Abstract
Transmembrane signaling mechanisms are critical for regulating the plasticity of neuronal connections underlying the establishment of long-lasting memory (e.g., Linden and Routtenberg, 1989, Brain Res Rev. 14: 279–296; Sossin, 1996, Trends Neurosci 19: 215–218; Mayr and Montminy, 2001, Nat Rev Mol Cell Biol. 2: 599–609; Chen et al., 2011, Nature 469: 491–497). One signaling mechanism that has received surprisingly little attention in this regard is the well-known Wnt transmembrane signaling pathway even though this pathway in the adult plays a significant role, for example, in postsynaptic dendritic spine morphogenesis and presynaptic terminal neurotransmitter release (Inestrosa and Arenas, 2010, Nature Rev Neurosci 11: 77–86).
The present report now provides the first evidence of Wnt signaling in spatial information storage processes. Importantly, this Wnt participation is specific and selective. Thus, spatial, but not cued, learning in a water maze selectively elevates the levels in hippocampus of Wnt 7 and Wnt 5a, but not the Wnt 3 isoform, indicating behavioral selectivity and isoform specificity. Wnt 7 elevation is subfield-specific: granule cells show an increase with no detectable change in CA3 neurons. Wnt 7 elevation is temporally specific: increased Wnt signaling is not observed during training, but is seen 7 days and, unexpectedly, 30 days later.
If the Wnt elevation after learning is activity-dependent, then it may be possible to model this effect in primary hippocampal neurons in culture. Here we evaluate the consequence of potassium or glutamate depolarization on Wnt signaling. This represents, to our knowledge, the first demonstration of an activation-dependent elevation of Wnt levels. Additionally, the novel finding emerged of an increased number of Wnt-stained puncta in neuritis suggestive of trafficking from the cell body to neuronal processes, probably dendrites. It is proposed that Wnt signaling pathways, both canonical and non-canonical, regulate long-term information storage in a behavioral-, cellular- and isoform-specific manner.
Keywords: water maze, hippocampus, dentate gyrus, synaptophysin, protein trafficking
Introduction
The Wnt (Wingless-Int) signaling pathway is a key regulator of morphogenesis and pattern formation during development. Wingless in Drosophila and its mammalian homologue Int-1 were first identified and characterized as secreted proteins of importance in establishing the pattern of development in the bodies of all multicellular organisms during embryogenesis (Nüsslein-Volhard and Wieschaus, 1980; Nusse et al., 1984). In addition to its critical role in early development, it is now known that in the adult nervous system the signaling Wnt proteins regulate neuronal functions such as presynaptic assembly, axon pathfinding, dendritic arborization and synaptogenesis (Li et al., 2005; Speese and Budnik, 2007; Salinas and Zou, 2008; Chiang et al., 2009; Korkut and Budnik, 2009; Inestrosa and Arenas, 2010).
Wnt signaling molecules belong to a family of lipid-modified secreted glycoproteins with molecular weight ranging from 38–41 kD. N-glycosylation and palmitoylation are major post-translational modifications necessary for Wnt maturation, sorting and secretion (Tanaka et al., 2002; Willert et al., 2003; Coudreuse and Korswagen, 2007). Upon secretion, Wnt proteins activate G protein coupled Frizzled (Fz) receptors that are found both pre- and postsynaptically. Despite the absence of evidence that Wnt signaling actually plays a role in spatial memory formation, a key malfunction in Alzheimer disease (AD), a curious counterfactual history has developed in which disorders of memory such as AD have nonetheless been thought to involve Wnt dysregulation (Pei et al., 1997; De Ferrari and Inestrosa, 2000; Caruso et al., 2006; Boonen et al., 2009; He and Shen, 2009; Shruster et al., 2010; Toledo and Inestrosa, 2010). To bridge this information gap, the present studies show that indeed this signaling system participates in long-term spatial information storage hence its dysfunction might well lead to devastating memory impairments.
Materials and Methods
Subjects
Fifty-day-old male Wistar rats (Charles River) weighing 250–300g at the start of the behavioral experiments were used. Animals were housed in groups of 3 with free access to water and rodent chow. The optimum temperature (22°C) and 12 hr light/dark cycle at the animal facility were maintained and controlled. Animal care and their use in behavioral procedures were in agreement with the guidelines of the National Institute of Health Guide; animal use protocols were approved by the Northwestern University Animal Care and Usage Committee.
Behavioral Training
Water Maze
The water maze apparatus consisted of a plastic circular container 1 m in diameter that was placed in the middle of a test room. The container was filled with water that was dyed using black water based nontoxic Tempera paint in order to allow the tracking of white rats. Water temperature was kept at 22°C. A fixed platform made of plastic (5 cm diameter) was submerged 2 cm below the water surface in the northwest quadrant of the pool. Three lamps aimed at the ceiling illuminated the test room. The following visual cues were hung on the walls: vertical black and white striped poster as well as white rectangle on a black background were located on the north wall, a black asterisk on a white background on the east wall, a black and white parallel vertical stripes and black circle on a white background on the south wall and a black triangle on a white background on the west wall. A video camera was mounted on the ceiling and was positioned in middle of the pool. An experimenter sat at the desk in front of the computer and was not visible from the pool. Performance in the water maze task was tracked and recorded using HVS software.
Animals were brought from the animal housing facility to the test room and handled for 4 min per day on 4 consecutive days to acclimate them to being handled. On the training day, each animal was placed in a holding cage in the testing room for 60 sec; it was then picked up carefully and placed in the water at randomly selected starting positions. Eight trials were conducted daily for 5 days. During each trial animals were allowed to swim for a maximum of 60 sec. After finding the submerged platform they were left on the platform for 30 sec, then removed and returned to a holding cage. There was a 30 sec interval between trials for each animal. If the animal was unable to find the hidden platform during 60 sec period, it was guided to the platform by the experimenter. In case of visible platform training, an orange ping-pong ball was mounted directly onto the platform and was visible from any location within the maze. In this situation the water maze was surrounded with black curtains in order to block visual access to the wall cues.
The probe test, in which the platform was removed from the pool, was performed 7 days after training. Animals were allowed to swim freely for 60 sec. During the probe test, time spent in annulus (circular area surrounding the platform) and in each of the 4 quadrants was recorded.
Immunostaining
Immunofluorescent staining for Wnt proteins was carried out according to Gogolla et al (2006). Briefly, after animals were sacrificed brains were removed and hemisected. One hemisphere was immediately transferred onto dry ice, frozen and later cryo-sectioned for 30 µm brain sections. The sections were post-fixed first with 4% PFA for 5 min then with 20% methanol/PBS solution for 5 min followed by permeabilization with 0.5% Triton X-100 in PBS overnight. 24 hr later sections were rinsed briefly with PBS and blocked using 20%BSA in PBS for 4 hr at room temperature followed by incubation in primary antibody solution (5%BSA/PBS) overnight at 4°C. The primary antibodies used were anti-Wnt 7a/b rabbit polyclonal (recognizes both ‘a’ and ‘b’ isoforms of Wnt 7, 1:250, Santa Cruz, CA) and anti-NeuN mouse monoclonal antibody (1:1000, Millipore, Billerica, MA). After the primary antibody incubation step, sections were washed with 5%BSA/PBS solution 3 times for 10 min each on a shaker at moderate speed and then incubated with secondary antibodies for 4 hr. Goat-anti rabbit Alexa 488 (1:500, Molecular probes, Carlsbad, CA) and Goat-anti mouse Alexa 594 (1:500, Molecular probes, Carlsbad, CA) secondary antibodies were used. Sections were rinsed briefly and mounted using hardest mounting medium with DAPI (Vector labs, Burlingame, CA). Wnt 7 staining was visualized with an Olympus BX61 fluorescent microscope and the images captured with an Olympus DP70 digital camera. When measuring Wnt 7 staining intensities, within granule and CA3 pyramidal cell bodies (30 cells per area selected), ROIs (∼10 µm2) were created using ImageJ software, and the mean intensity of grey values was measured inside these ROIs. Staining within one series was performed in parallel, and imaging settings were adjusted to be optimal for the entire set of samples belonging to one experiment. These settings were then kept unchanged for the acquisition of all images.
