Abstract
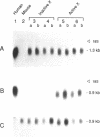
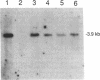
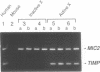
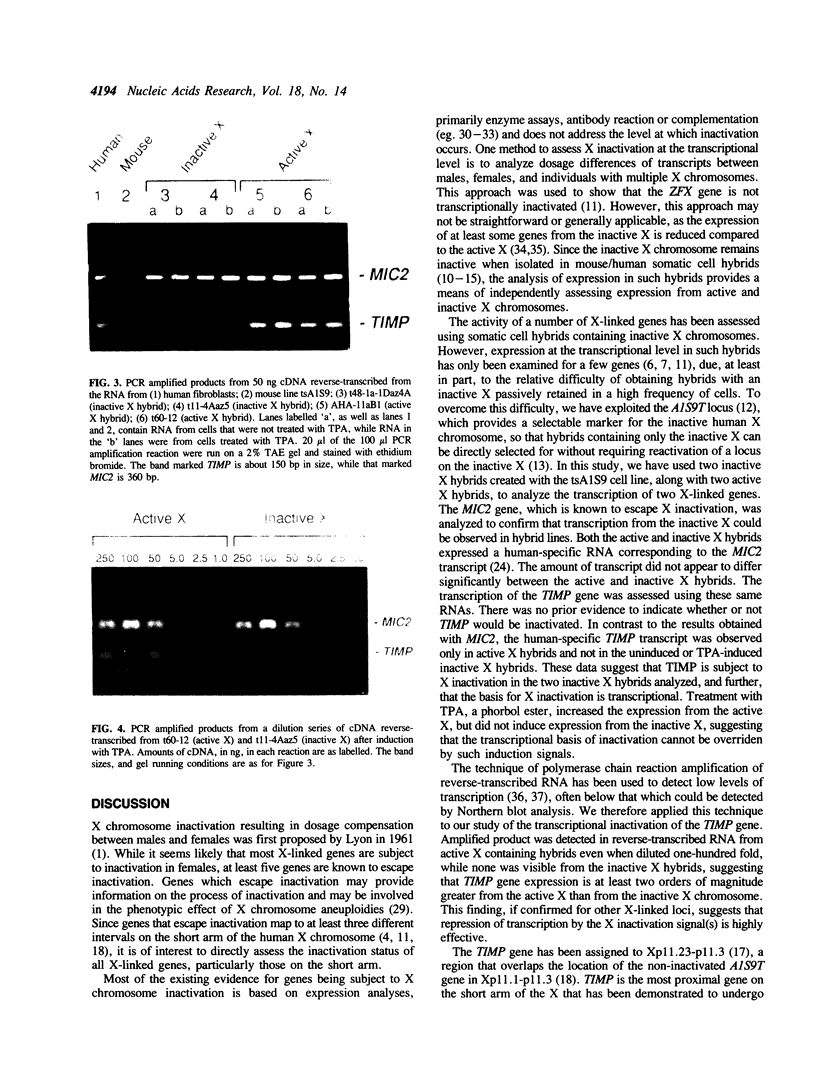
X chromosome inactivation results in the cis-limited inactivation of most, but not all, genes on one of the two X chromosomes in mammalian females. The molecular basis for inactivation is unknown. In order to examine the transcriptional activity of human X-linked genes, a series of mouse-human somatic cell hybrids under positive selection for the active or inactive human X chromosome has been created. Northern blot analysis of RNA from these hybrids showed that the human MIC2 gene, which is known to escape X inactivation, was transcribed in hybrids with either the active or inactive X chromosome. In contrast, the human TIMP gene was only transcribed in hybrids with an active human X chromosome. Further analysis using the polymerase chain reaction showed that there was at least one-hundred fold less transcription of the TIMP gene from the inactive X than from the active X chromosome. These findings demonstrate that the human TIMP gene is subject to X inactivation at the level of transcription, and illustrate the usefulness of the polymerase chain reaction to study the extent of X-linked gene repression by the process of X inactivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. J., Powers V. E., Munroe D. L., Sheinin R., Willard H. F. Gene on short arm of human X chromosome complements murine tsA1S9 DNA synthesis mutation. Somat Cell Mol Genet. 1989 Mar;15(2):173–178. doi: 10.1007/BF01535079. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Willard H. F. Localization of a gene that escapes inactivation to the X chromosome proximal short arm: implications for X inactivation. Am J Hum Genet. 1990 Feb;46(2):273–279. [PMC free article] [PubMed] [Google Scholar]
- Brown C. J., Willard H. F. Noninactivation of a selectable human X-linked gene that complements a murine temperature-sensitive cell cycle defect. Am J Hum Genet. 1989 Oct;45(4):592–598. [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coulombe B., Ponton A., Daigneault L., Williams B. R., Skup D. Presence of transcription regulatory elements within an intron of the virus-inducible murine TIMP gene. Mol Cell Biol. 1988 Aug;8(8):3227–3234. doi: 10.1128/mcb.8.8.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON R. G., NITOWSKY H. M., CHILDS B. DEMONSTRATION OF TWO POPULATIONS OF CELLS IN THE HUMAN FEMALE HETEROZYGOUS FOR GLUCOSE-6-PHOSPHATE DEHYDROGENASE VARIANTS. Proc Natl Acad Sci U S A. 1963 Sep;50:481–485. doi: 10.1073/pnas.50.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling S. M., Goodfellow P. J., Pym B., Banting G. S., Pritchard C., Goodfellow P. N. Molecular genetics of MIC2: a gene shared by the human X and Y chromosomes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):205–212. doi: 10.1101/sqb.1986.051.01.025. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Coulombe B., Castelino M., Skup D., Williams B. R. Characterization and expression of a murine gene homologous to human EPA/TIMP: a virus-induced gene in the mouse. EMBO J. 1987 Mar;6(3):651–657. doi: 10.1002/j.1460-2075.1987.tb04804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P., Pym B., Mohandas T., Shapiro L. J. The cell surface antigen locus, MIC2X, escapes X-inactivation. Am J Hum Genet. 1984 Jul;36(4):777–782. [PMC free article] [PubMed] [Google Scholar]
