Abstract
The aim of this paper is to review recent experimental and clinical publications on bone biology with respect to the optimal mechanical environment in the healing process of fractures and osteotomies. The basic postulates of bone fracture healing include static bone compression and immobilisation/fixation for three weeks and intermittent dynamic loading treatment afterwards. The optimal mechanical strain should be in the range of 100–2,000 microstrain, depending on the frequency of the strain application, type of bone and location in the bone, age and hormonal status. Higher frequency of mechanical strain application or larger number of repetition cycles result in increased bone mass at the healing fracture site, but only up to a certain limit, values beyond which no additional benefit is observed. Strain application and transition period from non-load-bearing to full load-bearing can be modified by implants allowing dynamisation of compression and generating strains at the fracture healing site in a controlled manner.
Introduction
Increasing population numbers result in larger numbers of fractures and consequently larger absolute numbers of complications. One of the most common complications of fracture healing is delayed union or non-union, resulting in prolonged treatment and severe costs [1, 2]. This is particularly frustrating in cases of elective osteotomy and/or limb lengthening. Treatment using external fixation in itself takes a long time and imposes a large physical and psychological burden upon a patient [2]. Delayed union in such circumstances additionally extends the healing time. The main goal of treatment is to shorten the bone healing period by different means, not only to provide shorter recovery for the patient but also to reduce the period of required immobilisation/fixation.
A bone is strained when subjected to external forces. Bone strains mediate an adaptive remodelling response of the bone cell population. A link between mechanical input and remodelling process is historically known as Wolff’s law of bone transformation [3–6]. The process of bone replacement and repair are going on continuously in the normal skeleton and the mechanisms involved in fracture healing are no different [7]. Historically, different modes of immobilisation/fixation have been used to enable better fracture/osteotomy (F/O) healing. It has been empirically found that no single method is suitable for all possible F/O in all bones of the body. In the clavicle it has been found that in many cases loose immobilisation with a figure-of-eight brace is sufficient. Other means of immobilisation include plaster cast, retention osteosynthesis (K-wires + plaster cast), osteosynthesis with screws plates (rigid plate, low-contact plate, locking plate), intramedullary nails and external fixators [1, 2]. Postoperative mobilisation can be done either in open kinetic chain or in closed kinetic chain tasks. Closed kinetic chain exercise may also include partial or full load-bearing [1].
The basic postulates of F/O healing include static bone compression and immobilisation/fixation with the goal of achieving sufficient stability at the F/O site [1, 2]. However, important questions regarding the optimal mechanical environment for F/O healing remain unanswered; very rigid fixation prolongs the healing time and may cause osteopenia due to excessive protection from stress and/or due to decreased bone blood perfusion [8–17]. The postoperative period is clinically guided only by loosely defined instructions of non-load-bearing, partial load-bearing and full load-bearing [18]. An important problem of load-bearing activities during rehabilitation is increased pain and poor control of actual mechanical strain produced at the F/O site [19].
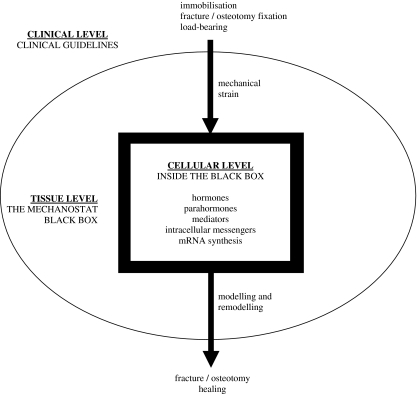
In recent decades experimental studies have contributed new knowledge about the role of mechanical loads in optimal F/O healing, but new experimental data may not be easily transferable into clinical practice. When skeletal physiology is concerned one can easily get lost in the abundance of scientific data from different observation levels. Generally there are three main observation levels: the cellular level, the tissue level and the observation of bone healing from the clinical perspective (Fig. 1). The aim of this paper is to review recent experimental and clinical publications on bone biology with the final goal of defining the optimal mechanical environment in the healing F/O process.
