Abstract
Purpose
Our aim was to evaluate the effectiveness of two different dosing regimens of human recombinant erythropoietin (rHu-EPO) for preoperative autologous blood collection in patients undergoing total hip arthroplasty (THA).
Methods
Prospective randomised trials in which erythropoietin 15,000 IU was administered intravenously twice a week or 30,000 IU once a week (total 90,000 IU) combined with ferrous II sulphate (Ferro-Gradumet 2) orally and compared with Ferro-Gradumet 2 alone.
Results
Although different dosing regimens of rHu-EPO administration during preoperative autologous blood donation have similar effects on the collection of two units of autologous blood, preoperative haemoglobin level and perioperative allogenic blood transfusion, a once weekly dose regimen of rHu-EPO was more convenient (although not statistically significantly) for patients.
Conclusion
We recommend the more practical and comfortable but yet highly effective therapeutic regimen with a single weekly intravenous administration of rHu-EPO for patients scheduled for THA.
Introduction
Patients undergoing total hip arthroplasty (THA) with baseline haemoglobin (Hb) level up to 130 g/L are usually unable to donate sufficient autologous blood to satisfy their transfusion requirements during and after surgery. More than 50% of such patients require additional allogenic blood transfusions [1, 2]. The goal of preoperative administration of recombinant human erythropoietin (rHu-EPO) is to increase erythropoiesis in patients who are donating blood for autologous use and therefore decrease the need for allogenic transfusions [3, 4]. Previous reports found that the pharmacological response to erythropoietin therapy is a function of dose and administration regimen and that repeated administration of rHu-EPO is more effective in stimulating the reticulocyte response than is single-dose administration of the same total amount of rHu-EPO [5]. Several studies suggest that if rHu-EPO is administered subcutaneously rather than intravenously (i.v.), a lower dose may be sufficient to maintain the haematocrit at a given level [6]. However, over the past 20 years, researchers recorded cases in which patients developed neutralising ant-erythropoietin antibodies, a rare complication after rHu-EPO administration to increase red-blood-cell (RBC) production in patients with the anaemia because of chronic renal failure. Such antibodies can cause pure RBC aplasia [7]. The incidence of antibody-mediated pure RBC aplasia was mostly related to subcutaneous administration of rHu-EPO [8, 9]. Optimal dose, interval and route of administration for rHu-EPO are yet to be established.
The aim of this study was is to evaluate the effectiveness of two different dosing regimens of rHu-EPO administered i.v. to avoid all possible complications of subcutaneous administration (15,000 IU i.v. twice a week or 30,000 IU i.v. once a week; total 90,000 IU) combined with ferrous II sulphate (Ferro-Gradumet 2) perorally and compared with administration of Ferro-Gradumet alone orally for the collection of two units of preoperative autologous blood thereby reducing the need for allogenic blood transfusions after primary THA.
Patients and methods
Patients
We studied 93 patients between 60 and 80 years of age who were scheduled for primary THA for osteoarthritis. All patients who were able to donate autologous blood preoperatively with Hb level between 105 and 130 g/L were suitable. Patients were enrolled after giving informed consent in accordance with the ethical committee of the hospital. Exclusion criteria were a history of bleeding disorder, seizures or uncontrolled hypertension, deep vein thrombosis, gastrointestinal bleeding within six month of surgery, malignancy, acute or chronic infection and consumption of cytostatic or immunosuppressant drugs. Iron was given orally to all patients as ferrous-2 sulphate (3 × 65 mg elementary iron) during the study, starting one week before the first autologous blood donation. THA in all patients was performed by the senior authors using a direct lateral approach. The rHu-EPO beta (Recormon®) used in the study was provided by F Hofmann –La Roche, Basel, Switzerland. For all patients, routine premedication consisted of midazolam per os (0.1 mg/kg) one hour before induction of anaesthesia and 500 ml of Ringer's solution before they were positioned upright for the induction of spinal anaesthesia at either L3/L4 or L2/L3 levels via midline approach. An isobaric solution of levobupivacain (3.0–3.5 ml) was administered using a 25 g or 27 g pencil-point needle.
