterial killing capacity (D) of K. pneumoniae-infected neutrophils from WT and CXCL1-/- deficient mice was determined by assessing extracellular and intracellular CFUs at 30, 60, and 180 mins post-infection with K. pneumoniae (MOI of 1). Relative phagocytosis (E) of K. pneumoniae-infected WT and CXCL1-/- neutrophils at 30 min post-treatment (MOI of 1). For experiments A-E, a total of 5-8 mice/group were used. (* indicates p<0.05 compared with CXCL1-/- neutrophils).
LTB4 administration augments survival, bacterial clearance, cellular recruitment, and expression of cytokines/chemokines in the lungs of CXCL1-/- mice following K. pneumoniae infection
Because CXCL1 regulates LTB4 as well as ROS and RNS during bacterial infections, we wanted to determine whether LTB4 regulates ROS- and RNS-dependent host immune mechanisms in K. pneumoniae-infected CXCL1-/- mice. We administered LTB4 i.t. to CXCL1-/- mice at 1 h post-K. pneumoniae challenge, based on an earlier report showing that this route was the most efficient for administering LTB4 in mice (24). Intriguingly, we observed that LTB4 treatment dramatically improved survival and bacterial clearance the lungs and bacterial dissemination in CXCL1-/- mice, but not WT mice, following K. pneumoniae infection (Fig.4 A-C). We next examined whether LTB4 administration affects pathologic consequences in the lungs of CXCL1-/- mice during Gram-negative bacterial pneumonia. Total leukocyte count (Fig. 4D), neutrophil count (Fig. 4E), expression of cytokines (TNF-α, IL-6) and chemokines (MIP-2 and LIX) were enhanced in CXCL1-/- mice upon LTB4 administration (Fig. 4F). These findings show that LTB4 can correct impaired host immune defects in CXCL1-/- mice following K. pneumoniae infection.
Figure 4. Impaired survival, bacterial clearance, neutrophil influx, and cytokine/chemokine production in the lungs of CXCL1-/- mice are restored by exogenous LTB4.
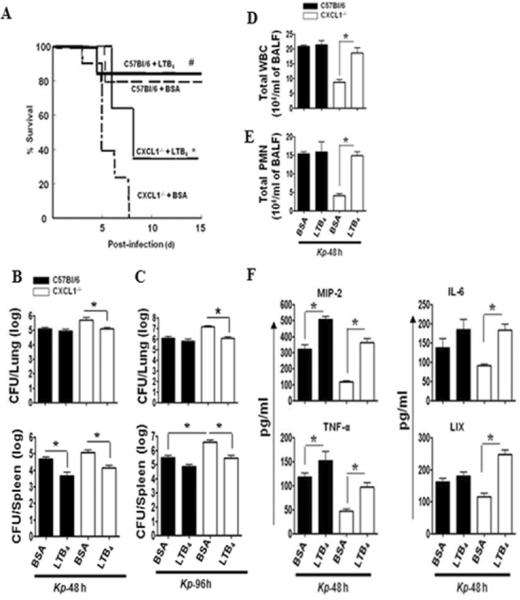
Mortality (A) in WT or CXCL1-/- mice infected with 103 CFUs of K. pneumoniae and administered with LTB4 (100 ng/mouse) or vehicle (BSA) 1 h later, and survival was assessed up to 15 days. Data are presented as % survival (n=20 mice/group) and analyzed using Wilcoxon signed-rank test. * indicates the difference between LTB4 or vehicle (BSA) control treated CXCL1-/- mice (p<0.05) and # indicates the difference between WT+LTB4 and CXCL1-/-+LTB4 mice.) Bacterial clearance (B, C) in the lungs and dissemination were examined in lung homogenates of LTB4 or BSA administered WT and CXCL1-/- mice at 48 and 96 h post-K. pneumoniae challenge (103 CFUs/mouse). Data are presented as Mean ± SE (n=5-6 mice/group). (* indicates p<0.05 compared with BSA (vehicle) administered mice.) Cellular infiltration (D,E) in airspaces at 48 h after i.t. treatment with LTB4 (100 ng/mouse) or vehicle (BSA) control. Concentrations of CXCL2/MIP-2, IL-6, TNF-α, and CXCL5/LIX (F) in BALF from CXCL1-/- or WT mice infected with 103 CFUs of K. pneumoniae and administered with LTB4 or vehicle (BSA). For experiments D-F, n=6-8 mice/group. (* indicates p<0.05 as compared to BSA administered mice).
