Abstract
Despite evidence that high-affinity GABAA receptor subunit mRNA and protein are present in dorsal root ganglia (DRG), low affinity currents dominate those detected in acutely dissociated DRG neurons in vitro. This observation raises the possibility that high affinity receptors are normally trafficked out of the DRG toward central and peripheral terminals. We therefore hypothesized that with time in culture, there would be an increase in high-affinity GABAA currents in DRG neurons. To test this hypothesis, we studied dissociated DRG neurons 2 hrs (acute) and 24 hrs (cultured) after plating with whole cell patch clamp techniques, western blot and qRT-PCR analysis. GABAA current density increases dramatically with time in culture in association with the emergence of two persistent currents with EC50’s of 0.25 ± 0.01 μM and 3.2 ± 0.02 μM for GABA activation. In a subpopulation of neurons, there was also an increase in the potency of GABA activation of the transient current from an EC50 of 78.16 ± 10.1 μM to 9.56 ± 1.3 μM with time in culture. A fraction of the high affinity current was potentiated by δ-subunit agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP). δ-subunit immunoreactivity was largely restricted to the cytosolic fraction in acute but the membrane fraction in cultured DRG neurons with no detectable change in δ-subunit mRNA. However, the emergence of a high affinity current blocked by THIP and insensitive to bicuculline was detected in a subpopulation of cultured neurons as well in association with an increase in ρ2 and 3-subunit mRNA in cultured DRG neurons. Our results suggest that high-affinity δ-subunit containing GABAA receptors are normally trafficked out of the DRG where they are targeted to peripheral and central processes. They also highlight that the interpretation of data obtained from cultured DRG neurons should be made with caution.
Introduction
γ-aminobuteric acid (GABA) is the major inhibitory neurotransmitter in the adult mammalian nervous system, mediating its principal effects via anion selective ionotropic GABAA receptors. In the central nervous system (CNS), GABAA receptor activation leads to a net anion influx resulting in membrane hyperpolarization. Recent evidence indicates that GABAA receptors underlie at least two distinct modes of inhibition. Classical synaptic inhibition is phasic, reflecting rapid increases in GABA in to the millimolar range, and is mediated by relatively rapidly desensitizing GABAA receptors which have low affinity for GABA (Mody et al., 1994). A second mode of inhibition is tonic, mediated by high affinity extrasynaptic GABAA receptors which are activated by GABA concentrations in the tens of nanomolar to a few micromolar range (Farrant and Nusser, 2005). GABAA receptor subunit composition dictates the biophysical and pharmacological properties of the receptor as well as the surface distribution. For example, γ2 subunit containing receptors are transient low affinity receptors targeted to synapses (Essrich et al., 1998) while δ subunit containing receptors are sustained high affinity extrasynaptic receptors (Nusser et al., 1998, Belelli et al., 2009).
GABAA signaling in primary afferent neurons is distinct in several respects from that observed in the CNS. First, because of the differential regulation of Cl−, GABAA receptor activation in primary afferents results in membrane depolarization (Sung et al., 2000). Second, there appears to be disagreement between mRNA and functional data, and anatomical evidence in support of presynaptic GABAA receptors, particularly on putative nociceptive afferents. On the one hand, a number of GABAA receptor subunits are expressed at relatively high levels in virtually all DRG neurons (Persohn et al., 1991). These expression data are consistent with electrophysiological data indicating that there is a relatively high GABAA current density in ~100% of neurons tested (Oyelese et al., 1997, White, 1990). On the other hand, anatomical evidence of presynaptic GABAA receptors on putative nociceptive afferents is relatively sparse (Todd, 1996). Third, there is evidence that GABAA receptors are transported to the periphery in primary afferents (Carlton et al., 1999), despite the absence of a source for high concentrations of GABA such as that released at synapses from GABAergic neurons. These observations raise the distinct possibility that extrasynaptic GABAA receptors are the dominant GABAA receptor subtype in nociceptive primary afferents.
However, previous analysis of GABAA-receptor mediated currents in acutely isolated sensory neuron somata suggests that these currents are dominated by relatively rapidly desensitizing low affinity receptors (Oyelese et al., 1997, Sung et al., 2000). Even currents evoked with low concentrations of GABA (i.e., 2.5 μM) desensitize (White, 1990). There is also evidence that these currents are potentiated by benzodiazepines (Witschi et al., 2011), which act at γ-subunit containing receptors that are normally targeted to synapses (McKernan et al., 1995). Furthermore, while it remains to be determined whether the amount of GABA released within the DRG is sufficient to activate low affinity receptors, it has been suggested that GABA signaling within the ganglia constitutes a normal component of sensory processing (Vit et al., 2009). This raises the possibility that the GABAA currents present in acutely isolated sensory neurons are the currents normally present in sensory somata in vivo. Thus, despite evidence that high affinity GABAA receptor subunits are expressed in DRG (Ma et al., 1993), there is little evidence that these receptors are detectable in acutely isolated DRG neurons. We therefore hypothesized that high affinity GABAA receptors are normally trafficked out of the ganglia. A key prediction of this hypothesis is that high affinity receptors should be detectable with time in culture as receptors normally targeted for peripheral and central processes are inserted in the plasma membrane as is observed with other transducers (Kirillova et al., 2011). The present study was designed to test this hypothesis. The biophysical and pharmacological properties of GABA receptor mediated currents were assessed in dissociated DRG neurons 2 hrs (acute) and 24 hrs (cultured) after plating with conventional whole cell patch clamp techniques. Semi-quantitative reverse-transcriptase polymerase chain reaction (sqRT-PCR) and Western blot analysis were used to further asses the basis for culture dependent changes in GABAA currents. Our results were generally consistent with our hypothesis that high affinity GABAA receptors are normally trafficked out of the ganglia, but also suggested that there are additional changes in subunit expression which appear to be associated with placing the neurons in culture.
Experimental procedures
Animals
Adult (180–280g) male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used for all experiments. Rats were housed in the University of Pittsburgh animal facility in groups of two on a 12:12 light dark schedule. Food and water were available ad libitum. All experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines as well as guidelines established by the International Association of the Study of Pain for the use of laboratory animals in research.