Study design
Patients were randomly assigned to three groups: 30 patients received 15,000 IU (average 200 IU/kg) rHu-EPO i.v. twice a week (on 17th, 13th, tenth, sixth and third day preoperatively and the second day postoperatively) and iron orally (group 1); 31 patients received 30,000 IU (average 400 IU/kg) rHu-EPO i.v. once a week (on 17th, 10th and 3rd day preoperatively) and iron perorally (group 2); 32 patients received only iron perorally (group 3). Donation of 12% of total blood volume was performed on the tenth and third preoperative days. The minimum Hb level (Hb) for donation was 110 g/L, according to current European guidelines for preoperative autologous donation [10]. The pre-study evaluation included thorough medical history and physical examination. Pre-study laboratory tests included complete blood count, reticulocyte count, serum chemistry studies, urine analysis, serum iron, total iron-binding capacity (TIBC), transferrin saturation and serum ferritin. At the time of each injection of rHu-EPO, (17th, 13th, tenth, sixth and third preoperative day for group 1; 17th, tenth and third preoperative day for groups 2 and 3, respectively), vital signs, haematological values (including reticulocytes) and serum chemistry (potassium) were assessed. If the Hb level was over 150 g/L, erythropoietin beta was not administered if the Hb level was less than 110 g/L, autologous blood was not donated. Adverse events, blood loss and transfusion data were collected for all patients by an anaesthetist. Criteria for perioperative transfusion (both autologous and allogenic) included Hb level ≤80 g/L and/or clinical symptoms of anaemia (increased heart rate or lower blood pressure despite fluid i.v. bolus). All patients received the same protocol for deep-venous thrombosis prophylaxis with low-molecular-weight heparin, and cefazolin (one gram) was administered i.v. 60 min before the procedure for perioperative infection prophylaxis. In all patients reinfusion drains were used. Reinfusion drains (for cell salvage) were used in the immediate postoperative period in the recovery room (first six hours) and were later converted to standard Hemovac drains. All drains were removed 48 hours after arthroplasty.
Laboratory studies
Samples were obtained on the 17th, 13th, tenth and third day preoperatively in groups 1 and 2 before each injection of rHu-EPO and in group 3 immediately before donation of autologous blood. Blood was taken on the day of surgery two hours preoperatively and six hours postoperatively, and on several postoperative days (first, second, third, tenth and 35th) from all patients. Ferrokinetic studies (serum iron, serum ferritin, TIBC and transferrin saturation) were measured before and at the end of the study (Table 1). The three groups were similar in terms of male/female ratio, age, height, weight, total blood volume, American Society of Anaesthesiologists (ASA) classification and baseline Hb level. Group 1 consisted of five male and 25 female patients, with mean age 68.43. Group 2 consisted of three male and 28 female patients with a mean age of 67.71 years. Group 3 consisted of three male and 29 female patients with a mean age of 66.31 years. The primary variable for measuring efficiency was the percentage of patients in each group requiring allogenic blood and the mean number of units given to each patient who received a transfusion. The secondary variable for measuring efficiency was percentage of patients in each group from whom the planned two units of autologous blood was collected, mean number of units collected per patients and a change in haematological parameters.
Table 1.