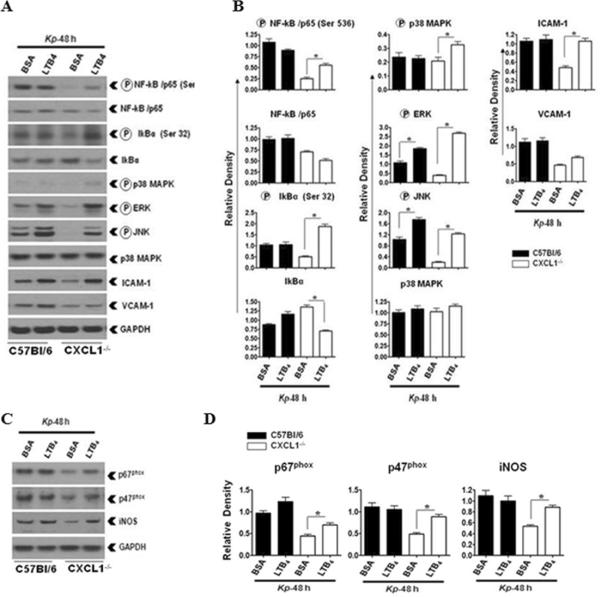
LTB4 administration improves activation of NF-κB, MAPKs, and expression of cellular adhesion molecules in the lungs of CXCL1-/- mice following K. pneumoniae infection
To explore LTB4-mediated host immune mechanisms in CXCL1-/- mice in more detail, we measured the activation of NF-κB, MAPKs, and expression of ICAM-1 and VCAM-1 in the lungs following K. pneumoniae infection. Subsequent to LTB4 administration in K. pneumonia-infected CXCL1-/- mice, we observed augmented activation of NF-κB and MAPKs along with increased expression of ICAM-1 in CXCL1-/- mice (Fig 5A-B). These findings demonstrate that augmentation of host immune mechanisms in K. pneumoniae-infected CXCL1-/- mice after LTB4 administration involve activation of NF-κB and MAPKs (JNK and ERK), and upregulation of cell adhesion molecules.
Figure 5. Defective K. pneumoniae-induced activation of NF-κB, MAPKs, and expression of ICAM-1 in the lungs of CXCL1-/- mice is corrected by LTB4.
Activation of NF-κB and MAPKs and expression of ICAM-1 and VCAM-1 (A) in K. pneumoniae-infected and LTB4 administered homogenized whole lungs at 48 h post-infection. WT and CXCL1-/- mice were infected with 103 CFU of K. pneumoniae cells via the i.t. route and treated with LTB4 (100 ng/mouse) or vehicle administration 1 h later. Data are presented as a representative of three independent blots/experiments. Densitometric analysis (B) of activation of NF-κB and MAPKs and expression of ICAM-1 and VCAM-1 in the lung homogenates after LTB4 or vehicle treatment. Data represent the means ± SEM of arbitrary densitometric units for each band from three independent blots/experiments. (* indicates p<0.05 as compared to BSA treated C57Bl6 or CXCL1-/- mice). Expression levels of p67phox, p47phox, and iNOS (C) in homogenized lungs of K. pneumoniae-infected and i.t.-treated with vehicle or LTB4 (100 ng) 1 h post-infection. This blot is representative of three independent blots. Densitometric analysis of iNOS, p67phox, and p47phox levels in lung (D) homogenates from three independent experiments (p<0.05). (* indicates p< 0.05 compared with C57Bl6 or CXCL1-/- vehicle (BSA) administered mice (n=5-6/group). For experiments A-D, a total of 6-9 mice/group was used.
