Abstract
A new low-field EPR approach for noninvasive measurements of myocardial oxygen tension and tissue acidity was developed. The approach was applied to monitor myocardial pO2 and pH in a model of global no-flow ischemia (30 min) and reperfusion (I/R) in isolated perfused rat hearts. The myocardial oxygen measurements were performed using deuterated Finland trityl radical probe. A rapid decrease of myocardial pO2 from 160 mmHg to about 2 ± 1 mmHg was observed within the first minute of ischemia followed by incomplete restoration of pO2 to 50 mmHg during 30 minutes of reperfusion. The lower oxygen concentration after ischemia was attributed to the 50% reduction in coronary flow after ischemia as a consequence of myocardial I/R damage. Myocardial pH measurements using a specially designed imidazoline pH-sensitive nitroxide showed severe myocardial acidification to pH 6.25 during 30 minutes of ischemia. Preconditioning of the hearts with two 5 minute periods of ischemia significantly reduced the acidification of myocardial tissue during sustained ischemia. Noninvasive EPR monitoring of myocardial oxygenation and pH may provide important insights into the mechanisms of I/R injury and a background for development of new therapeutic approaches.
Keywords: Myocardial ischemia, myocardial acidosis, electron paramagnetic resonance, EPR oximetry, triarylmethyl radical, pH-sensitive nitroxide
Introduction
Ischemic heart disease caused by insufficient blood supply to the myocardium is a major form of cardiovascular disease. Clinical interventions used to reintroduce blood flow to an ischemic region of the myocardium are accompanied by additional damage which is termed reperfusion injury (1). Myocardial ischemia leads to critical changes in the tissue microenvironment. Shortage in oxygen supply results in myocardial oxygen depletion (2) and reliance on anaerobic metabolism resulting in severe acidosis of myocardial tissue (3–4). The alterations in myocardial oxygenation and pH during ischemia and reperfusion correlate with the extent of ischemia and reperfusion (I/R) injury (4–6). Therefore, noninvasive monitoring of myocardial oxygenation and pH and their response to ischemia and reperfusion could provide important insights into the mechanisms of the injury and a background for development of new therapeutic approaches. Low-field electron paramagnetic resonance (EPR) techniques provide an opportunity for noninvasive monitoring of the tissue microenvironment due to sufficient depth of the radio frequency penetration into living tissue. The application of EPR for in vivo functional measurements requires the use of specially designed paramagnetic probes (7).
EPR oximetry methods are based on Heisenberg exchange interaction between the diradical molecule of oxygen and a spin probe which results in the broadening of the probe EPR line. Several types of paramagnetic probes have been used for cardiac EPR oximetry. Nitroxide radicals were the first soluble probes used for EPR measurements of ischemia-induced oxygen depletion in isolated rat hearts (2, 8). However, poor sensitivity of nitroxides to oxygen tensions below 20 mmHg and low stability in living tissues severely restricts their application. Several types of particulate probes, such as carbon-based materials (9–10), lithium phthalocyanine (11) and its derivatives (12–13), have been proposed for EPR oximetry. The main advantages of particulate probes are high functional sensitivity and stability in living tissues. The other type of spin probes used for EPR oximetry are triarylmethyl (TAM) radicals (14–16). TAMs demonstrate good stability in vivo and exhibit a very narrow EPR line sensitive to oxygen concentration. In contrast to particulate probes, the application of soluble TAMs provides an opportunity for spatially-resolved oxygen measurements by EPR-based imaging techniques. Recently we reported a synthetic procedure for large-scale synthesis of deuterated Finland trityl radical, dTAM (17). Herein we applied the dTAM spin probe for EPR monitoring of myocardial oxygenation in isolated perfused rat hearts with sensitivity to oxygen tension of 1 mmHg.
An EPR method for pH measurements is based on application of pH-sensitive nitroxide radicals of imidazole types (18–19). Reversible protonation of nitrogen atom N-3 in the radical heterocycle results in a significant change in hyperfine splitting constant, aN, providing a basis for pH measurements by EPR. Application of the method for in vivo pH measurements requires probes with sufficient aqueous solubility, stability in living tissue and functional sensitivity in the physiologically relevant pH range (20–21). In this work we applied a highly hydrophilic pH-sensitive radical with optimized functional sensitivity in the range of pH 5.6 – 7.6 and enhanced stability towards bioreduction to monitor ischemia-induced myocardial acidosis.
Methods
Spin probes
Deuterated Finland trityl radical, dTAM, was synthesized according to the procedure described previously (17). The pH-sensitive nitroxide radical bound to glutathione, R-SG, was synthesized by a procedure similar to that described by Woldman et al. (22) (see Supplement Information for the details).
Partition coefficients for dTAM probe
The solutions of dTAM (200 μM) in the presence of 0.1 M Na-phosphate buffer at various pH in the range from 6.0 to 8.0 were vigorously mixed with equal volume of octanol for 5 min and allowed to stand for another 5 min until separation of the phases was completed. The values of the partition coefficient, Kp, were calculated as a ratio of the integral intensities of the EPR spectra of dTAM probe in octanol and aqueous phases, correspondingly, and were found to be equal to 0.36±0.13 (pH 6.0), 0.02±0.01 (pH 6.5), and ≤ 0.003 (pH 7.0 and 8.0).
