Abstract
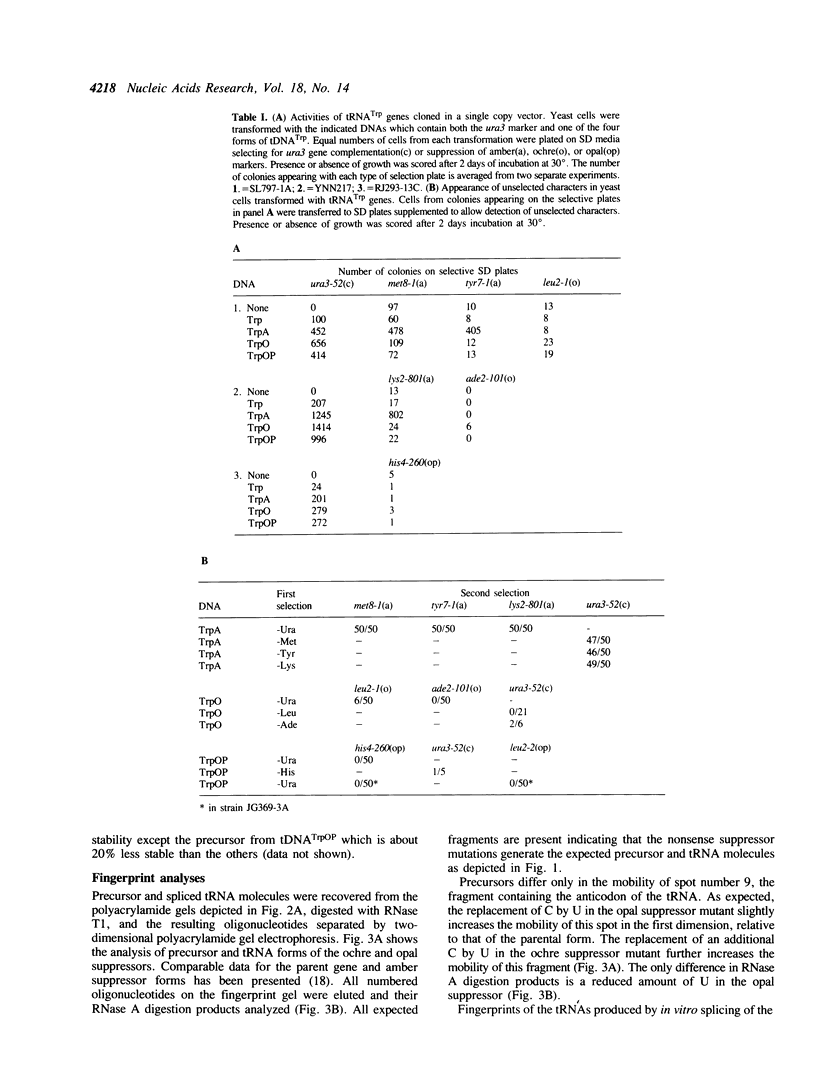
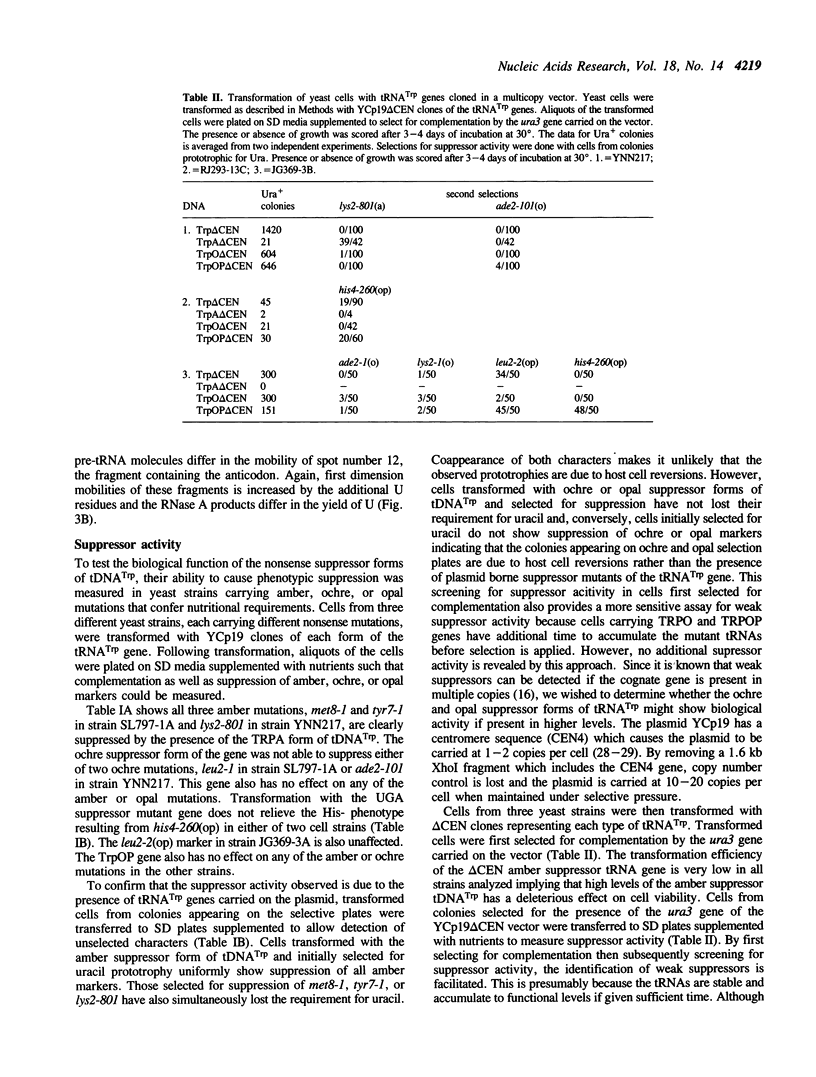
Naturally occurring suppressor mutants derived from tRNATrp genes have never been identified in S. cerevisiae. Oligonucleotide-directed mutagenesis was used to generate potential ochre and opal suppressors from a cloned tRNATrp gene. In vitro transcription analyses show the ochre suppressor form of the gene, TRPO, accumulates precursors and tRNA in amounts comparable to the parent. The opal suppressor, TRPOP, accumulates 4-5 fold less tRNA. Both forms of the gene are processed and spliced in vitro to produce tRNAs with the expected base sequences. The altered genes were subcloned into yeast vectors and introduced into yeast strains carrying a variety of amber, ochre, and opal mutations. When introduced on a CEN vector, neither ochre nor opal suppressor forms show suppressor activity. Deletion of the CEN region from the clones increases the copy number to 10-20/cell. The opal suppressor form shows moderate suppressor activity when the gene is introduced on this vector, however, the ochre suppressor form exhibits no detectable biological activity regardless of gene copy number. Northern blot analyses of the steady state levels of tRNATrp in cells containing the high copy-number clones reveal 20-100% increases in the abundance of tRNATrp.
Full text
PDF


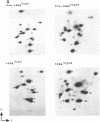
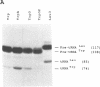
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S., Fitzgerald-Hayes M., Carbon J. Structural analysis and sequence organization of yeast centromeres. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1175–1185. doi: 10.1101/sqb.1983.047.01.133. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Enzymatic replacement of the anticodon of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1982 Mar 2;21(5):855–861. doi: 10.1021/bi00534a007. [DOI] [PubMed] [Google Scholar]
- Calderon I. L., Contopoulou C. R., Mortimer R. K. Isolation of a DNA fragment that is expressed as an amber suppressor when present in high copy number in yeast. Gene. 1984 Jul-Aug;29(1-2):69–76. doi: 10.1016/0378-1119(84)90167-7. [DOI] [PubMed] [Google Scholar]