Tissue preparation
Rats were deeply anesthetized with a subcutaneous injection (1mg/kg) of a cocktail containing ketamine (55mg/kg), xylazine (5.5 mg/kg), and acepromazine (1.1mg.kg) (ketamine was from Fort Dodge Animal Health, Fort Dodge, WI, USA; xylazine and acepromazine were from Phoenix Scientific Inc., St. Joseph, MO, USA). L4–5 DRG were harvested, enzymatically treated, mechanically dissociated, and plated on laminin and ornithine- coated (Invitrogen) cover-slips as previously described (Lu et al., 2006). After plating for two hours, cells were either flooded with a L-15 (Invitrogen) based media containing 10% fetal bovine serum, 5 mM HEPES, 5 mM glucose and 1000 units of penicillin-streptomycin (Invitrogen) or flooded with Minimal Essential Media (MEM, Invtirogen) containing 10% fetal bovine serum, 1000 units of penicillin-streptomycin, and 1x MEM vitamins (Invitrogen). Those flooded with L-15 based media were stored at room temperate and studied over the next 5–6 hours while those flooded with MEM-based media were placed in a CO2 (3%) incubator at 37°C for 24 hours prior to study: the former were considered acutely cultured and the latter were considered cultured neurons.
Electrophysiology
Whole-cell patch-clamp recordings were performed with a HEKA EPC10 amplifier (HEKA Eletronik GmbH, Lambrecht, Germany). Unless otherwise noted, data were acquired at 10 kHz and filtered at 2 kHz. Borosilicate glass (WPI, Sarasota, FL) electrodes were 2–3 MΩ when filled with the following solution (mM): CsCl 140, MgCl2 1, EGTA 11, HEPES 10, Mg-ATP 2, and GTP 1; pH was adjusted to 7.2 with Tris-base and osmolality was adjusted to 310 mOsm with sucrose. The bath solution contained the following (in mM): NaCl 130, KCl 3, CaCl2 2.5, MgCl2 0.6, HEPES 10, and glucose 10; pH was adjusted to 7.4 with Tris-base and osmolality was adjusted to 320 mOsm with sucrose. Neurons were held at −60 mV. Currents were evoked by 3 or 60 seconds of focal application of GABA or GABAA receptor agonists at different concentrations. The rule out the possibility that the KCl included in the bath solution contributed to the current evoked in response to GABAA receptor activation, GABA currents were measure in bath solution with and without K+. The results of this experiment indicate that there was no detectable influence of K+ flux to the current evoked by GABA (1, 10, and 100 μM). Current amplitudes were 12.1±1.0, 68.5±3.1 and 128.4±12.0 pA compared to 12.6±5.7, 49.7±6.8 and 139.3±22.3 (n = 4) in the presence and absence of 3 mM K+ in the bath solution, respectively.
Western blot
For the separation of membrane and cytosol fraction, the cells or tissue were ground in RIPA buffer, let it sit on ice for 20 minutes, and then spun down in a table-top centrifuge at 13,000rpm for 10 minutes. The supernatant was then loaded onto a sucrose buffer and spun in an ultra centrifuge at approximately 100,000g for an hour. The top 3/4 of the supernatant was used as the cytoplasm-enriched fraction. A pellet of membrane-enriched protein formed at the bottom of the tube. One μl of 10% SDS was added to the remaining supernantant and used to resuspend the pellet. Protein was separated on a 7.5–10% SDS-PAGE gel and blotted to nitrocellulose membrane (Amersham) with a Trans-Blot Transfer Cell system (Bio-Rad). Blots were blocked with 5% milk in TBS buffer (20 mM Tris, 150 mM NaCl, pH 7.4) at room temperature for 30 min. After decanting the blocking buffer, the blot was incubated with an antibody against the δ-subunit of the GABAA receptor (AB9752, Millipore: 1:500). The specificity of this antibody has been determined previously (Korpi et al., 2002). Membranes were incubated with the δ-subunit antibody overnight at 4°C at a 1:200 dilution. Immunoreactivity was detected using Enhanced Chemiluminescence (ECL, Amersham). Chemiluminescence was captured with a CCD camera (Las-3000, Fujifilm) and analyzed with Fuji software Multi Gauge.
RT-PCR
Total RNA was extracted from two groups of cells (2 hr (acute) and 24 hr (cultured) after plating) using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA synthesis was carried out using 1 μg total RNA with SuperScript II reverse transcriptase and an anchored oligo(dT) primer. SYBR Green was used to monitor amplification of template with primers on a real-time thermal cycler (Applied Biosciences) controlled by a PC running Prism 7000 SCS software. A melting curve was generated at the end of each experiment to asses for the presence of contamination. Gel electrophoresis was used to confirm the generation of a single PCR product of the expected size. The Standard curve and ΔΔCT methods were used to assess differences in relative expression levels following confirmation that 24hr culturing has no effect on comparator gene (e.g., GAPDH) expression levels. Primers for amplification of ρ1,2,3 genes were as follows: ρ1 F-GCAACATGGACTTCAGCCGGTTT, R-GGTCGTCTTCTGTGTAGGCATAGCTT, ρ2 F-TACCTGAGGCATTACTGGAGGGATGA, R-TTCTTCACCAGCCTGCCATCGAA, ρ3 F-TGGATCACACTGATGCTGGATGCT, R-GAATGCGGGTTTGTTTCGGTGAAGAG, those for amplification of δ were F-TCC CTG GGC TTT ACC TCA ATT, R-ACT AAT GGC CCT CTG TGC AAA while those for GAPDH were: F-GGCCTACATGGCCTCCAA, R-TGGAATTGTGAGGGAGATGCT.
Statistical analysis
Data are expressed as mean ± SEM unless otherwise stated. Differences between groups were assessed with a one-way ANOVA for multiple group comparisons (i.e., concentration response data) or a Students t-test to compare results from acute and cultured neurons, where p < 0.05 was considered statistically significant.