Isolated rat heart preparation
Male Sprague-Dawley rats (275 – 299 g) were used in accordance with The Ohio State University Institutional Lab Animal Care and Use Committee Guidelines. The animals were anesthetized with intraperitoneal injection of sodium pentobarbital, 120 mg/kg. After ensuring complete anesthesia the trachea was cannulated with a catheter attached to a rodent ventilator (Harvard Apparatus, Holliston, MA) to provide ventilation of lungs with room air. The right superficial jugular vein was exposed and sodium heparin, 800 U/kg, was injected. Midsternal thoracotomy was performed to expose the heart. The ascending aorta was rapidly cannulated, the heart was quickly excised and transferred to a perfusion setup (Fig. 1). Retrograde perfusion was initiated at constant pressure of 80 mmHg and 37 °C with modified Krebs-bicarbonate buffer. The perfusate buffer was constantly bubbled with a 95% O2 and 5% CO2 gas mixture. The buffer was prepared with deionized water and contained 11 mM glucose, 116 mM NaCl, 5 mM KCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3. To avoid formation of calcium carbonate precipitate, the CaCl2 was added to the solution after it was heated to 37 °C and equilibrated with CO2. Before the experiments the perfusate buffer solution was routinely filtered through a 0.45 μm cellulose nitrate filter.
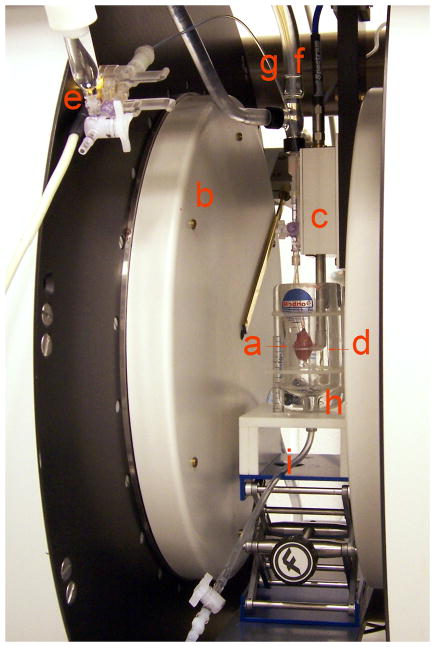
Fig. 1.
Experimental setup: (a) rat heart; (b) EPR magnet; (c) resonator; (d) resonator loop; (e) pressure transducer; (f) perfusate line; (g) hydraulic line; (h) temperature-controlled chamber; (i) perfusate drain.
Experimental setup
An experimental setup allowing for Langendorff heart perfusion, contractile parameter measurements and EPR data acquisition was assembled (Fig. 1). Isolated rat hearts were perfused directly in the loop of a 12 mm surface coil L-band EPR resonator. The measurements were performed using an L-band (1.2 GHz) EPR spectrometer (Magnettech, Germany). The spectrometer is equipped with automatic tuning and automatic coupling controls allowing for EPR measurements of moving objects. The hearts were maintained at 37 °C and relative humidity of approximately 100 %. Contractile function of the hearts was continuously monitored using a water-filled latex balloon inserted into the left ventricle (LV) and attached via hydraulic line to a pressure transducer (Memscap, Skoppum, Norway). The signal from the pressure transducer was amplified and recorded on a PowerLab four-channel strip chart recorder connected to a personal computer equipped with LabChart data acquisition software (ADInstruments, Colorado Springs, CO). The balloon volume was inflated at the beginning of the experiment to a LV end diastolic pressure of 5 mm Hg.
Measurements of myocardial pO2
Isolated rat hearts were perfused for 15 minutes to achieve stabilization of contractile function, and then 100 μM dTAM was added to perfusate buffer. The hearts were subjected to 30 minutes of global no-flow ischemia followed by 60 minutes of reperfusion. Oxygen measurements were performed in three isolated hearts. EPR spectra were continuously recorded with the following spectrometer settings: magnetic field sweep, 1 G; modulation amplitude, 12.8 mG; modulation frequency, 100 kHz; acquisition time, 10 s. Microwave power was adjusted to avoid saturation of EPR line, the attenuation was 15 dB. The experimental spectra were fitted by rational approximation of Voigt function given by Hui et al. (23). The non-linear least square fitting were performed by Levenberg-Marquardt algorithm using home designed MATLAB code. Oxygen concentration was calculated from the Lorentzian component of EPR spectrum linewidth using a calibration curve.
Calibration of pH-sensitive R-SG probe
Calibration of the probe was performed at 37 °C. The radical (1 mM) was dissolved in 5 mM phosphate buffer, and the solution pH was adjusted to the required value by addition of NaOH or HCl. L-band EPR spectra were acquired using the following spectrometer settings: magnetic field sweep, 80 G; modulation amplitude, 2.5 G; modulation frequency, 100 kHz; microwave power attenuation, 3 dB; acquisition time, 20 s. The observed hyperfine splitting, aN, was measured as half the distance between low- and high-field components of the EPR spectrum, and plotted as a function of pH. The pKa value of the radical was determined from fitting pH-dependence of aN with the equation: , where aN(RH+) and aN(R) – hyperfine splitting constants of protonated and unprotonated radical, correspondingly.
