Abstract
Background
Inhibition mediated by γ-aminobutyric acid type A (GABAA) receptors has long been considered an important target for a variety of general anesthetics. In the hippocampus, two types of phasic GABAA receptor-mediated inhibition coexist: GABAA,fast, which is expressed primarily at peri-somatic sites, and GABAA,slow, which is expressed primarily in the dendrites. Their spatial segregation suggests distinct functions: GABAA,slow may control plasticity of dendritic synapses, while GABAA,fast controls action potential initiation at the soma. We examined modulation of GABAA,fast and GABAA,slow inhibition by isoflurane at amnesic concentrations, and compared it to modulation by behaviorally equivalent doses of the GABAA receptor-selective drug etomidate.
Methods
Whole-cell recordings were conducted at near-physiological temperature from pyramidal cells in organotypic hippocampal cultures obtained from C57BL/6 x 129/SvJ F1 hybrid mice. GABAA receptor-mediated currents were isolated using glutamate receptor antagonists. GABAA,slow currents were evoked by electrical stimulation in the stratum lacunosum-moleculare. Miniature GABAA,fast currents were recorded in the presence of tetrodotoxin.
Results
100 µM isoflurane (approximately EC50,amnesia) slowed fast and slow inhibitory postsynaptic current decay by approximately 25%. Higher concentrations, up to 400 µM, produced proportionally greater effects without altering current amplitudes. The effects on GABAA,slow were approximately one-half those produced by equi-amnesic concentrations of etomidate.
Conclusions
Isoflurane enhances both types of phasic GABAA receptor-mediated inhibition to similar degrees at amnesic concentrations. This pattern differs from etomidate, which at low concentrations selectively enhances slow inhibition. These effects of isoflurane are sufficiently large that they may contribute substantially to its suppression of hippocampal learning and memory.
Introduction
Ionotropic γ-aminobutyric acid type A (GABAA) receptors are key mediators for many receptor-selective anesthetics, including etomidate and propofol.1 They are also an important target for benzodiazepines, which by targeting specific receptor subtypes can produce anxiolysis, sedation and amnesia at low doses, and unconsciousness at higher doses.2 GABAA receptors have been considered likely mediators of at least some components of the anesthetic state induced by inhaled agents, such as isoflurane.3 However, since alternative and/or complementary molecular mediators have been identified and defined using advanced neurobiological techniques for some end-points, such as immobility and hypnosis,4 and multiple forms of synaptic and non-synaptic inhibition mediated by GABAA receptors have been described,5,6 the role of GABAA receptors and inhibitory synapses in anesthesia is undergoing reassessment.
Some of the best evidence for a role of GABAA receptors in inhaled anesthetic-induced amnesia comes from studies of mice lacking the α1 subunit of the GABAA receptor. The α1 subunit is present at many inhibitory synapses in the hippocampus and neocortex and is instrumental in benzodiazepine-induced sedation and amnesia.7 Mice lacking this subunit, either globally or only in the hippocampus, are resistant to isoflurane-induced amnesia, as measured by fear conditioning to tone or context (no phenotype with respect to sedation was reported).8 These results thus support a role for synaptic α1-containing GABAA receptors in inhaled anesthetic suppression of learning and memory. However, studies of “knock-in” mice carrying isoflurane-resistant α1 or α2 subunits failed to find any change in the concentration of isoflurane required to impair learning.9,10 Moreover, inhibitory postsynaptic currents (IPSCs) mediated by α1- and α2-containing GABAA receptors at prototypical “fast inhibitory synapses” were reported to be relatively insensitive to isoflurane, compared to a tonic form of inhibition that exists in hippocampal neurons that utilizes receptors containing α5 GABAA receptor subunits.11
These results have thus called into question the behavioral significance of modulation of synaptic α1- and α2-containing receptors, or even synaptic inhibition itself, to isoflurane-induced amnesia. Inhibitory synapses do, however, display a remarkable diversity in their physiological, pharmacological, and anatomical characteristics5. Is it possible that other types of inhibitory synapses that utilize different subunits play a more important role in modulating memory than those formed by basket cells and other interneurons that impinge on the somata of pyramidal cells? In recent studies we showed that GABAA,slow, a form of synaptic inhibition that is prominent in the dendrites of pyramidal neurons and is particularly well suited to control synaptic plasticity, is mediated in part by α5 subunits of the GABAA receptor,12 and that amnesic concentrations of etomidate enhance GABAA,slow significantly more than GABAA,fast IPSCs.13 Here we report that amnesic concentrations of isoflurane also enhance GABAA,slow substantially, by approximately one-half of the modulation produced by equally effective (amnesic) concentrations of etomidate. Further, we show that in contrast to etomidate, isoflurane modulates both types of phasic inhibition to similar degrees. These finding suggest that modulation of GABAA,slow contributes to amnesia produced by both etomidate and isoflurane, and that for isoflurane an additional contribution may come from modulation of GABAA,fast.