LTB4 augments expression of NADPH oxidase components in the lungs of CXCL1-/- mice following K. pneumoniae infection
We next examined whether LTB4 administration in K. pneumoniae-challenged CXCL1-/- mice leads to increased expression of NADPH oxidase and iNOS. Our results show that LTB4 augments expression of p67phox, p47phox, and iNOS in CXCL1-/- mice (Fig 5C-D). On the other hand, LTB4 administration in K. pneumoniae-infected WT mice did not alter the expression levels of either NADPH oxidase components or iNOS (Fig. 5C-D).
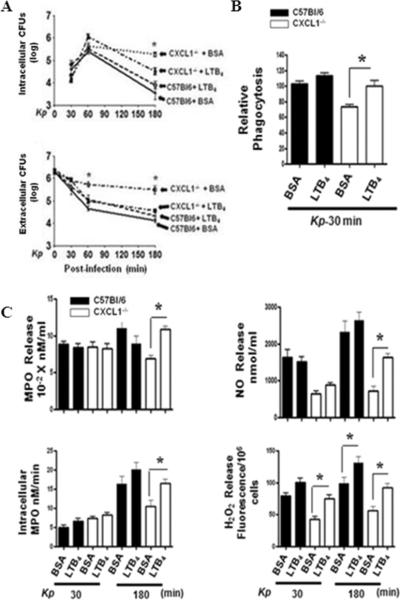
LTB4 treatment enhances phagocytosis, bacterial killing, and ROS/RNS production in neutrophils of CXCL1-/- mice following K. pneumoniae stimulation
The local restoration of components important for neutrophil bactericidal activity after i.t. LTB4 administration suggested that the deficit in bacterial killing in CXCL1-/- neutrophils was dependent on LTB4. In order to determine whether LTB4 treatment augments bacterial killing, we purified neutrophils from bone marrow by negative selection and infected them with K. pneumoniae. Our findings show that LTB4 treatment improved bacterial killing by CXCL1-/- neutrophils (Fig 6A). The mechanism for this appeared to be multi-factorial, as LTB4 administration led to enhanced phagocytosis of K. pneumoniae in CXCL1-/- neutrophils (Fig 6B), and increased neutrophil MPO activity and H2O2 production (Fig 6C). These findings suggest that LTB4 is critical for phagocytosis and bacterial clearance in CXCL1-/- neutrophils via oxidative stress.
Figure 6. Impaired bacterial killing, ROS and RNS generation, and phagocytosis in K. pneumoniae-stimulated CXCL1-/- neutrophils is restored by LTB4.
(A) Bacterial killing capacity of K. pneumoniae-infected, LTB4-treated neutrophils from WT and CXCL1-/- deficient mice was determined at 30, 60, and 180 mins by assessing intracellular and extracellular CFUs. Relative phagocytosis (B) of K. pneumoniae-infected WT and CXCL1-/- neutrophils at 30 min post-treatment (1 MOI). Relative phagocytosis index was calculated as described in Materials and Methods. MPO release, intracellular MPO, NO, and H2O2 production (C) in K. pneumoniae-infected WT and CXCL1-/- neutrophils at 30 and 180 mins post-LTB4 treatment. For experiments A-C, a total of 7-9 mice/group were used. (* indicates p<0.05 as compared to BSA treated neutrophils).
Discussion
Previous studies had shown that CXCL1 enhanced neutrophil recruitment to the lungs during infectious inflammation, as inhibition of CXCL1 by a blocking Ab resulted in attenuation of neutrophil migration to the airspaces after Escherichia coli LPS challenge in a rat model (7, 8). Furthermore, transgenic CXCL1 mice, which constitutively express CXCL1 within the lungs, have more neutrophil influx and less bacterial burden in their organs after challenge with K. pneumonia (44) . In subsequent studies, the signaling cascades associated with neutrophil-dependent bacterial clearance have been explored using CXCL1 gene-deleted mice (13, 28). Using CXCL1-/- mice, we reported previously that CXCL1 derived from both hematopoietic and resident cells is essential for expression of CXCL2/MIP-2 and CXCL5/LIX, and activation of NF-κB and MAPKs in the lung during Klebsiella infection (13).