EPR and electrochemical measurements of myocardial pH
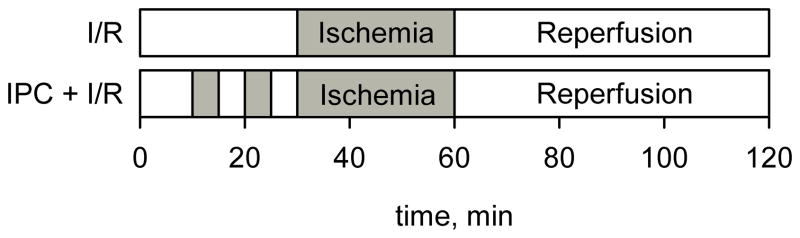
Isolated rat hearts were perfused for 30 minutes and then subjected to 30 minutes of global no-flow ischemia followed by 60 min of reperfusion. Preconditioned hearts were perfused for 10 minutes followed by two episodes of 5 minutes ischemia and 5 minutes of reperfusion before the main course of 30 minute ischemia as shown in Figure 2. The solution of R-SG spin probe (1.5 mM) in perfusate buffer was infused into the hearts through a side arm of the perfusate line upon onset of global no-flow ischemia. EPR spectra were continuously recorded with the following spectrometer settings: magnetic field sweep, 80 G; modulation amplitude, 2.5 G; modulation frequency, 100 kHz; microwave power attenuation, 3 dB; acquisition time, 20 s. The observed hyperfine splitting constant, aN, was measured, and the myocardial pH was calculated using calibration curve. Myocardial pH was measured in 6 control and 6 preconditioned hearts. The difference in pH between control and preconditioned hearts was assessed using one-way ANOVA.
Fig. 2.
Experimental protocols for the model of ischemia/reperfusion and ischemic preconditioning (IPC).
A combination pH electrode in 20 gauge needle (Warner Instruments, Hamden, CT) was used to validate the EPR method of pH measurements. In order to measure myocardial pH the electrode was inserted into the left ventricular wall.
Results
Measurements of myocardial oxygenation
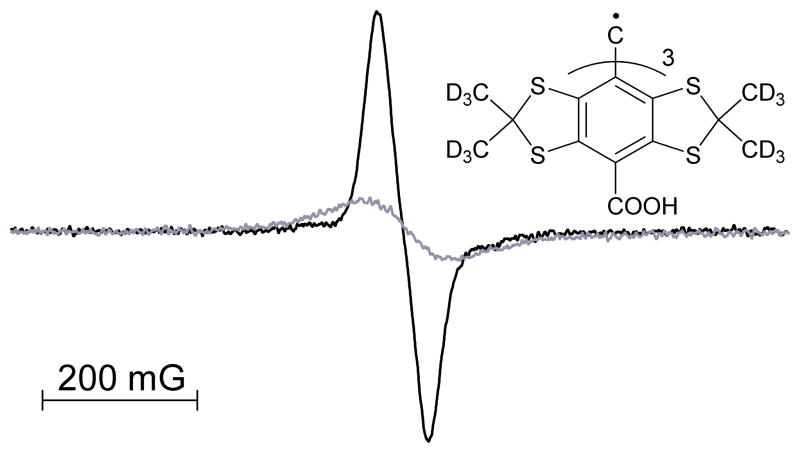
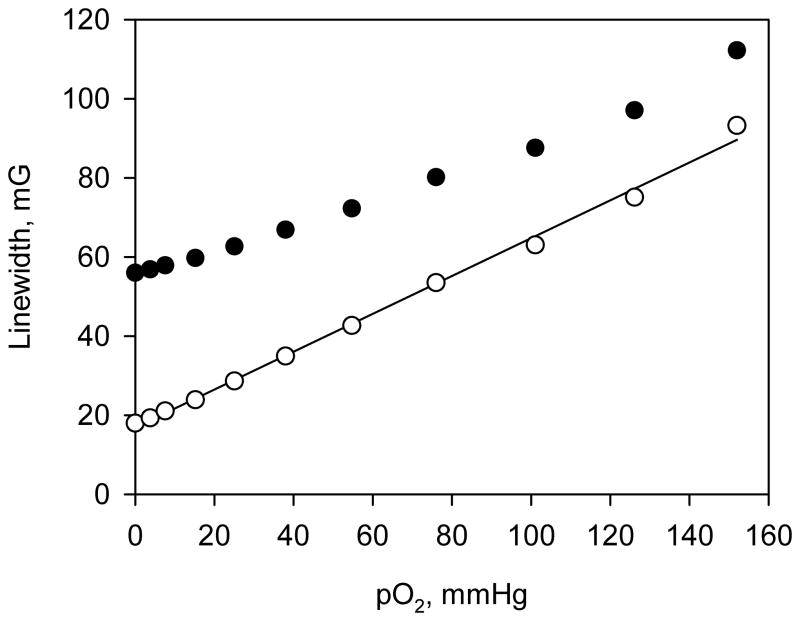
Deuterated Finland triarylmethyl radical, dTAM (see Fig. 3 for the structure), was used for EPR measurements of oxygen concentration. The EPR spectrum of the radical is represented by a single sharp line with peak-to-peak linewidth of 58 mG. Spin exchange interaction of the radical with molecular oxygen results in broadening of the spectral line providing a basis for EPR measurements of oxygen concentration (Fig. 3). An EPR spectral line can be described by a convolution of Lorentzian function with an effective Gaussian originated from unresolved spectral structure. Spin exchange interaction increases Lorentzian linewidth of the EPR signal. Figure 4 shows the dependence of peak-to-peak and Lorentzian linewidths of the dTAM EPR signal on oxygen concentration. Note that peak-to-peak linewidth of the probe EPR line deviates from linear dependence at low oxygen concentrations and is practically oxygen-insensitive below 10 mmHg of pO2. The Lorentzian linewidth of the EPR spectrum demonstrated a good linear dependence with the sensitivity equal to 0.53 mG per mmHg of O2 allowing for detection of oxygen tensions as low as 1 mmHg.