- Etcheverry T., Salvato M., Guthrie C. Recessive lethality of yeast strains carrying the SUP61 suppressor results from loss of a transfer RNA with a unique decoding function. J Mol Biol. 1982 Jul 15;158(4):599–618. doi: 10.1016/0022-2836(82)90251-0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell. 1985 Feb;40(2):381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan tRNA of Escherichia coli. Nature. 1970 Oct 3;228(5266):57–57. doi: 10.1038/228057a0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Ogden R., Johnson P., Abelson J., Dembeck P., Itakura K. Transcription and processing of a yeast tRNA gene containing a modified intervening sequence. Proc Natl Acad Sci U S A. 1980 May;77(5):2564–2568. doi: 10.1073/pnas.77.5.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Abelson J. The yeast tRNATyr gene intron is essential for correct modification of its tRNA product. Nature. 1983 Apr 21;302(5910):681–687. doi: 10.1038/302681a0. [DOI] [PubMed] [Google Scholar]
- Kim D., Johnson J. Construction, expression, and function of a new yeast amber suppressor, tRNATrpA. J Biol Chem. 1988 May 25;263(15):7316–7321. [PubMed] [Google Scholar]
- Koshland D., Kent J. C., Hartwell L. H. Genetic analysis of the mitotic transmission of minichromosomes. Cell. 1985 Feb;40(2):393–403. doi: 10.1016/0092-8674(85)90153-9. [DOI] [PubMed] [Google Scholar]
- Liebman S. W., Sherman F. Inhibition of growth by amber suppressors in yeast. Genetics. 1976 Feb;82(2):233–249. doi: 10.1093/genetics/82.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Sherman F., Stewart J. W. Isolation and characterization of amber suppressors in yeast. Genetics. 1976 Feb;82(2):251–272. doi: 10.1093/genetics/82.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. P., Aker M., Sitney K. C., Mortimer R. K. First position wobble in codon-anticodon pairing: amber suppression by a yeast glutamine tRNA. Gene. 1986;49(3):383–388. doi: 10.1016/0378-1119(86)90375-6. [DOI] [PubMed] [Google Scholar]
- MONIER R., STEPHENSON M. L., ZAMECNIK P. C. The preparation and some properties of a low molecular weight ribonucleic acid from baker's yeast. Biochim Biophys Acta. 1960 Sep 9;43:1–8. doi: 10.1016/0006-3002(60)90399-1. [DOI] [PubMed] [Google Scholar]