Fig. 1.
Schematic representation of the clinical level, the tissue level and the cellular level of skeletal mechanobiology
Bone response to mechanical stimuli at the cellular level
The idea that osteocytes act as mechanosensors has been suggested for a long time and gradually evidence for this idea has emerged [20]. Four decades ago a possible control mechanism of bone mechanobiology became apparent with the discovery of the piezoelectric effect in bone. In theory it was believed this effect could translate an environmental stimulus into a biologically recognisable signal controlling growth or resorptive processes. It was recognised that the action of the piezoelectric signal may be to alter the intracellular electrochemical environment [21]. Research in the last decades has confirmed the role of electric and electromagnetic fields in the regulation of extracellular matrix synthesis and stimulation of fracture repair [22]. Nevertheless, further research has significantly widened the spectrum of extracellular and intracellular transmitters involved in such responses and the attention has since focused more on individual mediators of mechanocellular response.
Today the research on bone response to mechanical stimuli at the cellular level features in the vast majority of publications in the field of bone mechanobiology [23]. According to the present state of knowledge, the mechanoresponsive cells in the bone include osteocytes and osteoblasts. It seems that bone cells respond to both mechanical deformation as well as fluid flow and loading in vivo always results in the combination of both physical stimuli [24].
Known mediators of mechanically induced bone formation include prostanoids PGE2 and PGI2, nitric oxide (NO) and glucose-6-phosphate dehydrogenase (G6PD) [24]. In vitro experiments on osteocytes and osteoblasts have shown NO and prostanoids increase following exposure to physiological levels of mechanical strain and fluid flow. Extracellular and intracellular signalling components involved in mechanotransduction include integrins, cytoskeleton, ion channels, hormone receptors and various signal transduction pathways, such as the mitogen-activated protein kinase (MAPK), the phosphoinositide 3-kinase (PI3K), the protein kinase A (PKA) and protein kinase C (PKC) pathways. Activation of these pathways leads to changes in gene expression regulating cell division and matrix synthesis [25].
On the hormonal level it has been shown that an early response of bone cells to mechanical loading stimuli results in the release of insulin-like growth factors (IGF) and that parathyroid hormone is also involved in adaptive bone remodelling due to mechanical stimuli [24]. It has also been found that reduced ability to maintain bone strength in postmenopausal women is a failure of the normal adaptive response to mechanical strain due to lower oestrogen concentrations and downregulation of oestrogen receptor alpha (ERα) expression in bone cells of postmenopausal women [26]. Bone in vivo undergoes an adaptive response to loading that is less effective in the absence of ERα and osteoblast-like cells require ERα to proliferate in response to mechanical strain in vitro. As ERα expression in osteoblasts and osteocytes depends on oestrogen concentration [27], a failure to maintain bone strength after menopause might be due to a reduction in the activity of ERα in bone cells, thereby limiting their anabolic response to mechanical loading and allowing a loss of bone tissue comparable to that associated with disuse [26].
Most recently attention has also turned to the bone cell surface receptor, low-density lipoprotein receptor-related protein 5 (LRP5) as one of the potential key regulators of bone mass [28]. While experimental data show the presence of certain LRP5 mutations is associated with different osteogenic response to loading, there is only a marginal gender-related role for normal LRP5 function in this loading-related response [29].
The review of present knowledge in the field of bone cellular response to mechanical stimuli thus shows a very complex picture. It has become clear that the bone cell response to mechanical strain cannot be entirely explained by a single mechanism. The knowledge gained gives a broad potential for future development of clinically effective modulators of osteogenic loading-related response. Nevertheless, so far detailed understanding of cellular mechanisms has not significantly affected standard clinical regimens of post F/O mechanical loading.
Tissue level mechanisms of bone adaptation to mechanical stimuli
While research at the cellular level is most abundant in the published literature, only understanding of the processes at the tissue level can bridge the knowledge gap between cellular mechanisms of F/O and empirical clinical findings.