Fig. 3.
EPR spectra of 0.1 mM dTAM in oxygen-free (black) and air saturated (grey) phosphate buffer. Insert: chemical structure of the dTAM spin probe.
Fig. 4.
Dependence of peak-to-peak (●) and Lorentzian (○) linewidth of dTAM EPR spectrum on oxygen concentration.
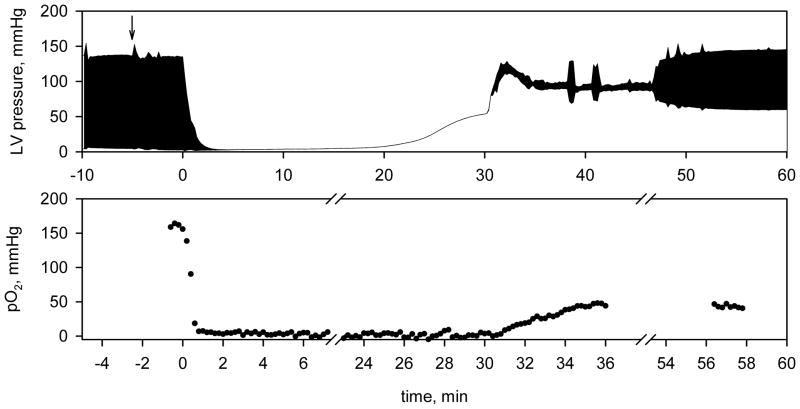
In order to measure myocardial oxygenation of isolated perfused hearts the dTAM spin probe was added to the perfusate buffer. Triarylmethyl radicals with three carboxylic groups are highly hydrophilic at neutral pH (octanol/water partition coefficient, Kp≤ 0.02, in a range of pH from 6.5 to 8.0, see Methods section) and are distributed in the extracellular compartments (15). Thus predominantly intravascular and interstitial localization of the dTAM probe in myocardial tissue is expected. The presence of dTAM in the buffer did not affect contractile parameters of the heart suggesting low if any toxicity of the probe. Figure 5 represents a typical curve of myocardial oxygenation during perfusion, global no-flow ischemia and reperfusion. The measured oxygen concentration in the heart was about 160 mmHg before ischemia and dropped to 2 ± 1 mmHg in less than one minute of global no-flow ischemia. Reperfusion resulted only in partial restoration of oxygenation. The concentration of oxygen in postischemic heart was measured to be about 50 mmHg and remained at the same level after contractile function was recovered. Apparently a major factor in lower oxygen concentration after ischemia is decreased oxygen supply due to reduction in the perfusate flow rate from 16 ml/min before ischemia to 8 ml/min after ischemia as a consequence of myocardial I/R injury. The integral intensity of the dTAM EPR spectrum in the isolated hearts decreased by about 50% during 30 minutes of global no-flow ischemia (data not shown) probably due to anaerobic reduction of the radical to the corresponding EPR silent triarylmethane (24).
Fig. 5.
(top) Left ventricular pressure of the isolated perfused rat heart. The heart was subjected to 30 minutes of global no-flow ischemia followed by reperfusion. Arrow indicates addition of 100 μM dTAM to the perfusate buffer. (bottom) Myocardial oxygenation measured by L-band EPR spectroscopy using the dTAM spin probe. The EPR spectra were fitted with Voigt function to determine Lorentzian linewidth, and oxygen concentration was calculated using calibration curve shown in Fig. 4. The EPR spectrometer settings were as follows: magnetic field sweep, 1 G; modulation amplitude, 12.8 mG; modulation frequency, 100kHz; microwave power attenuation, 15 dB; acquisition time, 10 s.
EPR monitoring of ischemia-induced myocardial acidosis
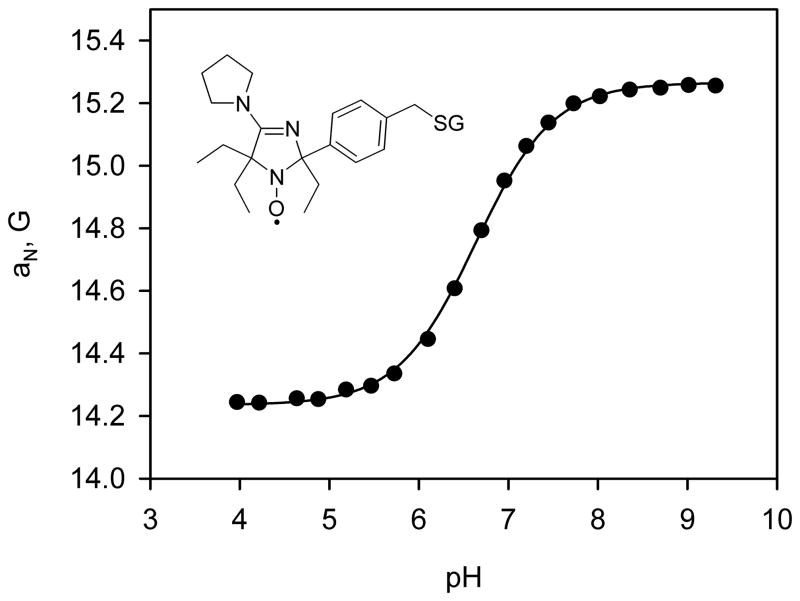
The pH-sensitive nitroxide radical, R-SG (see Fig. 6 for the structure), has been synthesized (see Supplement Information). The observed hyperfine splitting constant dependence of the radical on pH is shown in Fig. 6. The dependence is described by a standard titration curve with the pKa = 6.6 at 37°C allowing for pH measurements in the range from 5.6 to 7.6.