Results
GABAA currents in DRG neurons increase with time in culture
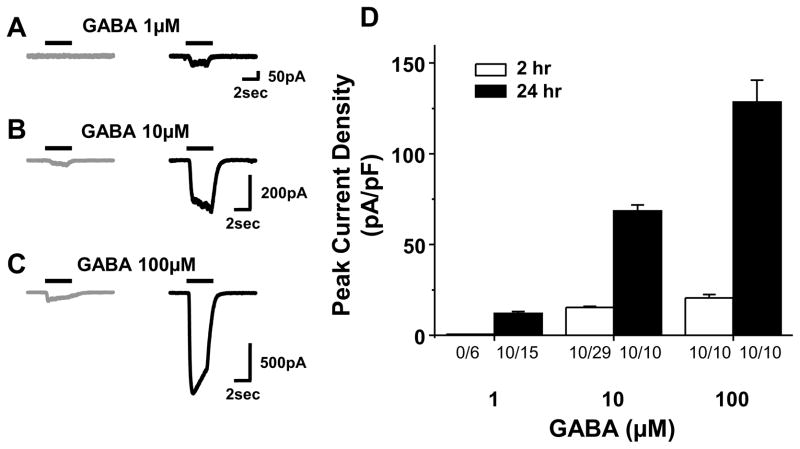
Because we are ultimately interested in the role of GABA signaling in primary afferent neurons in nociceptive processing, the focus of this study was on small diameter neurons, a population enriched in putative nociceptors (Lawson SN, 2002). Therefore the membrane capacitance of the neurons included in this study were less that 45 pF. Increasing concentrations of GABA (1, 10, and 100 μM) was used to evoke GABA currents in acutely dissociated DRG neurons (2hr after plating) and those cultured for 24 hours (Fig 1). While acutely dissociated neurons had no response to a brief (3 sec) application of 1 μM of GABA (0 of 6), after 24hrs in culture, a bicuculline-sensitive current was present in 10 of 15 DRG neurons tested, where the average current density was 2.1 ± 0.5 pA/pF (Fig. 1B, n=10). A response to 10 μM GABA was detectable in only 10 of 29 acutely dissociated DRG neurons, but was clearly manifest in all of the neurons studied after 24 hrs in culture: the current density in cultured neurons was significantly (n=10, p < 0.05) greater than that in acutely dissociated neurons, even when only data from responsive neurons were included in this analysis (Figure 1B). Likewise, while all of the acutely dissociated neurons tested (n = 10) were responsive to 100 μM GABA, current density in cultured neurons (128.5 ± 12.0 pA/pF) was significantly (p < 0.01) greater than in acutely dissociated neurons (20.5 ± 1.9 pA/pF, n = 10). While GABAB receptors are clearly present in DRG neurons (Menon-Johansson et al., 1993), and a GABAB receptor antagonist was not used in the majority of experiments, control experiments (n = 9 acute and 5 cultured) indicated that presence of the GABAB receptor antagonist, CGP35348 (10μM) had no influence on the magnitude or kinetics of inward currents evoked with GABA (data not shown).
Figure 1.
GABA-evoked currents from acutely dissociated (2hr in culture) and cultured (24hr in culture) DRG neurons. A–C: Representative examples of GABA-evoked currents recorded from acutely dissociated (gray) and cultured (black) DRG neurons. The horizontal bars indicate application of GABA in different concentration (1, 10, 100 μM). D: Averaged data from 10 responsive neurons in each group. Since none of the acutely dissociated neurons were responsive to 1 μM GABA, the current “amplitude” in this group is zero. Numbers beneith each bar are the proportion of neurons responsive to GABA at easch concentration.
Time dependent changes in high and low affinity GABAA currents
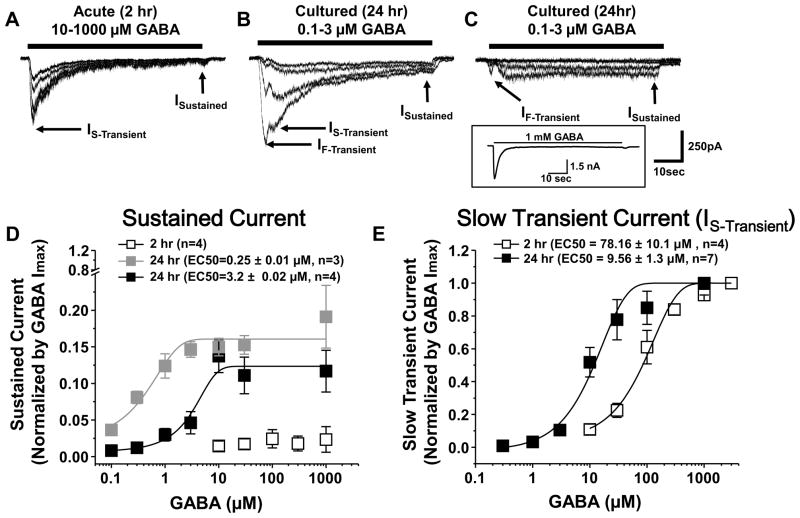
Our initial screen of GABA currents in DRG neurons suggested that there was an increase in GABA potency with time in culture. One of the unique features of extrasynaptic high affinity GABA receptors is that the currents are persistent. Therefore, to better characterize time-dependent changes in GABA potency as well as the presence of persistent currents in cultured DRG neurons, another group of neurons was studied with prolonged (1 min) application of additional concentrations of GABA. Ten μM GABA was used as starting dose on acutely dissociated neurons as none were responsive to 1 μM, while doses from 0.1μM were tested on cultured DRG neurons. There was a concentration dependent increase in the rate of current desensitization in acutely cultured DRG neurons (Figure 2A). Nevertheless even with 10 μM GABA, peak current (Ipeak = 198 ± 22.9 pA) was reduced by >98% by the end of a 1 minute GABA application (Isustain = 3.4 ± 0.2 pA, n = 5). In contrast, there was a significant (p < 0.05) increase in the sustained current (Isustain = 55.5 ± 6.8 pA) evoked with 10 μM GABA in cultured DRG neurons. This increase in sustained current also constituted a significantly larger fraction (15.5± 3.2 %) of peak current amplitude (Ipeak = 359 ± 126 pA, n=7, p < 0.05), despite the increase in peak current. Furthermore, there was little desensitization of the current evoked in cultured DRG neurons with 1 μM GABA (n = 7, Figure 2B and C). Dose response analysis of peak and sustained currents revealed up to three populations of DRG neurons defined by the presence transient and sustained currents with different potencies for GABA. One had sustained currents EC50 of 0.25 ± 0.01 μM (Figure 2B & D, n = 3) while the other had sustained currents with an EC50 of 3.2 ± 0.02 μM (Figure 2C & D, n = 4).
Figure 2.
