Abstract
Membranous nephropathy (MN) is a leading cause of nephrotic syndrome in adults and a significant cause of end-stage renal disease, yet current therapies are non-specific, toxic, and often ineffective. The development of novel targeted therapies requires a detailed understanding of the pathogenic mechanisms, but progress is hampered by the lack of a robust mouse model of disease. We report that DBA/1 mice as well as congenic FcγRIII−/− and FcRγ−/− mice immunized with a fragment of α3(IV) collagen developed massive albuminuria and nephrotic syndrome, due to subepithelial deposits of mouse IgG and C3 with corresponding basement membrane reaction and podocyte foot process effacement. The clinical presentation and histopathologic findings were characteristic of MN. Even though immunized mice produced genuine anti-α3NC1 autoAbs which bound to kidney and lung basement membranes, neither crescentic glomerulonephritis nor alveolitis ensued, likely due to the predominance of mIgG1 over mIgG2a and mIgG2b autoAbs. The ablation of activating IgG Fc receptors did not ameliorate injury, implicating subepithelial deposition of immune complexes and consequent complement activation as a major effector pathway. We have thus established an active model of murine MN. This model, leveraged by the availability of genetically engineered mice and mouse-specific reagents, will be instrumental for studying the pathogenesis of MN and for evaluating the efficacy of novel experimental therapies.
Keywords: autoimmune, autoantibody, mouse model, glomerulonephritis, subepithelial immune complexes, podocytes
Introduction
Various forms of glomerulonephritis (GN) collectively are a major cause of end-stage renal disease. A prominent pathogenic mechanism is the glomerular deposition or in situ formation of immune complexes (ICs), which induce tissue injury by activating complement and/or engaging Fc receptors on effector cells. The pattern of injury, effector mechanisms, histopathology findings, and clinical course are largely determined by the glomerular location of ICs (1).
Membranous nephropathy (MN) is a leading cause of nephrotic syndrome in adults. The hallmark feature is the subepithelial deposition of electron dense ICs containing Ag, IgG and complement with glomerular basement membrane (GBM) thickening and podocyte foot process effacement, but little glomerular inflammation. Subepithelial ICs form in situ when circulating Abs bind to Ags expressed on podocytes or to Ags planted at this site, though deposition of small circulating ICs is also possible (1, 2). Podocyte autoAgs include phospholipase A2 receptor, targeted by autoAbs in ~70% of patients with idiopathic MN (3), neutral endopeptidase, implicated in antenatal MN due to maternal alloimmunization (4), as well as aldose reductase, manganese superoxide dismutase, and probably other autoAgs (5, 6). Cationic bovine serum albumin, likely of dietary origin, has been identified as the Ag in some forms of early childhood MN (7). Pathogenesis of MN is driven by subepithelial ICs activating complement and sublythic podocyte injury by C5b-9, leading to proteinuria (8).
AutoAbs to α3α4α5(IV) collagen, the major component of the glomerular basement membrane (GBM), mediate rapidly progressive crescentic GN (9, 10). In anti-GBM Ab-mediated GN, including Goodpasture (GP) disease, autoAbs bind to conserved epitopes in the noncollagenous (NC1) domain of α3(IV) collagen (11–14). In Alport post-transplant nephritis, anti-GBM alloAbs bind to distinct epitopes in the NC1 domains of α3(IV) or α5(IV) collagen, present only in the kidney allograft (14–18). A characteristic finding of anti-GBM disease is the smooth linear deposition of IgG and often complement along the GBM, with a crescentic and necrotizing injury. Tissue-bound anti-GBM autoAbs elicit a type II inflammatory response, inducing complement- and Fcγ receptor-mediated recruitment and activation of inflammatory cells. The contribution of various effector pathways has not been fully elucidated.
Activating Fcγ receptors have been identified as essential mediators in some mouse models of Ab-mediated GN. Mice have three activating IgG Fc receptors: FcγRI, FcγRIII and FcγRIV (19). Each comprises an α chain associated with a common FcRγ subunit, which initiates signal transduction upon receptor cross-linking by ICs. Ablation of activating IgG Fc receptors in FcγR−/− mice prevents nephrotoxic nephritis induced by heterologous anti-GBM Abs (20, 21) and lupus nephritis in NZB/NZW mice (22), but does not protect against lupus nephritis in MRL/lpr mice (23) or cryoglobulinemia-associated membranoproliferative GN (24). The role of individual FcγRs has been studied in murine nephrotoxic nephritis, and variable requirements for FcγRI, FcγRIII, and FcγRIV were reported by different groups (25–28). However, the importance of activating FcγR’s in active models of Ab-mediated nephritis elicited by immunization with glomerular Ags has not been determined, as C57Bl/6 mice commonly used for these studies are relatively resistant to actively induced GN (29).
Our goal was to determine the contribution of activating IgG Fc receptors to the pathogenesis of murine autoimmune GN induced by immunization with a well-defined GBM autoAg. For this purpose, we chose to use DBA/1 mice due to their greater susceptibility to GN relative to C57Bl/6 mice (30, 31) and the availability of congenic FcγRIII−/− and FcRγ−/− mice (32). Wild type, FcγRIII−/− and FcRγ−/− mice immunized with the NC1 domain of α3(IV) collagen, regardless of the expression of activating Fcγ receptors, developed massive albuminuria and nephrotic syndrome, associated with subepithelial IgG and C3 deposits by immunofluorescence (IF) and electron microscopy (EM) and characteristic GBM spike reaction without crescentic injury. We have thus established a novel active model of murine MN, which will be valuable for new studies of the disease mechanisms and preclinical studies of experimental therapies.
Materials and Methods
Materials
The recombinant NC1 domain of human α3(IV) collagen (rh-α3NC1) was expressed in HEK293 cells and purified as described (33). Mouse kidney (Pel-Freez Biologicals, Rogers, AR) cortex basement membranes (BMs), including the GBM, were digested with bacterial collagenase (Worthington, Lakewood, NJ); the solubilized NC1 hexamers were purified by passage through a DE-52 ion-exchange column and gel-filtration chromatography (34). The α3α4α5NC1 and α1α2NC1 hexamers from mouse GBM were affinity-purified using immobilized mAb b14 (34). NC1 domains from mouse GBM were separated by reverse-phase HPLC on a C18 column (Vydac 201TP C18 10u) using a gradient of acetonitrile in 0.1% trifluoroacetic acid, as described (35). Rat IgG mAbs H11 (to α1NC1), H31 (to α3NC1), RH34 (to human α3NC1), RH42 (to α4NC1), b14 (to α5NC1), as well as murine mAbs TF51 (mIgG1), TF90 (IgG2a) and TF86 (IgG2b), binding to the NC1 domains of mouse α3α4α5(IV) collagen, were provided by Dr. Yoshikazu Sado.
Animal experiments
DBA/1 mice were purchased from The Jackson Laboratory. Breeding pairs of FcγRIII−/− and FcRγ−/− mice backcrossed onto the DBA/1 background for over 12 generations were obtained from Dr. Sandra Kleinau. Col4a3−/− mice expressing a human COL4A3/COL4A4 YAC transgene (36) were obtained from Dr. Laurence Heidet. B6.Col4a3−/− mice (37) were kindly provided by Dr. Jeffrey Miner (Washington University, St Louis, MO). Mice were housed in a specific pathogen-free facility with free access to food and water. All procedures were approved by IACUC and conducted in accordance with the Guidelines for Animal Care and Use Program of Vanderbilt University.
Groups of 4–6 wild type, FcγRIII−/− and FcRγ−/− DBA/1 mice, both males and females, between 6 and 10 weeks old, were immunized subcutaneously at two sites on the back with α3NC1 (30 μg in 50 μl PBS) emulsified in equal volume of Complete Freund’s Adjuvant (Sigma, Saint Louis, MO), then boosted three weeks later with the same amount of antigen in Incomplete Freund’s Adjuvant (Sigma, Saint Louis, MO). In control mice, the antigen was replaced by vehicle (PBS). Blood was collected every two weeks from the saphenous vein. Spot urine was collected every two weeks using a urine collection station3. Mice were regularly checked for signs of disease and were euthanized if they developed edema or ascites, or abnormally high BUN levels, or excessive loss of body weight (>10% in a week), or became lethargic. The remaining mice were killed at ~18 weeks post-immunization, except for several mice from each group sacrificed at 10 weeks for comparison purposes. Blood, kidneys and lungs were collected for further analyses. Three separate experiments were performed for each strain.
Evaluation of Kidney Function and Renal Histopathology
Urinary albumin excretion was measured in spot urine samples by capture ELISA using a mouse albumin quantitation kit (Bethyl, Montgomery, TX). Urine creatinine and blood urea nitrogen (BUN) were measured using Infinity creatinine and urea liquid stable reagents (Thermo Fisher Scientific, Middletown, VA), according to manufacturer’s protocols. Albuminuria was expressed as urinary albumin to creatinine ratio (ACR). The proteins in serum and urine samples were separated by SDS-PAGE electrophoresis under non-reducing conditions and stained with Coomassie Brilliant Blue. Total plasma cholesterol and triglycerides were measured by standard enzymatic assays at the Vanderbilt Mouse Metabolic Phenotyping Center. Portions of mouse kidneys or lungs were fixed in 10% buffered formalin, dehydrated through graded ethanols, embedded in paraffin, and kidney sections (2μm thick) were stained with periodic-acid Schiff (PAS) or Jones silver stain. Lung sections were stained with hematoxylin and eosin (H&E). At least 50 glomeruli from each mouse were observed to assess lesions. Mesangial proliferation, global or segmental sclerosis, spike formation, necrosis, or crescents were assessed. For transmission EM, kidney cortex was fixed in 4% paraformaldehyde in 0.1M cacodylate buffer (pH=7.4), post-fixed in aqueous 1.25% osmium tetroxide, dehydrated through an ethanol series, embedded in plastic, sectioned with a diamond knife, and stained with 4% uranyl acetate and lead citrate. All assessments were made without knowledge of the experimental group.