Ferrokinetic studies
| Collection time | Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|---|
| Fe (μg/L) | Baseline | 13.72 ± 3.54 (8.00–23.00) | 15.29 ± 4.04 (7.80–23.50) | 14.27 ± 3.95 (7.00–25.90) | 0.276 |
| At the end of the study | 12.09 ± 4.72 (7.60–32.00) | 12.71 ± 4.17 (8.64–31.00) | 11.37 ± 2.58 (7.32–28.00) | 0.4 | |
| TIBC (μg/L) | Baseline | 53.81 ± 6.16 (40.20–67.00) | 56.41 ± 8.22 (44.60–78.00) | 56.44 ± 4.26 (49.00–66.00) | 0.189 |
| At the end of the study | 52.53 ± 8.39 (40.10–87) | 54.15 ± 5.59 (46.50–72.00) | 55.42 ± 5.11 (45.60–65.00) | 0.22 | |
| Ferritin (ng/ml) | Baseline | 78.56 ± 12.6 (45.3–136.8) | 81.25 ± 9.8 (52.0–141.80) | 76.21 ± 10.2 (50.0–130.6) | 0.326 |
| At the end of the study | 62.7 ± 10.2 (40.6–124.8) | 58.7 ± 12.8 (46.4–130.80) | 70.7 ± 10.8 (59.7131.8) | 0.354 | |
| Transferrin saturation (%) | Baseline | 25.72 ± 5.49 (15.00–42.80) | 26.94 ± 6.64 (14.90–39.60) | 24.96 ± 6.39 (12.10–43.00) | 0.5 |
| At the end of the study | 21.72 ± 11.41 (10.90–77.00) | 20.17 ± 6.44 (13.30–33.90) | 20.53 ± 4.49 (11.60–32.20) | 0.239 |
Data shown as mean ± standard deviation, with minimal and maximal values in parentheses
Fe iron, TIBC total iron-binding capacity
Statistical methods
Demographic and analytical values are presented using descriptive statistics and expressed as mean ± standard deviation (SD) and medians, minimal and maximal observed values or percentages. The paired Student’ t test was used to determine significant differences within the group, and the Wilcox on test was used for data in which values could not be assumed to be normally distributed. The t test and the Mann–Whitney test were used to determine the statistical significance between groups. A p value <0.05 was considered statistically significant.
Results
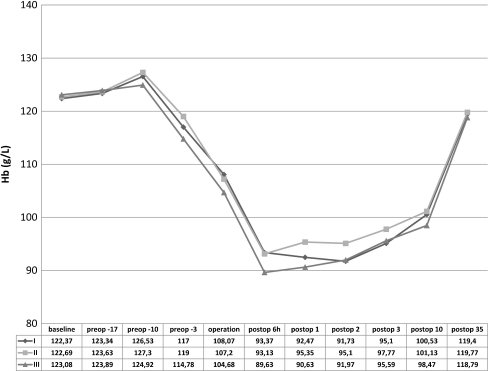
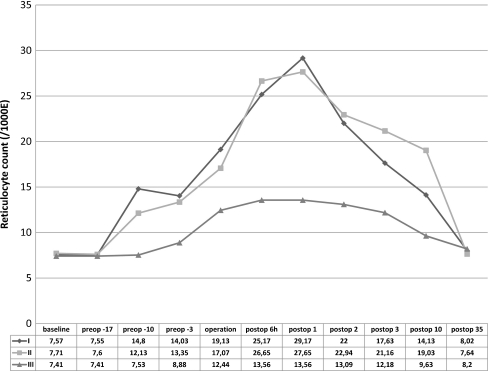
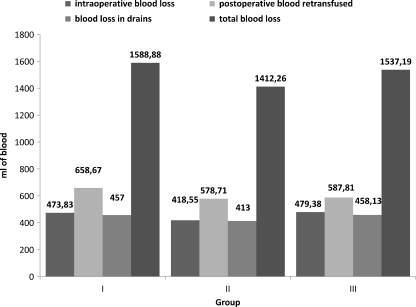
Patients in Group 1 were able to donate 58 U of the requested 60 U of autologous blood. Forty-nine of the 58 U were transfused, and nine U were discarded. Patients in group 2 were able to donate 61U of the requested 62 U of autologous blood. Forty-three of the 61U were transfused, and 18 U were discarded. Patients in group 3 were able to donate 58 U of the requested 64 U of autologous blood. Fifty of the 58 U were transfused, and eight U were discarded. The effect of erythropoietic activity on Hb level and reticulocyte count over time is shown in Figs. 1 and 2. Only one patient in group 2 had Hb level at baseline less than the recommended 110 g/L (105. g/L) for autologous donation but because of effective erythropoiesis stimulation, the patient was able to donate one U of autologous blood (at the time of first autologous blood donation, Hb level was 110 g/L). There were no significant differences in postoperative Hb levels between groups Mean reticulocyte count was not significantly different at initial assessment between groups. From the tenth preoperative day to the day of surgery, there was significant increase in reticulocyte count in both erythropoietin-treated groups. Reticulocyte count returned to baseline levels five weeks after surgery in all groups. Six hours after surgery and at the first postoperative day, patients who received erythropoietin (groups 1 and 2) had significantly higher values than did those in the no-erythropoietin group (3); p < 0.001, t test). Differences between reticulocyte count in groups 1 and 2 did not reach significance at any time. Intraoperative blood loss, postoperative blood reinfusion, blood loss in drains and total blood loss are shown in Fig. 3. Perioperative blood loss was similar among groups.