Currents elicited by prolonged application of GABA (1 minute) on acutely dissociated (A), and cultured (B and C) DRG neurons. Data were acquired at 100Hz and filtered at 50 Hz. Horizontal bars indicate application of GABA (10–1000 μM in A, and 0.1–3 μM in B and C). Arrows indicate the transient, both fast (IF-Transient) and slow (IS-Transient)) and sustained (ISustained) current. Current shown in A is typical of that observed in 4 other acutely dissociated neurons while those shown in B and C are typical of that observed in cultured DRG neurons with very high affinity (B, EC50 = 0.27 μM) and high affinity (C, EC50 = 3.2 μM) sustained GABA currents. Higher concentrations of GABA applied to the neuron in C (inset) resulted in a large transient current. Concentration-response relationtiops for sustained (D) and slow transient (E) currents evoked by GABA in acutely dissociated (2 hr in culture; open square, n=4) and cultured (24hr in culture; filled square, n=7) DRG neurons. Data from each neuron were normalized with respect to maximal inward current and subsquantly fitted to single Hill equation to generate the EC50 values indicated in the legend for Panels D and E.
There were also two transient currents detected in DRG neurons. One was a relatively slowly desensitizing current (IS-Transient) present in both acutely dissociated and cultured DRG neurons (Figure 2A and B). The second was a rapidly desensitizing current (IF-Transient) detectable in all cultured DRG neurons (Figure 2B and C). The EC50 for IF-Transient appeared to be consistent among cultured DRG neurons but was not determined because of contamination by IS-Transient in a significant number of neurons. The EC50 for activation of IS-Transient in acutely dissociated neurons was also consistent (78.16 ± 10.1 μM, Figure 2E). However, there were at least two populations of cultured neurons defined by the threshold for the activation of the IS-Transient. One was activated by GABA at concentrations as low as 1 μM (Figure 2B) while the other required considerably higher concentrations of GABA (Figure 2C, inset). The EC50 for the high affinity IS-Transient in cultured neurons (9.56 ± 1.3 μM, n = 7) was significantly (p < 0.01) lower than that of the lower affinity IS-Transient (91.5 ± 12.1 μM, n = 6) in cultured neurons or the transient current in acutely dissociated neurons (78.16 ± 10.1 μM, n=4, Figure 2E). There was no significant difference between acutely dissociated or cultured neurons with respect to the EC50 of the low affinity transient current. There did not appear to be a differential association of high and low affinity transient currents with high and low affinity sustained currents in cultured DRG neurons.
We did not pursue a pharmacological approach to tease apart the three components of the transient GABA current in cultured neurons. That said, because IF-Transient is activated at lower concentrations of GABA than either of the two IS-Transient currents and IF-Transient was not present in acutely dissociated neurons, we do not believe that IF-Transient is an artifact. As the decay of IF-Transient was obscured by IS-Transient, it was not possible to accurately determine the rate of decay of this current and consequently, no additional statistical analyses were performed. However, the decay rate of IS-Transient was comparable in acute and cultured neurons: 130 ± 10 ms vs 103 ± 8 ms, respectively.
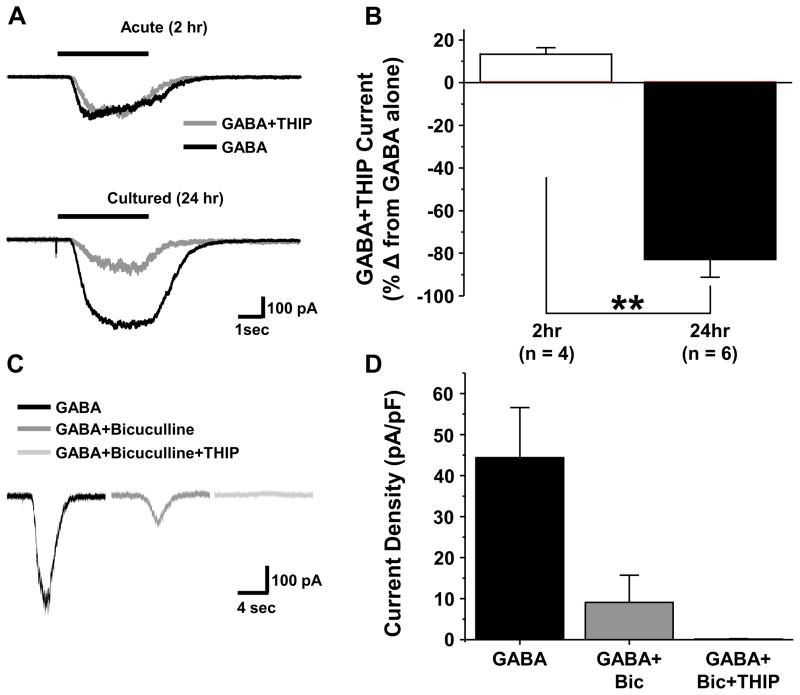
The emergence of a THIP sensitive current in cultured DRG neurons
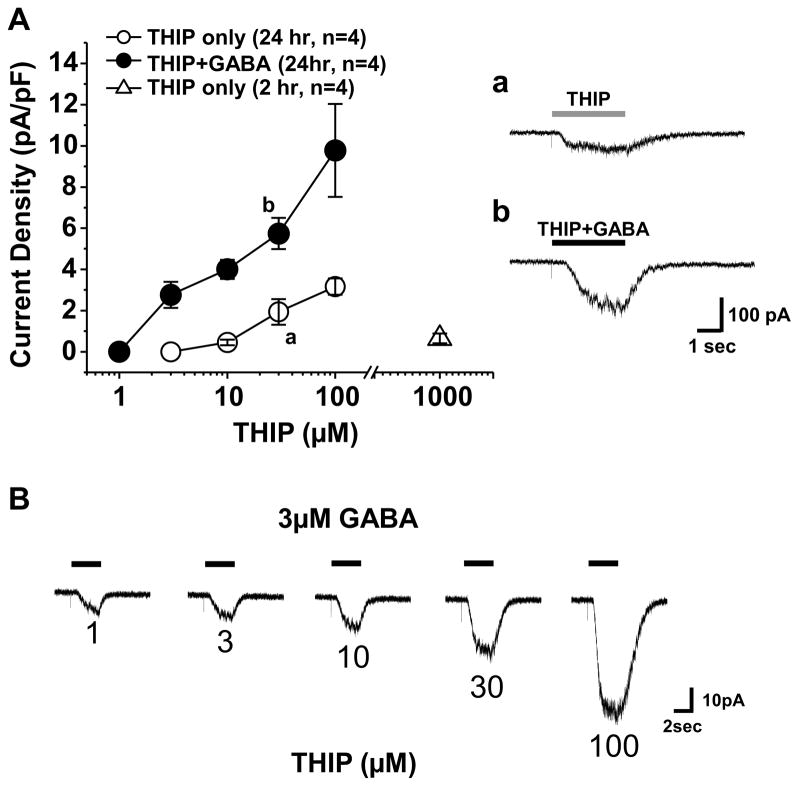
The preceding data indicate the emergence of high affinity non-desensitizing GABAA receptors with time in culture. The biophysical and pharmacological properties of these currents are consistent with those of δ-subunit containing GABA receptors that are located extrasynaptically in the CNS. To further explore the possibility that δ-subunit containing receptors contribute to the high affinity current observed in cultured DRG neurons, we assessed the impact of δ-subunit agonist, THIP, on acutely dissociated and cultured DRG neurons. Application of THIP alone for 3 sec resulted on a concentration-dependent increase in evoked current in cultured neurons, where the threshold for activation was ~ 10 μM (Figure 3A, n=4). In contrast, acutely dissociated DRG neurons were largely unresponsive to THIP at concentrations as high as 1mM (Figure 3A). The results of co-application of THIP with GABA were more complicated suggesting that in a subpopulation of neurons (6/11) a low concentration of THIP (1 μM) inhibited the GABA evoked current. However, at concentrations of THIP above 1 μM, THIP potentiated the GABA evoked current in every neuron tested. The THIP-induced potentiation of 3 μM of GABA was concentration dependent (Figure 3A and B).