Western Blotting
Immediately after completion of behavioral training, or immediately after the 7 and 30 day probe tests, animals were sacrificed, brains were removed and snap-frozen in ethanol/dry ice mixture. Hippocampi were dissected from the right hemisphere on ice. The tissue was homogenized in ice-cold buffer containing 20 mM Tris-HCl (pH 7.4), 50 mM NaF, 0.32 M sucrose, 2 mM EDTA, 2 mM EGTA, 0.2 mM sodium orthovanadate, 1 mM PMSF, and protein inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Proteins (0.1 −1µg of total protein per lane) were separated by 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked in 5% dry fat-free milk blocking solution in T-TBS for 1 hr at room temperature prior to application of primary antibodies. The primary antibodies employed included rabbit polyclonal Wnt 7a/b (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal Wnt 3 (1:300; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal Wnt 5a (1:300; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal β-catenin (1:1000; BD Biosciences, San Jose, CA) and mouse monoclonal GAPDH (1:5000; Ambion, Austin, TX). After incubation with primary antibodies overnight at 4°C, membranes were washed in TBS and T-TBS and incubated at room temperature for 1 hr with HRP-conjugated secondary antibodies (1:20,000; goat anti-mouse IgG; 1:20,000; goat anti-rabbit IgG, Pierce, Rockford, IL.). Human recombinant Wnt 7 (R&D systems, Minneapolis, MN) was used as a positive control (PC). Immunoreactivity was visualized using the Pico West SuperSignal chemiluminescent substrate of HRP (Pierce, Rockford, IL), and optical densities were quantified using NIH ImageJ software. The loading control values were obtained from the same membrane and measured optical densities of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Primary hippocampal cell culture
Hippocampal neuronal cultures were prepared from embryonic day 18 Sprague Dawley rats. Embryonic hippocampi were microdissected under the microscope. Tissues were trypsinized using 0.25% Trypsin and incubated at 37°C for 20 minutes. After trypsinization, DNA-aseI was added for 30 sec with the final concentration of 0.25mg/ml. The pellet was washed 3 times and gently triturated using fire polished glass Pasteur pipette. Dissociated cells were re-suspended in DMEM plating media with L-Glutamine containing 10% heat-inactivated fetal bovine serum and 1% antibiotic solution (104 U of penicillin G/ml, 10 mg streptomycin/ml and 25 µg amphotericin B/ml). Neurons were plated at a density of 100,000 cells/ml on 18 mm diameter poly-D-lysine coated glass coverslips. Three hours after plating the medium was replaced with serum-free Neurobasal feeding medium containing 2% B-27 supplement (Gibco) and L-Glutamine. On DIV3 Ara-C was added with the final concentration of 1µM to induce dividing cell death. The following day Ara-C containing medium was replaced with fresh Neurobasal/B27 medium. 14-day hippocamapal cells were used for all in vitro studies.
Immunocytochemistry
Primary hippocampal cells were fixed at room temperature with ice-cold 4% paraformaldehyde in PBS for 20 min. Membranes were permeabilized by incubation in 0.1% Triton X-100 in PBS for 15 min then incubated in blocking solution (3% normal goat serum in PBS) for 1 hr. Primary antibodies included: rabbit polyclonal antibodies for Wnt 7a/b (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) and a mouse monoclonal antibody for synaptophysin (1:500; Sigma, St Louis, MO). All antibodies were added overnight at 4°C. Cells were washed with PBS and then incubated for 2 hr in the presence of appropriate fluorescently tagged anti-mouse and anti-rabbit secondary antibodies (AlexaFluor 488 and 594, respectively; 1:500 dilution; Invitrogen/Molecular Probes, Carlsbad, CA). Coverslips were mounted onto the microscope slides using non-hardset mounting medium with DAPI (Vector labs, Burlingame, CA). Staining was visualized using the Olympus BX61 fluorescent microscope at 60X magnification and the images were captured with the Olympus DP70 digital camera. For the quantification of synaptophysin clusters, ROIs (∼25 µm2) were chosen using the Image J software and measurements for the number of synaptophysin positive clusters, cluster area and intensity per ROI were taken both proximally and distally (50–75 µm) along the same axonal branch.
Results
Are hippocampal Wnt levels selectively altered by different learning paradigms?
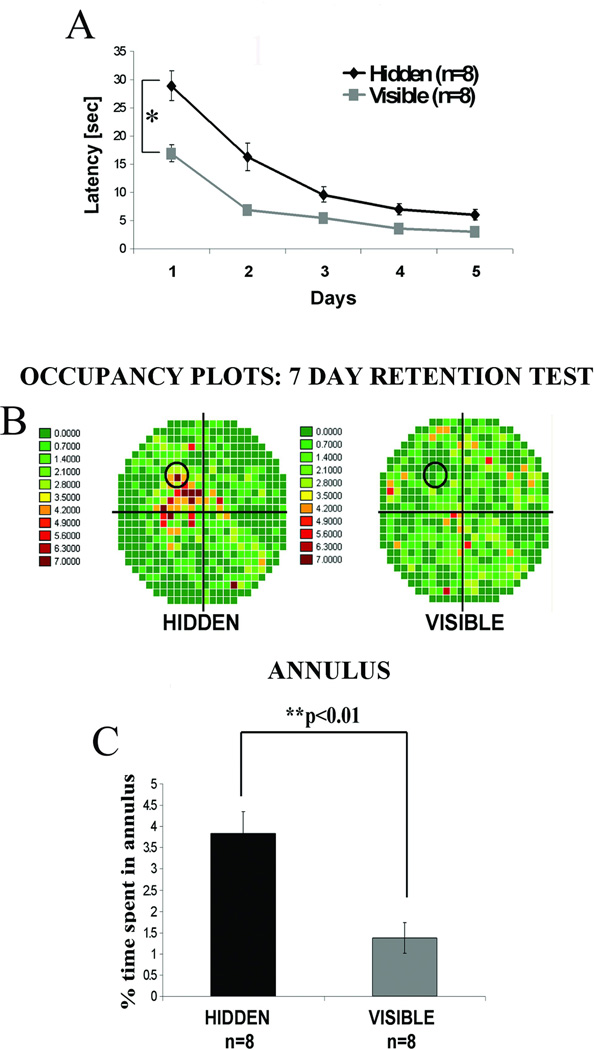
We compared hippocampal Wnt 7 levels in rats trained in 2 different learning paradigms in the water maze. In one, the hidden platform test, animals used extramaze contextual cues to learn platform location. In the other, the visible platform test, the animals used intramaze cues; thus, no spatial or contextual cues were required to locate the clearly marked visible platform. Apart from platform visibility, both groups were treated in the same manner; motor and sensory performance requirements were thus essentially identical. Though the hidden platform group (spatial learning) initially exhibited slower learning than the visible platform group (cued learning, Figure 1A; *p<0.05, repeated measures ANOVA), both learned to find the platform in the water maze to the same level of proficiency after 5 days of training. In contrast to acquisition rates, in the retention probe test given 7 days later, the hidden platform group demonstrated good recall of the platform location while visible platform animals exhibited poor recall as shown in the occupancy plots (Figure 1B). Quantification of time spent in the region of the platform or annulus during the probe test (Figure 1C) also demonstrated the superior spatial memory in the hidden platform group (**p<0.01, one-way ANOVA).
Figure 1. Long-term spatial memory is superior in animals trained in the hidden platform water maze test compared to those trained on the visible platform task.
Adult male Wistar rats were trained in water maze either on a spatial hidden (n=8) or a cued visible (n=8) platform task for 5 consecutive days using 8 training trials per day. 7 days after the last training day a probe test was carried in which the platform was removed and animals were allowed to swim freely in the maze for 60 sec. All animals were sacrificed immediately after the 7 day probe test for Western blotting or immunohistochemical analyses. (A) Average swim latencies in sec for 5 training days showing the significantly lower latencies for the visible platform group compared with the hidden platform animals (*p<0.05, repeated measures ANOVA). (B) Occupancy plots for the probe trial showing the percent time that both groups spent swimming in each quadrant. Increasing color intensity toward red indicates the region where rats spent the most time during probe trial. Animals trained on a hidden platform spent most of the time in the target quadrant where an escape platform was submerged during training trials, while the animals from a visible platform group spent an even amount of time swimming in all four quadrants. (C) Differences in 7 day retention between hidden and visible platform trained animals. Quantitative data showing the percent time spent in annulus (circular area surrounding the platform). The hidden platform group spent significantly more time in the annulus compared with the visible platform animals (**p<0.01, one-way ANOVA).
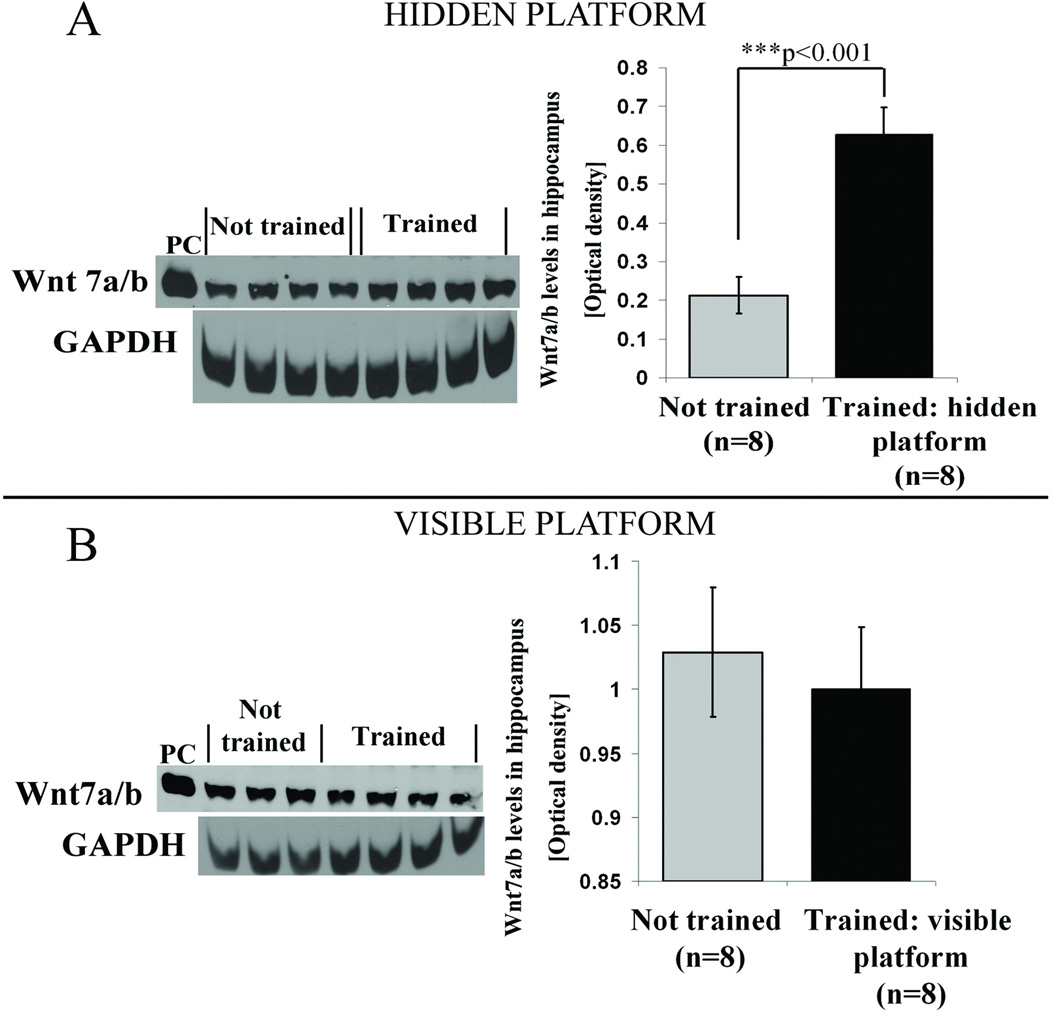
The clear distinction between retention for the hidden and visible platform location was directly reflected in the differential elevation of hippocampal Wnt 7 levels. Thus, Wnt 7 levels measured 7 days after acquisition were elevated in the hidden platform spatial memory group compared to not trained control group (Figure 2A, ***p<0.001, students t test). In contrast, no such elevation was detected in animals 7 days after visible platform training, as hippocampal Wnt levels were not different from not trained controls (Figure 2B, p =0.289, students t test).