Frost has reviewed the tissue-level mechanisms, embodied in the mechanostat theory and the “Utah paradigm” of skeletal physiology [23]. This paradigm explains that bone properties are based on genetically determined baseline conditions of bones that may later in life be subjected to different levels of loading. The paradigm acknowledges two basic tissue-level mechanisms: modelling by formation and resorption drifts can increase/decrease the strength of a particular bone and remodelling by basic multicellular units that turns bone over in small packets. Both tissue-level mechanisms act through the same set of cells and cellular mechanisms [30]. Genetically determined threshold ranges of these signals control both mechanisms: when bone strains exceed a certain threshold, modelling is switched on to strengthen the particular bone; when bone strains stay below a lower threshold range, remodelling can overpower modelling and the net result is reduced whole-bone strength.
The role of mechanical stimuli and strain during the initial callus formation is not clear, but empirical data show that maximal possible rigidity at the F/O site is desirable until mineralised callus is formed. Once the mineralised callus is formed and the modelling/remodelling phase has begun, mechanical strains can influence the remodelling and modelling phases of bone healing. With hard callus the process of disuse atrophy may at some point prevail over the healing activity if there is not sufficient mechanical strain present [23]. Preponderance of experimental evidence shows the strain should be in the range of 100–2,000 microstrain, depending on the age, hormonal status, type of bone and location in the bone and a particular strain/stress pattern over certain period of time (i.e. strain history rate) [31, 32]. Strain history rate can be considered as either time averaged strain values, peak strain values, amplitudes of oscillatory strain components, strain values causing microdamage or time rates of strain change.
In vivo measurements of bone strain in several studies on both animals and humans have been reviewed by Fritton and Rubin [33]. It was found that during normal walking the bones experience strains in the range of 1,000 microstrain, but during more vigorous exercise the strains can increase up to 3,000 microstrain. On the other hand, even strains as low as two orders of magnitude below physiological levels can stimulate large increases in bone mass if presented at 30 Hz [34–36]. Even a magnitude of less than 10 microstrain with high frequency biomechanical intervention can prevent bone loss associated with disuse and improve the bone mass [35, 36].
In animal experiments net bone gain was observed when controlled, sinusoidally varying loading was applied to mature cortical bone [6, 37–44]. Remarkably few loading cycles, i.e. 24–100 cycles per day over four to eight week periods, with frequencies in the range of 0.5–1 Hz resulted in up to 24% increase of bone cross-sectional area and up to 45% increase of bone mineral content [37–44].
Neither the size nor the bone composition were affected by any additional increase in the number of load cycles from 36 to 1,800 [41] or by prolonging the loading time from 100 s to 25 min [44]. In an eight week period of controlled dynamic loading, bone loss was observed with peak longitudinal strains below 0.001, whereas peak longitudinal strains above 0.001 were associated with substantial surface new bone formation [43].
Direct comparison of static and controlled cyclic loads with similar magnitude showed a 13% decrease of bone cross-sectional area and an increased porosity for static loads, while 24% increase in cross-sectional area for sinusoidally varying loads applied for 15 min in a day for eight weeks was observed [42]. Long bone fracture treatment for six weeks in a rabbit by constant compression (80 N) was compared to 8 N constant compression superimposed by 40 N cyclic loading treatment by machine [45, 46]. Union strength was evaluated with a torque test and energy absorption to failure test. During the first three weeks stronger union was produced by constant compression treatment, but later on cyclic loading resulted in stronger union [45, 46]. It was estimated that with the cyclic loading type of treatment 27% of the healing time was saved in comparison to constant compression [45, 46].