Figure 3.
Effects of THIP alone or in combination with GABA (3μM) on acutely dissociated and cultured DRG neurons. A. concentration-response relationships for THIP on acutely dissociated (Open triangle, n=4) and cultured (circle, n=4) DRG neurons. THIP evoked little if any detectable current in acutely dissociated DRG neurons at the highest concentration tested (1 mM). The combination of THIP with GABA (3 μM, filled Circles, n = 4), resulted in a leftward shift in the THIP concentration response curve, relative to that obtained with THIP alone (open Circle, n = 4); a and b are representitive currents evoked by THIP (30 μM) and combination of THIP (30 μM) with GABA (3 μM), respectively. B. Representative current evoked in response to a 3 second application of 3 μM GABA to a cultured DRG neuron in combination with increasing concentrations of THIP. The time of GABA application is indicated by the black bar. The THIP concentration, in μM is indicated below each current trace.
Redistribution of GABA δ-subunit with time in culture
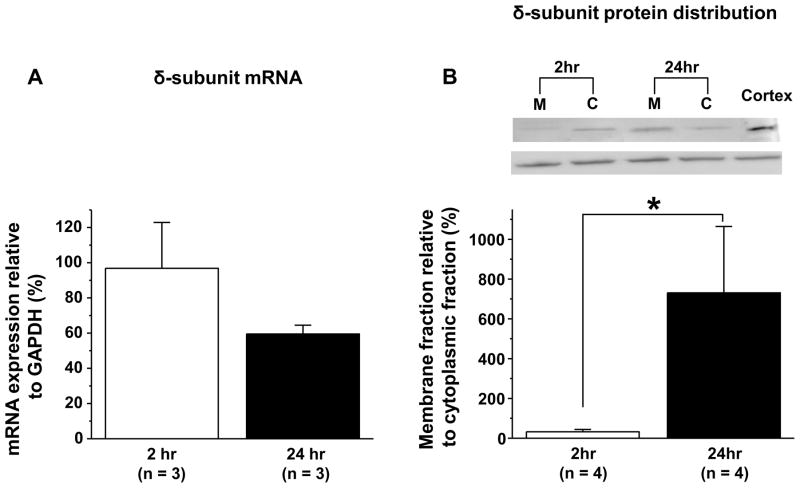
To further explore the possibility that the increase in high affinity GABA current with time in culture is due to an increase in δ-subunit containing receptors in the plasma membrane and whether this change reflects de novo expression of this subunit and/or translocation of receptors already present in acutely dissociated neurons, we performed two additional experiments. In the first, we employed sqRT-PCR to assess changes in δ subunit mRNA in acutely dissociated and cultured DRG neurons. Results from this analysis indicated that there was no detectable increase in δ-subunit expression (Figure 4A). Using the ΔΔCT method to compare the change in δ-subunit expression relative to the housekeeping gene GAPDH, the fold change at 24 hrs relative to acutely dissociated DRG was ~1 (n = 4). In the second experiment, we assessed δ-subunit like immunoreactivity levels in membrane and cytosolic fractions of the total protein harvested from acute and cultured DRG neurons. Results of this analysis revealed a shift in the distribution of the protein from cytosol at 2 hrs in culture to membrane at 24 hrs in culture (Figure 4B). This shift was significant (p < 0.01, n = 4).
Figure 4.
A change in distribution rather than a change in expression of the δ-subunit of the GABAA receptors in cultured DRG neurons. A. Semiquantitative (sq) real time PCR was used to assess δ-subunit mRNA levels in acutely dissociated and cultured DRG neurons. While expression was low, there was no detectable change in δ-subunit mRNA relative to that of the comparator GAPDH. B. Western blot analysis of cytosolic and membrane fractions of the total protein obtained from acutely dissociated and cultured DRG neurons revealed a significant (p < 0.01) increase in amount of membrane associated δ-subunit like immunoreactivity relative to that detected in the cytosol in cultured neurons. Inset, example of blot from one experiment in which half the neurons were harvested at 2hr and the other half at 24hr. Total protein from each harvest was processed in parallel to separate cytosolic and membrane fractions, and equal amounts of total protein was added to each lane as indicated. The cortex specimen in which δ-subunit is highly expressed was used to facilitate comparisons between blots and as a positive control for molecular weight of δ-subunit (50kDa) like immunoreactivity.
