Coral reefs are often referred to as the rainforests of the sea, although, as Davidson (1) points out, one could equally well call rainforests the coral reefs of the land. Regardless, these two ecosystems do indeed share several important attributes, most notably high diversity and severe declines worldwide over the last several decades. There are many differences, however, between reefs and rainforests, including the nature of the threats that they face. Whereas the trees that underpin rainforest ecosystems are largely lost by the direct activities of people (harvesting or land clearing), corals are declining through a far more diverse and less well understood concatenation of events and processes. Studies of the reefs of the north coast of Jamaica have provided scientists with some of the clearest examples of reef fragility and apparent collapse (2, 3), and consequently they have become a textbook case of environmental doom and gloom. In a paper by Edmunds and Carpenter (4) in this issue of PNAS, Jamaican reefs again play a central role, but for the first time in recent memory, reef recovery rather than continued decline seems at least possible. Their findings confirm the central importance of herbivory for the maintenance of healthy coral reefs, and provide surprising but encouraging evidence that formerly abundant organisms can linger at extremely low densities for nearly two decades, and then rebound.
Modern studies of Jamaican reefs began in the 1950s (5), and in the 1970s Jamaican reefs still appeared to be flourishing, despite the severe overfishing that almost certainly began decades to centuries earlier (6). Scientists working on these reefs at the time, myself included, acknowledged the absence of large fish, but never seriously considered the possibility that the corals themselves were potentially at risk. The first glimpse of possible vulnerability came with Hurricane Allen in 1980, which caused extensive damage at shallow to intermediate depths (7). The rapidly growing staghorn corals (Acropora cervicornis) that formerly dominated many reefs failed to recover as expected (8), in part because of the concentration of predators on survivors, and in part because of a disease that has decimated both A. cervicornis and its congener A. palmata. This disease (9), however, moved comparatively slowly—only recently has it been viewed as a major influence without apparent precedent in the last several thousand years (10, 11). In contrast, when disease struck the long-spined sea urchin Diadema antillarum in 1983, it did so with such speed, geographic scope, and lethality that it was immediately recognized for what it was—a biological disturbance of unprecedented scale with potentially enormous ecological effects (12).
By February 1984, the sea urchin had been virtually eliminated from all of its range, making this the most extensive and severe mass mortality ever reported for a marine organism.
Much of what we know about this extraordinary event comes from the efforts of Lessios (12–14), who discovered urchins dying en masse near the Atlantic outlet of the Panama Canal in January 1983. Seeing its significance, he alerted reef scientists scattered across the region (in the days before email) so that they would be ready for it should the mortality spread. And spread it did, at first slowly (only reaching Costa Rica to the west and Colombia to the east in June), but then with greater speed, reaching Bermuda in September (12). By February 1984, D. antillarum had been virtually eliminated from all of its range, with the exception of populations in the eastern Atlantic, making this the most extensive and severe mass mortality ever reported for a marine organism. Populations were reduced to at least 7% and often to less than 2% of their former numbers within a few days of the onset of symptoms; on reefs that used to be black with urchins (reported densities of up to 71 per m2), one could swim for an hour without seeing a single living individual (13). The speed with which this mortality occurred is part of the reason why, to this day, we still do not know the agent responsible, although the pattern of spread (largely following currents) and specificity (no other urchin was affected) make a pathogen a near certainty. (One cannot help but wonder how the Centers for Disease Control could cope with a comparably contagious and lethal pathogen carried by airplanes instead of currents.)
In natural experiments of this type, there are no true replicates, but the consistency of ecological responses around the region leaves little doubt that D. antillarum was a keystone species at the time. Indeed, the temporal link between the mass mortality and algal overgrowth provides some of the best evidence for the primarily top-down (herbivory) rather than bottom-up (nutrients) control of algal abundance on Caribbean reefs (ref. 15, but see ref. 16). The effects were most severe where overfishing had reduced numbers and sizes of herbivorous fishes (13), a pattern pointing to the importance of ecological redundancy for ecosystem health. In Jamaica, for example, enormous blooms of quickly colonizing seaweeds covered all dead substrates (which were still extensive on Jamaican reefs because of Hurricane Allen). These algae were later replaced by larger, longer living, less palatable algae (e.g., as in Fig. 1a), probably because of feeding preferences of fish grazers that, although reduced in numbers by overfishing, are nevertheless present and typically more selective than Diadema (17). Along the north coast of Jamaica, coral cover dropped from an average of 52% to 3% between 1977 and 1993, with seaweed abundance over the same period increasing from 4 to 92% (2). On less overfished reefs, the transition from coral to algal dominance has come later, sometimes suddenly in the context of other disturbances (18).