Figure 2. Seven days after completion of training spatial but not cued learning in water maze induces an increase in hippocampal Wnt 7 levels.
(A) Representative western blots and quantification results of protein band intensities for 4 animals from each group showing an elevation in Wnt 7 levels in the hidden platform trained group compared with not trained controls. Not trained control animals from the same batch were handled along with the trained animals but not exposed to the water maze. GAPDH was used as a loading control. Human recombinant Wnt 7 (R&D systems) protein was used as a positive control (PC). A significant increase in Wnt 7 levels was observed in animals trained on the hidden platform compared with not trained animals (***p<0.001, students t test). (B) Wnt 7 levels in animals from the visible platform group were not different from not trained controls.
Is the learning-induced elevation in Wnt levels isoform-specific?
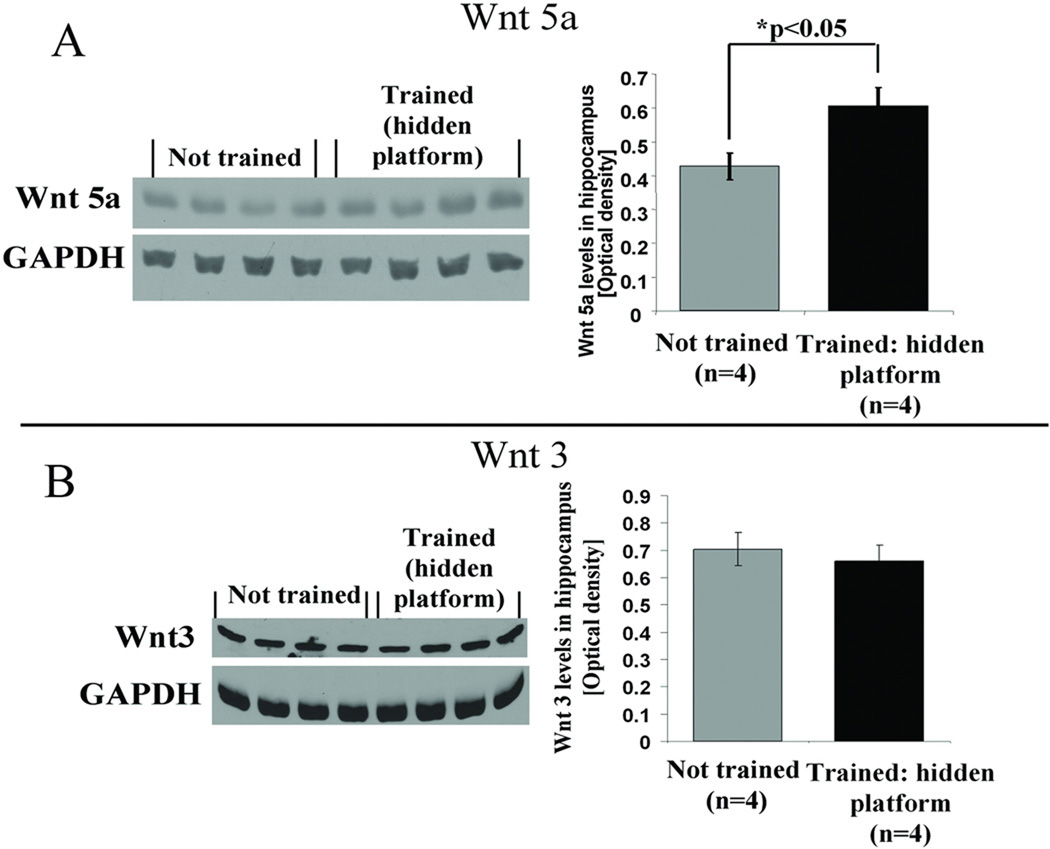
It has been suggested that different Wnt isoforms perform different functions within different compartments of the synapse (Chen et al., 2006; Cerpa et al., 2008; Gogolla et al., 2009). We therefore evaluated the participation of two isoforms other than Wnt 7, that are also known to be present within the hippocampus (Lie et al., 2005; Farias et al., 2009). Using animals from the same behavioral training protocol as described in Figure 1, hippocampal levels of Wnt 5a and Wnt 3 were measured in hidden platform trained animals and in controls that were handled but not trained. We found that there was an increase in Wnt 5a levels 7 days after completion of hidden platform training compared to the not trained control group (Figure 3 A, *p<0.05, student’s t test). In contrast to Wnt 5a, there was no detectable change in Wnt 3 levels (Figure 3B, p=0.243, student’s t test).
Figure 3. Spatial learning in a hidden platform water maze activates Wnt signaling in an isoform-specific manner.
(A) Wnt 5a levels were elevated in the hidden platform trained group, compared with not trained controls, 7 days after completion of 5 day water maze training. Quantification of protein band intensities, after standardizing using loading control values, revealed a significant increase in Wnt 5a levels as compared to not trained animals (***p<0.05, students t test). (B) Representative western blots for 4 animals from each group showing no change in Wnt 3 levels in the hidden platform trained group 7 days after completion of 5 day water maze training compared with not trained controls. Quantification results of protein band intensities showing that Wnt 3 levels, in contrast to Wnt 5 levels remain unchanged after spatial learning in a hidden platform water maze.
How long does the learning-induced elevation of Wnt 7 levels persist?
Prior evidence indicated that memory for the hidden platform can persist for 30 days (Ramirez-Amaya et al., 2001). We therefore asked whether the elevation of Wnt also persists for this duration.
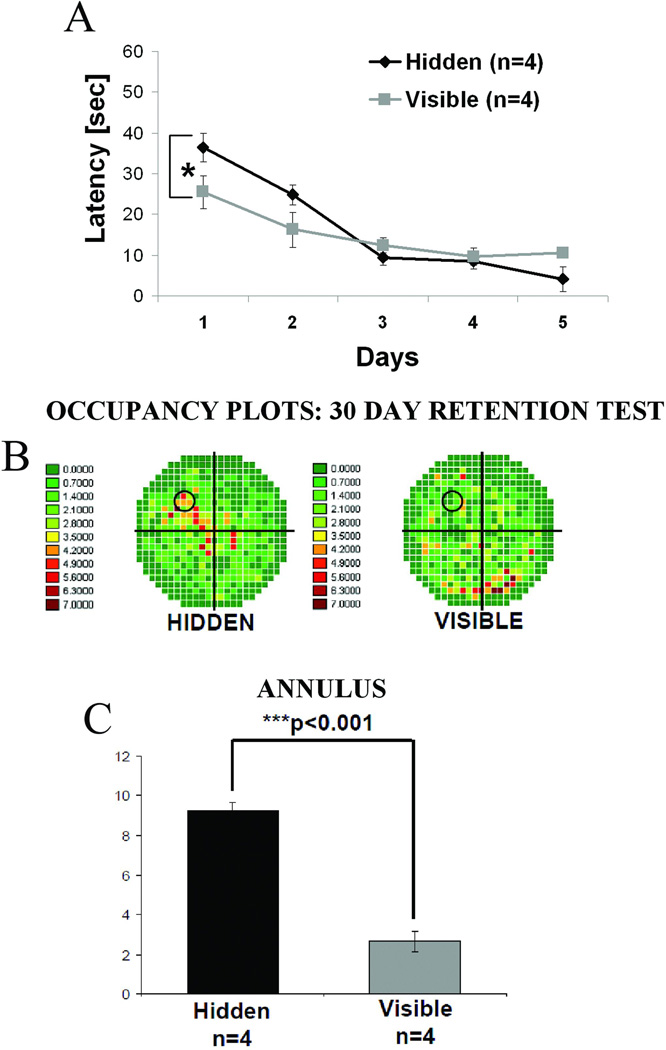
Wistar rats were trained in water maze on a hidden (n=4) or visible (n=4) platform for 5 consecutive days and the retention test was carried out 30 days after completion of training. While the visible platform group again exhibited more rapid acquisition than the hidden platform group during training (Figure 4A, *p<0.05, repeated measures ANOVA), 30 day retention was, as before, superior in the hidden platform relative to the visible platform group (Figure 4B and C). Animals from the hidden platform group still exhibited a strong memory for platform location 30 days after learning, as during the probe test they spent significantly more time swimming in the target quadrant or in the region of the annulus compared with the visible platform group animals (Figure 4C, **p<0.001, one-way ANOVA). Immediately after the probe test was completed, animals were sacrificed and western blotting was carried out on hippocampal tissue extracts.