Materials and Methods
All experiments conformed to the guidelines laid out in the Guide for the Care and Use of Laboratory Animals, and were conducted with the approval of the University of Wisconsin-Madison (Madison, WI) Animal Care and Use Committee. Organotypic hippocampal brain slice preparation, electrophysiology, materials and chemicals used, and recording conditions were similar to those described recently. Briefly, organotypic hippocampal slice cultures were prepared from 3 to 8-day-old C57Bl6/129-SvJ hybrid mice as described by Stoppini et al,14 maintained in an incubator at 36 °C in 5% CO2, and used between 10 and 14 days in culture, at which time hippocampal structures remained easily identifiable. Whole cell recordings were obtained from CA1 pyramidal neurons using borosilicate glass pipettes (KG33; Garner Glass, Claremont, CA) filled with a solution containing (in mM): CsCl 135 (for evoked IPSCs recordings, CsCl was partially replaced by 40 mM K-gluconate ), Na-HEPES 10, EGTA 10, MgATP 3, GTP 0.5, lidocaine N-ethyl bromide (QX-314) 5, pH=7.25. Signals were amplified using a MultiClamp 700A amplifier (Axon Instruments, Foster City, CA) and ClampEx software (Axon Instruments), filtered at 5 kHz, and sampled at 10 kHz using a Digidata 1322A (Axon Instruments). Open tip resistance ranged from 1.5–4 MΩ. Whole-cell access resistance was less than 15 MΩ before compensation by 50–80%. Slices were continuously superfused with artificial cerebrospinal fluid at 24±1°C (evoked responses) or 34±1°C (spontaneous IPSCs), saturated with carbogen (95%O2+5%CO2) and containing the glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 µM) and (2R)-amino-5-phosphonovaleric acid (APV, 40 µM) to block excitatory neurotransmission.
Chemicals and Drugs
All chemicals and drugs except for isoflurane were purchased from Sigma-Aldrich (St. Louis, MO). Isoflurane (Abbott Laboratories, Chicago, IL) was prepared from a saturated stock solution stored in 500 ml Teflon gas sampling bags (Fisher Scientific International Inc., Hampton, NH) and transferred to glass syringes for use. To minimize loss of isoflurane, polytetrafluoroethylene tubing was used to connect the glass syringe reservoirs and the recording chamber. The concentration of isoflurane in the recording chamber was measured using gas chromatography (Gow-Mac series 580 flame ionization detector gas chromatograph, 6’×18” stainless steel column packed with 0.2% carbowax 1500 on carbopak C, 60/80, Gow-MAC Instrument Company Bethlehem, PA). The detector and the column were set to 160 °C and 140 °C, respectively. A nitrogen flow of 30 mL/min resulted in retention times of 45s.
Determination of EC50,amnesia
To compare the effects of isoflurane and etomidate on IPSCs, the EC50,amnesia concentration of isoflurane was taken to be 0.28%,9 which corresponds to an aqueous concentration of 114µM,15 and the concentration of etomidate as 0.25 µM.16 These concentrations were determined using comparable fear-conditioning paradigms, and both in hybrid C57Bl6x129-SvJ mice – the same hybrid strain that we used for the present experiments.
Data analysis