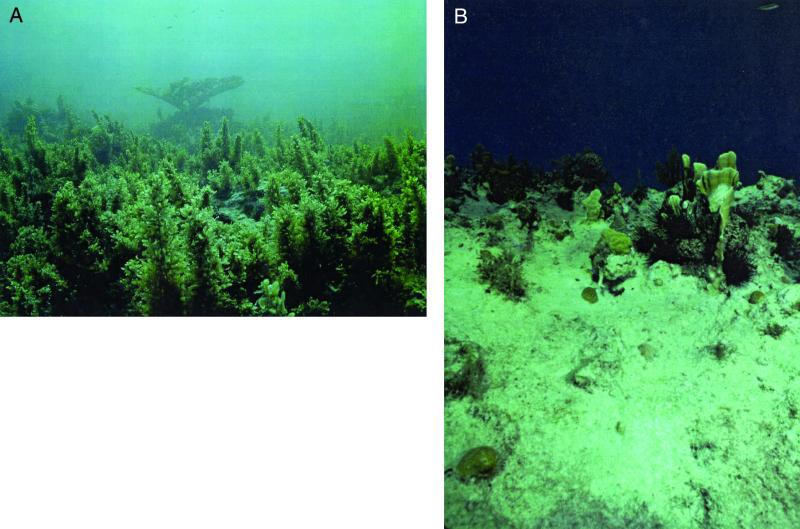
Figure 1.
Comparison of algal zone (A) and sea urchin zone (B). Edmunds and Carpenter (4) document the transition from the former to the latter at five sites along the north coast of Jamaica. Note abundance of the seaweed Sargassum in (A), and the presence of the sea urchin Diadema and coral recruits in (B). Photograph credits: (A) Robert C. Carpenter; (B) Peter J. Edmunds.
What was not anticipated by scientists was the very long-term failure of Diadema to recover because, like many sea urchins, it is highly fecund [12 million eggs annually (14)]. Graphs of the numbers and densities of Diadema through 1993 (14) look like an electrocardiogram of a patient in cardiac arrest (as indeed the reefs by then appeared to be in many places). Recently, however, there have been anecdotal observations of patches of Diadema in shallow water around the Caribbean and hints in Jamaica itself (4, 19) that recovery was possibly near at hand. What makes the paper by Edmunds and Carpenter important is that it documents what appears to be the beginning of a sustained recovery, not only for the urchins but also for the corals themselves. Although coral cover still remains low within zones of high urchin density, recruitment of corals [which had fallen to near zero on Jamaican reefs by 1993 (3)] is near levels not seen since the late 1970s (note recruits in Fig. 1b). Most importantly, this recruitment includes not only juveniles of weedy corals (that often recruit well under marginal circumstances), but also juveniles of major reef builders [e.g., Acropora and Montastraea, corals whose recruitment is typically sporadic and low even on healthy reefs (3)].
This development is good news for coral reefs regionally, but it also poses many general questions about when and how reefs (and other ecosystems) recover from massive disturbances. The transition of reefs from coral-dominated to algal-dominated ecosystems has been cast as an example of a transition between alternate stable states (18, 20–22), but, as Edmunds and Carpenter (4) note, the reverse transition from algal- to coral-dominated reefs has not been previously reported. The reality of alternate states has been much debated in the literature, and experimental tests are hard to come by because the temporal and spatial scales required for definitive answers are so large (20, 23). Regardless of where one comes down on the contentious issue of stability, however, feedback loops that tend to keep a system where it is, rather than moving to another state, can play critical roles in any recovery process.
Several such feedback loops appear to have operated in the context of the Diadema collapse. The first are Allee effects, that is, a threshold in numbers below which rates of population growth become negative rather than positive (24). Allee effects are inevitable in all organisms like Diadema that cannot self-fertilize and must spawn their gametes into the water; sperm swim well for their size, but the absolute distances they can travel are limited, so that eggs of isolated individuals remain unfertilized (25). Part of the failure of Diadema to recover almost certainly stems from this phenomenon; as Lessios reviews (13), recruitment typically continued after the mass mortality until upstream populations were themselves decimated, at which point it ceased. However, if this were the only process operating, it is hard to understand why Diadema continued to hover on the knife edge of very low densities for so long, rather than continuing to decline after 1984.
One possible explanation is that, somewhere, pockets of sufficient density continued to seed other areas with recruits, but that additional feedback loops continued to inhibit recovery. For example, Diadema juveniles appear to favor areas that are moderately well grazed (26), and macroalgal dominance resulting from the Diadema dieoff (especially in areas of overfishing) could have produced conditions that were unfavorable to recruitment. This idea is consistent with Lessios's finding that the sea urchin Echinometra (a potential competitor of Diadema as an adult) facilitated recruitment by juvenile Diadema, although why experimental manipulations of adult Diadema had no effect remains a mystery. It is also possible that sporadic outbreaks of disease among survivors regularly set back populations that were otherwise rebounding (27). Alternatively, an undetectably slow recovery of Diadema across the Caribbean has been underway, but was accelerated on the north coast of Jamaica by a pulse of recruitment because of some unknown coincidence of physically or biologically favorable conditions.