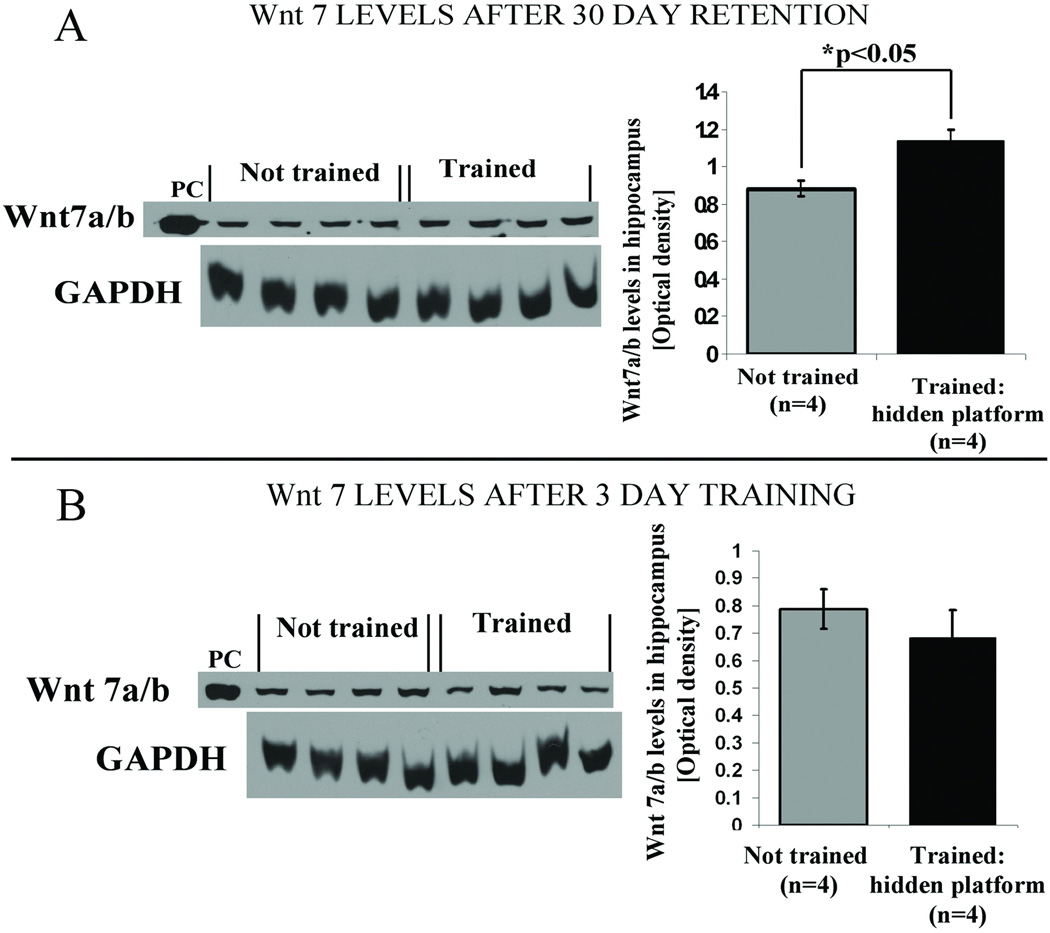
Figure 4. At 30 day retention interval strong spatial memory for platform location is observed in animals trained on the hidden platform.
Adult male Wistar rats were trained in water maze either on a hidden (n=4) or a visible (n=4) platform as in Figure 1. In this case, however, the probe test was carried out 30 days, rather than 7 days, after the completion of training. All animals were sacrificed immediately after the probe test. Control animals (n=4) were handled but not exposed to the water maze. (A) Average swim latencies in seconds for 5 training days showing the significantly lower latencies for the visible platform group compared with the hidden platform animals (*p<0.05, repeated measures ANOVA). (B) Occupancy plots for the probe trial showing the percent time that both groups spent swimming in each quadrant. Animals trained on a hidden platform spent most of the time in the target quadrant where an escape platform had previously been submerged during training trials; animals from the visible platform group did not show preference towards any quadrant. (C) Quantitative data showing the percent time spent in annulus. The hidden platform group spent significantly more time in the annulus even 30 days after training compared with the visible platform animals (**p<0.01, one-way ANOVA) indicating strong long-lasting retention of platform location.
It was found that 30 days after the 5-day training period Wnt 7 levels still remained significantly elevated in hidden platform trained animals as compared to the not trained control group (Figure 5A, *p<0.05 student’s t test). In the visible platform group, in contrast, we detected no change of Wnt 7 levels at 30 days (data not shown).
Figure 5. Hippocampal Wnt 7 levels remain significantly elevated 30 days after spatial learning.
(A) Representative western blot and quantification data showing elevated levels of hippocampal Wnt 7 for hidden platform trained animals after 30 day retention interval (*p<0.05, student’s t test). Wnt 7 levels in visible platform group were not different from not trained controls (data not shown). (B) There was no detectable effect of a 3-day training period on Wnt 7 levels. Quantification of western blots revealed no significant differences in Wnt 7 levels in the 3 day hidden platform group compared with not trained controls. Similar results were obtained for a 5 day training group (data not shown), suggestive of the absence of change in Wnt 7 levels during or immediately after spatial training.
In order to determine whether Wnt 7 levels were elevated during or immediately after water maze training, animals (n=4) were trained on the hidden platform task and sacrificed immediately after the 3rd or the 5th day of training. Interestingly, there was no detectable elevation in Wnt 7 levels compared with not trained controls in either the 3 day (Figure 5B, p=0.204, student’s t test) or 5 day (data not shown) group.
Is the learning – induced elevation in Wnt 7 levels hippocampal subfield-specific?
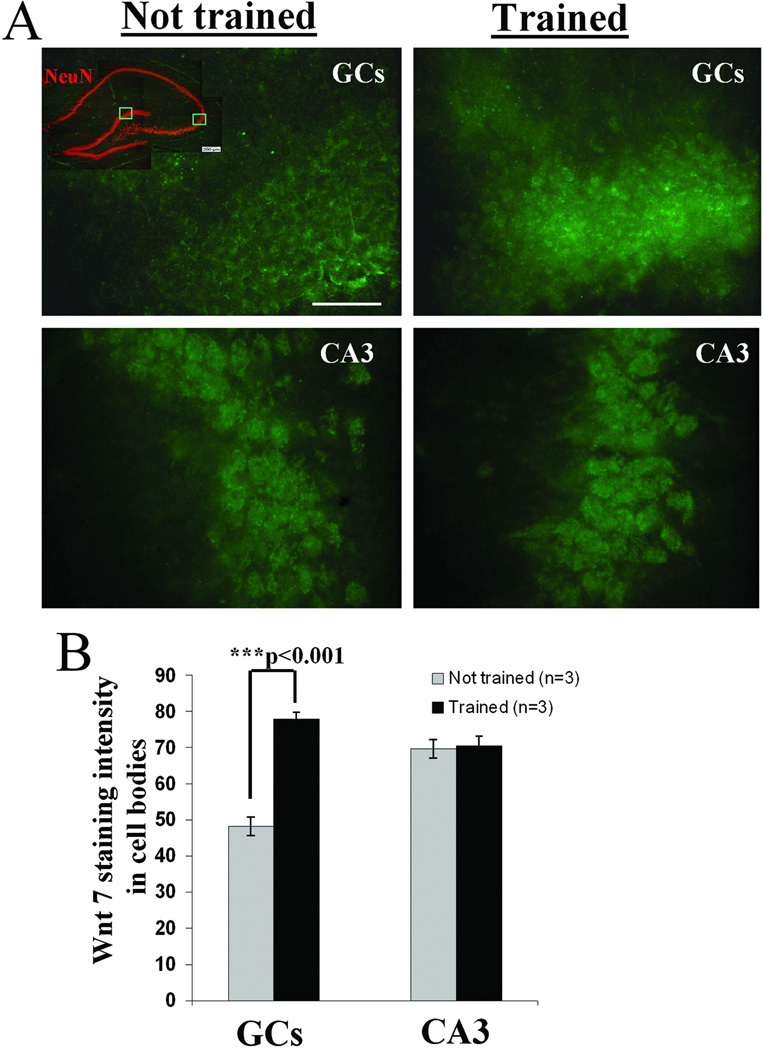
Given that each subfield of the hippocampus can be assigned different functions in the memory storage process (Jung and McNaughton, 1993; Bures et al., 1997; Hollup et al., 2001; Oh et al., 2003; Hunsaker et al., 2008; Nakashiba et al., 2009), it is important to determine whether the elevation in Wnt levels is restricted to particular cell types in particular hippocampal subfields. To address this issue immunohistochemical analysis of Wnt 7 was carried out in animals trained on the hidden platform test.