Direct and Indirect Immunofluorescence (IF)
Portions of snap-frozen mouse kidneys or lung embedded in OCT were cryosectioned (5 μm), fixed in acetone for 10 min at −20°C and blocked with 1% BSA. For direct IF, frozen sections were stained with FITC-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b (BD Bioscience Pharmingen, San Jose, California), or FITC-conjugated goat anti-mouse C3c (Nordic Immunology, Tilburg, Netherlands). For indirect IF staining of collagen IV chains, rat IgG mAbs were used as described (36). Staining with mAbs H11, H31 and RH34 was performed both with and without prior treatment of the sections with acid urea (6M urea in 0.1M glycine, pH 3.0). Rabbit anti-C5b9 (Abcam; Cambridge, MA) was used at 5 μg/ml. Secondary Abs were Alexa Fluor 488-conjugated goat anti-rabbit and anti-rat IgG (Invitrogen, Carlsbad, CA) or FITC-goat anti-rat IgG (BD Bioscience Pharmingen, San Jose, California). Sections were mounted with anti-fade reagent (Invitrogen, Carlsbad, CA) and examined under Nikon Eclipse E800 epifluorescence microscope. Photomicrographs were recorded with a charge-coupled device digital camera, using the same exposure settings for each primary antibody.
Analysis of Circulating and Kidney-Bound Mouse IgG (auto)Abs
Kidney-bound Abs were eluted from homogenized mouse kidney cortex with 0.1 M glycine, pH 2.8. Serum and kidney-eluted mIgG Abs were analyzed by ELISA. Maxisorp plastic plates were coated overnight with rh-α3NC1 (100 ng/well) or mouse GBM NC1 hexamers (300 ng/well) and blocked with 1% BSA. Mouse sera were diluted as indicated. Secondary antibodies were alkaline phosphatase-conjugated goat anti-mouse IgG (Rockland Immunochemicals, Gilbertsville, PA) and horseradish peroxidase-conjugated goat anti-mouse IgG1, IgG2a and IgG2b (Bethyl, Montgomery, TX). The dilutions of secondary Abs were chosen so that standard mAbs (TF51 for mIgG1, TF90 for mIgG2a, TF86 for mIgG2b at 1 μg/ml each) yielded comparable ELISA readings when assayed for binding to total mouse GBM NC1 hexamers. After addition of substrate for phosphatase (Sigma, Saint Louis, MO) or peroxidase (Biorad, Hercules, CA), color development was measured with a SpectraMax Plus 384 ELISA plate reader (Molecular Devices, Sunnyvale, CA).
Statistical Analyses
Data are shown as mean ± SEM. Statistical analyses were performed using GraphPad Prism version 5.01. The significance of differences among groups was evaluated by one-way analysis of variance (ANOVA) test, followed by post-hoc tests for pairwise comparisons. Survival curves were compared by log rank test. A p-value of less than 0.05 was considered to be statistically significant.
Results
Analyses of circulating anti-α3NC1 mIgG Abs
Circulating anti-α3(IV)NC1 mIgG Abs were detected in wild type, FcγRIII−/− and FcRγ−/−DBA/1 mice immunized with rh-α3NC1 at ~2 weeks post-immunization, reached maximum titers at 6–8 weeks, then declined (not shown). For all mIgG subclasses, serum levels of anti-α3NC1 Abs were essentially equal between wild type and FcγRIII−/− mice (Fig. 1A). Sera from FcRγ−/− mice had significantly less mIgG1 and a trend toward lower mIgG2b, consistent with reduced Ab responses in FcγR−/− mice immunized with other Ags (29). In all groups of mice, serum anti-α3NC1 Abs of mIgG1 subclass had higher titers than mIgG2a or mIgG2b Abs (Fig. 1B), suggesting a predominantly Th2-polarized immune response.
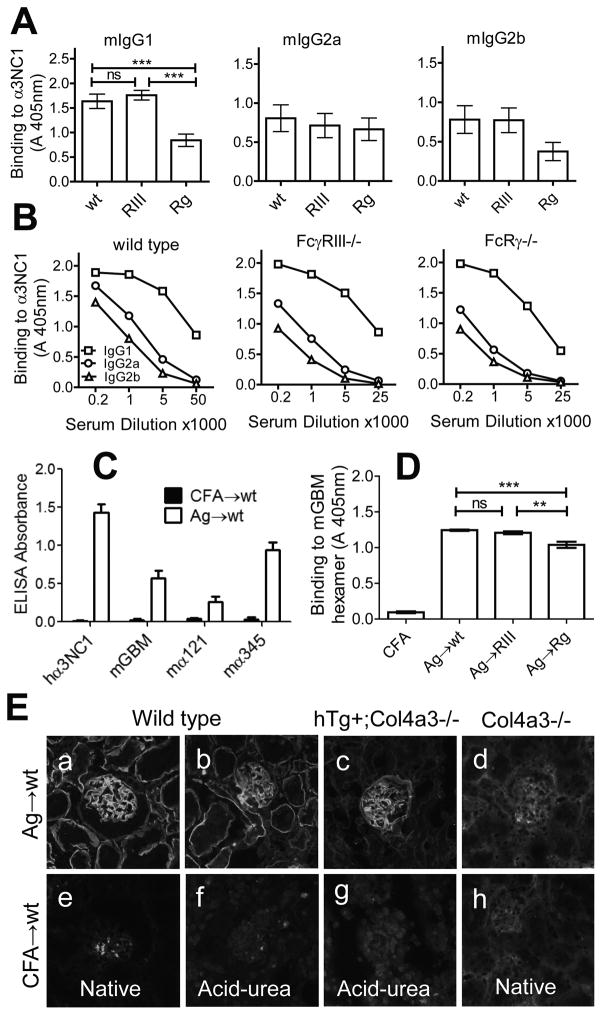
Figure 1. Characterization of serum mIgG (auto)Abs from α3NC1-immunized mice.
A. Circulating mIgG anti-α3NC1 Abs were assayed by indirect ELISA in plates coated with α3NC1 (100 ng/well). Mouse sera collected at 6 weeks post-immunization (n=12–14 mice per group) were diluted 1/5000 for analysis of mIgG1, and 1/1000 for mIgG2a and mIgG2b. Results show means and SEM. B. Titers of mIgG1 (squares), mIgG2a (circles) and mIgG2b (triangles) Abs to rh-α3NC1 (100 ng/well) were compared by indirect ELISA using serial dilutions of mouse sera. C. Specificity of circulating mIgG Abs from α3NC1-immunized mice (open bars, n=5) was analyzed by ELISA in plates coated with rh-α3NC1, total NC1 hexamers from mouse GBM, and affinity-purified mouse α1α2NC1 and α3α4α5 NC1 hexamers. Circulating Abs from CFA-immunized control mice (solid bars) had negligible binding to all Ags. Mouse sera were diluted 1/200. D. The levels of circulating mIgG autoAbs binding to total NC1 hexamers from mouse GBM (300 ng/well) were assayed by indirect ELISA. Mouse sera collected at 6 weeks post-immunization (n=6 per group) were diluted 1/100. The significance of differences among groups was assessed by one-way ANOVA followed by Bonferroni post tests for pairwise comparisons (ns, not significant; * p<0.05; ** p<0.01; *** p<0.001). E. Binding of serum mIgG Abs from mice immunized with α3NC1 (a–d) or adjuvant alone (e–h) to kidneys from wild type mice (a,b,e,f), Col4a3−/− mice expressing a human COL4A3 transgene (c,g), and Col4a3−/− mice (d,h) was evaluated by indirect IF staining.
Because mice were immunized with a heterologous Ag, we addressed whether the resulting mIgG Abs cross-reacted with endogenous autoAg from mouse BMs. Sera from α3NC1-immunized but not control mice contained mIgG Abs binding to mouse GBM NC1 hexamers a mixture of α1α2NC1 and α3α4α5NC1 hexamers in which the latter were the major target (Fig. 1C). Serum mIgG autoAbs cross-reactive with mouse GBM NC1 hexamers were found in all groups of α3NC1-immunized mice, but their titers were lower in FcRγ−/− mice (Fig. 1D). By indirect immunofluorescence, mIgG from immunized mouse sera stained the GBM and tubular BMs in wild-type mice, the GBM only in COL4A3/A4-humanized mice, but did not bind to kidney BMs in Col4a3−/− mice (Fig. 1E). This pattern mirrors the expression of α3(IV) collagen in mouse kidney BMs (36). We thus established that mIgG autoAbs can bind to α3α4α5(IV) collagen in mouse BMs under normal physiologic conditions.
Ablation of activating Fcγ receptors does not ameliorate the development of heavy albuminuria and nephrotic syndrome
Urinary albumin excretion (Fig 2A) and BUN levels (Fig 2B) in experimental mice were monitored for up to 18 weeks. A significant increase in albuminuria was first detected at 6 weeks after immunization with α3NC1 (Fig. 2A). Between 6 and 10 weeks post-immunization, ACR increased dramatically by several hundred-fold in all groups, rising slightly faster in FcγRIII−/−mice. In control mice immunized with adjuvant alone, ACR remained in the normal range during the experiment. After 10 weeks, ACR plateaued at high values in the nephrotic range in all groups of mice. By this time, heavy non-specific proteinuria and hypoalbuminemia developed in all α3NC1-immunized mice, but not in adjuvant-immunized control mice (Fig. 2C).