Our current study is the first to determine the mechanisms by which CXCL1 regulates innate immunity to Klebsiella-induced pneumonia. Our results demonstrate that: (i) CXCL1 mediates neutrophil accumulation in the lungs via LTB4 production during K. pneumoniae infection; (ii) neutrophil-depleted animals exhibit reduced LTB4, NADPH oxidase, and iNOS expression while neutrophil repletion in neutropenic mice enhances production of LTB4; iii) i.t. administration of LTB4 restores survival, neutrophil recruitment, cytokine/chemokine production, expression of NADPH oxidase components and iNOS, as well as activation of NF-κB and MAPKs in K. pneumoniae-infected CXCL1-/- mice; and iv) LTB4 treatment improves the expression of NADPH oxidase components and iNOS as well as bacterial killing capacity of K. pneumoniae-infected CXCL1-/- neutrophils. The model depicting the mechanisms underlying CXCL1-mediated neutrophil immunity during K. pneumoniae infection in the lung is shown in Fig. 7. During K. pneumoniae infection, CXCL1 is produced by local bone marrow-derived and resident cells, and causes neutrophil recruitment into the lungs. These recruited neutrophils produce LTB4, which activates NF-κB and MAPKs essential for cytokine (TNF-α and IL-6), chemokine (MIP-2 and LIX), and ROS/RNS production. In turn, these events are important for neutrophil-mediated bacterial clearance in the lungs. Cytokines/chemokines and ROS/RNS can also induce more CXCL1 via a positive feedback loop involving autocrine and paracrine mechanisms. Although some of the cascades are not validated by the current investigation, future studies are required to explore these cascades.
Figure 7. Scheme depicting CXCL1-dependent bacterial clearance in the lung following K. pneumoniae infection.
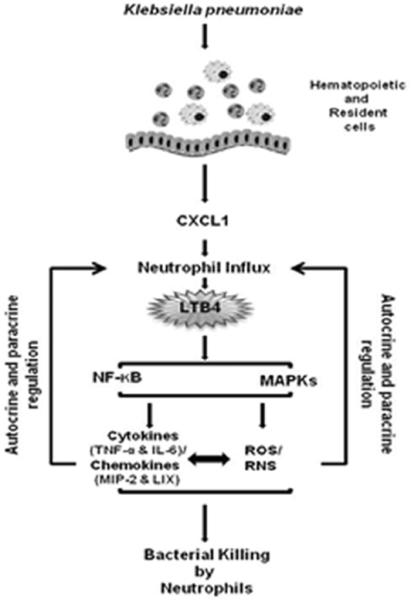
K. pneumoniae induces CXCL1 production by hematopoietic and resident cells, causing neutrophil accumulation in the lungs. Neutrophil influx into the lungs is important for LTB4 production by bone marrow-derived cells. LTB4 subsequently activates NF-κB and MAPKs, resulting in the production of cytokines/chemokines and the generation of ROS/RNS. Cytokines/chemokines and ROS/RNS regulate each other through signaling cascades. Cytokines/chemokines and ROS/RNS can regulate the production of LTB4 via a positive feedback loop. ROS/RNS ultimately lead to bacterial clearance from the lungs.
A lipid mediator produced via the 5-lipoxygenase (5-LO) pathway of arachidonic acid metabolism (45-47), LTB4 is the major player in the formation of oxygen and nitrogen free radicals in myeloid cells, including macrophages and neutrophils (48-51). In addition, LTB4 is an important neutrophil chemoattractant that has been shown to regulate chemotaxis, degranulation, release of lysosomal enzymes, and formation of oxygen free radicals in neutrophils (52, 53). Furthermore, antibody-mediated MIP-2 blocking negatively regulates LTB4 production, an example of crosstalk that can occur between chemokines and LTB4 (54). Our studies illustrate roles for CXCL1 in mediating both neutrophil recruitment and bacterial killing by ROS and RNS via regulation of endogenous LTB4 production following infection. Because of the presence of the LTB4 receptors, BLT-1 and BLT-2, in both myeloid, and lung resident cells, the effects of LTB4 could be mediated through both autocrine and paracrine mechanisms (21, 26, 55-57).