Data were analyzed offline on a personal computer using Mini Analysis (Synaptosoft, Decatur, GA) and ClampFit (Molecular Devices, Sunnyvale, CA). The threshold for event detection was set at three times the root mean square noise level. Spontaneous IPSCs were analyzed by automated event detection that acquires amplitude, 10–90% rise times, and the time to 63% decay. Evoked IPSCs were analyzed by fitting to a monoexponential function using least squares minimization of errors.
Statistics
Statistical analysis was performed using Microcal Origin (version 7, OriginLab Corporation, Northampton, MA) or GraphPad Prism (version 7, GraphPad Software, San Diego, CA), or Microsoft Excel (version 12.3.1, Microsoft Corporation, Redwood, WA). Data are presented as mean±SD. Statistical comparisons were made using one-tailed Student’s t-tests when a strong expectation existed based on existing literature (e.g. slowing of decay by isoflurane and etomidate), or z-tests when testing for deviation of normalized values (iso/control) from unity. Effects were considered significant at p<0.05.
Results
Isoflurane slows the decay of GABAA,slow IPSCs
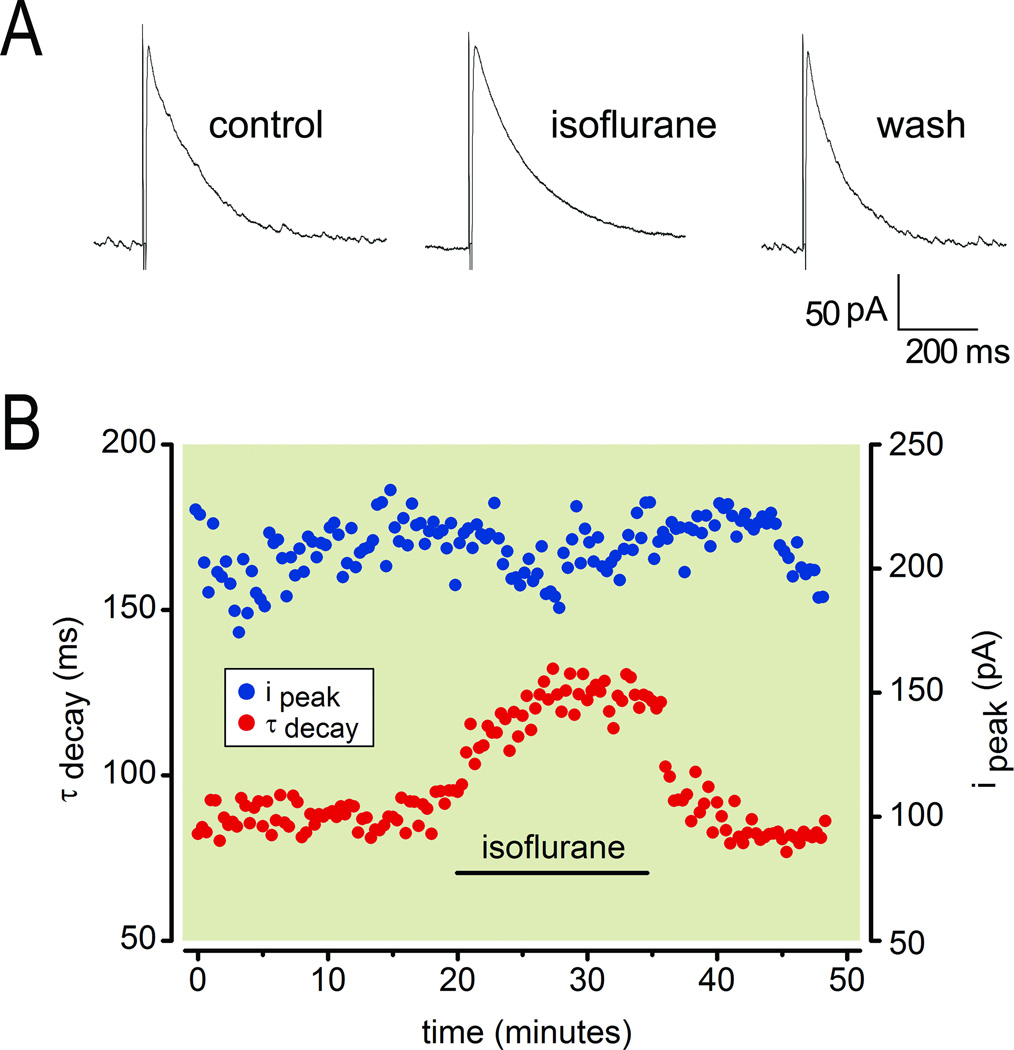
As reported previously,13 electrical stimulation in stratum lacunosum-moleculare of the OTChip in the presence of glutamate receptor blockers evoked GABAergic IPSCs with rise and decays times characteristic of GABAA,slow. These evoked responses were reversibly slowed by 200 µM isoflurane (Fig. 1A), a concentration that is equivalent to 0.5% isoflurane in vivo, which is the lowest concentration that is reliably amnesic.9 Both the time from drug application to peak effect and the time from drug termination to recovery of baseline responses were rapid in the OTChip, typically less than 10 minutes (Fig. 1B). Analysis of evoked responses during drug wash in and washout revealed that 200 µM isoflurane reversibly slowed IPSC decay (Fig. 1B, red symbols) but did not alter IPSC amplitude (Fig. 1B, blue symbols).
Fig.1.
Isoflurane slows the decay of γ-aminobutyric acid type A,slow (GABAA,slow) IPSCs. (A) Whole-cell patch clamp recording of IPSCs in a CA1 pyramidal cell under control conditions, in the presence of 200 µM isoflurane, and after washout (all at 24 °C). The time constant of decay (in ms) and the peak amplitude (in pA) were 85 and 211 (control), 124 and 210 (isoflurane), 82 and 209 (washout). (B) Time series of the experiment illustrating the effect of isoflurane on the decay of GABAA,slow. Note lack of effect on the amplitude and rapid onset and offset of isoflurane’s effect.
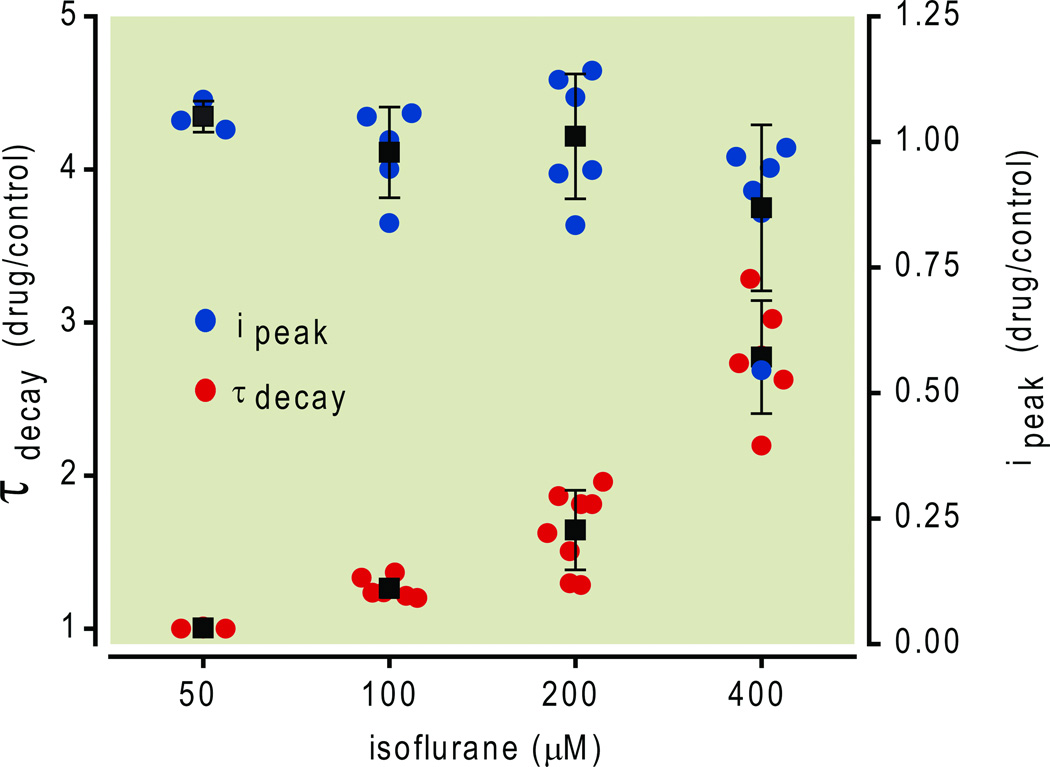
We explored the concentration dependence of isoflurane’s effect on evoked GABAA,slow IPSCs by applying isoflurane at concentrations ranging from 55 µM (which is approximately one-half EC50-amnesia)9 to 400 µM (approximately 3.5 times EC50-amnesia, or 1.25 times EC50-movement). As illustrated in Fig. 2, 55 µM isoflurane had no measurable effect, but higher concentrations caused IPSC decay to be slowed in a concentration-dependent fashion. At a concentration of 100 µM, which is slightly less than the EC50,amnesia concentration, isoflurane slowed IPSC decay by a statistically significant extent (70 ± 11 ms control vs. 86 ± 9 ms isoflurane, p = 0.001, n=4, one-tailed paired Student’s t-test).