Fig. 6.
pH-Dependence of the observed hyperfine splitting constant, aN, of the R-SG spin probe. EPR spectra were acquired at 37 °C. The solid line is the fit of experimental data with standard titration curve yielding pKa=6.6, aN(RH+)=14.24 G and aN(R)=15.27 G. Insert: chemical structure of the R-SG spin probe.
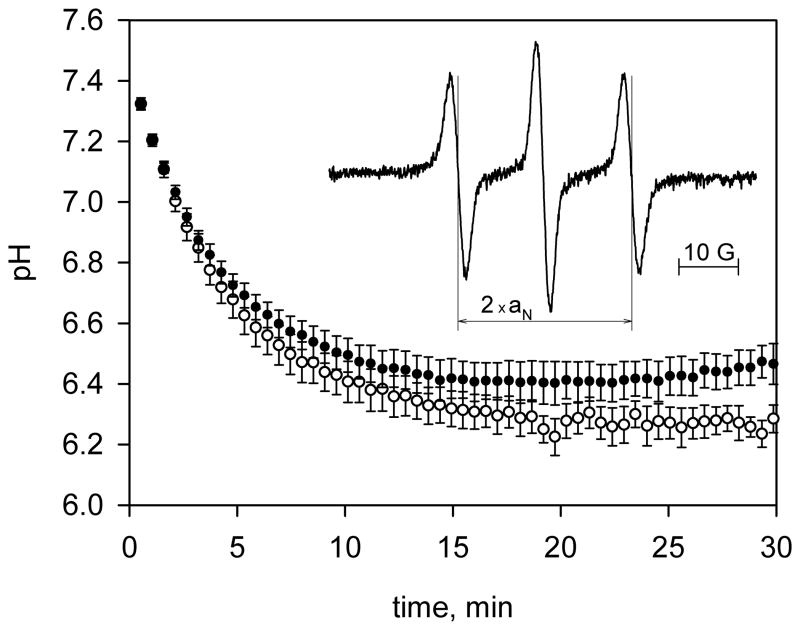
We utilized the probe for EPR monitoring of ischemia-induced acidosis in control and ischemia preconditioned isolated rat hearts. The radical demonstrated extraordinary high stability in myocardial tissue losing only about 10% of its initial EPR intensity during the 30 minute experiment. A representative EPR spectrum of the R-SG spin probe in the heart after 10 minutes of ischemia is shown in Fig. 7 (insert). The spectrum demonstrates a hyperfine splitting constant, aN = 14.62 G, which corresponds to myocardial pH 6.4. As shown in Fig. 7 global no-flow ischemia resulted in acidification of myocardial tissue down to pH 6.25 in control and 6.4 in preconditioned hearts. The difference in pH between control and preconditioned with ischemia hearts was found to be significant (P < 0.05). The presence of R-SG in the ischemic hearts did not affect their postischemic recovery supporting low toxicity of the probe.
Fig. 7.
Ischemia-induced myocardial acidosis of control (○) and ischemia preconditioned (●) rat hearts measured by L-band EPR spectroscopy using R-SG spin probe. The solution of R-SG, 1.5 mM, in perfusate buffer was infused into the hearts through a side arm of the perfusate line upon onset of global ischemia. EPR spectra were continuously recorded with acquisition time, 20 s. Data are mean ± S.E.; n = 6. Insert: EPR spectrum of R-SG in a heart acquired after 10 minutes of global no-flow ischemia. The measured hyperfine splitting constant, 14.62 G, corresponds to pH value 6.4.
Discussion
Molecular oxygen plays a central role in the pathology of cardiac I/R injury to myocardium. Noninvasive monitoring of oxygenation within the heart could provide important insight into the mechanisms of injury. In the present work, we employed the deuterated triarylmethyl radical, dTAM, for EPR oximetry of isolated perfused rat hearts. To date this is the first application of triarylmethyl radical probe for EPR monitoring of oxygenation in the heart. Previously several groups have reported application of nitroxide radicals (8, 25) and glucose char (9) for cardiac EPR oximetry. The particulate probe lithium phtalocyanine, LiPc, has been used in numerous EPR oximetric studies on isolated perfused hearts (26–28) and in vivo (29–32). Friedman et al. (27) used LiPc probe for EPR study of myocardial oxygenation of isolated rat hearts perfused at constant flow. The concentration of oxygen in the perfused hearts was found to be about 185 mmHg and fell to less than 1 mmHg with 3 minutes of ischemia. Reperfusion after ischemia resulted in a myocardial hyperoxygenation as a result of reduced oxygen demand by ischemia-affected tissue. Hyperoxygenation of myocardial tissue was also observed during the reperfusion phase in the model of regional ischemia in vivo (29–32).