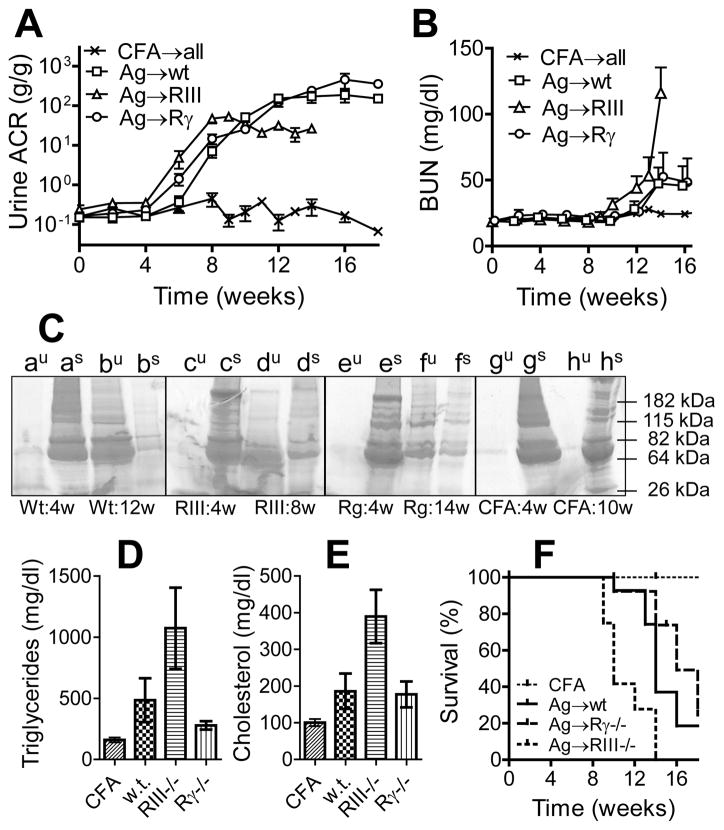
Figure 2. Mice immunized with α3NC1 develop heavy proteinuria and nephrotic syndrome independent of Fcγ receptors.
Renal function was monitored in wild type mice (squares), FcγRIII−/− mice (triangles) and FcRγ−/− mice (circles) immunized with rh-α3NC1 (n=15–16 mice in each group, from three separate experiments). Mice in the control group (x), were immunized with adjuvant alone (n=14; including 4–5 mice for each strain). A: Urinary albumin creatinine ratio (means and SEM). B: Blood urea nitrogen levels (means and SEM). C: SDS-PAGE analysis of urine (au–hu) and serum (as–hs) samples (2μl/lane) from α3NC1-immunized wild type mice (a–b), FcγRIII−/− mice (c–d), FcRγ−/− mice (e–f) and CFA-immunized control mice (g–h), collected before the onset of albuminuria and when mice were euthanized. Serum triglycerides (D) and total cholesterol (E) were assayed at 10 weeks after immunization with rh-α3NC1 or adjuvant. The graphs show means and SEM (n=7–9 mice in each group). Differences among groups were significant by one-way ANOVA. F. Survival curves show the proportion of mice not euthanized due to nephropathy-associated morbidity. The difference between FcγRIII−/− mice and either wild type or FcRγ−/− mice was significant by log-rank test.
BUN levels, normal until ~10 weeks post-immunization, started rising progressively in some mice with heavy proteinuria (Fig. 2B), often in association with a sudden increase in body weight due to edema and ascites. At 10 weeks, serum triglycerides (Fig. 2D) and total cholesterol (Fig. 2E) were also increased in α3NC1-immunized mice. Overall, these clinical findings are characteristic of nephrotic syndrome, and the affected mice were euthanized. Morbidity developed earlier in FcγRIII−/− mice, but the survival curves for wild type and FcRγ−/− mice were not significantly different (Fig. 2F). Male mice suffered from these adverse consequences of prolonged heavy proteinuria earlier than females (Fig. 3). These results show that DBA/1 mice immunized with α3NC1 develop very heavy albuminuria and eventually nephrotic syndrome by a mechanism that does not require expression of activating Fcγ receptors.
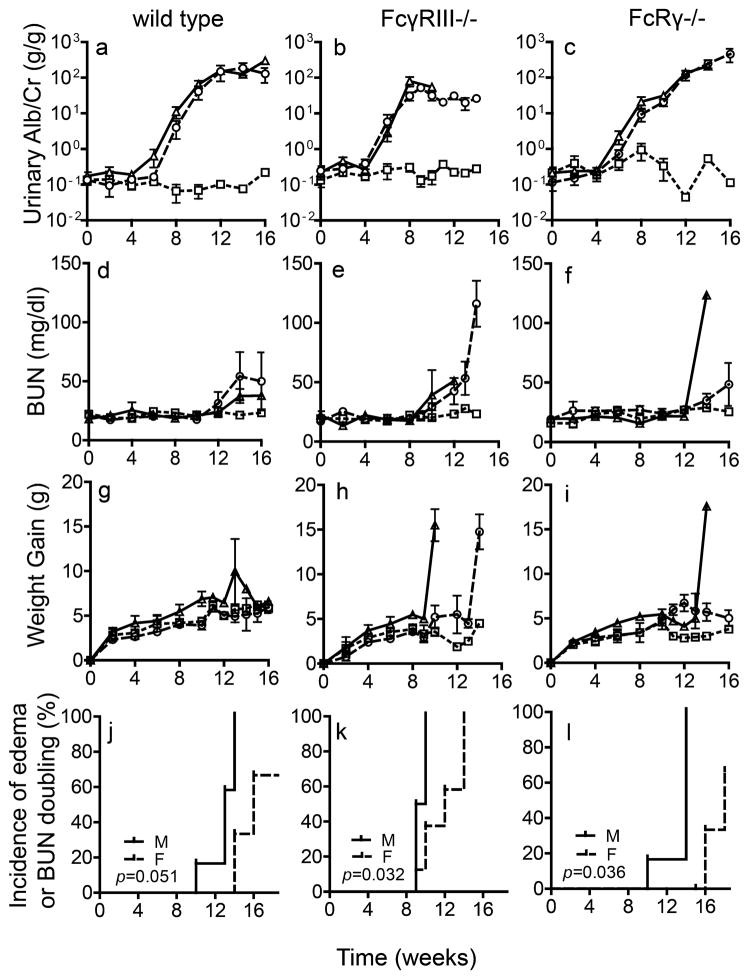
Figure 3.
For each strain, the time course for albuminuria (a–c), BUN (d–f), changes in body weight (g–i), and the incidence of morbidity requiring euthanasia (j–l) were compared among male (triangles) and female (circles) mice immunized with rh-α3NC1 and adjuvant-immunized control mice of either sex (squares). The kinetics of albuminuria was similar between males and females in all mouse strains (a–c). The increase in BUN levels (d–f) was associated with a sudden increase in body weight (g–i) due to development of edema and ascites in nephrotic mice. Males (continuous line) developed edema and ascites and/or their BUN levels doubled earlier than female (dashes) mice (j–l). The significance of differences in the survival curves was assessed by log rank test.
Kidney histopathology is typical of membranous nephropathy
PAS-stained kidney sections (Fig. 4A, a–h) from α3NC1-immunized mice showed GBM thickening but minimal glomerular inflammation and few crescents (5% ± 3% in wild type mice and less than 2% ± 1% in FcγRIII−/− and FcRγ−/− mice, the difference not significant by one-way ANOVA). Jones silver stain (Fig. 4, i-l) showed prominent GBMs with capillary wall spikes (inset) characteristic of MN. Transmission EM (Fig. 4B) revealed severe irregular thickening of the GBM due to subepithelial electron-dense deposits surrounded by the expanded matrix, and diffuse effacement of podocyte foot processes in α3NC1-immunized mice.
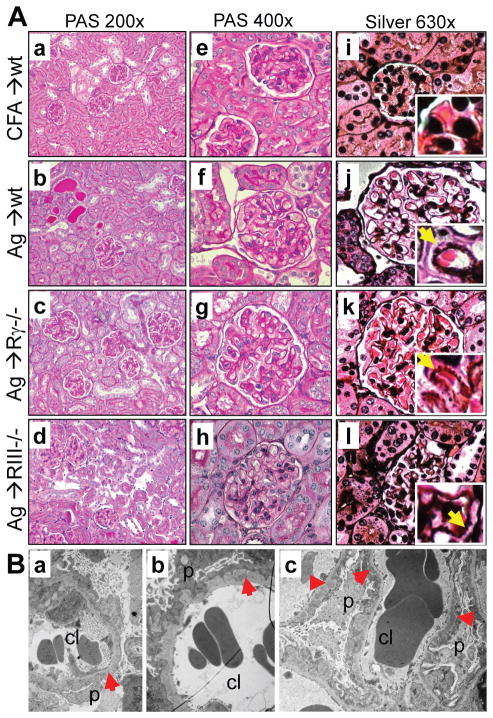
Figure 4. GBM thickening with formation of spikes and subepithelial electron-dense deposits are characteristic of membranous nephropathy.
A. Kidney sections from adjuvant-immunized control mice and wild type, FcγRIII−/− and FcRγ−/− mice immunized with rh-α3NC1, stained with PAS (a–h) or Jones silver (i–l) show GBM thickening but little glomerular inflammations or crescents, with occasional proteinaceous casts and mild interstitial fibrosis and inflammation. Jones silver stain shows GBM spikes (inset, arrows). Original magnification: 200× (a–d), 400× (e–h), 630× (i–l). B. Transmission EM shows subepithelial electron-dense deposits (red arrowheads), thickened GBM, and podocyte foot process effacement in α3NC1-immunized wild type (a), FcγRIII−/− (b) and FcRγ−/− (c) mice. Original magnification: 2850×.