- Grant S. G., Chapman V. M. Mechanisms of X-chromosome regulation. Annu Rev Genet. 1988;22:199–233. doi: 10.1146/annurev.ge.22.120188.001215. [DOI] [PubMed] [Google Scholar]
- Graves J. A., Gartler S. M. Mammalian X chromosome inactivation: testing the hypothesis of transcriptional control. Somat Cell Mol Genet. 1986 May;12(3):275–280. doi: 10.1007/BF01570786. [DOI] [PubMed] [Google Scholar]
- Kahan B., DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. doi: 10.1073/pnas.72.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Willard H. F., Nussbaum R. L., Romeo G., Puck J. M., Davies K. E. Report of the committee on the genetic constitution of the X chromosome. Cytogenet Cell Genet. 1989;51(1-4):384–437. doi: 10.1159/000132801. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Der Kaloustian V. M., Nyhan W. L., Yough W. J., Childs B. X-linked hypoxanthine-guanine phosphoribosyl transferase deficiency: heterozygote has two clonal populations. Science. 1968 Apr 26;160(3826):425–427. doi: 10.1126/science.160.3826.425. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Shapiro L. J., Norum R. A., Mohandas T., Axelman J., Dabora R. L. Differential expression of steroid sulphatase locus on active and inactive human X chromosome. Nature. 1982 Oct 28;299(5886):838–840. doi: 10.1038/299838a0. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Mareni C., Axelman J. Derepression with decreased expression of the G6PD locus on the inactive X chromosome in normal human cells. Cell. 1982 Jun;29(2):595–600. doi: 10.1016/0092-8674(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Hellkuhl B., Grzeschik K. H., Shapiro L. J. Expression of an X-linked gene from an inactive human X chromosome in mouse-human hybrid cells: further evidence for the noninactivation of the steroid sulfatase locus in man. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6759–6763. doi: 10.1073/pnas.77.11.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon N., Korn N., DeMars R. A-11: cell type-specific and single-active-X transcription controls of newly found gene in cultured human cells. Somat Cell Mol Genet. 1988 Nov;14(6):541–552. doi: 10.1007/BF01535309. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Schwartz H. E., Moser G. C., Holmes S., Meiss H. K. Assignment of temperature-sensitive mutations of BHK cells to the X chromosome. Somatic Cell Genet. 1979 Mar;5(2):217–224. doi: 10.1007/BF01539162. [DOI] [PubMed] [Google Scholar]
- Shapiro L. J., Mohandas T., Weiss R., Romeo G. Non-inactivation of an x-chromosome locus in man. Science. 1979 Jun 15;204(4398):1224–1226. doi: 10.1126/science.156396. [DOI] [PubMed] [Google Scholar]
- Wang T. S., Pearson B. E., Suomalainen H. A., Mohandas T., Shapiro L. J., Schröder J., Korn D. Assignment of the gene for human DNA polymerase alpha to the X chromosome. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5270–5274. doi: 10.1073/pnas.82.16.5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Durfy S. J., Mahtani M. M., Dorkins H., Davies K. E., Williams B. R. Regional localization of the TIMP gene on the human X chromosome. Extension of a conserved synteny and linkage group on proximal Xp. Hum Genet. 1989 Feb;81(3):234–238. doi: 10.1007/BF00278995. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]