The critical role of NADPH oxidase in host immunity is clearly shown by the immune deficiency syndrome chronic granulomatous disease, which is caused by an autosomal deficiency in NADPH oxidase that renders individuals vulnerable to life-threatening bacterial infections (58). Bacterial factors are known to induce oxidative stress in tissues. as observed in gastric epithelium during H. pylori infection as well as in the lungs following bacterial infection (59-62). In CXCL1-/- mice we saw reduced expression and activation of NADPH oxidase components p67phox and p47phox following K. pneumoniae infection. Thus, as we observed reduced LTB4 in CXCL1-/- mice upon K. pneumoniae infection, we favor the interpretation that LTB4 is responsible for the production of ROS and RNS via the activation of NADPH oxidase.
Our studies suggest that the reduction in NADPH oxidase and iNOS expression observed in the lungs of CXCL1-/- mice following K. pneumoniae infection is due to either attenuated neutrophil accumulation and/or activation of bone marrow/resident cells in the lung. Interestingly, these findings demonstrate a predominant role for neutrophils in producing LTB4 and different components of NADPH oxidase and iNOS in the lungs. The bone marrow chimeras and neutrophil repletion experiments additionally support the conclusion that neutrophils are the major contributor of LTB4 in the lung. Although our studies clearly established roles for neutrophils in mediating LTB4 production, LTB4 can be produced by other bone marrow cells including AMs or lung-resident cells (23, 26). Our findings confirm this as neutrophil depletion did not completely abolish LTB4 levels in the lung after K. pneumoniae infection (Fig 2). As LTB4 concentrations were decreased following Gr1 depletion (Fig. 2A), the majority of lung LTB4 did appear to be either directly or indirectly produced by neutrophils.
We focused on neutrophils in CXCL1-/- mice because of the critical importance of this unique cell type in K. pneumoniae clearance (44). In prior studies, we demonstrated that depletion of neutrophils resulted in modest changes in the production of CXCL1 and no change in the levels of CXCL2 and CXCL5 in the lungs following K. pneumoniae infection. These results are consistent with the fact that myeloid cells other than neutrophils and/or resident cells are involved in CXCL1, CXCL2, and CXCL5 production during intrapulmonary K. pneumoniae challenge (13). Conversely, we observed a substantial reduction in TNF-α levels in the lungs of neutrophil-depleted WT and CXCL1−/− mice (13). While it has been shown previously that TNF-α can induce the synthesis of LTB4, it is likely that neutrophil-derived TNF-α can induce LTB4 synthesis in the lungs via autocrine and/or paracrine mechanisms due to the presence of TNF receptors, such as TNFR1 and TNFR2 on both myeloid and resident cells in the lung (63, 64).
Interestingly, a single intrapulmonary administration of LTB4 corrected neutrophil recruitment and bacterial clearance in K. pneumoniae-infected CXCL1-/- mice (Fig. 4). In line with this observation, earlier studies have shown that LTB4 is important for macrophage, but not neutrophil, mediated host immunity via recruitment (24). This conclusion is supported by the fact that, unlike macrophage influx, neutrophil recruitment to the lungs was unaffected following pneumococcal infection in mice (24). Our results show LTB4 restored CXCL1-dependent cytokine and chemokine production in the lungs, suggesting that LTB4 interaction with its G-protein coupled receptors (BLT-1 and BLT-2) induces downstream signaling leading to cytokine/chemokine production. The production of cytokines and chemokines is mediated by transcription factors, including NF-κB and MAPKs including ERK, p38 and JNK kinases (13, 29, 32, 65, 66). In this regard, investigations have unequivocally demonstrated that NF-κB and MAPK activation is pivotal to inflammation during bacterial pneumonia (13, 29, 32, 65, 66).
An earlier study with K. pneumoniae infection in macrophages showed that LTB4 induced phosphorylation of p47phox and enhanced bacterial killing (24). In the current investigation, we demonstrated that isolated neutrophils produce substantial LTB4 and express both NADPH oxidase and iNOS. We also found that LTB4 treatment improved bacterial killing, phagocytosis, and the production of oxidants in K. pneumoniae-infected CXCL1-/- neutrophils. Our results are in agreement with reported findings that LTB4 can augment neutrophil phagocytosis of K. pneumoniae via Fc- and complement-mediated mechanisms (41).