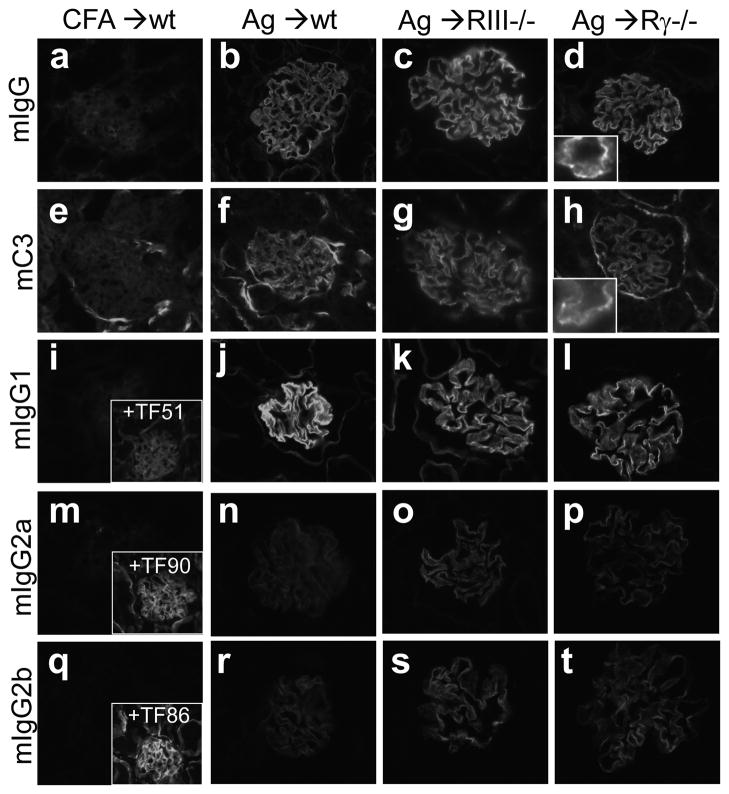
Glomerular deposition of mIgG and mC3 was analyzed by direct IF staining. In all groups of α3NC1-immunized mice, glomerular capillary loops showed intense staining (3+) for mIgG, in a granular pattern characteristic of MN, with an underlying linear GBM pattern typical for anti-GBM autoAbs (Fig. 5, b–d). Weak linear staining for mIgG was also observed in tubular BMs. C3 was deposited in a fine granular pattern along the glomerular capillary wall and focally in tubular BMs (Fig. 5, f–h). Analysis of kidney-bound mIgG subclasses showed very intense staining for mIgG1 (Fig. 5, j–l) and weaker staining for mIgG2a (Fig. 5, n–p) and mIgG2b (Fig. 5, r–t).
Figure 5. Granular deposition of mIgG and mC3 in the glomerular capillary loops.
Direct IF microscopy shows kidneys from adjuvant- immunized control mice (a,e,i,m,q) and wild type (b,f,j,n,r), FcγRIII−/− (c,g,k,o,s) and FcRγ−/− (d,h,l,p,t) mice euthanized at 10 weeks after immunization with rh-α3NC1. Sections were stained with fluorophore-conjugated Abs to mIgG (a–d), mC3c (e–h), mIgG1 (i–l), mIgG2a (m–p), mIgG2b (q–t). Original magnification 400×. The granular staining pattern is apparent at high magnification shown in the inset (d, h). Indirect immunofluorescence staining of kidney sections from the CFA control mice with mIgG1 mAb TF51 (i, inset), mIgG2a mAb TF90 (m, inset) and mIgG2b mAb TF86 (q, inset), all specific for mouse α3α4α5 NC1 domains, provided a reference for assessing the intensity of staining for each mIgG subclass deposited in the kidneys.
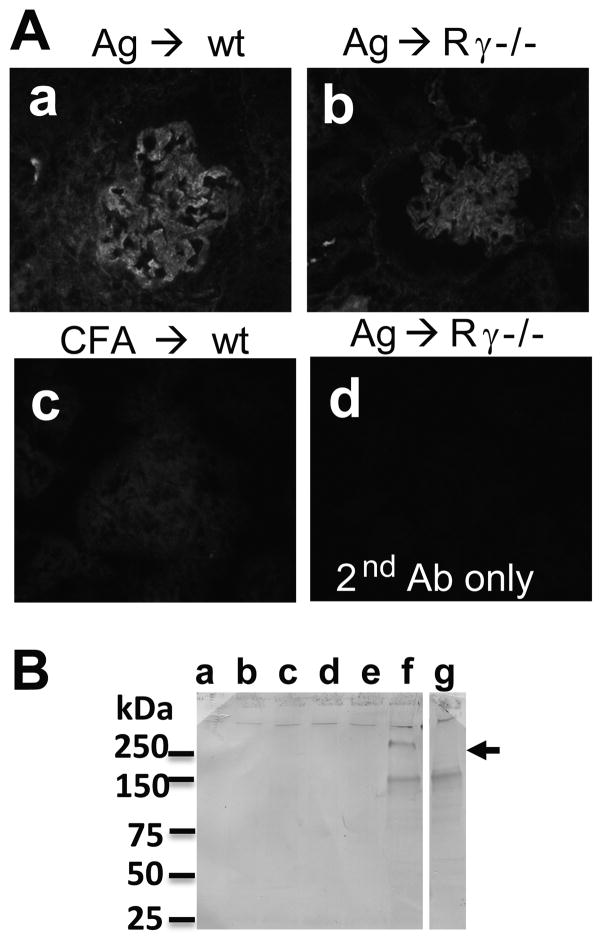
Indirect IF staining revealed C5b-9 deposition in the capillary loops of α3NC1-immunized but not CFA-immunized mice (Fig. 6A). Urinary excretion of C5b-9 was evaluated by Western blot analysis. Rabbit Abs to mC5b-9 specifically stained a ~250 kDa band (arrow) in urine samples collected at 8–10 weeks (but not at 0–6 weeks) from α3NC1-immunized mice, but not CFA-immunized control mice (Fig. 6B).
Figure 6. Kidney deposition and urinary excretion of mC5b-9.
A. Indirect IF staining shows granular GBM deposition of mC5b-9 in α3NC1-immunized wild type (a) and FcRγ−/− (b) mice, but not in adjuvant-immunized mice (c). Staining was negative when the primary Ab was omitted (d). B. Western blot analysis of mouse urine samples (5 μl/lane) separated by SDS-PAGE under non-reducing conditions using Abs to mC5b-9 showed specific staining of a ~250 kDa band (arrow) in urine collected at 8 weeks (lane f), but not at 0, 2, 4, or 6 weeks after immunization with α3NC1 (lanes b–e, respectively), nor in urine collected at 8 weeks after immunization with CFA (lane a). Urine from a 7-months old B6.Col4a3−/− mouse with heavy albuminuria (ACR = 103) was used as an additional control. The band at ~150 kDa was also stained when the primary Ab was omitted, suggesting it is due to secondary Ab cross-reacting with excreted mIgG in mouse urine.
Analyses of kidney-eluted mIgG autoAbs
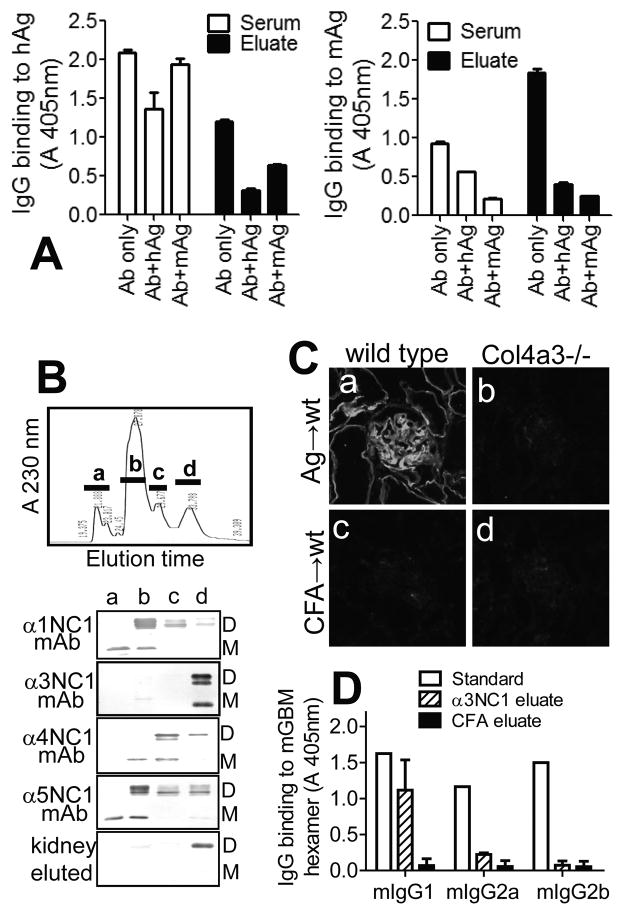
We next characterized the specificity of mIgG Abs eluted from mouse kidneys. Compared to serum Abs, kidney-eluted Abs had greater reactivity toward total mouse GBM NC1 hexamers (Fig. 7A, right) and less toward rh-α3NC1 (Fig. 7A, left). The Ags in solution inhibited the binding of eluted Abs to rh-α3NC1 by 50–75% and to mouse GBM hexamers by ~80%, confirming the cross-reactivity of kidney-bound mIgG autoAbs. In contrast, mouse GBM hexamers in solution inhibited the binding of serum Abs to immobilized mouse GBM hexamers but not to rh-α3NC1, indicating that the bulk of circulating Abs specifically target rh-α3NC1. Using murine NC1 domains separated by reverse-phase HPLC, we further established that kidney-eluted mIgG Abs reacted exclusively with mouse α3NC1 (Fig. 7B), thus ruling out an epitope spreading to other NC1 domains. By indirect IF, kidney-eluted Abs bound in a linear pattern to kidney BM from wild type mice, but did not stain Col4a3−/− mouse kidneys (Fig. 7C). The latter suggests that intermolecular epitope spreading to normal podocyte Ags (other than α3(IV) collagen) is unlikely. However, we cannot rule out the production of autoAbs to podocyte Ags induced or up-regulated during disease, or to neo-epitopes generated by oxidative modification of podocyte or GBM proteins. By ELISA, kidney-eluted IgG autoAbs binding to mouse GBM NC1 hexamers were predominantly mIgG1 (Fig. 7D), as found for serum Abs (Fig. 1), and consistent with the analysis of kidney-bound mIgG subclasses by direct IF staining (Fig. 5). Overall, these results show that kidney-bound mIgG autoAbs are a subset of serum Abs that specifically target α3NC1 epitopes shared by the human and mouse Ags, and which are accessible in native NC1 hexamers of α3α4α5(IV) collagen.