A ρ containing subunit may also contribute to the increase in GABA current with time in culture
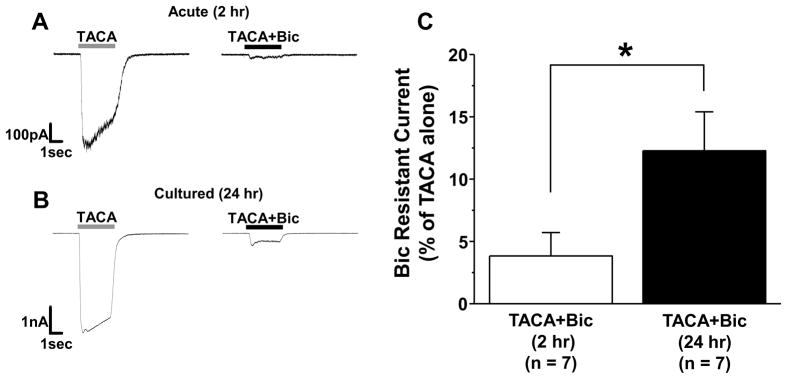
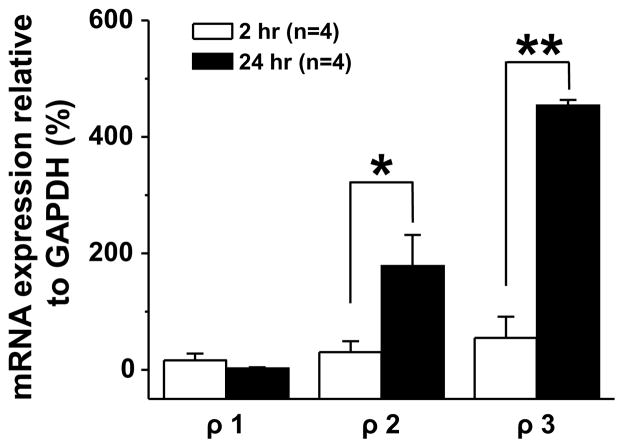
To further explore the properties of the high affinity GABA current blocked by THIP (Fig 5), we first quantified the fraction of current blocked in acute and cultured DRG neurons. THIP had no detectable influence on the response of acutely dissociated neurons to 10 μM GABA (n = 4). In contrast, in the 6 of 11 cultured neurons in which a THIP induced block of GABA current was detected, 1 μM THIP blocked 68.2 ± 9.4 % of current density evoked with 3 μM GABA (n=6). Because of the competing impact of inhibition and potentiation, only 13.4 ± 5.2 % of 3 μM GABA evoked current (n=3) was blocked by 3 μM THIP. Because of evidence that THIP is able to block ρ-subunit containing GABA receptors (Qian and Dowling, 1994) in addition to activating δ-subunit containing receptors, we explored the possibility that the THIP-blocked current was mediated by a ρ-subunit containing receptor. This involved four additional experiments. In the first, we determined the extent to which the high affinity current was blocked by bicuculline, given evidence that ρ-subunit containing GABAA receptors are resistant to this competitive antagonist (Chebib and Johnston, 1999). Cultured neurons with a bicuculline resistant current constituted a minority of DRG neurons, as bicuculline (50 μM) completely blocked GABA (3 μM) evoked current in 34 out of 50 neurons tested. The bicuculline resistant current observed in the remaining 16 neurons was 20.7 ± 5.2 % of the current evoked by GABA alone. Second, to confirm that the current blocked by THIP was resistant to bicuculline, we assessed the fraction of bicuculline resistant current evoked with 3 μM GABA that was blocked by 1 μM THIP. The results of this analysis indicated that the bicuculline resistant current was blocked by THIP (Figure 5C and D). Third, we tested the effects of a second, non-selective GABAA receptor agonist trans-4-aminocrotonic acid (TACA) on cultured DRG neurons, given evidence that this compound activates receptors with and without the ρ subunit (Johnston et al., 1975). The TACA evoked current in acutely dissociated DRG neurons was almost completely blocked by bicuculline (97.5 ± 0.03 %, n=7, Figure 6A & C). In contrast, a significantly (p < 0.05) greater fraction (12.3 ± 3.1 %, n = 7) of the TACA-evoked current in cultured DRG neurons was resistant to bicuculline (Figure 6B & C). Fourth, we performed sqRT-PCR analysis on ρ-subunit expression levels in acutely dissociated and cultured DRG neurons. With time in culture, we detected significant increases in ρ2 and ρ3 with no detectable change in ρ1 (Figure 7).
Figure 5.
The emergence of a THIP inhibited current in cultured DRG neurons. A. GABA evoked-current in an acutely dissociated (10 μM, Top Traces) and cultured (3 μM, Bottom Traces) DRG neurons without (black traces) and with (gray traces) application of THIP (1 μM). B. Pooled data, plotted as a percent of baseline (GABAalone) from acute (2hr in culture stimulated with 10 μM GABA, n = 6) and cultured (24hr in culture, stimulated with 3 μM GABA, n = 6) DRG neurons. C. Current inhibited by THIP (1 μM) is resistant to bicuculline (50 μM). Current evoked by GABA (3 μM) before (black trace) or after the application of bicuculline (50 μM, dark gray trace), or the combination of bicucullline + THIP (50 μM + 1 μM, light gray trace). D. Pooled current density data for each test condition from three cultured neurons studied with this protocol that had bicuculline resistant current. Bic is bicuculline and ** is p < 0.01.
Figure 6.
Bicuculline resistant current evoked by TACA in cultured DRG neurons. TACA- (100 μM) evoked current in an acutely dissociated (A) and cultured (B) DRG neuron before (TACA, gray horizontal bar) and after (TACA+Bic, black horizontal bar) application of bicuculline (Bic, 100 μM). C. The fraction of peak current evoked with the application of 100 μM TACA resistant to bicuculline (100 μM) was significantly (p < 0.05) greater in cultered DRG neurons (n = 7) than that in in acutely dissociated (n = 7) DRG neurons.
Figure 7.
Increase in ρ-subunit expression in cultured DRG neurons. Semiquantitative real-time PCR was used to assess ρ-subunit expression levels in acutely dissociated and cultured DRG neurons. Pooled data revealed significant increass both in ρ2 and ρ3 with no detectable change in ρ1 (n = 4 per group). * is p < 0.05 and ** is p < 0.01).