Fig.2.
Summary of isoflurane effects on evoked γ-aminobutyric acid type A,slow (GABAA,slow) inhibitory postsynaptic currents (IPSCs). Isoflurane 55 – 400 µ M did not alter IPSC peak amplitude but did prolong decay. All data were obtained at 24 °C and are plotted as mean ± SD.
Isoflurane also slows the decay of GABAA,fast IPSCs
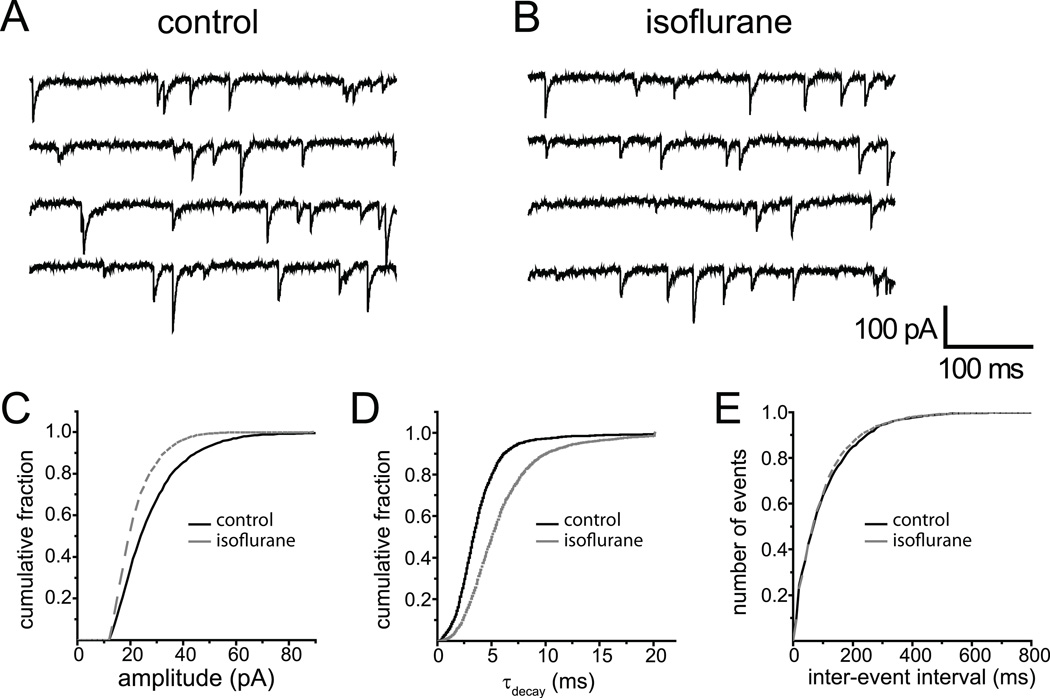
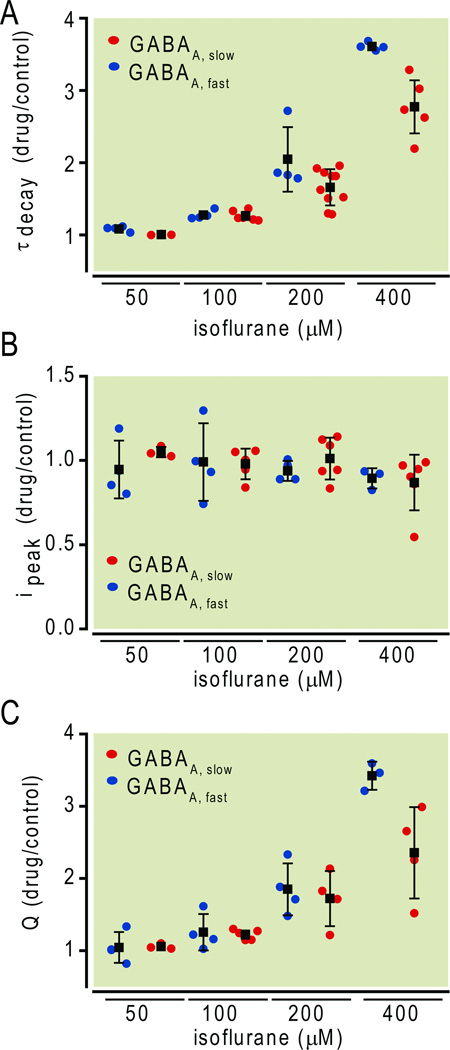
To measure effects of this same range of isoflurane concentrations on GABAA,fast IPSCs, we studied tetrodotoxin-resistant miniature IPSCs, of which more than 99.9% display the rapid rise and decay characteristic of this class.13,17 A representative example of the effect of 200 µM isoflurane is shown in Fig. 3. Comparison of raw traces in the absence vs. presence of isoflurane (Fig. 3A vs. 3B) shows little obvious effect. A synopsis of all miniature IPSCs recorded from this cell suggests a slight reduction in the peak amplitude (Fig. 3C), and a slowing of the decay (Fig. 3D), without any change in their frequency (Fig. 3E). A summary of all similar experiments over a range of isoflurane concentrations confirmed a dose-dependent effect on the decay of miniature IPSCs (Fig. 4A) but revealed no effect on their amplitude (Fig. 4B). For comparison, Figure 4 also presents the effects of isoflurane on the same parameters of evoked GABAA,slow responses. The decay rates of both types of IPSCs were slowed dose-dependently between 100 and 400 µM isoflurane (Fig. 4A). Since the peak amplitude was largely unaffected in this concentration range (Fig. 4B), charge transfer was increased for both types of IPSCs (Fig. 4C). At 400 µM, i.e. four times EC50 amnesia, it increased charge transfer more for fast than slow IPSCs (p<0.05, two-tailed Student’s t-test, GABAA,fast n=3, GABAA,slow n=4). At other concentrations, there were no significant differences (p>0.05, two-tailed Student’s t-test, GABAA,fast n=4 at all concentrations, GABAA,slow n=3, 5, and 4 at 50, 100, and 200 µM respectively).