Figure 7. Specificity of kidney-bound mIgG autoAbs.
A. ELISA shows the binding of serum (open bars) and kidney-eluted (closed bars) mIgG Abs to immobilized rh-α3NC1 (hAg, left) and total NC1 hexamers from mouse GBM (mAg, right), and the inhibition of this binding by hAg (5 μg/ml) or mAg (50 μg/ml) in solution. B. NC1 domains from mouse GBM were separated by reverse-phase HPLC on a C18 column (top), and the composition of the major peaks (a–d) was analyzed by Western blotting with chain-specific mAbs (bottom). Kidney-eluted mIgG autoAbs reacted only with the fraction containing murine α3NC1 (M=monomers; D=dimers). C. Indirect IF staining shows the binding of mIgG eluted from the kidneys of α3NC1-immunized mice (a–b) or control mice (c–d) to kidney cryostat sections from wild type mice (a,c) and Col4a3−/− Alport mice (b,d). D. ELISA analysis of subclasses of mIgG eluted from kidneys at ~10 weeks after immunization with rh-α3NC1 (diagonal lines) or CFA (solid bars) showed the prevalence of mIgG1 autoAbs binding to immobilized mouse GBM NC1 hexamers. Anti-NC1 mAbs TF51, TF90 and TF86 (1 μg/ml each) were used as standards for mIgG1, mIgG2a and mIgG2b, respectively (open bars). A similar distribution of mIgG subclasses was found for kidney-eluted autoAbs binding to rh-α3NC1 (not shown).
Expression of collagen IV chains and glomerular deposition of extrinsic Ag
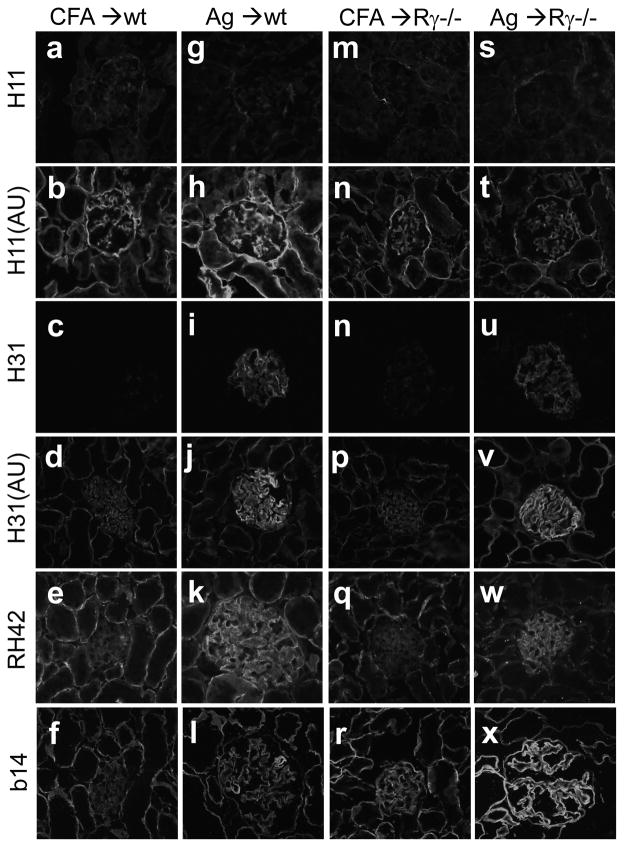
Increased production of collagen IV and/or other BM components by injured podocytes alters the GBM composition in experimental MN (38, 39). We analyzed the expression of collagen IV chains by indirect IF. In α3NC1-immunized mice, the GBM was stained more intensely by mAbs to α3, α4 and α5(IV) collagen chains, whereas the staining of tubular BMs was largely unchanged compared to control mice(Fig. 8, d–f, j–l, p–r, v–x). Glomerular expression of α1(IV) collagen, mostly in a mesangial pattern, was not altered (Fig. 8, b,h,n,t). These results reveal that α3-immunized mice have increased production and/or reduced turnover of α3α4α5(IV) collagen in the GBM, while α1α2(IV) collagen does not appear to contribute to GBM expansion in this model.
Figure 8. Increased collagen IV deposition in the GBM.
Kidneys from wild type (a–l) and FcRγ−/− (m–x) mice at 10 weeks after immunization with adjuvant (a–f, m–r) or rh-α3NC1 (g–l, s–x) were analyzed by indirect IF microscopy. Acetone-fixed cryostat sections were stained with mAbs H11 specific for α1NC1 (a,g,m,s), H31 specific for α3NC1 (c,i,n,u), RH42 specific for α4NC1 (e,k,q,w), and b14 specific for α5NC1 (f,l,r,x). Some sections were treated with acid urea (AU) before staining with mAbs H11 (b,h,n,t) and H31 (d,j,p,v). Original magnification 400×.
Unexpectedly, we found that mAb H31 specifically stained the GBM (but not tubular BMs) in native kidney sections from α3NC1-immunized mice (Fig. 8i,u). As the staining pattern in kidneys from control mice shows, mAb H31 targets an α3NC1 epitope that is normally cryptic in native NC1 hexamers in tissues (Fig. 8c,n) but is unmasked by acid-urea treatment (Fig. 8d,p). This finding implies that endogenous α3(IV) collagen in the GBM is altered during the disease and/or that rh-α3NC1 monomers deposit in the glomeruli a possibility addressed next.
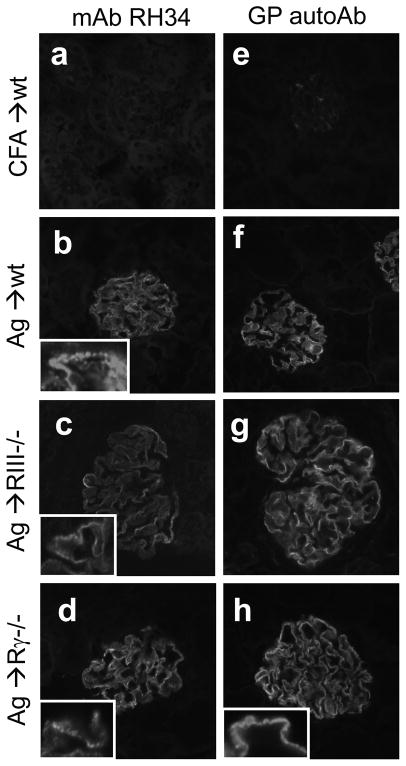
Indirect immunofluorescence staining using mAb RH34, which specifically binds to human but not mouse α3NC1 (36), showed granular deposition of rh-α3NC1 in the glomerular capillary loops, but no staining of tubular BMs (Fig. 9; b–d). Staining with GP autoAbs, which do not bind to α3(IV) collagen in native mouse BMs, produced the same results (Fig. 9; f–h). Overall, the co-localization of rh-α3NC1 with IgG and C3 in the capillary loops suggests that the extrinsic Ag used for immunization may participate in the formation of subepithelial IC deposits.
Figure 9. Deposition of rh-α3NC1 in the glomerular capillary loops.
Indirect IF staining shows the binding of mAb RH34 (a–d) and affinity-purified human GP autoAbs (e–h) to kidneys sections from adjuvant-immunized control mice (a,e) and from wild type mice (b,f), FcγRIII−/−mice (c,g) and FcRγ−/− mice (d,h) at 10 weeks after immunization with rh-α3NC1. A detailed view at higher magnification illustrates the granular staining pattern (insets). Control sections stained with secondary Ab but omitting the primary Ab were negative (not shown). Binding of mAb RH34 was slightly reduced but not abolished by acid urea treatment (not shown). Original magnification 400×.
Absence of lung inflammation despite mIgG binding to alveolar BMs
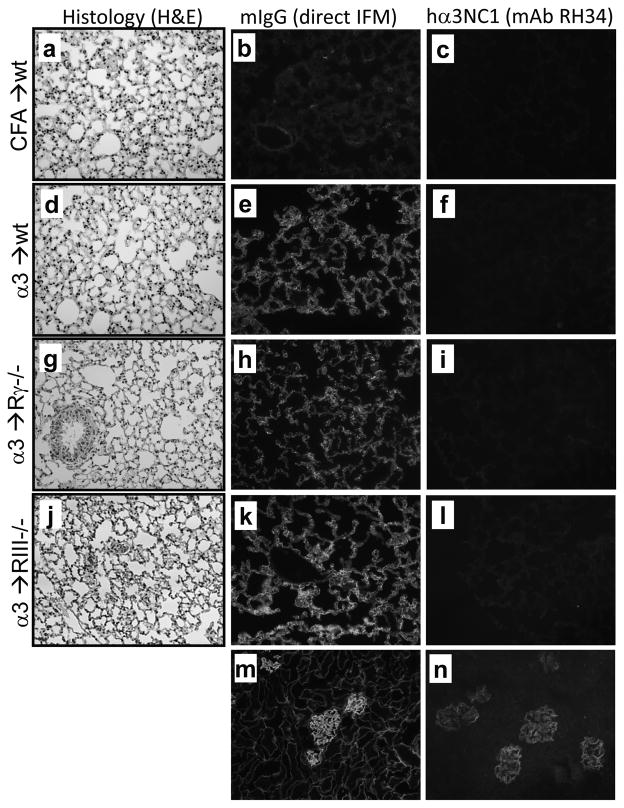
Because α3(IV) collagen is a normal component of alveolar BMs and is targeted by autoAbs mediating pulmonary hemorrhage in GP patients, we assessed the lung phenotype in experimental mice. In α3NC1-immunized and control mice, lungs appeared grossly normal, without overt hemorrhage. By light microscopy, H&E-stained lung sections showed normal alveoli with no inflammation (Fig. 10; d,g,j). Direct immunofluorescence staining showed strong linear mIgG deposition along the alveolar BMs in α3NC1-immunized but not in control mice (Fig. 10; e,h,k). Negative staining by mAb RH34 indicated the absence of rh-α3NC1 in lungs (Fig. 10; f,i,l). These results show that mIgG autoAbs bound to alveolar BMs in vivo but did not induce inflammation.