Fig. 1.
Haemoglobin values (means) during the study
Fig. 2.
Change in mean reticulocyte count from baseline during the study
Fig. 3.
Perioperative blood loss
Transfusion
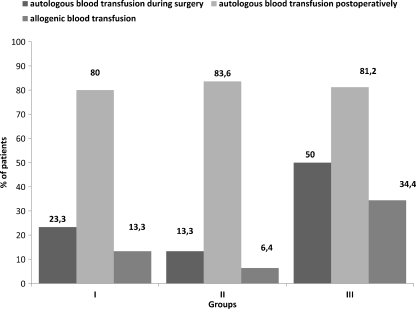
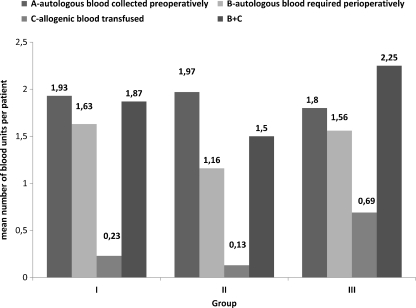
The incidence of blood transfusion is shown in Fig. 4. Only two patients in group 2 did not receive a transfusion. There were no patients who received an allogenic blood transfusion intraoperatively. The highest proportion of patients requiring blood transfusion intraoperatively (autologous) was in group 3: 16 (50%) patients, compared with seven (23,3%) and four (13.3%) in groups 1 and 2, respectively; (3:1,2 p = 0.0315, chi-square test). There was no significant difference in postoperative consumption of autologous blood between groups. Of patients in group 3, 34.4% received allogenic blood postoperatively compared with 13.3% and 6.4% in groups 1 and 2, respectively (3:1, 2 p = 0.013, 3: 2 p = 0 < 0.001, chi-square test). The difference in allogenic transfusion rates between groups 1 and 2 (13.3– 6.4%) did not reach significance (p = 0.09, chi-square test). Mean number of blood units is shown in Fig. 5. The mean number of units of allogenic blood transfused per patient was 0.23 ± 0.49 (0–3), 0.13 ± 0.51 (0–2) and 0.69 ± 1.23 (0–4) in groups 1, 2 and 3, respectively (3:1,2 p = 0.006, t test). The mean number of total transfused units (autologous and allogenic) was 1.87 ± 0.86 (0–5), 1.5 ± 0.78 (0–4) and 2.25 ± 1.24 (0–5) in groups 1, 2, and 3, respectively (3:1, 2 p = 0.013, t test). There was no significant difference among groups at baseline or at the end of the study between serum iron, TIBC, ferritin and transferrin saturation. However, serum iron, ferritin and transferrin saturation at the end of study significantly decreased compared with baseline level (p < 0.05, t test for paired samples).
Fig. 4.
Incidence of blood transfusion
Fig. 5.