Immunostained representative images of granule cells and CA3 neurons shown in Figure 6A illustrate the higher intensity of Wnt 7 in granule cells of hidden platform trained animals sacrificed after the 7 day probe test compared with not trained controls (quantified in Figure 6B, ***p<0.001, One-way ANOVA). In contrast, in the CA3 pyramidal cells we could not detect any change in Wnt 7 levels (p=0.831, One-way ANOVA). These results indicate a region-selective increase of Wnt 7 restricted to hippocampal granule cells after formation of long-term spatial memory.
Figure 6. Spatial learning elevates Wnt 7 levels in hippocampus in a subfield-specific manner.
Adult male Wistar rats were trained in water maze on a hidden platform as described (see Figure 1). Trained (n=3) and not trained (n=3) animals were sacrificed for immunostaining immediately after the 7 day probe test. (A) After comparison of immunostaining for Wnt 7 in granule cells (GCs) and CA3 pyramidal cells, only GCs showed a significant effect of training. Scale: 50µM. (B) Quantification of Wnt 7 staining intensities (described in methods) showed higher levels of Wnt 7 staining in the granule cells of the hidden platform group compared with not trained controls (***p<0.001, One-way ANOVA); no such difference was detected when CA3 cells were considered.
Is the elevation of Wnt 7 levels an activity-dependent process?
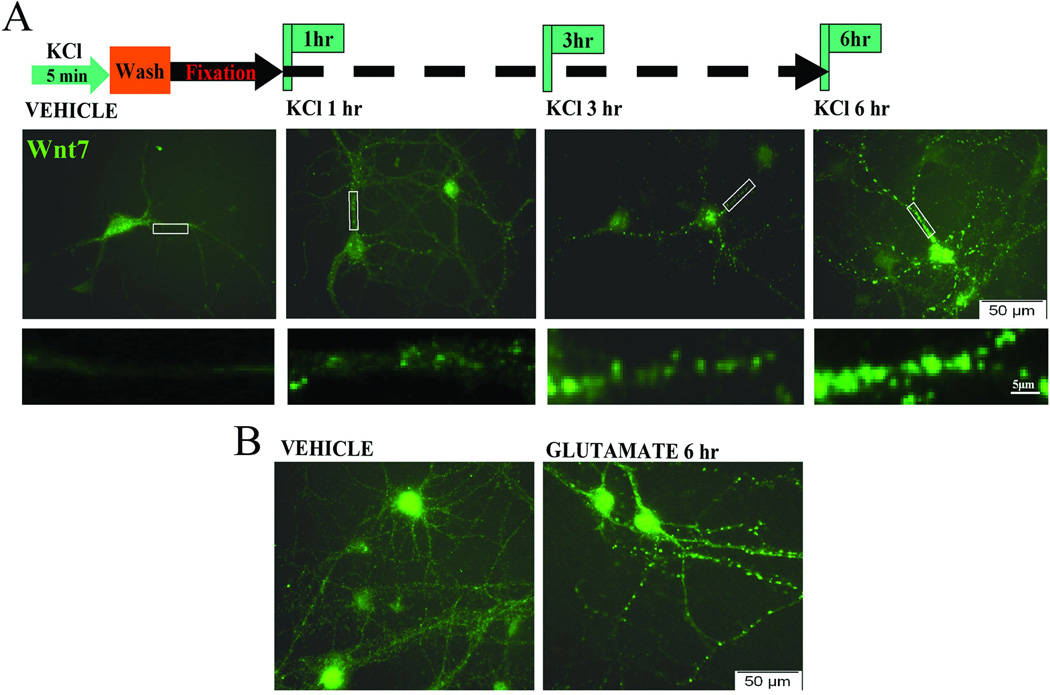
The elevated levels of Wnt 7 observed after learning might be due to an activity-dependent process in hippocampus. Indeed, learning of a spatial task specifically activates hippocampal granule cells, as well as CA1 and CA3 pyramidal cells (Jung and McNaughton, 1993; Moser et al., 2008).
To determine whether neuronal activation would elevate Wnt 7 levels, mature primary hippocampal neurons were cultured using previously described protocols (Chen et al., 2006; Ataman et al., 2008; Varela-Nallar et al., 2009; Singh et al., 2010) to study the activating effects of potassium or glutamate on Wnt signaling. The cells were exposed to 50 mM KCl for just 5 min, after which the KCl was washed out and then at different time points after the depolarization the cells were fixed. Under resting conditions (vehicle) Wnt 7 is primarily expressed in the cell cytoplasm and is present at low levels in processes in a diffuse form (Figure 7A). After depolarization by KCl, there is a time-dependent increase in levels of Wnt 7 both in the cell body and its processes. There is also a change from diffuse to punctate Wnt 7 staining, which is apparent at 6 hr after the 5 min KCl depolarization (see Figure 7A). Application of 200 µM glutamate for 5 min to primary hippocampal neurons produced a similar change as observed with KCl (Figure 7B); it increased levels and redistribution of Wnt 7 at 6 hr post-activation.
Figure 7. Neuronal activation causes increase in Wnt 7 and enhances its trafficking.
Primary hippocampal neurons were treated with high molar (50 mM) KCl or 200 µM glutamate for 5 min. The cells were fixed at different time intervals after the 5 min exposure to KCl or glutamate. (A) Staining for Wnt 7 after KCl activation revealed time dependent increase in Wnt 7 clusters in the neuronal processes with the most robust increase in Wnt 7 clusters 6 hrs post-treatment compared with vehicle treated control cells. Lower panel images show magnified branches from each condition suggesting that the cluster size and staining intensity increases based on the time interval after depolarization. Scale: 5µM. (B) Similar pattern of Wnt 7 distribution was observed after activation of primary hippocampal neurons with glutamate. This is the representative image showing Wnt 7 clusters within neuronal processes 6 hr after 5 min exposure to glutamate.
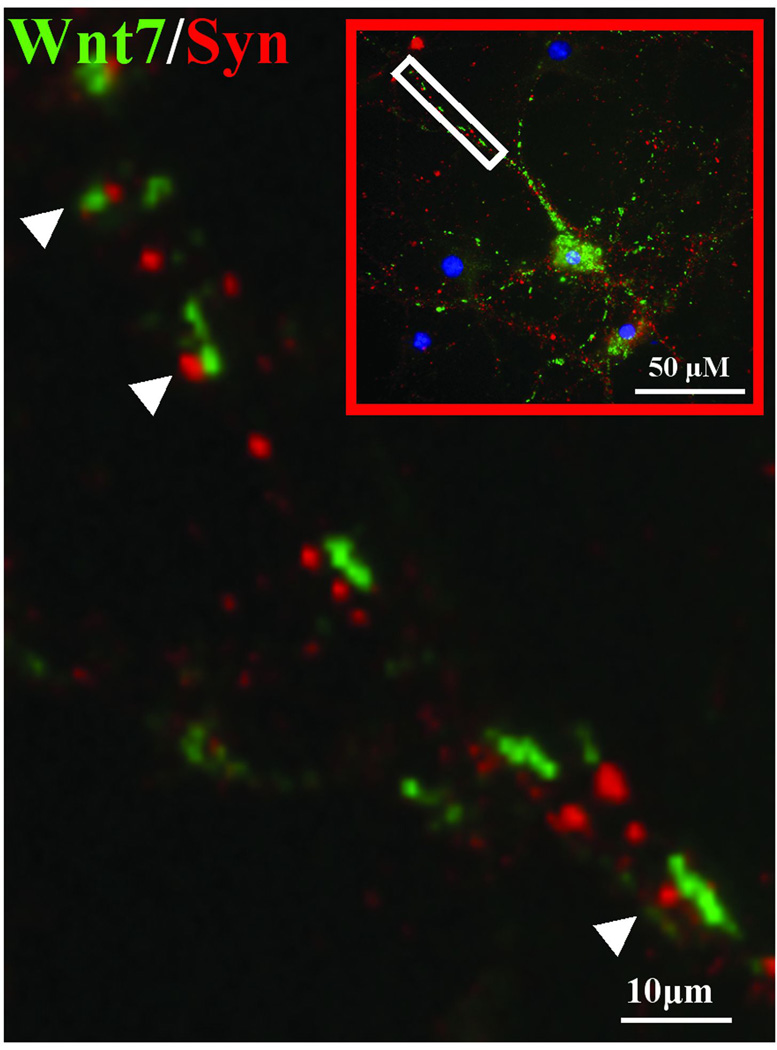
To identify the cellular compartment where the Wnt 7 puncta were localized, we double stained neurons with Wnt 7 and synaptophysin, a presynaptic vesicle marker protein. Strikingly, there was no detectable co-localization between the two proteins (Figure 8) indicating that Wnt clusters are localized in compartments within the neuron that do not contain synaptophysin, most likely dendrites. We noticed at high magnification that Wnt 7 containing clusters were at times in close proximity to the synaptophysin -immunopositive puncta (Figure 8, white arrowheads along the magnified branch). This suggests the possibility that the Wnt containing puncta may be in dendritic spines that are directly apposed to presynaptic terminals.
Figure 8. After neuronal depolarization Wnt 7 and synaptophysin are not co-localized and this may suggest that they are expressed in different neuronal processes.