Figure 10. In vivo binding of mIgG autoAbs to alveolar BMs does not induce lung inflammation.
Lungs from adjuvant-immunized control mice (a–c) and α3NC1-immunized wild type (d–f), FcγR−/− (g–i), and FcγRIII−/− (j–l) mice were collected at ~10–14 weeks post-immunization. Paraffin sections were stained by H&E and observed by light microscopy (a,d,g,j). In vivo binding of mIgG to alveolar BMs was evaluated by direct IF (b,e,h,k). Indirect IF staining for rh-α3NC1 using mAb RH34 was negative in all lung sections (c,f,i,l). Shown for comparison is the deposition of mIgG (m) and RH34 staining (n) in kidneys from FcγRIII−/−mice at 10 weeks after immunization with rh-α3NC1. Original magnification 200×.
Discussion
Membranous nephropathy is a common cause of nephrotic syndrome in adults. Up to 40% of patients develop end-stage renal disease (40). Current treatments are non-specific, toxic, and often ineffective (41, 42). Development of novel targeted therapies requires a thorough understanding of the pathogenic mechanisms. Rat Heymann nephritis has emerged as the best model for studying the disease mechanisms because it faithfully recapitulates human MN (43). However, further progress is hampered by the lack of a mouse model that can take full advantage of the availability of genetically engineered strains and mouse-specific reagents.
In this study, we describe an active mouse model of membranous nephropathy induced by immunization with a fragment of α3(IV) collagen, a normal GBM component and target of GP autoAbs. Wild type, FcγRIII−/− and FcRγ−/− DBA/1 mice immunized with rh-α3NC1 developed massive albuminuria followed by hypoalbuminemia, hyperlipidemia, and often edema typical features of nephrotic syndrome. Evaluation of kidney histopathology revealed GBM thickening with subepithelial spikes, granular deposits of mIgG and mC3 along the glomerular capillary loops by IF, with subepithelial electron-dense immune complexes deposits, and diffuse effacement of podocyte foot processes by EM. These clinical and histopathologic findings recapitulate the hallmark features of membranous nephropathy.
The susceptibility of mouse strains to experimental GN induced by immunization with rh-α3NC1 has been compared by Hopfer et al (30). C57Bl/6 develop milder glomerular injury than DBA/1 mice, while AKR and NOD mice are relatively resistant, despite mIgG deposition in the kidneys. Consistent with our findings, DBA/1 mice exhibit linear-granular GBM staining for mIgG and mC3 and electron-dense subepithelial deposits at 11 weeks post-immunization. A higher proportion of crescents (~23% vs ~5% in our model) may be the result of a more aggressive immunization protocol (mice were immunized in the footpad and boosted three times). Whether the kidney phenotype in mouse strains studied by Hopfer et al recapitulates human MN cannot be ascertained, because albuminuria measurements or other features of nephrotic syndrome have not been reported.
Properties of anti-α3NC1 mIgG Abs: impact on the renal phenotype
Because IgG (auto)Abs are central mediators of both anti-GBM/GP disease and MN, we have characterized in detail the properties of circulating and tissue-bound mIgG Abs elicited by immunization with rh-α3NC1. We showed that bona fide mIgG anti-α3NC1 autoAbs are produced, which cross-react with native mouse GBM NC1 hexamers in vitro and bind to mouse GBM and alveolar BM in vivo. Hence, these mIgG autoAbs must bind to α3NC1 epitopes accessible in the murine α3α4α5NC1 hexamers, similar to mIgG Abs produced in Col4a3−/− mice immunized with rh-α3NC1 and to hIgG alloAbs mediating post-transplant anti-GBM nephritis in autosomal recessive Alport syndrome (16). In contrast, GP autoAb cross-react only with isolated mouse α3NC1 monomers or dimers, but do not bind to native mouse α3α4α5NC1 hexamers, which are extensively cross-linked and thus protected against autoAb-induced dissociation (44–46). Although we cannot rule out that mice also produced GP-like autoAbs, such autoAbs would not bind to mouse tissues in vivo. Production of mIgG autoAbs that bind in vivo to murine BMs containing α3α4α5(IV) collagen indicates that immune self-tolerance toward endogenous α3(IV) collagen is breached in mice immunized with α3NC1 monomers.
Even though anti-α3NC1 mIgG autoAbs bound to GBM and alveolar BMs, they did not mediate crescentic glomerulonephritis or alveolitis, suggesting a limited ability to elicit inflammation. The simplest explanation is the prevalence of mIgG1 over mIgG2a and mIgG2b among anti-α3NC1 autoAbs. Only mIgG2a and mIgG2b but not mIgG1 mediate neutrophil influx and glomerular injury in a mouse model of acute neutrophil-mediated glomerular inflammation (25). In particular, anti-α3NC1 mAbs of mIgG1 subclass which bind to mouse GBM in vivo are not nephritogenic (47). The lattice structure of collagen IV limits the maximum amount and density of autoAbs that can bind to the NC1 domains. Therefore, competition by mIgG1 could reduce the density of GBM-bound IgG2a and IgG2b autoAbs below the threshold required for efficient crosslinking of activating FcγRs on effector cells, while augmenting signaling through the inhibitory IgG receptor, FcγRIIB.
Though present in smaller amounts in mouse sera and kidneys, mIgG2a and mIgG2b may be essential mediators of murine MN. Only mIgG2a and mIgG2b but not mIgG1 are complement-fixing, and α3NC1-immunized mice had glomerular deposition of mC3 and C5b-9. C5b-9 mediated podocyte injury is considered essential for development of proteinuria in experimental and possibly human MN (8, 48). We note that two mouse strains resistant to α3NC1-induced GN, AKR and NOD (30), are naturally deficient in complement C5 (49). Because the ablation of activating FcγRs did not prevent the development of heavy albuminuria and nephrotic syndrome in our model, the major effector pathway of subepithelial ICs is most likely complement activation, thus implying a role for mIgG2a and mIgG2b.
As mIgG1 is the murine equivalent of hIgG4, our model recapitulates the prevalence of hIgG4 autoAbs in idiopathic MN (50). Since mIgG1 is associated with Th2 and mIgG2a with Th1 polarization, the prevalence of mIgG1 in α3NC1-immunized DBA/1 mice implies a Th2 bias. Th subsets affect the pattern of injury and outcomes in GN, with Th1 predominance implicated in proliferative and crescentic glomerulonephritis, and Th2 predominance associated with membranous nephropathy (51). In idiopathic MN, Th2 cytokines increase and stimulate B cells to produce IgG4 (52, 53). A strong Th2 polarization has been reported in murine MN induced by cationic BSA (54). Th2 deviation induced by disruption of WSX-1 gene, which encodes a subunit of IL-27R, changed the renal pathophysiology from proliferative to membranous lupus in MRL/lpr mice (55). Hence, the development of murine MN may depend on features of the antigen and host factors that favor a Th2 polarization.
Formation of subepithelial ICs and the role of GBM antigens in MN
Given the central role of subepithelial ICs in pathogenesis of MN, we addressed their origin in our model. Co-localization of the antigen (rh-α3NC1) with mIgG and mC3 in the GBM (though not in kidney tubular or alveolar BMs) is consistent with a planted antigen mechanism or deposition of small circulating ICs. Glomerular deposition of extrinsic antigens from circulation occurs in other active models of MN, such as rats immunized with human brush border antigen and rabbits immunized with rat tubular antigen (56, 57). Prototypical planted antigens are exogenous cationic proteins, which bind to negative sites in the GBM and on podocytes. Cationic BSA is an antigen in early childhood MN (7), and repeated injections with cationized BSA induce experimental MN (58, 59). Human α3NC1 has a high isoelectric point of (pI ~ 9), which may contribute to its deposition in glomeruli. Alternatively, hα3NC1 may bind directly to its receptors on the podocyte cell surface, such as α3β1 or αvβ3 integrins (60, 61).
Because α3(IV) collagen deposited in the GBM is normally expressed by podocytes (62), subepithelial ICs may also form when anti-α3NC1 autoAbs bind to nascent α3(IV) collagen, before it is stably incorporated into the GBM. Indeed, the increased deposition of α3α4α5(IV) collagen in the GBM implies augmented production of α3(IV) collagen by podocytes in murine MN. Several GBM Ags have been implicated in various models of MN. Administration of Abs to GBM heparan sulfate proteoglycans into pre-sensitized rats induces subepithelial IC deposits and MN (63). Anti-laminin Abs play a central role in HgCl2-induced nephropathy in Brown-Norway rats, including formation of subepithelial deposits and heavy proteinuria (64). These studies raise the question whether GBM Ags may likewise be involved in human MN.