Fig.3.
Effect of isoflurane on γ-aminobutyric acid type A,fast (GABAA,fast) inhibitory postsynaptic currents (IPSCs) at 34 °C. Miniature IPSCs under control conditions (A) and in the presence of 200 µM isoflurane (B). Summary of isoflurane effects on amplitude (C), decay kinetics (D) and inter-event interval (E) of GABAA,fast miniature IPSCs in this single cell.
Fig.4.
Comparison of isoflurane effects on fast and slow inhibitory postsynaptic currents (IPSCs). (A) Isoflurane slowed the decay of both fast and slow IPSCs in a concentration-dependent manner. (B) Isoflurane did not alter IPSC amplitude. (C) Isoflurane increased charge transfer for both types of IPSCs. At 400 µM, i.e., four times EC50 amnesia, it increased charge transfer more for fast than slow IPSCs (p < 0.05, two-tailed Student’s t-test, GABAA,fast n = 3, GABAA,slow n = 4). At other concentrations, there were no significant differences (p > 0.05, two-tailed Student’s t-test, GABAA,fast n = 4 at all concentrations, GABAA,slow n = 3, 5, 4, at 50, 100, 200 µM respectively). Data are plotted as mean ± SD. Note that results were obtained at 24 °C and 34 °C for GABAA,slow and GABAA,fast, respectively.
Temperature-sensitivity of modulation
The effects of anesthetics on molecular targets are often studied in vitro at less than physiologic temperatures. However, these observations can be extrapolated to the in vivo situation by taking into account the temperature-induced changes of gas solubility in aqueous solutions.15 No difference in the effect of enflurane, an isomer of isoflurane, on GABAA,fast synaptic inhibition was found between room and body temperature. 18 Less is known about temperature-dependent changes of injectable anesthetic effects. We tested the temperature-dependence of intravenous anesthetics by comparing the effect of etomidate on the decay of GABAA,slow IPSCs at 24 °C and 34 °C. As expected, IPSC decay became faster with increasing experimental temperature (Q10 = 3.3 ± 0.9). Nevertheless, the concentration-dependent relative slowing of the decay by etomidate remained constant (Table 1). Therefore we combined data obtained at 24 °C and 34 °C in Fig. 5.
Table 1.
Etomidate modulation of GABAA,slow kinetics does not vary with temperature. Data are presented as mean ± SD.
| [etomidate] (µM) |
T (0C) | n | τcontrol (ms) |
τdrug (ms) |
Ratio (%) (τdrug/τcontrol) |
|---|---|---|---|---|---|
| 0.125 | 34±1 24±1 |
4 5 |
28 ± 10 78 ± 54 |
33 ± 11 97 ± 63 |
119 ± 11% 126 ± 9% |
| 0.25 | 34±1 24±1 |
7 6 |
30 ± 10 82 ± 49 |
55 ± 21 150 ± 72 |
199 ± 52% 196 ± 41% |
| 0.5 | 34±1 24±1 |
4 6 |
22 ± 4 101 ± 48 |
70 ± 27 226 ± 110 |
314 ± 85% 253 ± 49% |
| 1.0 | 34±1 24±1 |
4 3 |
26 ± 7 76 ± 26 |
104 ± 14 283 ± 150 |
441 ± 53% 363 ± 78% |
GABAA,slow- γ-aminobutyric acid type A, slow
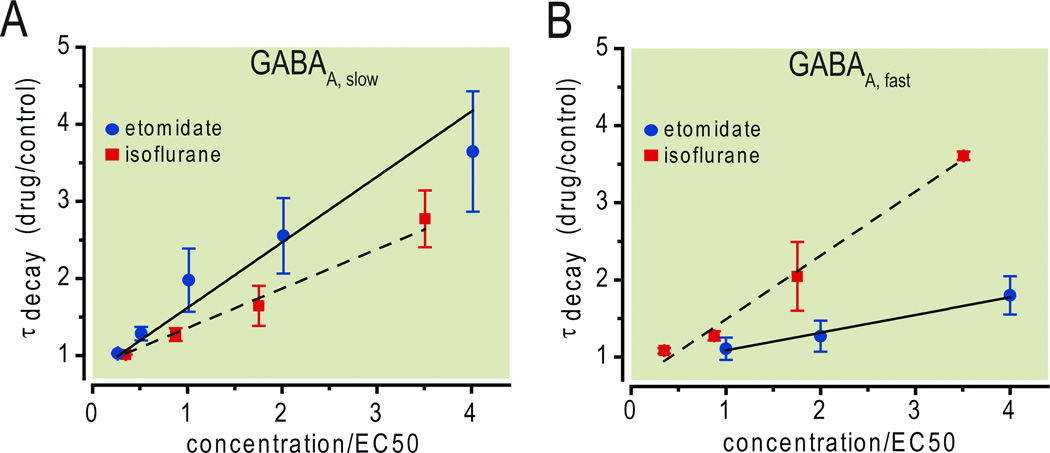
Fig.5.