The image was taken 6 hr after a 5 min 50 mM KCl induced depolarization of primary hippocampal neurons. The figure shows that both Wnt 7 (green) and synaptophysin (red) are seen in cell processes yet high magnification image of the neuronal branch (white box) shows the absence of co-localization. Moreover, Wnt 7 and synaptophysin immunopositive puncta are at times opposing each other and are in close proximity (white arrowheads).
Does Wnt 7 treatment of primary hippocampal neurons alter distribution of the presynaptic vesicle protein synaptophysin?
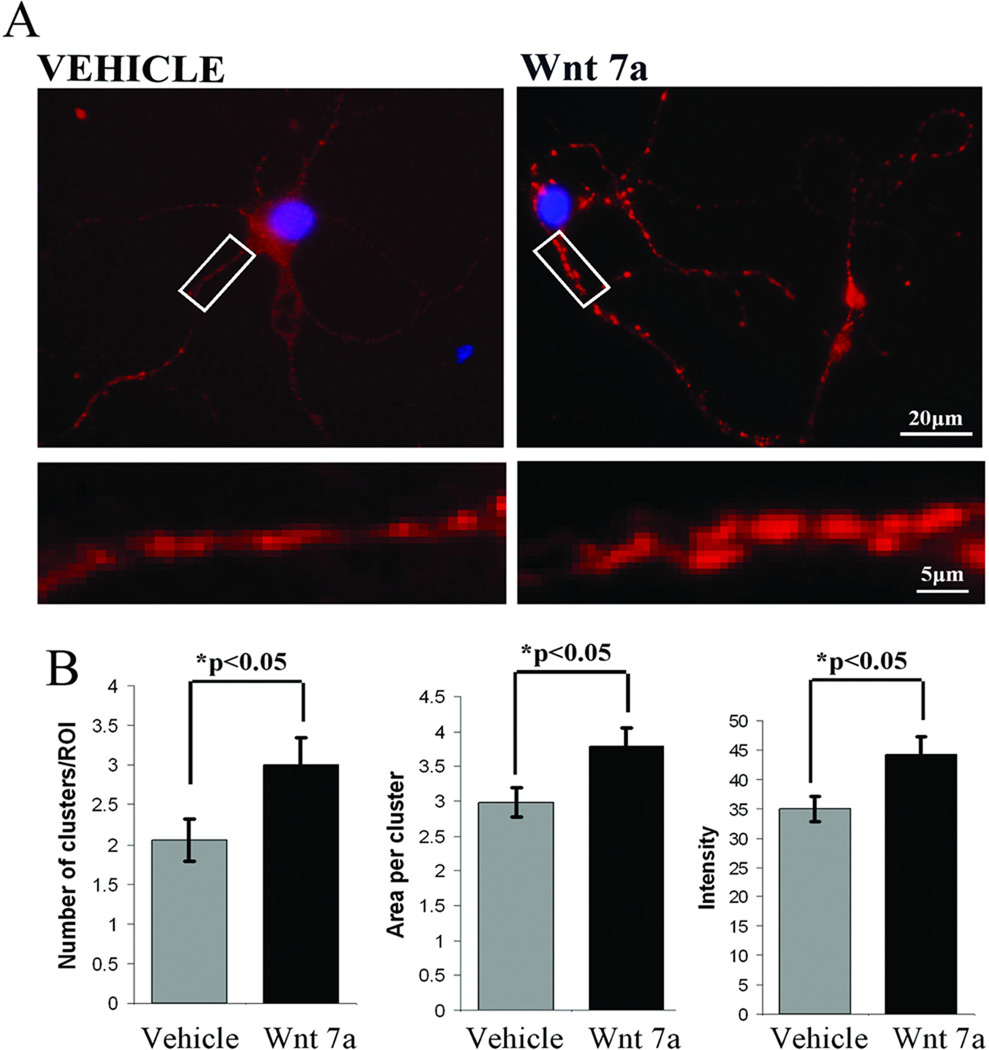
Learning as well as potassium or glutamate depolarization elevates Wnt 7 levels in neurons. In hippocampal cells in culture there was a qualitatively detectable increase in the number of Wnt 7 immunopositive puncta apposed to synaptophysin – positive presynaptic terminals. Because it has been proposed that Wnt 7 molecules may be released from the post-synaptic side and, acting retrogradely, bind to the pre-synaptic transmembrane Frizzled receptors to regulate presynaptic functions (Cerpa et al., 2008), we determined whether exogenous Wnt 7 application would influence synaptophysin distribution. The primary hippocampal neuronal cultures were treated with 0.5 µg/ml of human recombinant Wnt 7a for 3 hr. An increase in synaptophysin immunopositive puncta after Wnt 7a treatment was in fact observed when compared with vehicle treated control cells (Figure 9A). Quantification of staining (see Methods) showed that there was a significant increase in the number, area and intensity of synaptophysin clusters in the case of Wnt 7a treatment compared with vehicle treated control cells (Figure 9B, *p<0.05 student’s t test).
Figure 9. Exposure of primary hippocampal neurons to Wnt 7 facilitates synaptophysin clustering.
(A) Primary hippocampal neurons were treated with human recombinant Wnt 7a (0.5 ug/ml) for 3 hr. Lower panel images show magnified branches from each condition suggesting that the cluster size and staining intensity for synaptophysin increases after Wnt 7a treatment that points to the potential role of Wnt 7 in the regulation of pre-synaptic function. (B) Quantification of synaptophysin cluster number, area and staining intensity (described in methods) after 3 hr Wnt 7a treatment of primary hippocampal neurons revealed a significant increase in all three parameters compared with vehicle treated cells (*p<0.05 student’s t test).
Discussion
The present report revealed several new findings. First, Wnt signaling participates in long-lasting spatial memory formation processing. Second, an activity-dependent transport of Wnt 7 occurs from the cell body to neuronal processes, provisionally labeled as dendrites. Third, Wnt levels remain elevated 30 days after training. While claims for persistent biochemical change have been made based on the use of inhibitors of post-translational modification of synaptic proteins (Shema et al., 2007; Holahan and Routtenberg, 2008), the present findings are the first to our knowledge to actually demonstrate a persistent biochemical alteration in synaptic protein levels. Fourth, Wnt participation appears to be selective for particular Wnt isoforms. This is likely to confer specificity on the selection of the Wnt signaling pathways within particular synaptic compartments of identified brain regions set into motion by the particular learning process.
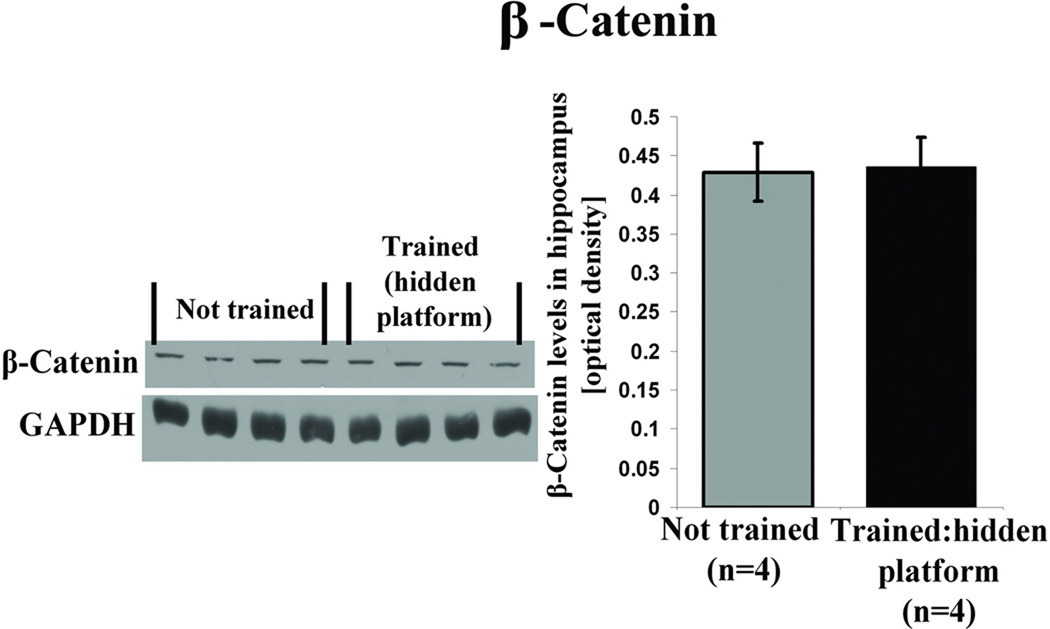
Here we consider 3 major intracellular Wnt signal transduction pathways that may be triggered by learning. In the classic canonical pathway, binding of secreted Wnt to its Frizzled receptor causes the activation of a downstream cascade that inhibits glycogen synthase kinase 3 beta (GSK3β) mediated phosphorylation of β-catenin. This prevents proteasomal degradation of β-catenin, facilitating its translocation to the nucleus and transcriptional activation of Wnt target genes (Salinas, 2005; Gao and Chen, 2010). In the present study we found a persistent elevation in Wnt 7 levels after spatial learning. In this scenario if Wnt 7 engages the classic canonical Wnt signaling pathway, then inactivation of GSK3β via its enhanced phosphorylation and increase in β-catenin levels are predicted. The absence of a change in β-catenin levels in the present study at 7 days after water maze training in the hidden platform group (Figure 10), while, at the same time, Wnt levels are increasing, suggests that this pathway may not be engaged during the development of long-lasting spatial memory.
Figure 10. Spatial learning does not change hippocampal β-catenin levels.