In constant pressure models of I/R a reduced coronary flow rate is observed during reperfusion of postischemic myocardium. The decrease in oxygen delivery leads to a lower myocardial oxygen tension compared to preischemic levels (33). Reoxygenation of ischemic tissue results in generation of reactive oxygen species which are one of the major factors in the pathology of myocardial reperfusion injury (33–36). Thus, the low oxygen levels due to coronary flow during early reperfusion may have a protective effect in the postischemic heart.
The oxygen tensions measured in the present work are in reasonable agreement with previously reported data for isolated perfused rat hearts. The oxygen tension was about 160 mmHg before ischemia, dropped to 2 ± 1 mmHg during global no-flow ischemia and only partially restored to about 50 mmHg during reperfusion (see Fig. 5). The observed decrease in oxygenation of the postischemic heart was apparently due to reduction in perfusate flow rate as a consequence of I/R damage to the myocardium. The rate of oxygen depletion after the onset of ischemia was found to be significantly higher than that measured using LiPc probes (27–28, 33). The myocardial oxygen was largely depleted in less than a minute of global no-flow ischemia in this work compared to 3 – 10 minutes as reported in refs. (27–28). Angelos et al. (33) observed fast depletion of myocardial oxygen down to 30 mmHg during the first minute of ischemia and further gradual decrease in myocardial oxygen tension over a 20 minute period of ischemia. Most likely these differences are explained by the difference in response times of the oxygen-sensitive probes used. The soluble triarylmethyl radical dTAM responds to changes in oxygen concentration instantly while the response time of the particulate probe, LiPc, could be longer since the molecules of oxygen have to diffuse into the solid.
Anaerobic metabolism during ischemia results in acidification of myocardial tissue. The crucial role of tissue pH in myocardial ischemia and reperfusion is well documented but still contains many areas of controversy. 31P-NMR of inorganic phosphate is the most commonly employed method for noninvasive measurements of myocardial pH. However the NMR method of pH measurement has several limitations such as lack of functional resolution, 0.2 – 0.3 pH units, the variability of inorganic phosphate concentration with cellular metabolism, and dependence of its chemical shift on ionic strength (37). The EPR method of myocardial pH measurements developed in the present work utilizes the specially designed pH probe, R-SG. The pH-sensitive imidazoline nitroxide is bound to hydrophilic membrane-impermeable tripeptide, glutathione, which provides aqueous solubility of the probe and prevents its diffusion into the highly reducing intracellular environment. Extracellular localization of the probe in combination with the steric protection of its paramagnetic nitroxyl fragment by bulky ethyl groups (38) results in extraordinary stability and minimal toxicity of the probe in myocardial tissue. Meanwhile transsarcolemmal proton movement during ischemia results in fast equilibration of intra- and extracellular pH as measured by 31P NMR using endogenous intracellular inorganic phosphate and exogenously-added extracellular phenylphosphonate, respectively (39). The data observed using R-SG pH probe demonstrate severe myocardial acidification to pH about 6.25 during 30 minutes of global no-flow ischemia. Preconditioning of the hearts with two 5-minute periods of ischemia significantly reduced the acidification of myocardial tissue during sustained ischemia. The observed results are in a good agreement with previously reported data obtained using glass microelectrodes and 31P-NMR (40–41).
In summary, the present work demonstrates the capability of low-field EPR for functional measurements of myocardial tissue microenvironment using specially designed paramagnetic probes. The developed approaches allow for noninvasive monitoring of myocardial oxygenation and pH in isolated rat heart and have potential for in vivo applications. Moreover, these spectroscopic approaches can be extended to an imaging modality allowing for pO2- (15–16) and pH-mapping (42–43). Monitoring of these parameters and their response to ischemia and reperfusion could provide important insights into the mechanisms of the injury and a background for development of new therapeutic approaches.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL089036 and HL3834, and RFFI grant 10-04-91331. The authors thank Dr. Andrey Bobko for the technical assistance and helpful discussions.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Kuppusamy P, Chzhan M, Vij K, Shteynbuk M, Lefer DJ, Giannella E, Zweier JL. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart: a technique for imaging tissue metabolism and oxygenation. Proc Natl Acad Sci U S A. 1994;91(8):3388–3392. doi: 10.1073/pnas.91.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regan TJ, Effros RM, Haider B, Oldewurtel HA, Ettinger PO, Ahmed SS. Myocardial ischemia and cell acidosis: Modification by alkali and the effects on ventricular function and cation composition. Am J Cardiol. 1976;37(4):501–507. doi: 10.1016/0002-9149(76)90388-x. [DOI] [PubMed] [Google Scholar]