Discussion
The present experiment was designed to begin to test the hypothesis that a high affinity extrasynaptic GABAA receptor is normally targeted to central and peripheral processes of primary afferents. Toward that end, we studied acutely dissociated and cultured DRG neurons. The major observations of this study were as follows: 1) there is a dramatic increase in GABA current density with time in culture; 2) there is a dramatic leftward shift in the concentration-response curve to GABA with time in culture; 3) the high affinity GABA evoked currents observed after 24 hrs in culture are non-desensitizing and are activated by the δ-subunit containing receptor agonist THIP; 4) despite no detectable change in δ-subunit expression, there was a shift in the distribution of δ-subunit-like immunoreactivity from the cytosolic fraction at 2 hr in culture to the membrane fraction at 24 hr in culture; 5) there is an increase in bicuculline-insensitive high affinity GABA current with time in culture that is also activated by the mixed agonist TACA; 6) there is an increase in expression of ρ2- and ρ3-subunits in cultured DRG neurons. These results are consistent with our central hypothesis, but suggest that there are also cell culture-induced changes in GABA currents detectable after 24 hrs in culture.
The increase in current density, changes in biophysical and pharmacological properties of the GABA evoked currents, and δ-subunit movement from cytosol to cell membrane with time in culture, are all consistent with our initial hypothesis that high-affinity extrasynaptic GABAA receptors are normally expressed by DRG neurons but trafficked out of the cell body. Nevertheless, the dissociation process clearly involves tissue damage, which might also be the cause of the changes observed with time in culture. Previous data indicate that ATF3 mRNA, a marker for peripheral nerve injury, is detectable within hours of culturing sensory neurons (Dussor et al., 2003). Others have used longer term (days) cultures of neurons as a model for peripheral nerve injury, documenting changes in proteins, such as voltage-gated Na+ channels, in cultured neurons that are also observed following peripheral nerve injury (Fjell et al., 1999). We also obtained evidence in the present study that at least some of the increase in high affinity GABAA current observed in culture was due to an increase in the expression of ρ-subunits (see below). Preliminary analysis of ρ subunit mRNA levels in L5 ganglia harvested 7 days after L5 spinal nerve ligation revealed no significant changes in mRNA levels for any of the 3 ρ subunits (data not shown), suggesting that either the time course for the changes in ρ subunit expression are rapid and transient or that changes in ρ-subunit expression detected in culture are due to unique aspects of cell culture (such as exposure to bovine serum, etc). Nevertheless, the apparent impact of ρ-subunit containing receptors was relatively small (between 12 and 20% of the total GABAA current). Furthermore, there are a number of lines of evidence to suggest that the dissociated sensory neuron cell body is a reasonable model of the afferent terminal, given evidence that receptors, second messenger cascades and effector proteins that appear to contribute to the activation and/or sensitization of afferent terminals in situ, are present in the dissociated cell body (Spigelman et al. 2001). The results of the present study suggest that while potential changes in protein expression due to cell culture per se cannot be ignored, and that acutely cultured neurons is a viable way to mitigate the impact of injury-induced changes in afferent properties, the use of even longer term culture can be used to assess the properties of proteins not normally targeted and/or rapidly inserted in the plasma membrane of the sensory neuron cell body.
Available evidence suggests that extrasynaptic GABAA receptors are composed of two α subunits (α4 or α6) combined with two β and the δ subunit (Farrant and Nusser, 2005). The EC50 for sustained GABA currents we observed in cultured DRG neurons are remarkably similar to those reported for receptors composed of α6β3δ (0.49 μM) and of α4β3δ (2.28 μM) heterologously expressed in Xenopus oocytes (Storustovu and Ebert, 2006). Extrapolating from heterologous expression studies should always be done with caution. However, additional evidence that THIP possesses both high potency and efficacy at receptors composed of α4β3δ combination of subunits (Adkins et al., 2001, Brown et al., 2002) and that α4- subunit mRNA is detected in both rat (unpublished observation) and human DRG neurons (Maddox et al., 2004), makes it tempting to speculate that at least some of the high affinity currents described in the present study reflect activity at receptors composed of this combination of subunits. On the other hand, the observation of a leftward shift of EC50 for transient currents in a subpopulation of cultured neurons suggests that even low affinity GABAA receptors are modified with time in culture reflecting either changes in subunit assembly (Peng et al., 2002) or post-translational modifications. Additional experiments will be needed to determine why this shift in transient current only occurs in a subpopulation of DRG neurons. Nevertheless, the observation highlights yet another feature of the heterogeneity among DRG neurons.
We have suggested that at least some of the high affinity current present in cultured DRG neurons reflect activity at GABAA receptors containing a ρ-subunit based on the pharmacological properties of at least a fraction of the currents observed and the presence of ρsubunit mRNA. Inhibition by THIP and resistance to bicuculline are characteristic of ρ-subunit containing GABAA receptor currents described in previous studies (Qian and Dowling, 1994, Bormann and Feigenspan, 1995). ρ-subunit containing receptors have historically been viewed as a unique subclass of ionotropic GABA receptor because this subunit is able to form functional homomeric receptors (Zhang et al., 1995, Enz and Cutting, 1999). While there is evidence that ρ-subunit containing receptors are present in the spinal cord (Johnston et al., 1975) and cerebellum (Drew et al., 1984), they have been most extensively characterized in retina bipolar cells, where they modulate the transmission of visual signals between bipolar and ganglion cells (Ichinose and Lukasiewicz, 2002, Jones and Palmer, 2009). Our data suggesting that ρ-subunits are expressed in sensory neurons in the absence of tissue injury are consistent with previous expression data (Zheng et al., 2003). That these receptors are functional in peripheral terminals of nociceptive afferents is suggested by the observation that peripheral administration of the selective agonist CACA is antinociceptive (Reis and Duarte, 2007). However, considerably more work is needed to characterize the function of these receptors both in the presence and absence of tissue injury.