Observations from our current investigation have translational importance in identifying new avenues to augment host immunity in patients with nonfunctional CXCL1 who have bacterial pneumonia. In this regard, three single nucleotide polymorphisms in human CXCL1 have been reported (67), although their association with host immune defects has not yet been examined. While MIP-2 and TNF-α were augmented in WT mice by exogenous LTB4 at a concentration of 100 ng/mouse, LTB4 did not affect neutrophil immunity in WT mice (Fig 4). To augment neutrophil immunity, numerous cytokines, including IFN-γ and G-CSF could be administered locally rather than systemically. The chemotactic lipid, LTB4 has advantages over cytokine proteins because it is less immunogenic and more cost effective than the cytokines, and can be successfully administered locally.
Acknowledgments
We thank Sergio Lira at Mount Sinai Medical Center for providing CXCL1-/- strain. We would like to thank Rachel Zemans and Ken Malcolm at National Jewish Health and Dan Chisenhall and Pete Mottram at LSU for critical reading of the manuscript. We also thank Laboratory of Lung Biology members Theivanthiran Balamayooran, Liliang Jin and Kanapathipillai Jeyagowri for helpful discussions and critical reading of the manuscript.
This work was supported by a Scientist Award from the Flight Attendant Medical Research Institute (YCSA-062466); and grants from the NIH (R01 HL-091958 and R01 HL-091958S1) to SJ
List of Abbreviations
- ICAM-1
Intracellular cell-adhesion molecule-1
- LIX
Lipopolysaccharide-induced CXC chemokines
- KC
Keratinocyte cell derived chemokines
- MIP-2
macrophage inflammatory protein-2
- VCAM-1
Vascular cell-adhesion molecule-1
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- BALF
Bronchoalveolar lavage fluid
- MPO
Myeloperoxidase
References
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fein AM. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis. 1999;28:726–729. doi: 10.1086/515218. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JP, 3rd, Martinez FJ. Community-acquired pneumonia. Curr Opin Pulm Med. 1998;4:162–172. [PubMed] [Google Scholar]
- 4.Abraham E. Neutrophils and acute lung injury. Critical Care Medicine. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 5.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozic CR, Kolakowski LF, Jr., Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 8.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- 9.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol. 1995;58:359–364. doi: 10.1002/jlb.58.3.359. [DOI] [PubMed] [Google Scholar]
- 11.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32:531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72:7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol. 2010;185:6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Patel S, Jyoti A, Keshari RS, Verma A, Barthwal MK, Dikshit M. Nitric oxide-mediated augmentation of neutrophil reactive oxygen and nitrogen species formation: Critical use of probes. Cytometry A. 2010;77:1038–1048. doi: 10.1002/cyto.a.20975. [DOI] [PubMed] [Google Scholar]
- 15.Marriott HM, Jackson LE, Wilkinson TS, Simpson AJ, Mitchell TJ, Buttle DJ, Cross SS, Ince PG, Hellewell PG, Whyte MK, Dockrell DH. Reactive oxygen species regulate neutrophil recruitment and survival in pneumococcal pneumonia. Am J Respir Crit Care Med. 2008;177:887–895. doi: 10.1164/rccm.200707-990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakazato T, Sagawa M, Yamato K, Xian M, Yamamoto T, Suematsu M, Ikeda Y, Kizaki M. Myeloperoxidase is a key regulator of oxidative stress mediated apoptosis in myeloid leukemic cells. Clin Cancer Res. 2007;13:5436–5445. doi: 10.1158/1078-0432.CCR-07-0481. [DOI] [PubMed] [Google Scholar]
- 17.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 18.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 19.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albina JE, Mastrofrancesco B. Modulation of glucose metabolism in macrophages by products of nitric oxide synthase. Am J Physiol. 1993;264:C1594–1599. doi: 10.1152/ajpcell.1993.264.6.C1594. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Galan E, Gomez-Hernandez A, Vidal C, Martin-Ventura JL, Blanco-Colio LM, Munoz-Garcia B, Ortega L, Egido J, Tunon J. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81:216–225. doi: 10.1093/cvr/cvn277. [DOI] [PubMed] [Google Scholar]