- Masson J. M., Meuris P., Grunstein M., Abelson J., Miller J. H. Expression of a set of synthetic suppressor tRNA(Phe) genes in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6815–6819. doi: 10.1073/pnas.84.19.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Masson J. M., Kleina L. G., Abelson J., Miller J. H. Construction of two Escherichia coli amber suppressor genes: tRNAPheCUA and tRNACysCUA. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6548–6552. doi: 10.1073/pnas.83.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure G. A., Robinson G. W., Naumovski L., Friedberg E. C. Partial suppression of an ochre mutation in Saccharomyces cerevisiae by multicopy plasmids containing a normal yeast tRNAGln gene. J Mol Biol. 1985 May 5;183(1):31–42. doi: 10.1016/0022-2836(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Raymond G. J., Bryant P. K., 3rd, Nelson A., Johnson J. D. Large-scale isolation of covalently closed circular DNA using gel filtration chromatography. Anal Biochem. 1988 Aug 15;173(1):125–133. doi: 10.1016/0003-2697(88)90169-8. [DOI] [PubMed] [Google Scholar]
- Raymond K. C., Raymond G. J., Johnson J. D. In vivo modulation of yeast tRNA gene expression by 5'-flanking sequences. EMBO J. 1985 Oct;4(10):2649–2656. doi: 10.1002/j.1460-2075.1985.tb03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel M. C., Abelson J. Effect of intron mutations on processing and function of Saccharomyces cerevisiae SUP53 tRNA in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2663–2673. doi: 10.1128/mcb.6.7.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher J., Grosjean H., de Henau S., Finelli J., Buckingham R. H. Construction of a UGA suppressor tRNA by modification in vitro of yeast tRNACys. Eur J Biochem. 1984 Jan 2;138(1):77–81. doi: 10.1111/j.1432-1033.1984.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Normal yeast tRNA(CAGGln) can suppress amber codons and is encoded by an essential gene. J Mol Biol. 1986 Dec 20;192(4):725–735. doi: 10.1016/0022-2836(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Willis I., Frendewey D., Nichols M., Hottinger-Werlen A., Schaack J., Söll D. A single base change in the intron of a serine tRNA affects the rate of RNase P cleavage in vitro and suppressor activity in vivo in Saccharomyces cerevisiae. J Biol Chem. 1986 May 5;261(13):5878–5885. [PubMed] [Google Scholar]
- Winey M., Mendenhall M. D., Cummins C. M., Culbertson M. R., Knapp G. Splicing of a yeast proline tRNA containing a novel suppressor mutation in the anticodon stem. J Mol Biol. 1986 Nov 5;192(1):49–63. doi: 10.1016/0022-2836(86)90463-8. [DOI] [PubMed] [Google Scholar]