Adult male Wistar rats were trained in water maze on a hidden platform as described (see Figure 1). Trained (n=4) and not trained (n=4) animals were sacrificed for western blot analysis immediately after the 7 day probe test. This figure shows representative western blot and quantification data illustrating no change in hippocampal β-catenin levels for hidden platform trained animals after the 7 day retention compared with not trained animals (p =0.913, students t test).
In a recent report by Maguschak and Ressler (2011) that appeared after our paper was submitted for publication, β-catenin participation in regulation of fear memory is described. The authors found that while Wnt 1 mRNA levels are decreasing initially after fear-conditioning there is no change in total β-catenin levels nor in phosphorylated form of GSK3β (Maguschak and Ressler, 2008) that points to the fact that the classic canonical pathway does not participate in post-learning information processing, consistent with our results.
Even though they did not find change in total β-catenin levels the authors did see a time-dependent increase in the phosphorylated form of β-catenin at Tyr-654 residue. This phosphorylation of Tyr-654 residue placed in the last armadillo repeat of β-catenin is not mediated by the serine/threonine GSK3β and decreases its binding to E-cadherin.
There are, however, some essential issues with the findings of Maguschak and Ressler (2011) that warrant concern. First, these authors studied the role of Wnt 1 mRNA in basolateral amygdala (BLA) in contextual fear conditioning. One major issue is the use of Wnt mRNA to assess dynamic changes of Wnt levels in Wnt 1 signaling. mRNA levels do not correspond in a 1:1 fashion with protein levels so the actual amount of change in Wnt signaling protein molecules cannot be estimated. Because of this limitation it is not possible to directly compare their results with the present findings.
Further caution is also appropriate when considering the impairment in 48 hr fear memory after their BLA injection of DKK-1. This protein is a non-specific Wnt signaling inhibitor and will thus block Wnt signaling for all Wnt isoforms. The potential role of a particular isoform cannot be inferred from any blockade effects observed with DKK-1. The role of other Wnt isoforms, including Wnt 7, in fear memory formation will require consideration. Nonetheless, given the isoform specificity of the present results and the approach used herein it would suggest the possibility that Wnt signaling, if monitored directly in the amygdala, might also participate in contextual fear memory. This then would point to the importance of this signaling mechanism in different memory systems regulated by different brain structures.
In the divergent Wnt canonical pathway Wnt-Fz interaction and inhibition of the GSK-3β activity blocks phosphorylation of MAP-1B, a major phosphoprotein component of the neuronal cytoskeleton involved in axonal extension (DiTella et al., 1996; Edelmann et al., 1996; Lucas et al., 1998). This pathway does not engage β-catenin stabilization or the regulation of gene transcription. Decreased phosphorylation of MAP-1B leads to destabilization of microtubules and subsequent remodeling of axonal terminals (Vandecandelaere et al., 1996; Gonzalez-Billault et al., 2004). If elevated levels of hippocampal Wnt 7 seen in the present study are important for long-lasting memory then the role of Wnt signaling in structural remodeling previously associated with long-term memory would therefore be pivotal.
Specific support for this remodeling hypothesis comes from two different lines of research. In one, Gogolla et al., (2009) showed that Wnt signaling can regulate hippocampal mossy fiber (MF) terminal remodeling in the CA3 subfield after exposure of mice to prolonged environmental enrichment. But this study, in contrast to the present one, does not speak to the issue of learning nor to the role of Wnt signaling in memory, even though it links Wnt signaling to MF remodeling.
In the other line of research a selective increase in presynaptic structural remodeling of the MFs was seen after hidden but not visible, platform training (Rekart et al., 2007). The MF growth only occurs days after acquisition and not during or immediately after learning of the spatial task. This post-learning hippocampal mossy fiber remodeling therefore parallels in both space and time the elevated Wnt levels observed here.
It is interesting that in the present study Wnt 7 increases in granule cells but not in CA3 pyramidal neurons. These findings are open to different interpretations but the one that we favor assumes that elevated levels of Wnt 7 in granule cells and presumably their dendrites strengthens connectivity of granule cell/perforant path synapses leading to further enhancement of granule cell activity that promotes activity-dependent growth of their axons in CA3 (Adams et al., 1997; Escobar et al., 1997).
A third signaling pathway, the non-conventional Wnt canonical pathway (Inestrosa and Arenas, 2010), regulates exocytotic processes at the synapses but does not regulate gene transcription or structural presynaptic remodeling. It has been suggested that Wnts released from the postsynaptic compartment regulate vesicle recycling and neurotransmitter release from presynaptic nerve terminals in a retrograde manner (Ahmad-Annuar et al., 2006). Our studies on Wnt distribution in hippocampal primary cell cultures and earlier findings (Chen et al., 2006; Ataman et al., 2008; Varela-Nallar et al., 2010) on acute brain slices and Drosophila neuromuscular junction preparations, provide new evidence consistent with Wnt regulation of pre- and post-synaptic plasticity (Tang, 2007).
Indeed, several lines of evidence support the idea that Wnt signaling could regulate long-lasting memory by its control of specific synaptic functions. First, Wnt 7 function has been associated with the regulation of large MF presynaptic terminal remodeling in hippocampus as well as in cerebellum, possibly through microtubule destabilization (Hall et al., 2000; Gogolla et al., 2009).
Second, Wnt 7 regulates the trafficking of the α7 nicotinic acetylcholine receptors to presynaptic terminals in hippocampal neurons (Farias et al., 2007).
Third, Wnt 7 increases the clustering of synapsin 1 in cerebellar neurons. Clustering of synapsin 1 after Wnt 7 application is particularly prominent at areas of the axon that probably represent future synapses (Lucas and Salinas, 1997; Hall et al., 2000).
Fourth, Wnt 7 increases the number of clusters of presynaptic proteins in addition to synaptophysin such as synaptotagmin and SV-2 with the maximal effect after 1 hr incubation of hippocampal neurons with the Wnt-conditioned medium (Cerpa et al., 2008). In the present study we found that primary hippocampal neurons treated for 3 hr with recombinant Wnt 7a protein show an increase in the number, area and intensity of synaptophysin positive clusters mimicking effects of secreted Wnt. These results suggest that Wnt 7 regulates presynaptic function in primary hippocampal neurons.
Fifth, double-mutant mice lacking Wnt-7a and Dishevelled 1, an important Wnt signaling phosphoprotein downstream from Wnt, showed impaired neurotransmitter release suggesting a role for Wnt 7 in synaptic transmission (Ahmad-Annuar et al., 2006).
Sixth, neuronal activation with KCl or glutamate induces a translocation of Wnt 7 leading to an increase in distinct Wnt 7 labeled puncta in the neurites of hippocampal cells. Because the Wnt 7 clusters occur in neuronal processes that do not stain for synaptophysin but are located in processes subjacent to the presynaptic structures, we suppose that the Wnt 7 is within dendrites. Recent data from our laboratory using viral-mediated labeling of neurons confirms this hypothesis (unpublished observations). If so, this newly described movement of Wnt 7 may parallel other somato-dendritic trafficking such as seen with Arc after LTP or learning (Steward et al., 1998; Huang et al., 2007; Miyashita et al., 2009).
Seventh, while Wnt 7 has been associated with the regulation of pre-synaptic function, Wnt 5a in contrast promotes clustering of postsynaptic plasticity-related proteins, induces de novo dendritic spine formation and increases the size of preexisting spines (Farias et al., 2009). A brief application of Wnt 5a to primary hippocampal cell cultures also increases the amplitude of fEPSPs (Varela-Nallar et al., 2010). Based on these findings it is tempting to think that elevated levels of Wnt 5a in hippocampus seen here 7 days after training could contribute to the storage and perpetuation of spatial memories via regulation of postsynaptic processes, thereby complementing the presynaptic role of Wnt 7.
Eighth, it has been demonstrated that inhibition of endogenous hippocampal Wnt 3 strongly attenuates both hippocampal LTP and adult granule cell neurogenesis (Lie et al., 2005; Chen et al., 2006). The effect of spatial learning in the present study points to roles of Wnt 7 and Wnt 5a but not, however, Wnt 3 in spatial information storage processes within hippocampus, indicating the selective participation of Wnt isoforms in different aspects of synaptic function.
The persistence of a biochemical change in brain 30 days after completion of training, as seen here in elevated Wnt 7 levels was surprising. Earlier studies on Wnt signaling showed that blockade of Wnt reduced the magnitude of late- but not early LTP phase. Conversely, pre-incubation of hippocampal slices in presence of Wnt, potentiated LTP (Chen et al., 2006; Speese and Budnik, 2007). However, the time frame for these findings was only measured in hours.
A number of studies have reported, however, on the persistent role of kinases in the maintenance and retrieval of stored information (Lovinger et al., 1987; Matthies and Reymann, 1993; Pastalkova et al., 2006; Holahan and Routtenberg, 2008). Recently, significant attention has been paid to the role of PKMzeta in memory persistence, which is present in dendrites (product of an alternative transcription of PKCzeta gene lacking regulatory subunit). Several forms of long-term memory are blocked when locally inhibiting PKMzeta with the inhibitory peptide ZIP in different brain regions weeks after training (Sacktor et al., 1993; Serrano et al., 2008; Kwapis et al., 2009; Hardt et al., 2010; von Kraus et al., 2010). However, even though suggestive, no direct measurements have been made of either its presumed elevated activity levels weeks after training or its inhibition at that time by ZIP. In contrast, the present study points to an increase in Wnt 7 a month after learning, demonstrating a persistent long-lasting change of Wnt 7 protein levels in hippocampus. To our knowledge this finding is the first to show long-lasting persistence of an increase in protein level in brain a month after spatial learning. Whatever the underlying mechanism that this persistent change in Wnt 7 is mediating, the possibility exists that it is playing a memory-sustaining role. As with any such discovery, understanding its significance poses the next challenge.