- 22.Mehrabian M, Allayee H. 5-lipoxygenase and atherosclerosis. Curr Opin Lipidol. 2003;14:447–457. doi: 10.1097/00041433-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun. 2010;78:2264–2271. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 26.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickman-Davis JM, O'Reilly P, Davis IC, Peti-Peterdi J, Davis G, Young KR, Devlin RB, Matalon S. Killing of Klebsiella pneumoniae by human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2002;282:L944–956. doi: 10.1152/ajplung.00216.2001. [DOI] [PubMed] [Google Scholar]
- 28.Shea-Donohue T, Thomas K, Cody MJ, Aiping Z, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124. doi: 10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 31.Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, Worthen GS. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 32.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte Chemoattractant Protein 1 Regulates Pulmonary Host Defense via Neutrophil Recruitment during Escherichia coli Infection. Infect Immun. 2011;79:2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thamilselvan V, Menon M, Thamilselvan S. Oxalate-induced activation of PKC-alpha and -delta regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2009;297:F1399–1410. doi: 10.1152/ajprenal.00051.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kupfner JG, Arcaroli JJ, Yum HK, Nadler SG, Yang KY, Abraham E. Role of NF-kappaB in endotoxemia-induced alterations of lung neutrophil apoptosis. J Immunol. 2001;167:7044–7051. doi: 10.4049/jimmunol.167.12.7044. [DOI] [PubMed] [Google Scholar]
- 35.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. Myeloid differentiation protein-2-dependent and -independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2009;40:701–709. doi: 10.1165/rcmb.2008-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maccarrone M, Bari M, Battista N, Finazzi-Agro A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- 37.Maccarrone M, Bari M, Lorenzon T, Bisogno T, Di Marzo V, Finazzi-Agro A. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. J Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- 38.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- 39.Lin JC, Chang FY, Fung CP, Yeh KM, Chen CT, Tsai YK, Siu LK. Do neutrophils play a role in establishing liver abscesses and distant metastases caused by Klebsiella pneumoniae? PLoS One. 2010;5:e15005. doi: 10.1371/journal.pone.0015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loof TG, Goldmann O, Gessner A, Herwald H, Medina E. Aberrant inflammatory response to Streptococcus pyogenes in mice lacking myeloid differentiation factor 88. Am J Pathol. 2010;176:754–763. doi: 10.2353/ajpath.2010.090422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mancuso P, Nana-Sinkam P, Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2001;69:2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White-Owen C, Alexander JW, Sramkoski RM, Babcock GF. Rapid whole-blood microassay using flow cytometry for measuring neutrophil phagocytosis. J Clin Microbiol. 1992;30:2071–2076. doi: 10.1128/jcm.30.8.2071-2076.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hampton MB, Vissers MC, Winterbourn CC. A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J Leukoc Biol. 1994;55:147–152. doi: 10.1002/jlb.55.2.147. [DOI] [PubMed] [Google Scholar]
- 44.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, Standiford TJ. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 45.Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- 46.Ford-Hutchinson AW. Regulation of leukotriene biosynthesis. Cancer Metastasis Rev. 1994;13:257–267. doi: 10.1007/BF00666096. [DOI] [PubMed] [Google Scholar]
- 47.Sala A, Folco G. Neutrophils, endothelial cells, and cysteinyl leukotrienes: a new approach to neutrophil-dependent inflammation? Biochem Biophys Res Commun. 2001;283:1003–1006. doi: 10.1006/bbrc.2001.4865. [DOI] [PubMed] [Google Scholar]
- 48.Peters-Golden M. Expanding roles for leukotrienes in airway inflammation. Curr Allergy Asthma Rep. 2008;8:367–373. doi: 10.1007/s11882-008-0057-z. [DOI] [PubMed] [Google Scholar]