Isoflurane and etomidate differ in their modulation of phasic inhibition. At equi-amnesic concentrations, etomidate enhanced γ-aminobutyric acid type A,slow (GABAA,slow) more than GABAA,fast inhibitory postsynaptic currents (A). The converse was true for isoflurane (B). The linear fits are based on unweighted least squares minimization using the mean values at each concentration. Their slopes for GABAA,slow are 0.85 (etomidate) and 0.51 (isoflurane), and for GABAA,fast are 0.23 (etomidate) and 0.83 (isoflurane). Note that results were obtained at 34 °C for etomidate. For isoflurane, 24 °C and 34 °C were used for GABAA,slow and GABAA,fast, respectively. The EC50 amnesia was considered to be 114 µM (0.28%) for isoflurane and 0.25 µM for etomidate (see Materials and Methods for details). Data are plotted as mean ± SD.
Discussion
We found that isoflurane, at concentrations that effectively block hippocampal memory formation in vivo, prolonged both types of GABAA–ergic phasic inhibition in the murine hippocampus. The effect profile, however, differed substantially from that of the more selective GABAA–ergic drug etomidate: isoflurane affected GABAA,slow less and GABAA,fast more than etomidate at comparably amnesic concentrations. We discuss the relevance of these findings within the framework of GABAA receptor-mediated modulation of hippocampal memory formation.
Two types of phasic inhibition
The existence of (at least) two types of phasic GABAA –ergic inhibitory currents, which were originally discovered in the hippocampus, is now well-established in many brain areas.6 In the hippocampus, the slow time course of decay of GABAA,slow (30–70 ms, as opposed to 3–8 ms for GABAA,fast at 36°C) is its most striking characteristic. Its slow time course, in combination with its dendritic localization, places this slow synaptic current into an ideal position to balance the equally slow time course of dendritic excitation mediated by the N-methyl-D-aspartate (NMDA) receptor-mediated component of glutamatergic synaptic input.19 Given the critical role of NMDA receptors in initiating many forms of synaptic plasticity, including long-term potentiation, GABAA,slow-mediated inhibition is thus well suited to control synaptic plasticity – and by extension, hippocampus-dependent learning and memory.
Recent research has highlighted the importance of synchronized fast inhibitory currents for the generation of brain rhythms in the γ-frequency range 20 suggesting that pharmacologic modulation of GABAA,fast might similarly have direct consequences for altering higher brain function.21
Differential effects of isoflurane and etomidate
We reported recently that amnestic concentrations of etomidate enhance GABAA,slow phasic inhibition more than GABAA,fast, and concluded that etomidate-induced conscious amnesia may be due, in large part, to the preferential enhancement of GABAA,slow IPSCs. Previous observations linking etomidate-induced amnesia to tonic GABAA-ergic inhibition, specifically to receptors containing the α5 subunit (discovered by using α5 knock-out mice),22 are compatible with this interpretation, as GABAA,slow phasic inhibition is also mediated in part by α5-containing receptors.12,23 An assumption underlying this reasoning is that since etomidate is a comparatively selective GABAA receptor agonist, its effects on higher cognitive function are likely to result from the sum of its effects on various types of GABAA receptor-mediated inhibition. However, the precise quantitative contributions of each of the three distinct forms of GABAA-ergic inhibition (two phasic, one tonic) to sedation, amnesia, hypnosis and immobility in response to etomidate are yet to be determined.