Wnt signaling enhancement observed here is selective and calibrated with regard to behavioral task, time-dependency and molecular specificity. Wnt protein levels in hippocampus demonstrate task-specific up-regulation in animals trained on the hidden platform version of the water maze, but not in the visible platform task. These elevated levels are seen 7 and 30 days after the retention test but not during or immediately after acquisition. We propose, therefore, that Wnt signaling in hippocampus selectively and specifically participates in long-term spatial information storage. Furthermore, presynaptic and postsynaptic elements are coordinately organized by different Wnt signaling systems to alter synaptic efficacy underlying information storage. As a useful coordinating hypothesis the following sequence of events involving Wnt 7 participation may be considered: 1) spatial information processing activates granule cells (GCs) in hippocampus (Jung and McNaughton, 1993); 2) learning-induced activity causes Wnt 7 molecules to be shuttled to GC dendritic processes; 3) retrograde Wnt signaling from GC dendrites onto the perforant path presynaptic Frizzled receptors then induces a clustering of synaptophysin and other presynaptic proteins enhancing synaptic transmission; 4) this would strengthen perforant path synaptic connectivity with GC dendrite synapses in the middle and/or outer molecular layer of the dentate gyrus; 5) persistently elevated activation of GCs would enhance the level of information flow within hippocampal circuits (Henze et al., 2002).
The role of Wnt signaling in the neuropathology of memory impairment (e.g. Alzheimer’s disease) has been considered. Evidence points to the dysregulation in AD brain of Wnt signaling components such as β-catenin and GSK-3β, (Pei et al., 1997; Zhang et al., 1998) and Wnt interacting proteins, e.g. Notch 1, PS1 (Berezovska et al., 1998; Murayama et al., 1998). Genetic variation in the Wnt co-receptor LRP6 (low-density lipoprotein receptor related protein 6) is also linked to late-onset AD (De Ferrari et al., 2007). Moreover, Wnt signaling may have an essential role in the processing of the amyloid precursor protein and in Aβ induced neurotoxicity (Phiel et al., 2003; Alvarez et al., 2004). The present findings, which establish an important role for Wnt signaling in memory processes, may now pave the way for evaluating Wnt dysfunction as a novel causal mechanism in the etiology of memory disorders.
Acknowledgements
This work was supported by research grant NIMH MH 54326-07. The authors are grateful to Dr N. Gogolla for providing advice on Wnt immunostaining protocol for brain tissue sections.
References
- Adams B, Lee M, Fahnestock M, Racine RJ. Long-term potentiation trains induce mossy fiber sprouting. Brain Res. 1997;775:193–197. doi: 10.1016/s0006-8993(97)01061-5. [DOI] [PubMed] [Google Scholar]
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros I, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez AR, Godoy JA, Mullendorff K, Olivares JH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovska O, Xia MQ, Hyman BT. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:738–745. doi: 10.1097/00005072-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer’s disease: up or down, that is the question. Ageing Res Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Bures J, Fenton AA, Kaminsky Y, Zinyuk L. Place cells and place navigation. Proc Natl Acad Sci USA. 1997;94:343–350. doi: 10.1073/pnas.94.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso A, Motolese M, Lacovelli M, Caraci F, Copani A, Nicoletti F, Terstappen GC, Gaviraghi G, Caricasole A. Inhibition of the canonical Wnt signaling pathway by apolipoprotein E4 in PC12 cells. J Neurochem. 2006;98:364–371. doi: 10.1111/j.1471-4159.2006.03867.x. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-beta to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development. 2009;136:1273–1282. doi: 10.1242/dev.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse D, Korswagen HC. The making of Wnt: new insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Bain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Frieze F, Avila ME, Major MB, Myers A, Saez K, Henriguez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTella MC, Feiguin F, Carri N, Kosik KS, Caceres A. MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J Cell Sci. 1996;109:467–477. doi: 10.1242/jcs.109.2.467. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Zervas M, Costello P, Roback L, Fischer I, Hammarback JA, Cowan N, Davies P, Wainer B, Kucherlapati R. Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc Natl Acad Sci USA. 1996;93:1270–1275. doi: 10.1073/pnas.93.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar ML, Barea-Rodriguez EJ, Derrick BE, Reyes JA, Martinez JL., Jr Opioid receptor modulation of mossy fiber synaptogenesis: independence from long-term potentiation. Brain Res. 1997;751:330–335. doi: 10.1016/s0006-8993(96)01373-x. [DOI] [PubMed] [Google Scholar]
- Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GG, Valles AS, Colombres M, Godoy JA, Toledo EM, Lucas RJ, Barrantes FJ, Inestrosa NC. Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27:5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Coroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Staining protocol for organotypic hippocampal slice cultures. Nat Protoc. 2006;1:2452–2456. doi: 10.1038/nprot.2006.180. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Billault C, Jimenez-Mateos EM, Caceres A, Diaz-Nido J, Wandosell F, Avila J. Microtubule-associated protein 1B function during normal development, regeneration, and pathological conditions in the nervous system. J Neurobiol. 2004;58:48–59. doi: 10.1002/neu.10283. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hardt O, Migues PV, Hastings M, Wong J, Nader K. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2010;20:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J Neurosci. 2009;29:6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Holahan M, Routtenberg A. The protein kinase C phosphorylation site on GAP-43 differentially regulates information storage. Hippocampus. 2008;18:1099–1102. doi: 10.1002/hipo.20486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci. 2001;21:1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a, b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Korkut C, Budnik V. WNTs tune up the neuromuscular junction. Nat Rev Neurosci. 2009;10:627–634. doi: 10.1038/nrn2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter MJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Routtenberg AR. The role of protein kinase C in long-term potentiation: a testable model. Brain Res Rev. 1989;14:279–296. doi: 10.1016/0165-0173(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Wong KL, Murakami K, Routtenberg A. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 1987;436:177–183. doi: 10.1016/0006-8993(87)91573-3. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. β-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Wnt signaling in amygdala-dependent learning and memory. J Neurosci. 2011;31:13057–13067. doi: 10.1523/JNEUROSCI.3248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H, Reymann KG. Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. Neuroreport. 1993;4:712–714. doi: 10.1097/00001756-199306000-00028. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Murayama M, Tanaka S, Palacino J, Murayama O, Honda T, Sun X, Yasutake K, Nihonmatsu N, Wolozin B, Takashima A. FEBS Lett. 1998;433:73–77. doi: 10.1016/s0014-5793(98)00886-2. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Tanaka T, Tung YC, Braak E, Igbal K, Grundke-Igbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–8. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Balderas I, Sandoval J, Escobar ML, Bermudez-Rattoni F. Spatial long-term memory is related to mossy fiber synaptogenesis. J Neurosci. 2001;21:7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekart JL, Sandoval CJ, Bermudez-Rattoni F, Routtenberg A. Remodeling of hippocampal mossy fibers is selectively induced seven days after the acquisition of a spatial but not a cued reference memory task. Learn Mem. 2007;14:416–421. doi: 10.1101/lm.516507. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC. Retrograde signalling at the synapse: a role for Wnt proteins. Biochem Soc Trans. 2005;33:1295–1298. doi: 10.1042/BST0331295. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney L, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. Plos Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Shruster A, Eldar-Finkelman H, Melamed E, Offen D. Wnt signaling pathway overcomes the disruption of neuronal differentiation of neural progenitor cells induced by oligomeric amyloid β-peptide. J Neurochem. 2010;116:522–529. doi: 10.1111/j.1471-4159.2010.07131.x. [DOI] [PubMed] [Google Scholar]
- Singh AP, Vijayraghavan K, Rodrigues V. Dendritic refinement of an identified neuron in the Drosophila CNS is regulated by neuronal activity and Wnt signaling. Development. 2010;137:1351–1360. doi: 10.1242/dev.044131. [DOI] [PubMed] [Google Scholar]
- Sossin WS. Mechanisms for the generation of synapse specificity in long-term memory: the implications of a requirement for transcription. Trends Neuorsci. 1996;19:215–218. doi: 10.1016/0166-2236(96)20016-5. [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kitagawa Y, Kadowaki T. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. J Biol Chem. 2002;277:12816–12823. doi: 10.1074/jbc.M200187200. [DOI] [PubMed] [Google Scholar]
- Tang SJ. The Synaptic Wnt Signaling Hypothesis. Synapse. 2007;61:866–868. doi: 10.1002/syn.20434. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15:272–285. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Vandecandelaere A, Pedrotti B, Utton MA, Calvert RA, Bayley PM. Differences in the regulation of microtubule dynamics by microtubule associated proteins MAP1B and MAP2. Cell Motil. Cytoskelet. 1996;35:134–146. doi: 10.1002/(SICI)1097-0169(1996)35:2<134::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Nallar L, Grabowski CP, Alfaro IE, Alvarez AR, Inestrosa NC. Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural Dev. 2009;4:41. doi: 10.1186/1749-8104-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kraus LM, Sacktor TC, Francis JT. Erasing sensorimotor memories via PKMzeta inhibition. PloS One. 2010;5:e11125. doi: 10.1371/journal.pone.0011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]