The reasons underlying this apparent recovery of Diadema, documented at five sites spanning 8 km of coastline (4), will probably always be unclear, but, by publishing these data now, Edmunds and Carpenter give reef scientists the time to monitor and experiment in ways that are likely to increase our understanding of the recovery process. Will recruitment success spread rapidly across the Caribbean, and will it follow the paths of currents (as the apparent pathogen did)? Can recruitment be facilitated locally by algal removal or removal of potential predators on juvenile urchins (which, possibly significantly, are nearly absent because of overfishing on the north coast of Jamaica)? Will, indeed, this apparent recovery persist at all, much less spread?
Apart from the intrinsic scientific interest of these questions, one has to hope that the answer to this last question is yes, because algal overgrowth is hardly the only threat that these reefs face. All reef growth is a balance between growth and death, so that any increase in the former increases the future ability (28) of Caribbean reefs to cope with threats that still remain. These threats include other diseases, bleaching associated with global warming, the direct effects of elevated CO2, and declines in water quality associated with detrimental land use policies, any of which may ultimately prove to be catastrophic. Nevertheless, the report by Edmunds and Carpenter (4) is the best news to emerge from Caribbean reefs in decades, and any good news is welcome indeed.
Footnotes
See companion article on page 5067.
References
- 1.Davidson O G. The Enchanted Braid. New York: Wiley; 1998. [Google Scholar]
- 2.Hughes T P. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 3.Hughes T P, Tanner J E. Ecology. 2000;81:2250–2263. [Google Scholar]
- 4.Edmunds P J, Carpenter R C. Proc Natl Acad Sci USA. 2001;98:5067–5071. doi: 10.1073/pnas.071524598. . (First Published March 27, 2001; 10.1073/pnas.071524598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goreau T F. Ecology. 1959;40:67–89. [Google Scholar]
- 6.Jackson J B C. Coral Reefs. 1997;16,Suppl.:S23–S32. [Google Scholar]
- 7.Woodley J D, Chornesky E A, Clifford P A, Jackson J B C, Kaufman L S, Knowlton N, Lang J C, Pearson M P, Porter J W, Rooney M C, et al. Science. 1981;214:749–755. doi: 10.1126/science.214.4522.749. [DOI] [PubMed] [Google Scholar]
- 8.Knowlton N, Lang J C, Rooney M C, Clifford P. Nature (London) 1981;294:251–252. [Google Scholar]
- 9.Richardson L L. Trends Ecol Evol. 1998;13:438–443. doi: 10.1016/s0169-5347(98)01460-8. [DOI] [PubMed] [Google Scholar]
- 10.Aronson R B, Precht W F, Macintyre I G. Coral Reefs. 1998;17:223–230. [Google Scholar]
- 11.Greenstein B J, Curran H A, Pandolfi J M. Coral Reefs. 1998;17:249–261. [Google Scholar]
- 12.Lessios H A, Robertson D R, Cubit J D. Science. 1984;226:335–337. doi: 10.1126/science.226.4672.335. [DOI] [PubMed] [Google Scholar]
- 13.Lessios H A. Annu Rev Ecol Syst. 1988;19:371–393. [Google Scholar]
- 14.Lessios H A. Proc R Soc London B. 1995;259:331–337. [Google Scholar]
- 15.Hughes T P, Szmant A M, Steneck R, Carpenter R, Miller S. Limnol Oceanogr. 1999;44:1583–1586. [Google Scholar]
- 16.Lapointe B E. Limnol Oceanogr. 1999;44:1586–1592. [Google Scholar]
- 17.Morrison D. Ecology. 1988;69:1367–1382. [Google Scholar]
- 18.Ostrander G K, Armstrong K M, Knobbe E T, Gerace D, Scully E P. Proc Natl Acad Sci USA. 2000;97:5297–5302. doi: 10.1073/pnas.090104897. . (First Published May 2, 2000; 10.1073/pnas.090104897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aronson R B, Precht W F. Limnol Oceanogr. 2000;45:251–255. [Google Scholar]
- 20.Knowlton N. Am Zool. 1992;32:674–682. [Google Scholar]
- 21.Done T J. Hydrobiologia. 1992;247:121–132. [Google Scholar]
- 22.Nyström M, Folke C, Moberg F. Trends Ecol Evol. 2000;15:413–417. doi: 10.1016/s0169-5347(00)01948-0. [DOI] [PubMed] [Google Scholar]
- 23.Petraitis P S, Latham R E. Ecology. 1999;80:429–442. [Google Scholar]
- 24.Courchamp F, Clutton-Brock T, Grenfell B. Trends Ecol Evol. 1999;14:405–410. doi: 10.1016/s0169-5347(99)01683-3. [DOI] [PubMed] [Google Scholar]
- 25.Levitan D R, Petersen C. Trends Ecol Evol. 1995;10:228–231. doi: 10.1016/S0169-5347(00)89071-0. [DOI] [PubMed] [Google Scholar]
- 26.Bak R P M. Proc 5th Intl Coral Reef Congr. 1985;5:267–272. [Google Scholar]
- 27.Forcucci D. Bull Mar Sci. 1994;54:917–928. [Google Scholar]
- 28.Knowlton, N. (2001) Proc. Natl. Acad. Sci. USA98, in press.