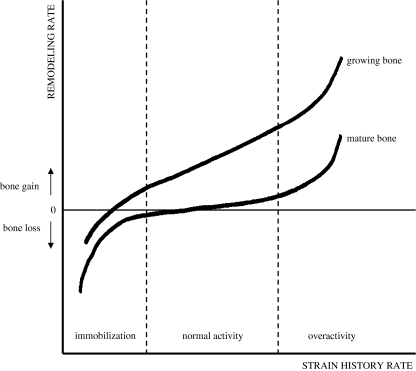
The influence of strain history rate on remodelling rate at the tissue level has thus been investigated in several clinical and laboratory studies [24]. The point of common agreement is that dynamic loads induce higher remodelling rates than static loads [37–44]. The absence of any apparent sensitivity to static strain is consistent with the absence of any natural requirement for the skeleton to adapt to static loads. Excessive strain due to premature acting of external forces (load-bearing and/or weight-bearing) can endanger the fracture healing process because of macroscopic movement of bone fragments or even refracture of the hard callus. On the other hand, insufficient strains could cause remodelling mechanisms to prevail over the modelling drifts and the net result would be removal of callus with delayed or failed bone healing [23]. Mechanical strain should be in the range of 100–2,000 microstrain and it shows saturation kinetics as no more than 100 strain repetitions a day are sufficient to achieve the maximal healing potential of the tissue (Fig. 2).
Fig. 2.
Strain history rate and remodelling of mature and growing bone. Adapted from [47]
Clinical guidelines on optimal fracture/osteotomy treatment
Clinical guidelines on optimal F/O healing depend on several mechanical factors that can influence the F/O healing process: the fracture geometry, the type of fracture, the fracture gap and the magnitude, direction and history of the interfragmentary movement [1, 2, 18]. All of these global factors determine the local strain distribution and thereby provide the mechanobiological signals for the repair processes and the cellular reactions [25]. The strains are achieved by active load-bearing of bones under supervised conditions. The problem lies in the factors that influence these strain magnitudes: the age and agility of the patient, patients’ compliance, stability of immobilisation and the cumulative overall daily load-bearing activity [1]. With the maximum loads achieved the ideal immobilisation/fixation method should allow sufficient strain magnitudes without compromising the stability.
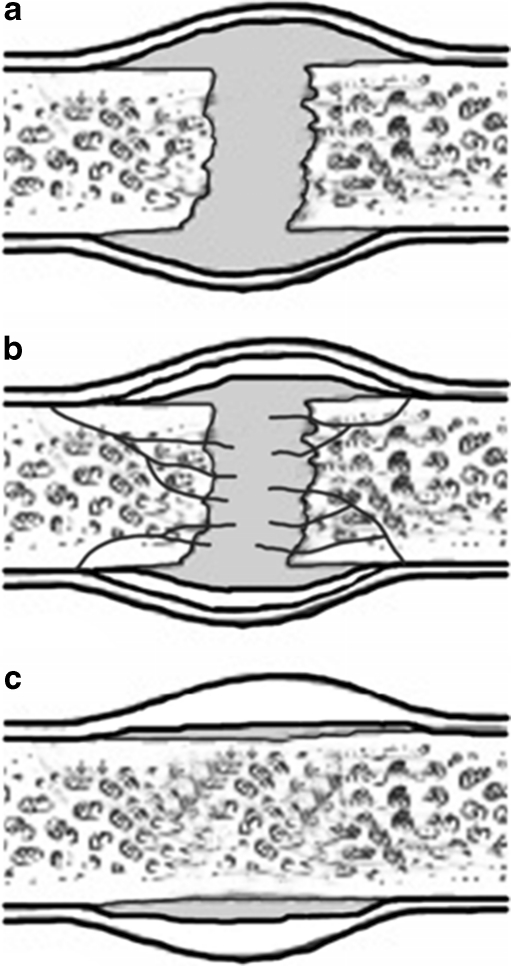
The solidity of the healed F/O must be sufficient not to endanger the fracture site stability by action of external forces. In this regard rigid compression is considered ideal for the initial phases of F/O healing (Fig. 3) until calcified, i.e. “hard” callus is formed [2]. Later, gradually larger amplitudes of load-bearing are allowed as it is generally believed that dynamisation promotes better healing [1, 2, 18]. It could not be proven that dynamisation accelerated the healing process. Nevertheless, with dynamised intramedullary fixation there was more impaction and significantly reduced gap between the fragments. Therefore, clinically the dynamisation effect may influence both the residual gap reduction and bone remodelling but it does not shorten the healing time itself [48].