A possible role of α3(IV) collagen in the pathogenesis of some forms of MN is suggested by the association between anti-GBM/GP disease and MN. At least 25 cases have been reported, reviewed in (65). Anti-GBM/GP disease can either precede or is followed by MN, though simultaneous presentation is more common. In these patients, the linear GBM IgG staining found on the renal biopsy is likely due to classic GP autoAbs (anti-α3NC1), typically positive by serology. Much less is known about how subepithelial ICs form. They may consist of GP autoAbs bound to podocyte-associated α3(IV) collagen, or to nascent α3(IV) collagen not yet incorporated in the GBM, or to α3(IV) collagen fragments released from the GBM. The same mechanisms may account for the formation of subepithelial IC in our mouse model.
Comparison with other models of experimental MN
Our mouse model recapitulates hallmark features of human MN as well as rat Heymann nephritis, the “gold standard” for experimental MN. Compared to the widely used passive model, active Heymann nephritis more closely resembles human disease because it involves the host’s own immune system. Its major disadvantages are longer duration, much greater variability, and animal discomfort due to footpad immunizations (66). By comparison, in our active mouse model, nephropathy is induced more rapidly and reliably. In addition, unlike rats, mouse models can take advantage of existing transgenic mice and mouse-specific reagents.
Development of a robust murine model of MN has been a work in progress for decades (66). Early models had various limitations, such as minimal or transient proteinuria or complement-independence. Severe proteinuria, diffuse GBM thickening, subepithelial deposits and GBM spikes are induced in susceptible mouse strains by repeated injections with cationized BSA (58). However, this model is cumbersome, requiring numerous intravenous injections (every other day for 6 weeks). Characteristic features of MN are also induced in mice after passive immunization with sheep Abs to murine podocytes (6). This model may depend on the quality of the heterologous antisera and/or the immunogen preparation used, since other groups have found that sheep Abs to mouse podocyte antigens cause little glomerular damage in wild type mice despite in vivo binding to glomerular capillary wall (67).
We anticipate that the murine MN model described herein, leveraged by the availability of numerous strains of knockout mice, will help address new questions about the disease patho-mechanisms, such as the role of various pathways of complement activation. A potential drawback is the possible need for backcrossing mice to the DBA/1 background. Knockout mice are most often available on the C57Bl/6 background. In our hands, C57Bl/6J mice immunized with α3NC1 according to the protocol used in DBA/1 mice developed moderate albuminuria by 12–18 weeks (ACR ranging from 1.64 to 2.39, compared to 0.01–0.03 in control mice, n=3 mice in each group) but no features of nephrotic syndrome (data not shown). The susceptibility of other mouse strains to experimental MN is being addressed in ongoing studies. Nonetheless, our model of active MN in DBA/1 mice is readily usable for testing the effectiveness on novel targeted drugs and ideal for preclinical studies of experimental therapies.
Acknowledgments
This work was supported by grant R01 DK080799 (to D.B.B.) from the National Institutes of Health. F.O. has been supported in part by a postdoctoral fellowship (11POST7300008) from the American Heart Association South-East Affiliate. M.B. has been a short-term trainee in the Vanderbilt Medical Student Research Training Program in Nephrology and Hypertension. Electron microscopy was performed by the Washington University O’Brien Center for Kidney Disease Research, supported by NIH grant P30 DK079333. Silver staining was performed at the Histology and Morphometry Core of the Vanderbilt University O’Brien Kidney Disease Center, supported by the grant P50 DK039261.
We thank Dr. Billy Hudson for providing cells expressing rh-α3NC1, Dr. Elena Tchekneva for access to the urine collection station, Ms. Ellen Donnert for performing the Jones silver stain, and Ms. Anjana Hassan for technical support.
Abbreviation used
- BM
basement membrane
- EM
electron microscopy
- GBM
glomerular basement membrane
- GN
glomerulonephritis
- GP
Goodpasture
- IC
immune complexes
- IF
immunofluorescence
- mIgG
mouse IgG
- MN
membranous nephropathy
- NC1
non-collagenous domain 1
Footnotes
E. Tchekneva, M. Dikov, V. Kadkina, and D. Polosukhina, “Collection station for accelerated collection of the specimens from laboratory animals”, United States Patent and Trade Mark Office, Publication Number 20110239953, 2011.
References
- 1.Nangaku M, Couser WG. Mechanisms of immune-deposit formation and the mediation of immune renal injury. Clin Exp Nephrol. 2005;9:183–191. doi: 10.1007/s10157-005-0357-8. [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis. 2010;56:157–167. doi: 10.1053/j.ajkd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschenes G, Ronco PM. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–2060. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 5.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, et al. Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol. 2010;21:507–519. doi: 10.1681/ASN.2008121259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer-Schwesinger C, Dehde S, Klug P, Becker JU, Mathey S, Arefi K, Balabanov S, Venz S, Endlich KH, Pekna M, et al. Nephrotic syndrome and subepithelial deposits in a mouse model of immune-mediated anti-podocyte glomerulonephritis. J Immunol. 2011;187:3218–3229. doi: 10.4049/jimmunol.1003451. [DOI] [PubMed] [Google Scholar]
- 7.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschenes G, Remuzzi G, Ulinski T, Ronco P. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med. 2011;364:2101–2110. doi: 10.1056/NEJMoa1013792. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham PN, Quigg RJ. Contrasting roles of complement activation and its regulation in membranous nephropathy. J Am Soc Nephrol. 2005;16:1214–1222. doi: 10.1681/ASN.2005010096. [DOI] [PubMed] [Google Scholar]
- 9.Borza DB. Autoepitopes and alloepitopes of type IV collagen: role in the molecular pathogenesis of anti-GBM antibody glomerulonephritis. Nephron Exp Nephrol. 2007;106:e37–43. doi: 10.1159/000101791. [DOI] [PubMed] [Google Scholar]
- 10.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Mechanisms of Disease: Alport’s syndrome, Goodpasture’s Syndrome, and Type IV Collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 11.Borza DB, Netzer KO, Leinonen A, Todd P, Cervera J, Saus J, Hudson BG. The Goodpasture autoantigen: Identification of multiple cryptic epitopes on the NC1 domain of the alpha3(IV) collagen chain. J Biol Chem. 2000;275:6030–6037. doi: 10.1074/jbc.275.8.6030. [DOI] [PubMed] [Google Scholar]
- 12.Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, Langeveld JP, Hudson BG. The Goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem. 1999;274:11267–11274. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- 13.Hellmark T, Burkhardt H, Wieslander J. Goodpasture Disease. Characterization of a single conformational epitope as the target of pathogenic autoantibodies. J Biol Chem. 1999;274:25862–25868. doi: 10.1074/jbc.274.36.25862. [DOI] [PubMed] [Google Scholar]
- 14.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JS, Kashtan CE, Turner AN, Heidet L, Hudson BG, Borza DB. The alloantigenic sites of alpha 3alpha 4alpha 5(IV) collagen: Pathogenic X-linked Alport allo-antibodies target two accessible conformational epitopes in the alpha 5NC1 domain. J Biol Chem. 2007;282:10670–10677. doi: 10.1074/jbc.M611892200. [DOI] [PubMed] [Google Scholar]
- 16.Wang XP, Fogo AB, Colon S, Giannico G, Abul-Ezz SR, Miner JH, Borza DB. Distinct epitopes for anti-glomerular basement membrane Alport alloantibodies and Goodpasture autoantibodies within the noncollagenous domain of {alpha}3(IV) collagen: A Janus-faced antigen. J Am Soc Nephrol. 2005;16:3563–3571. doi: 10.1681/ASN.2005060670. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Torre A, Shield CF, 3rd, Zamborsky ED, Werner MC, Suchin E, Wolf G, Helmchen UM, van den Heuvel LP, Grossman R, et al. Identification of alpha3, alpha4, and alpha5 chains of type IV collagen as alloantigens for Alport posttransplant anti-glomerular basement membrane antibodies. Transplantation. 2000;69:679–683. doi: 10.1097/00007890-200002270-00038. [DOI] [PubMed] [Google Scholar]