By contrast, isoflurane modulates numerous targets in addition to the GABAA-ergic system.3 In order to determine whether isoflurane’s modulation of any individual component is strong enough to contribute substantially to amnesia, we compared its effects, under identical experimental conditions, to changes induced by etomidate. Our results indicate that enhancement of GABAA,slow by isoflurane – approximately one-half of etomidate’s effect at behaviorally equivalent concentrations (Fig. 5A) – is indeed strong enough to contribute substantially. Since GABAA,slow is mediated largely by GABAA receptor subunits that contain β3 subunits,24 the recent finding that mice carrying a forebrain-specific knockout of the β3 subunit are resistant to the amnestic effect of isoflurane provides additional support for a role of GABAA,slow in isoflurane-induced amnesia.25 Surprisingly, that same study reported that this selective knockout did not influence etomidate-induced amnesia. The explanation for this lack of effect for etomidate is unclear, but it may reflect the restriction of the knockout to principal (excitatory) cells,26 whereas anesthetic-sensitive GABAA,slow IPSCs are also found in interneurons,27 where they provide essential timing information through cross-frequency coupling between inhibitory circuits that oscillate at theta- and gamma frequencies.28
The other component of phasic inhibition, GABAA,fast – which was barely affected by an amnesic concentration of etomidate – was enhanced by isoflurane to an even greater extent than GABAA,slow (Fig. 5B). To the extent that modulation of GABAA,fast can influence synaptic plasticity, perhaps by altering somatic spiking and back-propagation of action potentials into the dendrites,29 modulation of synaptic GABAA receptors would then play an even greater role in isoflurane-induced amnesia than indicated by comparing only effects of isoflurane and etomidate on GABAA,slow. Since isoflurane modulates both components of phasic inhibition, whereas etomidate preferentially modulates GABAA,slow IPSCs,13 this result supports the concept that different agents may achieve similar end points by distinct but partially overlapping mechanisms.30
A number of assumptions underlie the approach that we used for quantifying the contribution of GABAA receptors to isoflurane-induced amnesia. We believe them to be reasonable, and there are supporting data for each one. Nevertheless, to the extent that they represent simplifications of a complex system, and the underlying physiological basis of memory as well as the clinical relevance of fear conditioning-based models remain incompletely understood, they should be recognized as limitations to the present study. First, we measured drug effects on only a limited set of inhibitory processes. Even within just the hippocampus there exist numerous classes of inhibitory interneurons that differ in their firing patterns, physiological characteristics, and anatomical projections.31 They communicate among themselves and impinge on pyramidal neurons using a physiologically and pharmacologically diverse set of inhibitory synapses as well as non-synaptic tonic inhibition, and the different forms of inhibition display differential sensitivity to anesthetic drugs.11 Although we have presented evidence that slow dendritic IPSCs are well suited to control synaptic plasticity,6,13 the precise means by which any form of inhibition controls synaptic plasticity, and learning and memory, remains unclear. Second, to establish a “fractional contribution” of one specific class of anesthetic targets, there must be a linear summation of effects, or at least not a strongly synergistic or antagonistic contribution from different targets. Other investigators have examined this question, at least in relation to end-points other than amnesia, and have concluded that synergy does exist for some types of receptor-specific agents, but the deviation from additivity is usually small and for inhaled anesthetics it is generally absent. 32,33 Third, drug concentrations required to suppress learning depend on the types of learning – in humans34 and in animal models.9 The present study compared the effects of isoflurane and etomidate on only one type of learning – fear conditioning to context, a paradigm that depends upon the hippocampus and amygdala.35 Other paradigms or types of learning that engage this same circuitry differently, or that depend on different brain structures, require different drug levels for suppression. Since additional drug targets come into play at higher concentrations, it is possible – even likely – that the precise contributions of the different targets will vary with learning task even for a relatively specific drug such as etomidate. Nevertheless, with these caveats, the present data show that isoflurane modulates GABAA receptor-mediated inhibition sufficiently strongly even at low “amnesic” concentrations that this receptor family is expected to contribute substantially to this behavioral effect.
Other targets of isoflurane that may contribute to amnesia
Our quantitative comparison of effects of isoflurane and etomidate on hippocampal inhibition indicates that modulation of GABAA,slow alone by isoflurane is insufficient to cause amnesia. What other targets might contribute? Among the many possible targets, the slow depolarization mediated by NMDA receptors (the glutamatergic excitatory counterpart of GABAA,slow) is an attractive complementary target.36–38 In this “integrative” view, modest effects on the excitatory component (reduction in glutamate release,39,40 and a postsynaptic block of the NMDA receptor-mediated current37,41,42 ) paired with a modest but functionally important enhancement of GABAA,slow-mediated hyperpolarization, may cooperatively disrupt synaptic plasticity. The quantitative approach that we employed here may prove useful in establishing which of the multiple targets influenced by isoflurane actually do play important roles.
Final Boxed Summary Statement.
What we already know about this topic
Isoflurane enhances synaptic inhibition by potentiation of GABAergic synaptic transmission
The role of this mechanism in amnesia produce by sub-anesthetic concentrations of isoflurane is unclear
What this article tells us that is new
Isoflurane at amnestic concentrations prolonged both fast and slow forms of phasic GABAa receptor-mediated inhibition in mouse hippocampal neurons
Enhancement of GABA-mediated synaptic inhibition by isoflurane does contribute substantially to isoflurane-induced amnesia
Acknowledgments
The authors thank Chong Lor, BS (Associate Research Specialist); Pee Yang, student; and Mark Perkins, BS (Senior Research Specialist), for technical assistance, and Kirsten Martin for secretarial assistance (all at the Department of Anesthesiology, University of Wisconsin-Madison School of Medicine and Public Health, Madison Wisconsin).