Mean units collected per patient and transfused per patient
The use of erythropoietin in this study was generally well tolerated, with few adverse reactions: nausea in four (6.5%), pyrexia in four (6.5%) and headache in three (4.9%) patients. Erythropoietin therapy was not discontinued in any patient because of these reactions. Postoperative complications consisted of five wound hematomas (no operative evacuation was needed).
Discussion
This study suggest that the two regimens compared, of beta rHu-EPO i.v. (six-dose vs. three-dose regimen of the same total weekly doses) for three weeks enabled collection of two units of autologous blood and reduced allogenic blood transfusion in patients undergoing THA. These results are in agreement with the studies of Green et al. and Rosencher et al. [11, 12]. Both the six-dose and three-dose regimens were associated with better haematological parameters on the day of surgery and lower overall requirement for transfusion of allogenic blood when compared with the no-erythropoietin-treated group. Previous major double-blind, placebo controlled trials with a daily regimen of rHu-EPO for ten preoperative and five postoperative days showed benefit from rHu-EPO in orthopaedic surgical patients by increasing total RBC mass and reducing perioperative allogenic blood transfusion [13, 14]. The trial by Goldberg et al. demonstrated that weekly 600 IU/kg dosing regimen of rHu-EPO was similar to the daily 300 IU/kg regimen with respect to safety and avoidance of allogenic transfusion in patients scheduled to undergo major orthopaedic arthroplasty [15]. After these trials, the weekly dose regimen was approved as the standard regimen for orthopaedic surgery. Contrary to that study, several trials demonstrated that more frequent administration could be more effective than less frequent administration. Cody et al. reported that initial high peak levels from high once weekly doses may be wasted, as erythropoietin receptors on progenitor cells in bone marrow may become saturated; when these receptor are again free for binding, the level of serum erythropoietin will fall. Frequent administration of small amounts of rHu-EPO could maintain a more constant low but more effective level of serum erythropoietin [16]. Changes of reticulocyte count is one of the powerful predictors of responsiveness to rHu-EPO treatment [17, 18]. In our study, mean reticulocyte count slightly increased for several days after the beginning of erythropoietin treatment compared with baseline level in both erythropoietin groups, and this result is consistent with the findings of Ramakrishnan et al. [19] They found that after repeated erythropoietin administration, reticulocyte count steadily began to increase until the peak level was reached after 200–300 hours. Then the count began declining, eventually reaching the baseline level. Nonerythropoietin-treated patients received allogenic blood transfusion postoperatively at a higher rate (34.4%) than patients who had a twice-weekly (13.3%) and once-weekly (6.4%) regimens. Although there was no significant difference between groups 1 and 2 in percentage of allogenic blood transfusions, there was a slightly lower demand for allogenic blood transfusion in group 2 (single weekly dose). The average overall consumption of transfused blood (autologous and allogenic) was significantly higher (2.25 ± 1.24 U/patient) compared with both erythropoietin-treated groups. These results are in agreement with previous studies, which demonstrated that preoperative Hb level was one of the strongest predictors of perioperative allogenic blood transfusion in perisurgical setting [20, 21].
Many authors have shown the superiority of the subcutaneous over the i.v. route of rHEpo administration for more sustained serum levels over time (12–18 h) and lesser dose requirements [22–24]. However, subcutaneous injection can be painful; also, nearly all patients who had antibody-mediated pure RBC aplasia received erythropoietin administration by the subcutaneous route [25, 26]. For this reason, and for short-term administration, Lee et al. [27] believe that using the i.v. route is better for patients with autologous blood donation, as it allows a more reliable serum level to be achieved and maintained. Several clinical studies [28] analysed the relationship between erythropoietin, iron and erythropoietic response to anaemia. In our study, the relatively weak response of erythropoiesis to 90,000 IU rHu-EPO (approximately 6 x 200 IU/kg or 3 x 400 IU) could be explained by the chosen doses of erythropoietin beta and iron supplementation. Although all baseline iron parameters (including serum iron, TIBC and saturation of transferrin) were within normal ranges, with no intergroup differences, iron stores were not sufficient because the iron requirements exceed the available supply during rHu-EPO administration and accelerated erythropoiesis. Approximately 200 mg/day (as used in this study) is a standard regimen of iron supplementation [17].