- 18.Brainwood D, Kashtan C, Gubler MC, Turner AN. Targets of alloantibodies in Alport anti-glomerular basement membrane disease after renal transplantation. Kidney Int. 1998;53:762–766. doi: 10.1046/j.1523-1755.1998.00794.x. [DOI] [PubMed] [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Wakayama H, Hasegawa Y, Kawabe T, Hara T, Matsuo S, Mizuno M, Takai T, Kikutani H, Shimokata K. Abolition of anti-glomerular basement membrane antibody-mediated glomerulonephritis in FcRgamma-deficient mice. Eur J Immunol. 2000;30:1182–1190. doi: 10.1002/(SICI)1521-4141(200004)30:4<1182::AID-IMMU1182>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Ueda S, Ohno H, Hamano Y, Tanaka M, Shiratori T, Yamazaki T, Arase H, Arase N, Karasawa A, et al. Resistance of Fc receptor- deficient mice to fatal glomerulonephritis. J Clin Invest. 1998;102:1229–1238. doi: 10.1172/JCI3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Watanabe N, Akikusa B, Kurasawa K, Matsumura R, Saito Y, Iwamoto I, Saito T. Fc receptor-independent development of autoimmune glomerulonephritis in lupus-prone MRL/lpr mice. Arthritis Rheum. 2003;48:486–494. doi: 10.1002/art.10813. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Muhlfeld AS, Wietecha TA, Peutz-Kootstra CJ, Kowalewska J, Yi K, Spencer M, Pichaiwong W, Nimmerjahn F, Hudkins KL, et al. Deletion of activating Fcgamma receptors does not confer protection in murine cryoglobulinemia-associated membranoproliferative glomerulonephritis. Am J Pathol. 2009;175:107–118. doi: 10.2353/ajpath.2009.081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgini A, Brown HJ, Lock HR, Nimmerjahn F, Ravetch JV, Verbeek JS, Sacks SH, Robson MG. Fc gamma RIII and Fc gamma RIV are indispensable for acute glomerular inflammation induced by switch variant monoclonal antibodies. J Immunol. 2008;181:8745–8752. doi: 10.4049/jimmunol.181.12.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii T, Hamano Y, Ueda S, Akikusa B, Yamasaki S, Ogawa M, Saisho H, Verbeek JS, Taki S, Saito T. Predominant role of FcgammaRIII in the induction of accelerated nephrotoxic glomerulonephritis. Kidney Int. 2003;64:1406–1416. doi: 10.1046/j.1523-1755.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarzi RM, Davies KA, Claassens JW, Verbeek JS, Walport MJ, Cook HT. Both Fcgamma receptor I and Fcgamma receptor III mediate disease in accelerated nephrotoxic nephritis. Am J Pathol. 2003;162:1677–1683. doi: 10.1016/s0002-9440(10)64302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura A, Yuasa T, Ujike A, Ono M, Nukiwa T, Ravetch JV, Takai T. Fcgamma receptor IIB-deficient mice develop Goodpasture’s syndrome upon immunization with type IV collagen. A novel murine model for autoimmune glomerular basement membrane disease. J Exp Med. 2000;191:899–906. doi: 10.1084/jem.191.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopfer H, Maron R, Butzmann U, Helmchen U, Weiner HL, Kalluri R. The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. Faseb J. 2003;17:860–868. doi: 10.1096/fj.02-0746com. [DOI] [PubMed] [Google Scholar]
- 31.Xie C, Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol. 2004;172:5047–5055. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 32.Kleinau S, Martinsson P, Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000;191:1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG. Type IV collagen of the glomerular basement membrane: Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- 34.Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, Borza DB. Loss of alpha3/alpha4(IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to alpha5alpha6(IV) collagen associated with longer renal survival in Col4a3−/− Alport mice. J Am Soc Nephrol. 2006;17:1962–1969. doi: 10.1681/ASN.2006020165. [DOI] [PubMed] [Google Scholar]
- 35.Saus J, Wieslander J, Langeveld JP, Quinones S, Hudson BG. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988;263:13374–13380. [PubMed] [Google Scholar]
- 36.Heidet L, Borza DB, Jouin M, Sich M, Mattei MG, Sado Y, Hudson BG, Hastie N, Antignac C, Gubler MC. A Human-Mouse Chimera of the alpha3alpha4alpha5(IV) Collagen Protomer Rescues the Renal Phenotype in Col4a3(−/−) Alport Mice. Am J Pathol. 2003;163:1633–1644. doi: 10.1016/s0002-9440(10)63520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002;160:721–730. doi: 10.1016/S0002-9440(10)64892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y, Butkowski R, Burke B, Kleppel MM, Crosson J, Katz A, Michael AF. Differential expression of basement membrane collagen in membranous nephropathy. Am J Pathol. 1991;139:1381–1388. [PMC free article] [PubMed] [Google Scholar]
- 39.Minto AW, Kalluri R, Togawa M, Bergijk EC, Killen PD, Salant DJ. Augmented expression of glomerular basement membrane specific type IV collagen isoforms (alpha3–alpha5) in experimental membranous nephropathy. Proc Assoc Am Physicians. 1998;110:207–217. [PubMed] [Google Scholar]
- 40.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3:905–919. doi: 10.2215/CJN.04321007. [DOI] [PubMed] [Google Scholar]
- 41.Glassock RJ. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am J Kidney Dis. 2004;44:562–566. [PubMed] [Google Scholar]
- 42.Waldman M, Austin HA., 3rd Controversies in the treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2009;5:469–479. doi: 10.1038/nrneph.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cybulsky AV, Quigg RJ, Salant DJ. Experimental membranous nephropathy redux. Am J Physiol Renal Physiol. 2005;289:F660–671. doi: 10.1152/ajprenal.00437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo W, Wang XP, Kashtan CE, Borza DB. Alport alloantibodies but not Goodpasture autoantibodies induce murine glomerulonephritis: protection by quinary crosslinks locking cryptic alpha3(IV) collagen autoepitopes in vivo. J Immunol. 2010;185:3520–3528. doi: 10.4049/jimmunol.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borza DB, Bondar O, Colon S, Todd P, Sado Y, Neilson EG, Hudson BG. Goodpasture autoantibodies unmask cryptic epitopes by selectively dissociating autoantigen complexes lacking structural reinforcement: Novel mechanisms for immune privilege and autoimmune pathogenesis. J Biol Chem. 2005;280:27147–27154. doi: 10.1074/jbc.M504050200. [DOI] [PubMed] [Google Scholar]
- 46.Vanacore RM, Ham AJ, Cartailler JP, Sundaramoorthy M, Todd P, Pedchenko V, Sado Y, Borza DB, Hudson BG. A role for collagen IV cross-links in conferring immune privilege to the Goodpasture autoantigen: structural basis for the crypticity of B cell epitopes. J Biol Chem. 2008;283:22737–22748. doi: 10.1074/jbc.M803451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, Borza DB, Braley H, Holdsworth SR, Kitching AR. Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 2009;20:2518–2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol. 2005;16:1195–1204. doi: 10.1681/ASN.2004121098. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson UR, Muller-Eberhard HJ. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967;125:1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira DB. Membranous nephropathy: an IgG4-mediated disease. Lancet. 1998;351:670–671. doi: 10.1016/S0140-6736(97)04122-6. [DOI] [PubMed] [Google Scholar]
- 51.Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- 52.Kuroki A, Iyoda M, Shibata T, Sugisaki T. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68:302–310. doi: 10.1111/j.1523-1755.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 53.Hirayama K, Ebihara I, Yamamoto S, Kai H, Muro K, Yamagata K, Kobayashi M, Koyama A. Predominance of type-2 immune response in idiopathic membranous nephropathy. Cytoplasmic cytokine analysis. Nephron. 2002;91:255–261. doi: 10.1159/000058401. [DOI] [PubMed] [Google Scholar]
- 54.Wu CC, Chen JS, Chen SJ, Lin SH, Chen A, Chang LC, Sytwu HK, Lin YF. Kinetics of adaptive immunity to cationic bovine serum albumin-induced membranous nephropathy. Kidney Int. 2007;72:831–840. doi: 10.1038/sj.ki.5002426. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, et al. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 56.Edgington TS, Glassock RJ, Dixon FJ. Autologous immune-complex pathogenesis of experimental allergic glomerulonephritis. Science. 1967;155:1432–1434. doi: 10.1126/science.155.3768.1432. [DOI] [PubMed] [Google Scholar]
- 57.Barabas AZ, Lannigan R. Immune-complex nephritis in the rabbit produced by injections of rat renal tubular fraction 3 antigen. Br J Exp Pathol. 1981;62:94–102. [PMC free article] [PubMed] [Google Scholar]
- 58.Chen JS, Chen A, Chang LC, Chang WS, Lee HS, Lin SH, Lin YF. Mouse model of membranous nephropathy induced by cationic bovine serum albumin: antigen dose-response relations and strain differences. Nephrol Dial Transplant. 2004;19:2721–2728. doi: 10.1093/ndt/gfh419. [DOI] [PubMed] [Google Scholar]
- 59.Border WA, Ward HJ, Kamil ES, Cohen AH. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 1982;69:451–461. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borza CM, Borza DB, Pedchenko V, Saleem MA, Mathieson PW, Sado Y, Hudson HM, Pozzi A, Saus J, Abrahamson DR, et al. Human podocytes adhere to the KRGDS motif of the alpha3alpha4alpha5 collagen IV network. J Am Soc Nephrol. 2008;19:677–684. doi: 10.1681/ASN.2007070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borza CM, Pozzi A, Borza DB, Pedchenko V, Hellmark T, Hudson BG, Zent R. Integrin {alpha}3beta1, a Novel Receptor for {alpha}3(IV) Noncollagenous Domain and a Trans-dominant Inhibitor for Integrin {alpha}vbeta3. J Biol Chem. 2006;281:20932–20939. doi: 10.1074/jbc.M601147200. [DOI] [PubMed] [Google Scholar]
- 62.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009;20:1471–1479. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makino H, Lelongt B, Kanwar YS. Nephritogenicity of proteoglycans. II. A model of immune complex nephritis. Kidney Int. 1988;34:195–208. doi: 10.1038/ki.1988.165. [DOI] [PubMed] [Google Scholar]
- 64.Icard P, Pelletier L, Vial MC, Mandet C, Pasquier R, Michel A, Druet P. Evidence for a role of antilaminin-producing B cell clones that escape tolerance in the pathogenesis of HgCl2-induced membranous glomerulopathy. Nephrol Dial Transplant. 1993;8:122–127. [PubMed] [Google Scholar]
- 65.Basford AW, Lewis J, Dwyer JP, Fogo AB. Membranous nephropathy with crescents. J Am Soc Nephrol. 2011;22:1804–1808. doi: 10.1681/ASN.2010090923. [DOI] [PubMed] [Google Scholar]
- 66.Jefferson JA, Pippin JW, Shankland SJ. Experimental Models of Membranous Nephropathy. Drug Discov Today Dis Models. 2010;7:27–33. doi: 10.1016/j.ddmod.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao L, Haas M, Pippin J, Wang Y, Miwa T, Chang A, Minto AW, Petkova M, Qiao G, Song WC, et al. Focal and segmental glomerulosclerosis induced in mice lacking decay-accelerating factor in T cells. J Clin Invest. 2009;119:1264–1274. doi: 10.1172/JCI36000. [DOI] [PMC free article] [PubMed] [Google Scholar]