- 49.Peters-Golden M, Henderson WR., Jr. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 50.Jackson WT, Froelich LL, Boyd RJ, Schrementi JP, Saussy DL, Jr., Schultz RM, Sawyer JS, Sofia MJ, Herron DK, Goodson T, Jr., Snyder DW, Pechous PA, Spaethe SM, Roman CR, Fleisch JH. Pharmacologic actions of the second-generation leukotriene B4 receptor antagonist LY293111: in vitro studies. J Pharmacol Exp Ther. 1999;288:286–294. [PubMed] [Google Scholar]
- 51.Santus P, Sola A, Carlucci P, Fumagalli F, Di Gennaro A, Mondoni M, Carnini C, Centanni S, Sala A. Lipid peroxidation and 5-lipoxygenase activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:838–843. doi: 10.1164/rccm.200404-558OC. [DOI] [PubMed] [Google Scholar]
- 52.Gaudreault E, Stankova J, Rola-Pleszczynski M. Involvement of leukotriene B4 receptor 1 signaling in platelet-activating factor-mediated neutrophil degranulation and chemotaxis. Prostaglandins Other Lipid Mediat. 2005;75:25–34. doi: 10.1016/j.prostaglandins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Pettipher ER, Salter ED, Breslow R, Raycroft L, Showell HJ. Specific inhibition of leukotriene B4 (LTB4)-induced neutrophil emigration by 20-hydroxy LTB4: implications for the regulation of inflammatory responses. Br J Pharmacol. 1993;110:423–427. doi: 10.1111/j.1476-5381.1993.tb13827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4). Eur J Immunol. 2006;36:2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- 55.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 56.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serezani CH, Aronoff DM, Sitrin RG, Peters-Golden M. FcgammaRI ligation leads to a complex with BLT1 in lipid rafts that enhances rat lung macrophage antimicrobial functions. Blood. 2009;114:3316–3324. doi: 10.1182/blood-2009-01-199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seger RA. Advances in the diagnosis and treatment of chronic granulomatous disease. Curr Opin Hematol. 2010 doi: 10.1097/MOH.0b013e32834115e7. [DOI] [PubMed] [Google Scholar]
- 59.Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- 60.Dovhanj J, Kljaic K, Smolic M, Svagelj D. NADPH and iron may have an important role in attenuated mucosal defense in Helicobacter pylori infection? Mini Rev Med Chem. 2010;10:1309–1315. doi: 10.2174/138955710793564160. [DOI] [PubMed] [Google Scholar]
- 61.Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infect Immun. 2001;69:5991–5996. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadikot RT, Zeng H, Yull FE, Li B, Cheng DS, Kernodle DS, Jansen ED, Contag CH, Segal BH, Holland SM, Blackwell TS, Christman JW. p47phox deficiency impairs NF-kappa B activation and host defense in Pseudomonas pneumonia. J Immunol. 2004;172:1801–1808. doi: 10.4049/jimmunol.172.3.1801. [DOI] [PubMed] [Google Scholar]
- 63.Camussi G, Tetta C, Bussolino F, Baglioni C. Tumor necrosis factor stimulates human neutrophils to release leukotriene B4 and platelet-activating factor. Induction of phospholipase A2 and acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine O2-acetyltransferase activity and inhibition by antiproteinase. Eur J Biochem. 1989;182:661–666. doi: 10.1111/j.1432-1033.1989.tb14876.x. [DOI] [PubMed] [Google Scholar]
- 64.Conti P, Reale M, Barbacane RC, Bongrazio M, Panara MR, Fiore S. The combination of interleukin 1 plus tumor necrosis factor causes greater generation of LTB4, thromboxanes and aggregation on human macrophages than these compounds alone. Prog Clin Biol Res. 1989;301:541–545. [PubMed] [Google Scholar]
- 65.Batra S, Balamayooran G, Sahoo MK. Nuclear factor-kappaB: a key regulator in health and disease of lungs. Arch Immunol Ther Exp (Warsz) 2011;59:335–351. doi: 10.1007/s00005-011-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178:1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamura Y, Sakasegawa Y, Omi K, Kishida H, Asada T, Kimura H, Tokunaga K, Hachiya NS, Kaneko K, Hohjoh H. Association study of the chemokine, CXC motif, ligand 1 (CXCL1) gene with sporadic Alzheimer's disease in a Japanese population. Neurosci Lett. 2005;379:149–151. doi: 10.1016/j.neulet.2004.12.056. [DOI] [PubMed] [Google Scholar]