Conclusion
Although different dosing regimens of the same amount of rHu-EPO administration during preoperative autologous blood donation have had similar effects on the collection of two units of autologous blood, preoperative Hb level and perioperative allogenic blood transfusion, a once-weekly dose regimen of rHu-EPO was more convenient (although not statistically significant) and probably more comfortable for patients, with the assumption, therefore, that for the greater number of patients, visiting a hospital twice a week was more inconvenient, costly and psychologically and physically demanding. For this reason, we suggest the more practical and comfortable but yet highly effective therapeutic regimen with single weekly i.v. administration of rHu-EPO for patients scheduled for THA.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Mercuriali F, Biffi E, Inghilleri G, Vinci A. Low hematocrit: limiting factor in autologous blood predonation program. In: Genetet B, Habibi B, editors. Castelli D. Paris: Transfusion in Europe Paris ISBT; 1989. [Google Scholar]
- 2.Young SW, Marsh DJ, Akhavani MA, Walker CG, Skinner JA. Attitudes to blood transfusion post arthroplasty surgery in the United Kingdom: a national survey. Int Orthop. 2008;32(3):325–329. doi: 10.1007/s00264-007-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercuriali F, Zanella A, Barosi G, Inghilleri G, Biffi E, Vinci A, Colotti MT. Use of erythropoietin to increase the volume of autologous blood donated by orthopedic patients. Transfusion. 1993;33(1):55–60. doi: 10.1046/j.1537-2995.1993.33193142311.x. [DOI] [PubMed] [Google Scholar]
- 4.Colomina MJ, Bago J, Pellise F, Godet C, Villanueva C. Preoperative erythropoietin in spine surgery. Eur Spine J. 2004;13(Suppl 1):S40–S49. doi: 10.1007/s00586-004-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung WK, Goon BL, Guilfoyle MC, Wacholtz MC. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther. 1998;64(4):412–423. doi: 10.1016/S0009-9236(98)90072-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman JS, Reda DJ, Fye CL, Goldfarb DS, Henderson WG, Kleinman JG, Vaamonde CA. Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis. Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients. N Engl J Med. 1998;339(9):578–583. doi: 10.1056/NEJM199808273390902. [DOI] [PubMed] [Google Scholar]
- 7.Peces R, Torre M, Alcazar R, Urra JM. Antibodies against recombinant human erythropoietin in a patient with erythropoietin-resistant anemia. N Engl J Med. 1996;335(7):523–524. doi: 10.1056/NEJM199608153350717. [DOI] [PubMed] [Google Scholar]
- 8.Eckardt KU, Casadevall N. Pure red-cell aplasia due to anti-erythropoietin antibodies. Nephrol Dial Transplant. 2003;18(5):865–869. doi: 10.1093/ndt/gfg182. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, Varet B, Mayeux P. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346(7):469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 10.Co E. Guide to the preparation, use and quality assurance of blood components. 3. Strassbourg: Council of Europe Press; 1997. [Google Scholar]
- 11.Green WS, Toy P, Bozic KJ. Cost minimization analysis of preoperative erythropoietin vs autologous and allogeneic blood donation in total joint arthroplasty. J Arthroplast. 2010;25(1):93–96. doi: 10.1016/j.arth.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Rosencher N, Kerkkamp HE, Macheras G, Munuera LM, Menichella G, Barton DM, Cremers S, Abraham IL. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43(4):459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 13.Laupacis A, Feagan B, Wong C. Effectiveness of perioperative recombinant human erythropoietin in elective hip replacement. COPES Study Group. Lancet. 1993;342(8867):378. doi: 10.1016/0140-6736(93)91527-S. [DOI] [PubMed] [Google Scholar]
