Abstract
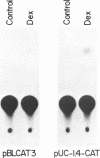
The rat cytochrome P450 CYP2B2 gene encodes one of the two major phenobarbital-inducible forms of hepatic microsomal cytochrome P-450. The sequence of a 1.4 Kb DNA segment from the 5' flanking region of this region [Jaiswal, A., Rivkin, E. and Adesnik, M. Nucl. Acids. Res. 15: 6755 (1987)] reveals the presence of a pentadecameric oligonucleotide sequence, located approximately 1.3 Kb upstream of the transcription initiation site, which is highly similar to the sequences of glucocorticoid response elements (GREs) that mediate the hormone-dependent transcriptional activation of many other genes. The putative GRE in the CYP2B2 gene 5' flanking region is shown to be functional by demonstrating that segments of DNA that contain it, including one that is only 25bp long, are capable of conferring dexamethasone inducibility on a chloramphenicol acetyltransfer-ase gene whose transcription is driven by the Herpes virus thymidine kinase gene promoter. Moreover, binding of a protein contained in a rat liver nuclear extract to a 25 bp synthetic DNA segment that contains the putative GRE was demonstrated in a gel mobility shift assay. This binding was specifically competed away by a DNA segment that contains the murine mammary tumor virus long terminal repeat which encompasses several well characterized GRE elements. The implications of these findings for the in vivo regulation of the P450IIB2 gene by glucocorticoids are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Atchison M., Adesnik M. A cytochrome P-450 multigene family. Characterization of a gene activated by phenobarbital administration. J Biol Chem. 1983 Sep 25;258(18):11285–11295. [PubMed] [Google Scholar]
- Corcos L., Weiss M. C. Phenobarbital, dexamethasone and benzanthracene induce several cytochrome P450 mRNAs in rat hepatoma cells. FEBS Lett. 1988 Jun 6;233(1):37–40. doi: 10.1016/0014-5793(88)81351-6. [DOI] [PubMed] [Google Scholar]
- Dannan G. A., Guengerich F. P., Kaminsky L. S., Aust S. D. Regulation of cytochrome P-450. Immunochemical quantitation of eight isozymes in liver microsomes of rats treated with polybrominated biphenyl congeners. J Biol Chem. 1983 Jan 25;258(2):1282–1288. [PubMed] [Google Scholar]
- Dong Y., Poellinger L., Okret S., Hög J. O., von Bahr-Lindström H., Jörnvall H., Gustafsson J. A. Regulation of gene expression of class I alcohol dehydrogenase by glucocorticoids. Proc Natl Acad Sci U S A. 1988 Feb;85(3):767–771. doi: 10.1073/pnas.85.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Song B. J., Hardwick J. P. Pregnenolone 16 alpha-carbonitrile-inducible P-450 gene family: gene conversion and differential regulation. Mol Cell Biol. 1986 Aug;6(8):2969–2976. doi: 10.1128/mcb.6.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Transcriptional regulation of rat liver epoxide hydratase, NADPH-Cytochrome P-450 oxidoreductase, and cytochrome P-450b genes by phenobarbital. J Biol Chem. 1983 Jul 10;258(13):8081–8085. [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Comparison of human mouse P1450 upstream regulatory sequences in liver- and nonliver-derived cell lines. Mol Endocrinol. 1987 Apr;1(4):312–320. doi: 10.1210/mend-1-4-312. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., Rivkin E., Adesnik M. 5' flanking sequence of the gene for rat hepatic cytochrome P450e. Nucleic Acids Res. 1987 Aug 25;15(16):6755–6755. doi: 10.1093/nar/15.16.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Langer S. J., Ostrowski M. C. Negative regulation of transcription in vitro by a glucocorticoid response element is mediated by a trans-acting factor. Mol Cell Biol. 1988 Sep;8(9):3872–3881. doi: 10.1128/mcb.8.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Omiecinski C. J. Tissue-specific expression of rat mRNAs homologous to cytochromes P-450b and P-450e. Nucleic Acids Res. 1986 Feb 11;14(3):1525–1539. doi: 10.1093/nar/14.3.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiecinski C. J., Walz F. G., Jr, Vlasuk G. P. Phenobarbital induction of rat liver cytochromes P-450b and P-450e. Quantitation of specific RNAs by hybridization to synthetic oligodeoxyribonucleotide probes. J Biol Chem. 1985 Mar 25;260(6):3247–3250. [PubMed] [Google Scholar]
- Oosterom R., Verleun T., Zuiderwijk J., Lamberts S. W. Growth hormone secretion by cultured rat anterior pituitary cells. Effects of culture conditions and dexamethasone. Endocrinology. 1983 Aug;113(2):735–741. doi: 10.1210/endo-113-2-735. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. L., McQuiddy P., Kasper C. B. Induction of the hepatic mixed-function oxidase system by synthetic glucocorticoids. Transcriptional and post-transcriptional regulation. J Biol Chem. 1987 Jan 5;262(1):326–332. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Strähle U., Klock G., Schütz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa Y., Mizukami Y., Sogawa K., Fujii-Kuriyama Y. Gene structure of a major form of phenobarbital-inducible cytochrome P-450 in rat liver. J Biol Chem. 1985 Jul 5;260(13):7980–7984. [PubMed] [Google Scholar]
- Weiner F. R., Czaja M. J., Jefferson D. M., Giambrone M. A., Tur-Kaspa R., Reid L. M., Zern M. A. The effects of dexamethasone on in vitro collagen gene expression. J Biol Chem. 1987 May 25;262(15):6955–6958. [PubMed] [Google Scholar]
- Yamazoe Y., Shimada M., Murayama N., Kato R. Suppression of levels of phenobarbital-inducible rat liver cytochrome P-450 by pituitary hormone. J Biol Chem. 1987 May 25;262(15):7423–7428. [PubMed] [Google Scholar]
- Yuan P. M., Ryan D. E., Levin W., Shively J. E. Identification and localization of amino acid substitutions between two phenobarbital-inducible rat hepatic microsomal cytochromes P-450 by micro sequence analyses. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1169–1173. doi: 10.1073/pnas.80.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]