Fig. 3.
Initial phases of fracture/osteotomy healing include [1]: a Inflammatory phase (0–3 days after the injury) with fracture haematoma, inflammatory response, granulation tissue formation. b Reparative phase (4 days to months after the injury) with angiogenesis, soft callus, lamellar bone deposition and hard callus formation. c Remodelling phase (months to years after the injury) with changes of the bone shape
Experimental data has also focused on the question of whether vibrations applied at very low magnitudes may be sensed directly by transmission of the signal through the skeleton or whether muscle activity modulates, and perhaps amplifies, the externally applied mechanical stimulus [49]. Current data indicate that the anabolic and anti-catabolic effects of whole-body vibrations on the skeleton are unlikely to require muscular activity to become effective. Even high-frequency signals that induce bone matrix deformations less than 5 microstrain can promote bone formation in the absence of muscular activity [49].
All clinical guidelines on F/O healing therefore at some point in time allow partial and later full load-bearing [18]. There exist general recommendations depending on the type of fracture, the particular bone location and the healing process as seen by the imaging studies [1, 2]. However, there is no reliable device to guide and/or apply the amount of optimal mechanical strain in each particular patient at a given point in time.
Conclusions
Experimental data show that once the calcified callus is formed the process of disuse atrophy may at some point prevail despite the healing activity if there is not sufficient mechanical strain present. The optimal mechanical strain should be in the range of 100–2,000 microstrain, depending on the frequency of the strain application, type of bone and location in the bone, age and hormonal status. Higher frequency of mechanical strain application or larger number of repetition cycles result in increased bone mass at the healing fracture site, but only up to certain limit, values beyond which no additional benefit is observed. Thus, in animal experiments a combination of constant compression treatment in the first three weeks with an intermittent dynamic loading treatment of long bone F/O later on appears to be a quicker and better treatment option than static compression alone. Stability of immobilisation and sufficient compression remain the predisposing factors for the healing process to start successfully. However, in the future the transition period from non-load-bearing to full load-bearing could be better guided with implants allowing controlled dynamisation of compression in terms of frequency and stress magnitude.
Scientific research has not yet given a definite answer on whether absolute parameters of strain frequencies/magnitudes exist for all patients. Exact values have not and might never be determined in exact absolute numbers, and these parameters may have to be titrated in every individual patient according to some objective criteria of healing progress. It would be desirable to have implants that allow controlled dynamisation in terms of frequency and stress magnitude. Such a device could act as an embedded adjunct to presently known implants and generate strains at the F/O healing site in a controlled manner in the course of fracture healing. In addition, it could also measure the bone elastic modulus and response to given mechanical stimuli in order to provide biomechanical feedback (and not only morphological imaging feedback) in the process of F/O healing. Proper design, clinical benefit and cost-effectiveness of such devices has yet to be determined.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Wood GW. Fractures and dislocation, general principles of fracture treatment. In: Canale ST, Beaty JH, editors. Campbell’s operative orthopaedics. 11. Philadelphia: Mosby; 2007. pp. 3018–3085. [Google Scholar]
- 2.Rüedi TP, Buckley RE, Moran CG. AO principles of fracture management. New York: Thieme; 2007. [Google Scholar]