- 14.Faris PM, Ritter MA, Abels RI. The effects of recombinant human erythropoietin on perioperative transfusion requirements in patients having a major orthopaedic operation. The American Erythropoietin Study Group. J Bone Joint Surg Am. 1996;78(1):62–72. doi: 10.2106/00004623-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg MA, McCutchen JW, Jove M, Cesare P, Friedman RJ, Poss R, Guilfoyle M, Frei D, Young D. A safety and efficacy comparison study of two dosing regimens of epoetin alfa in patients undergoing major orthopedic surgery. Am J Orthop (Belle Mead NJ) 1996;25(8):544–552. [PubMed] [Google Scholar]
- 16.Cody J, Daly C, Campbell M, Donaldson C, Khan I, Vale L, Wallace S, Macleod A (2005) Frequency of administration of recombinant human erythropoietin for anaemia of end-stage renal disease in dialysis patients. Cochrane Database Syst Rev (3):CD003895. doi:10.1002/14651858.CD003895.pub2 [DOI] [PubMed]
- 17.Avall A, Hyllner M, Bengtson JP, Carlsson L, Bengtsson A. Recombinant human erythropoietin in preoperative autologous blood donation did not influence the haemoglobin recovery after surgery. Acta Anaesthesiol Scand. 2003;47(6):687–692. doi: 10.1034/j.1399-6576.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Ruixo JJ, Krzyzanski W, Hing J. Pharmacodynamic analysis of recombinant human erythropoietin effect on reticulocyte production rate and age distribution in healthy subjects. Clin Pharmacokinet. 2008;47(6):399–415. doi: 10.2165/00003088-200847060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan R, Cheung WK, Wacholtz MC, Minton N, Jusko WJ (2004) Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol 44(9):991–1002. doi:10.1177/0091270004268411 [DOI] [PubMed]
- 20.Salido JA, Marin LA, Gomez LA, Zorrilla P, Martinez C. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am. 2002;84-A(2):216–220. doi: 10.2106/00004623-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Prasad N, Padmanabhan V, Mullaji A. Blood loss in total knee arthroplasty: an analysis of risk factors. Int Orthop. 2007;31(1):39–44. doi: 10.1007/s00264-006-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung W, Minton N, Gunawardena K. Pharmacokinetics and pharmacodynamics of epoetin alfa once weekly and three times weekly. Eur J Clin Pharmacol. 2001;57(5):411–418. doi: 10.1007/s002280100324. [DOI] [PubMed] [Google Scholar]
- 23.Goodnough LT, Monk TG, Andriole GL. Erythropoietin therapy. N Engl J Med. 1997;336(13):933–938. doi: 10.1056/NEJM199703273361307. [DOI] [PubMed] [Google Scholar]
- 24.Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35(9):1672–1678. doi: 10.1124/dmd.107.015248. [DOI] [PubMed] [Google Scholar]
- 25.Rossert J, Casadevall N, Eckardt KU. Anti-erythropoietin antibodies and pure red cell aplasia. J Am Soc Nephrol. 2004;15(2):398–406. doi: 10.1097/01.ASN.0000107561.59698.42. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, McKoy JM, Kim B, Lyons EA, Trifilio SM, Raisch DW, Evens AM, Kuzel TM, Schumock GT, Belknap SM, Locatelli F, Rossert J, Casadevall N. Pure red-cell aplasia and epoetin therapy. N Engl J Med. 2004;351(14):1403–1408. doi: 10.1056/NEJMoa040528. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Yoon KS, Park JS, Kang SB, Do SH, Kim JY. Recombinant human erythropoietin using preoperative autologous donation in lumbar stenosis operations. J Korean Soc Spine Surg. 1999;6(3):5. [Google Scholar]
- 28.Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96(3):823–833. [PubMed] [Google Scholar]