- 4.Niwano S, Tojo T. Systemic acidosis in acute myocardial ischemia--cause or result of life-threatening ventricular arrhythmia? Circ J. 2010;74(9):1794–1795. doi: 10.1253/circj.cj-10-0665. [DOI] [PubMed] [Google Scholar]
- 5.Mast F, Elzinga G. Metabolic recovery of acidotic rabbit cardiac muscle: effects of low pH and oxygen shortage. Circ Res. 1989;65(4):909–916. doi: 10.1161/01.res.65.4.909. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115(14):1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 7.Khramtsov VV, Zweier JL. Functional in vivo EPR Spectroscopy and Imaging Using Nitroxide and Trityl Radicals. In: Hicks R, editor. Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. John Wiley & Sons, Ltd; 2010. pp. 537–566. [Google Scholar]
- 8.Zweier JL, Kuppusamy P. Electron paramagnetic resonance measurements of free radicals in the intact beating heart: a technique for detection and characterization of free radicals in whole biological tissues. Proc Natl Acad Sci U S A. 1988;85(15):5703–5707. doi: 10.1073/pnas.85.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zweier JL, Chzhan M, Ewert U, Schneider G, Kuppusamy P. Development of a highly sensitive probe for measuring oxygen in biological tissues. J Magn Reson B. 1994;105(1):52–57. doi: 10.1006/jmrb.1994.1099. [DOI] [PubMed] [Google Scholar]
- 10.Vahidi N, Clarkson RB, Liu KJ, Norby SW, Wu M, Swartz HM. In vivo and in vitro EPR oximetry with fusinite: a new coal-derived, particulate EPR probe. Magn Reson Med. 1994;31(2):139–146. doi: 10.1002/mrm.1910310207. [DOI] [PubMed] [Google Scholar]
- 11.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90(12):5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilangovan G, Manivannan A, Li H, Yanagi H, Zweier JL, Kuppusamy P. A naphthalocyanine-based EPR probe for localized measurements of tissue oxygenation. Free Radic Biol Med. 2002;32(2):139–147. doi: 10.1016/s0891-5849(01)00784-5. [DOI] [PubMed] [Google Scholar]
- 13.Pandian RP, Dolgos M, Dang V, Sostaric JZ, Woodward PM, Kuppusamy P. Structure and Oxygen-Sensing Paramagnetic Properties of a New Lithium 1, 8, 15, 22-Tetraphenoxyphthalocyanine Radical Probe for Biological Oximetry. Chem Mater. 2007;19:3545–3552. [Google Scholar]
- 14.Ardenkjaer-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, Golman K. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson. 1998;133(1):1–12. doi: 10.1006/jmre.1998.1438. [DOI] [PubMed] [Google Scholar]
- 15.Elas M, Williams BB, Parasca A, Mailer C, Pelizzari CA, Lewis MA, River JN, Karczmar GS, Barth ED, Halpern HJ. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn Reson Med. 2003;49(4):682–691. doi: 10.1002/mrm.10408. [DOI] [PubMed] [Google Scholar]
- 16.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, Cook JA, Golman K, Ardenkjaer-Larsen JH, Subramanian S, Mitchell JB. Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A. 2002;99(4):2216–2221. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhimitruka I, Grigorieva O, Zweier JL, Khramtsov VV. Synthesis, structure and EPR characterization of deuterated derivatives of Finland trityl radical. Bioorganic & Medicinal Chemistry Letters. 2010;20:3946–3949. doi: 10.1016/j.bmcl.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khramtsov VV, Weiner LM, Grigor’ev IA, Volodarsky LB. Proton exchange in stable nitroxyl radicals. EPR studies of the pH of aqueous solutions. Chem Phys Lett. 1982;91:69–72. [Google Scholar]
- 19.Keana JFW, Acarregui MJ, Boyle SLM. 2,2-Disubstituted-4,4-dimethylimidazolidinyl-3-oxy nitroxides indicators of aqueous acidity through variation of aN with pH. J Am Chem Soc. 1982;104:827–830. [Google Scholar]
- 20.Khramtsov VV. Biological imaging and spectroscopy of pH. Curr Org Chem. 2005;9:909–923. [Google Scholar]
- 21.Potapenko DI, Foster MA, Lurie DJ, Kirilyuk IA, Hutchison JM, Grigor’ev IA, Bagryanskaya EG, Khramtsov VV. Real-time monitoring of drug-induced changes in the stomach acidity of living rats using improved pH-sensitive nitroxides and low-field EPR techniques. J Magn Reson. 2006;182(1):1–11. doi: 10.1016/j.jmr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Woldman YY, Semenov SV, Bobko AA, Kirilyuk IA, Polienko JF, Voinov MA, Bagryanskaya EG, Khramtsov VV. Design of liposome-based pH sensitive nanoSPIN probes: nano-sized particles with incorporated nitroxides. Analyst. 2009;134(5):904–910. doi: 10.1039/b818184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui AK, Armstrong BH, Wray AA. Rapid Computation of Voigt and Complex Error Functions. Journal of Quantitative Spectroscopy & Radiative Transfer. 1978;19(5):509–516. [Google Scholar]
- 24.Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher JL. Oxidative and reductive metabolism of tris(p-carboxyltetrathiaaryl)methyl radicals by liver microsomes. Chem Res Toxicol. 2009;22(7):1342–1350. doi: 10.1021/tx9001379. [DOI] [PubMed] [Google Scholar]