Funding
This work was supported by Grants GM55719 and NS056411 from the National Institutes of Health (Bethesda, MD), by the University of Wisconsin Department of Anesthesiology Research and Development Fund (Madison, WI), and by the Ralph M. Waters, M.D., Distinguished Chair endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph U, Crestani F, Mohler H. GABA(A) receptor subtypes: Dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 3.Hemmings HC, Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 5.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Capogna M, Pearce RA. GABA A,slow: Causes and consequences. Trends Neurosci. 2011;34:101–112. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 8.Sonner JM, Cascio M, Xing Y, Fanselow MS, Kralic JE, Morrow AL, Korpi ER, Hardy S, Sloat B, Eger EI, 2nd, Homanics GE. Alpha 1 subunit-containing GABA type A receptors in forebrain contribute to the effect of inhaled anesthetics on conditioned fear. Mol Pharmacol. 2005;68:61–68. doi: 10.1124/mol.104.009936. [DOI] [PubMed] [Google Scholar]
- 9.Sonner JM, Werner DF, Elsen FP, Xing Y, Liao M, Harris RA, Harrison NL, Fanselow MS, Eger EI, 2nd, Homanics GE. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology. 2007;106:107–113. doi: 10.1097/00000542-200701000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Werner DF, Swihart A, Rau V, Jia F, Borghese CM, McCracken ML, Iyer S, Fanselow MS, Oh I, Sonner JM, Eger EI, 2nd, Harrison NL, Harris RA, Homanics GE. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring alpha2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J Pharmacol Exp Ther. 2011;336:134–144. doi: 10.1124/jpet.110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus. Journal of Neurophysiology. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai S, Perouansky M, Pearce RA. Amnestic concentrations of etomidate modulate GABAA,slow synaptic inhibition in hippocampus. Anesthesiology. 2009;111:766–773. doi: 10.1097/ALN.0b013e3181b4392d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 15.Franks NP, Lieb WR. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br J Anaesth. 1993;71:65–76. doi: 10.1093/bja/71.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Benkwitz C, Liao M, Laster MJ, Sonner JM, Eger EI, 2nd, Pearce RA. Determination of the EC50 amnesic concentration of etomidate and its diffusion profile in brain tissue: Implications for in vitro studies. Anesthesiology. 2007;106:114–123. doi: 10.1097/00000542-200701000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Banks MI, Li TB, Pearce RA. The synaptic basis of GABAA,slow. J Neurosci. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antkowiak B, Heck D. Effects of the volatile anesthetic enflurane on spontaneous discharge rate and GABA(A)-mediated inhibition of Purkinje cells in rat cerebellar slices. J Neurophysiol. 1997;77:2525–2538. doi: 10.1152/jn.1997.77.5.2525. [DOI] [PubMed] [Google Scholar]
- 19.Kanter ED, Kapur A, Haberly LB. A dendritic GABAA-mediated IPSP regulates facilitation of NMDA-mediated responses to burst stimulation of afferent fibers in piriform cortex. J Neurosci. 1996;16:307–312. doi: 10.1523/JNEUROSCI.16-01-00307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferys JG, Traub RD, Whittington MA. Neuronal networks for induced '40 Hz' rhythms. Trends Neurosci. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 21.Whittington MA, Jefferys JG, Traub RD. Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro. Br J Pharmacol. 1996;118:1977–1986. doi: 10.1111/j.1476-5381.1996.tb15633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- 24.Hentschke H, Benkwitz C, Banks MI, Perkins MG, Homanics GE, Pearce RA. Altered GABAA,slow inhibition and network oscillations in mice lacking the GABAA receptor beta3 subunit. J Neurophysiol. 2009;102:3643–3655. doi: 10.1152/jn.00651.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rau V, Oh I, Liao M, Bodarky C, Fanselow MS, Homanics GE, Sonner JM, Eger EI., 2nd Gamma-aminobutyric acid type A receptor beta3 subunit forebrain-specific knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2011;113:500–504. doi: 10.1213/ANE.0b013e3182273aff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson C, Hardy SL, Werner DF, Hileman SM, Delorey TM, Homanics GE. New insight into the role of the beta3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci. 2007;8:85. doi: 10.1186/1471-2202-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukatch HS, MacIver MB. Physiology, pharmacology, and topography of cholinergic neocortical oscillations in vitro. J Neurophysiol. 1997;77:2427–2445. doi: 10.1152/jn.1997.77.5.2427. [DOI] [PubMed] [Google Scholar]
- 28.Banks MI, White JA, Pearce RA. Interactions between distinct GABA(A) circuits in hippocampus. Neuron. 2000;25:449–457. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- 29.Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Pittson S, Himmel AM, MacIver MB. Multiple synaptic and membrane sites of anesthetic action in the CA1 region of rat hippocampal slices. BMC Neurosci. 2004;5:52. doi: 10.1186/1471-2202-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafer SL, Hendrickx JF, Flood P, Sonner J, Eger EI., 2nd Additivity versus synergy: a theoretical analysis of implications for anesthetic mechanisms. Anesth Analg. 2008;107:507–524. doi: 10.1213/ane.0b013e31817b7140. [DOI] [PubMed] [Google Scholar]
- 33.Hendrickx JF, Eger EI, 2nd, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506. doi: 10.1213/ane.0b013e31817b859e. [DOI] [PubMed] [Google Scholar]
- 34.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI., 2nd The concentration of isoflurane required to suppress learning depends on the type of learning. Anesthesiology. 2001;94:514–519. doi: 10.1097/00000542-200103000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 36.Ming Z, Knapp DJ, Mueller RA, Breese GR, Criswell HE. Differential modulation of GABA- and NMDA-gated currents by ethanol and isoflurane in cultured rat cerebral cortical neurons. Brain Res. 2001;920:117–124. doi: 10.1016/s0006-8993(01)03044-x. [DOI] [PubMed] [Google Scholar]
- 37.Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318:434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 39.Westphalen RI, Hemmings HC., Jr. Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- 40.Winegar BD, MacIverr MB. Isoflurane depresses hippocampal CA1 glutamate nerve terminals without inhibiting fiber volleys. BMC Neurosci. 2006;7:5. doi: 10.1186/1471-2202-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Sousa SL, Dickinson R, Lieb WR, Franks NP. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Solt K, Eger EI, 2nd, Raines DE. Differential modulation of human N-methyl-D-aspartate receptors by structurally diverse general anesthetics. Anesth Analg. 2006;102:1407–1411. doi: 10.1213/01.ane.0000204252.07406.9f. [DOI] [PubMed] [Google Scholar]