- 3.Wolff J. The classic: on the significance of the architecture of the spongy substance for the question of bone growth: a preliminary publication. 1869. Clin Orthop Relat Res. 2011;469:3077–3078. doi: 10.1007/s11999-011-2041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanyon LE. Experimental support for the trajectorial theory of bone structure. J Bone Joint Surg Br. 1974;56:160–166. [PubMed] [Google Scholar]
- 5.Lanyon LE, Baggott DG. Mechanical function as an influence on the structure and form of bone. J Bone Joint Surg Br. 1976;58-B:436–443. doi: 10.1302/0301-620X.58B4.1018029. [DOI] [PubMed] [Google Scholar]
- 6.Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, Akeson WH. The effect of prolonged physical training on the properties of long bone: a study of Wolff’s Law. J Bone Joint Surg Am. 1981;63:780–787. [PubMed] [Google Scholar]
- 7.McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60-B:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 8.Baggott DG, Goodship AE, Lanyon LE. A quantitative assessment of compression plate fixation in vivo: an experimental study using the sheep radius. J Biomech. 1981;14:701–711. doi: 10.1016/0021-9290(81)90053-1. [DOI] [PubMed] [Google Scholar]
- 9.Chao EY, Kasman RA, An KN. Rigidity and stress analyses of external fracture fixation devices–a theoretical approach. J Biomech. 1982;15:971–983. doi: 10.1016/0021-9290(82)90015-X. [DOI] [PubMed] [Google Scholar]
- 10.Lewallen DG, Chao EY, Kasman RA, Kelly PJ. Comparison of the effects of compression plates and external fixators on early bone-healing. J Bone Joint Surg Am. 1984;66:1084–1091. [PubMed] [Google Scholar]
- 11.Terjesen T. Bone healing after metal plate fixation and external fixation of the osteotomized rabbit tibia. Acta Orthop Scand. 1984;55:69–77. doi: 10.3109/17453678408992316. [DOI] [PubMed] [Google Scholar]
- 12.Cheal EJ, Hayes WC, White AA, 3rd, Perren SM. Stress analysis of compression plate fixation and its effects on long bone remodeling. J Biomech. 1985;18:141–150. doi: 10.1016/0021-9290(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 13.Court-Brown CM. The effect of external skeletal fixation on bone healing and bone blood supply. An experimental study. Clin Orthop Relat Res. 1985;201:278–289. [PubMed] [Google Scholar]
- 14.Hart MB, Wu JJ, Chao EY, Kelly PJ. External skeletal fixation of canine tibial osteotomies. Compression compared with no compression. J Bone Joint Surg Am. 1985;67:598–605. [PubMed] [Google Scholar]
- 15.Holmström T, Paavolainen P, Slätis P, Karaharju E. Effect of compression on fracture healing. Plate fixation studied in rabbits. Acta Orthop Scand. 1986;57:368–372. doi: 10.3109/17453678608994414. [DOI] [PubMed] [Google Scholar]
- 16.Bastiani G, Aldegheri R, Renzi Brivio L. Dynamic axial fixation. A rational alternative for the external fixation of fractures. Int Orthop. 1986;10:95–99. doi: 10.1007/BF00267748. [DOI] [PubMed] [Google Scholar]
- 17.Aalto K, Holmström T, Karaharju E, Joukainen J, Paavolainen P, Slätis P. Fracture repair during external fixation. Torsion tests of rabbit osteotomies. Acta Orthop Scand. 1987;58:66–70. doi: 10.3109/17453678709146345. [DOI] [PubMed] [Google Scholar]
- 18.Kunnamo I. Evidence-based medicine guidelines. Chichester: Wiley; 2005. [Google Scholar]
- 19.Handoll HH, Parker MJ, Sherrington C (2003) Mobilisation strategies after hip fracture surgery in adults. Cochrane Database Syst Rev 1:CD001704 [DOI] [PubMed]
- 20.Skerry TM. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys. 2008;473:117–123. doi: 10.1016/j.abb.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Marino AA, Becker RO. Piezoelectric effect and growth control in bone. Nature. 1970;228:473–474. doi: 10.1038/228473a0. [DOI] [PubMed] [Google Scholar]
- 22.Ciombor DM, Aaron RK. The role of electrical stimulation in bone repair. Foot Ankle Clin. 2005;10:579–593. doi: 10.1016/j.fcl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Frost HM. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004;74:3–15. doi: 10.1043/0003-3219(2004)074<0003:AUOBPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 25.Liedert A, Kaspar D, Augat P, Ignatius A, Claes L. Mechanobiology of bone tissue and bone cells. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in cells and tissues. Moscow: Academia; 2005. [PubMed] [Google Scholar]