There is now extensive evidence indicating that δ-subunit containing GABAA receptors are widely expressed throughout the central nervous system (Brickley et al., 1996, Bai et al., 2001, Drasbek and Jensen, 2006, Takahashi et al., 2006) where they contribute to the persistent or tonic inhibition crucial in regulating neuronal excitability (Hausser and Clark, 1997, Hamann et al., 2002). As previously mentioned, one of the most striking ways that GABAA signaling in primary afferent neurons is distinct from that in the CNS is due to the differential regulation of Cl− which is maintained at elevated levels in sensory neurons into adulthood (Alvarez-Leefmans et al., 1988, Sung et al., 2000). The result is that GABAA receptor activation in primary afferents drives membrane depolarization, a process referred to as primary afferent depolarization (PAD), rather than membrane hyperpolarization. In the absence of tissue injury, PAD is still thought to be inhibitory as a result of depolarization-induced inactivation of voltage-gated Na+ channels and membrane shunting (Price et al., 2009). However, following tissue injury there is a shift in spinal GABA signaling, such that the GABAA receptor agonist muscimol, which is analgesic in the absence of tissue injury, exacerbates inflammatory hyperalgesia, at least at low doses, and the GABAA receptor antagonist gabazine becomes antinociceptive (Anseloni and Gold, 2008). Interestingly, benzodiazepines, thought to act at γ-subunit containing synaptic receptors, were analgesic both in the presence and absence of tissue injury (Anseloni and Gold, 2008, Knabl et al., 2008). We hypothesized that at least two changes were needed to account for these paradoxical results: a) a depolarizing shift in the Cl− equilibrium potential that was b) localized to the site of action of high affinity extrasynaptic GABAA receptors. The presence of high affinity extrasynaptic receptors in primary afferents, as suggested by the results of the present study, is consistent with this hypothesis. Additional experiments will be needed to determine whether there are localized changes in intracellular Cl− concentration in response to tissue injury.
Highlights.
GABAA currents in DRG neurons change with time in culture
High affinity “extrasynaptic” GABAA currents are increased
δ-subunit containing receptors already present are trafficked to the membrane
An increase in ρ-subunit expression and functional receptors is also detected
Acknowledgments
The authors would like to thank Yi Zhu and Dr. Steve Prescott for helpful comments during the preparation of this manuscript. This work was supported by NIH grant NS063010 (MSG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–738. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497 (Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–722. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Drew CA, Johnston GA, Weatherby RP. Bicuculline-insensitive GABA receptors: studies on the binding of (−)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984;52:317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Dussor GO, Price TJ, Flores CM. Activating transcription factor 3 mRNA is upregulated in primary cultures of trigeminal ganglion neurons. Brain Res Mol Brain Res. 2003;118:156–159. doi: 10.1016/s0169-328x(03)00335-8. [DOI] [PubMed] [Google Scholar]
- Enz R, Cutting GR. GABAC receptor rho subunits are heterogeneously expressed in the human CNS and form homo- and heterooligomers with distinct physical properties. Eur J Neurosci. 1999;11:41–50. doi: 10.1046/j.1460-9568.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res. 1999;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. GABA transporters regulate inhibition in the retina by limiting GABA(C) receptor activation. J Neurosci. 2002;22:3285–3292. doi: 10.1523/JNEUROSCI.22-08-03285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GA, Curtis DR, Beart PM, Game CJ, McCulloch RM, Twitchin B. Cis- and trans-4-aminocrotonic acid as GABA analogues of restricted conformation. J Neurochem. 1975;24:157–160. doi: 10.1111/j.1471-4159.1975.tb07642.x. [DOI] [PubMed] [Google Scholar]
- Jones SM, Palmer MJ. Activation of the tonic GABAC receptor current in retinal bipolar cell terminals by nonvesicular GABA release. J Neurophysiol. 2009;102:691–699. doi: 10.1152/jn.00285.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova I, Rausch VH, Tode J, Baron R, Janig W. Mechano- and thermosensitivity of injured muscle afferents. J Neurophysiol. 105:2058–2073. doi: 10.1152/jn.00938.2010. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Mohler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: recruitment of gamma 2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. [PubMed] [Google Scholar]
- Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol. 2006;577:169–190. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res. 2004;149:143–151. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Wafford K, Quirk K, Hadingham KL, Harley EA, Ragan CI, Whiting PJ. The pharmacology of the benzodiazepine site of the GABA-A receptor is dependent on the type of gamma-subunit present. J Recept Signal Transduct Res. 1995;15:173–183. doi: 10.3109/10799899509045215. [DOI] [PubMed] [Google Scholar]
- Menon-Johansson AS, Berrow N, Dolphin AC. G(o) transduces GABAB-receptor modulation of N-type calcium channels in cultured dorsal root ganglion neurons. Pflugers Arch. 1993;425:335–343. doi: 10.1007/BF00374184. [DOI] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Rizzo MA, Waxman SG, Kocsis JD. Differential effects of NGF and BDNF on axotomy-induced changes in GABA(A)-receptor-mediated conductance and sodium currents in cutaneous afferent neurons. J Neurophysiol. 1997;78:31–42. doi: 10.1152/jn.1997.78.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994;14:4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis GM, Duarte ID. Involvement of chloride channel coupled GABA(C) receptors in the peripheral antinociceptive effect induced by GABA(C) receptor agonist cis-4-aminocrotonic acid. Life Sci. 2007;80:1268–1273. doi: 10.1016/j.lfs.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Gold MS, Light AR. Electrophysiological Recording Techniques in Pain Research. In: Kruger L, editor. Methods in Pain Research. New York: CRC Press; 2001. pp. 147–168. [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Mashimo T, Uchida I. GABAergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport. 2006;17:1331–1335. doi: 10.1097/01.wnr.0000230515.86090.bc. [DOI] [PubMed] [Google Scholar]
- Todd AJ. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. Eur J Neurosci. 1996;8:2492–2498. doi: 10.1111/j.1460-9568.1996.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Sundberg C, Rubi B, Maechler P, Liu C, Puntel M, Lowenstein P, Castro M, Jasmin L. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol Pain. 2009;5:42. doi: 10.1186/1744-8069-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. GABAA-receptor-activated current in dorsal root ganglion neurons freshly isolated from adult rats. J Neurophysiol. 1990;64:57–63. doi: 10.1152/jn.1990.64.1.57. [DOI] [PubMed] [Google Scholar]
- Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31:8134–8142. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pan ZH, Zhang X, Brideau AD, Lipton SA. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc Natl Acad Sci U S A. 1995;92:11756–11760. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Xie W, Zhang J, Strong JA, Wang L, Yu L, Xu M, Lu L. Function of gamma-aminobutyric acid receptor/channel rho 1 subunits in spinal cord. J Biol Chem. 2003;278:48321–48329. doi: 10.1074/jbc.M307930200. [DOI] [PubMed] [Google Scholar]