- 25.Baker JE, Froncisz W, Joseph J, Kalyanaraman B. Spin label oximetry to assess extracellular oxygen during myocardial ischemia. Free Radic Biol Med. 1997;22(1–2):109–115. doi: 10.1016/s0891-5849(96)00289-4. [DOI] [PubMed] [Google Scholar]
- 26.Friedman BJ, Grinberg OY, Isaacs KA, Walczak TM, Swartz HM. Myocardial oxygen tension and relative capillary density in isolated perfused rat hearts. J Mol Cell Cardiol. 1995;27(12):2551–2558. doi: 10.1006/jmcc.1995.0042. [DOI] [PubMed] [Google Scholar]
- 27.Friedman BJ, Grinberg OY, Isaacs KA, Ruuge EK, Swartz HM. Effect of repetitive ischemia on myocardial oxygen tension in isolated perfused and hypoperfused rat hearts. Magn Reson Med. 1996;35(2):214–220. doi: 10.1002/mrm.1910350213. [DOI] [PubMed] [Google Scholar]
- 28.Ilangovan G, Liebgott T, Kutala VK, Petryakov S, Zweier JL, Kuppusamy P. EPR oximetry in the beating heart: myocardial oxygen consumption rate as an index of postischemic recovery. Magn Reson Med. 2004;51(4):835–842. doi: 10.1002/mrm.20000. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Zuo L, Cardounel AJ, Zweier JL, He G. Characterization of in vivo tissue redox status, oxygenation, and formation of reactive oxygen species in postischemic myocardium. Antioxid Redox Signal. 2007;9(4):447–455. doi: 10.1089/ars.2006.1389. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, He G. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol. 2007;293(3):H1442–1450. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]
- 31.Khan M, Mohan IK, Kutala VK, Kotha SR, Parinandi NL, Hamlin RL, Kuppusamy P. Sulfaphenazole protects heart against ischemia-reperfusion injury and cardiac dysfunction by overexpression of iNOS, leading to enhancement of nitric oxide bioavailability and tissue oxygenation. Antioxid Redox Signal. 2009;11(4):725–738. doi: 10.1089/ars.2008.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Cai M, Xu Y, Swartz HM, He G. Late phase ischemic preconditioning preserves mitochondrial oxygen metabolism and attenuates post-ischemic myocardial tissue hyperoxygenation. Life Sci. 2011;88(1–2):57–64. doi: 10.1016/j.lfs.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelos MG, Kutala VK, Torres CA, He G, Stoner JD, Mohammad M, Kuppusamy P. Hypoxic reperfusion of the ischemic heart and oxygen radical generation. Am J Physiol Heart Circ Physiol. 2006;290(1):H341–347. doi: 10.1152/ajpheart.00223.2005. [DOI] [PubMed] [Google Scholar]
- 34.Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem. 1989;264(32):18890–18895. [PubMed] [Google Scholar]
- 35.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61(3):461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Kutala VK, Khan M, Angelos MG, Kuppusamy P. Role of oxygen in postischemic myocardial injury. Antioxid Redox Signal. 2007;9(8):1193–1206. doi: 10.1089/ars.2007.1636. [DOI] [PubMed] [Google Scholar]
- 37.Pietri S, Martel S, Culcasi M, Delmas-Beauvieux MC, Canioni P, Gallis JL. Use of diethyl(2-methylpyrrolidin-2-yl)phosphonate as a highly sensitive extra- and intracellular 31P NMR pH indicator in isolated organs. Direct NMR evidence of acidic compartments in the ischemic and reperfused rat liver. J Biol Chem. 2001;276(3):1750–1758. doi: 10.1074/jbc.M008023200. [DOI] [PubMed] [Google Scholar]
- 38.Kirilyuk IA, Bobko AA, Grigor’ev IA, Khramtsov VV. Synthesis of the tetraethyl substituted pH-sensitive nitroxides of imidazole series with enhanced stability towards reduction. Org Biomol Chem. 2004;2(7):1025–1030. doi: 10.1039/b400252k. [DOI] [PubMed] [Google Scholar]
- 39.Clarke K, Stewart LC, Neubauer S, Balschi JA, Smith TW, Ingwall JS, Nedelec JF, Humphrey SM, Kleber AG, Springer CS., Jr Extracellular volume and transsarcolemmal proton movement during ischemia and reperfusion: a 31P NMR spectroscopic study of the isovolumic rat heart. NMR Biomed. 1993;6(4):278–286. doi: 10.1002/nbm.1940060407. [DOI] [PubMed] [Google Scholar]
- 40.Asimakis GK, Inners-McBride K, Medellin G, Conti VR. Ischemic preconditioning attenuates acidosis and postischemic dysfunction in isolated rat heart. Am J Physiol. 1992;263(3 Pt 2):H887–894. doi: 10.1152/ajpheart.1992.263.3.H887. [DOI] [PubMed] [Google Scholar]
- 41.Lundmark JA, Trueblood N, Wang LF, Ramasamy R, Schaefer S. Repetitive acidosis protects the ischemic heart: implications for mechanisms in preconditioned hearts. J Mol Cell Cardiol. 1999;31(4):907–917. doi: 10.1006/jmcc.1998.0931. [DOI] [PubMed] [Google Scholar]
- 42.Khramtsov VV, Caia GL, Shet K, Kesselring E, Petryakov S, Zweier JL, Samouilov A. Variable Field Proton-Electron Double-Resonance Imaging: Application to pH mapping of aqueous samples. J Magn Reson. 2010;202(2):267–273. doi: 10.1016/j.jmr.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Efimova OV, Sun Z, Petryakov S, Kesselring E, Caia GL, Johnson D, Zweier JL, Khramtsov VV, Samouilov A. Variable Radio Frequency Proton-Electron Double-Resonance Imaging: application to pH mapping of aqueous samples. J Magn Reson. 2011;209(2):227–232. doi: 10.1016/j.jmr.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.