- 26.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland MK, Hui DU, Rao LG, Wylie JN, Murray TM. Immunohistochemical localization of the estrogen receptor in human osteoblastic SaOS-2 cells: association of receptor levels with alkaline phosphatase activity. Bone. 1996;18:361–369. doi: 10.1016/8756-3282(96)00016-6. [DOI] [PubMed] [Google Scholar]
- 28.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 29.Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V high bone mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49:184–193. doi: 10.1016/j.bone.2011.03.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jee WSS. Integrated bone tissue physiology: anatomy and physiology. In: Cowin SC, editor. Bone mechanics handbook. 2. Boca Raton: CRC; 2001. pp. 1–68. [Google Scholar]
- 31.Meyer U, Meyer T, Wiesmann HP. The effect of magnitude and frequency of interfragmentary strain on the tissue response to distraction osteogenesis. J Oral Maxillofac Surg. 1999;57:1331–1339. doi: 10.1016/S0278-2391(99)90872-8. [DOI] [PubMed] [Google Scholar]
- 32.Frost HM, Meyer U, Joos U, Jensen OT. Dental alveolar distraction osteogenesis and the Utah paradigm. In: Jensen OT, editor. Alveolar distraction osteogenesis. Carol Stream: Quintessence; 2002. pp. 1–16. [Google Scholar]
- 33.Fritton SP, Rubin CT. In vivo measurement of bone deformations using strain gauges. In: Cowin SC, editor. Bone mechanics handbook. 2. Boca Raton: CRC; 2001. pp. 8–34. [Google Scholar]
- 34.Rubin CT, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 35.Rubin CT, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 36.Rubin CT, Turner AS, Müller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 37.Churches AE, Howlett CR, Waldron KJ, Ward GW. The response of living bone to controlled time-varying loading: method and preliminary results. J Biomech. 1979;12:35–45. doi: 10.1016/0021-9290(79)90007-1. [DOI] [PubMed] [Google Scholar]
- 38.Lanyon LE, Paul IL, Rubin CT, Thrasher EL, DeLaura R, Rose RM, Radin EL. In vivo strain measurements from bone and prosthesis following total hip replacement. An experimental study in sheep. J Bone Joint Surg Am. 1981;63:989–1001. [PubMed] [Google Scholar]
- 39.Churches AE, Howlett CR. Functional adaptation of bone in response to sinusoidally varying controlled compressive loading of the ovine metacarpus. Clin Orthop Relat Res. 1982;168:265–280. [PubMed] [Google Scholar]
- 40.O’Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodelling. J Biomech. 1982;15:767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 41.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 42.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 43.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 44.Lanyon LE, Rubin CT, Baust G. Modulation of bone loss during calcium insufficiency by controlled dynamic loading. Calcif Tissue Int. 1986;38:209–216. doi: 10.1007/BF02556712. [DOI] [PubMed] [Google Scholar]
- 45.Panjabi MM, White AA, 3rd, Wolf JW., Jr A biomechanical comparison of the effects of constant and cyclic compression on fracture healing in rabbit long bones. Acta Orthop Scand. 1979;50:653–661. doi: 10.3109/17453677908991288. [DOI] [PubMed] [Google Scholar]
- 46.Wolf JW, Jr, White AA, 3rd, Panjabi MM, Southwick WO. Comparison of cyclic loading versus constant compression in the treatment of long-bone fractures in rabbits. J Bone Joint Surg Am. 1981;63:805–810. [PubMed] [Google Scholar]
- 47.Carter DR. Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int. 1984;36(Suppl):S19–S24. doi: 10.1007/BF02406129. [DOI] [PubMed] [Google Scholar]
- 48.Kempf I, Leung K, Grosse A. Practice of intramedullary locked nails: scientific basis and standard techniques. Berlin: Springer; 2002. pp. 47–48. [Google Scholar]
- 49.Judex S, Rubin CT. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact. 2010;10:3–11. [PMC free article] [PubMed] [Google Scholar]