Abstract
Engineering of the membrane-like tissue structures to be utilized in highly dynamic loading environments such as the cardiovascular system has been a challenge in the past decade. Scaffolds are critical components of the engineered tissue membranes and allow them being formed in vitro and remain secure in vivo when implanted in the body. Several approaches have been taken to develop scaffolds for tissue membranes. However, all methods entail limitations due to structural vulnerability, short-term functionality, and mechanical properties of the resulted membrane constructs. To overcome these issues, we have developed a novel hybrid scaffold made of an extra thin layer of metal mesh tightly enclosed by biological matrix components. This approach retains all the advantages of using biological scaffolds while developing a strong extracellular matrix that can stand various types of loads after implantation inside the body.
Introduction
Engineering of the membrane-like tissue structures with an ability to remodel and regenerate is currently an unresolved subject in the field of tissue engineering. Several attempts with minimal success have been made to create functional viable membrane tissues such as heart valve leaflets with the ability to grow, repair, and remodel.1–5 These approaches were mainly unsuccessful due to structural vulnerability, short-term functionality, and mechanical properties of the membrane constructs.
Scaffolds are critical components of the engineered tissues that allow them to be formed in vitro and remain secure in vivo when being implanted in a host. Several approaches have been taken to develop scaffolds for tissue membranes. The most widely used method involves biodegradable naturally derived or synthetic polymers,4,6–8 where the polymer eventually degrades by normal metabolic activity, while the biological matrix is formed. To have a viable tissue, the rate of scaffold degradation should be proportional to the rate of tissue formation to guarantee mechanical stability over time.9,10 The poor control of enzymatic degradation and low mechanical performance are two major limitations of naturally derived polymers.11 In contrast, synthetic polymers can be prepared precisely with regard to structure and function. However, most of them produce toxic chemicals when they degrade in vivo, and due to lack of receptor-binding ligands, they may not provide a good environment for adhesion and proliferation of cells.12
The other approach is to create scaffolds from decellularized xenogenic tissues, which has some advantages over polymeric materials. Decellularized tissues provide a unique scaffold, which is essentially composed of extracellular matrix (ECM) proteins that serve as an intrinsic template for the cells.13,14 However, the process of decellularization cannot completely remove the trace of cells and their debris. These remnants not only increase the potential of an immunogenic reaction but also result in increased tissue susceptibility to calcification.15,16
The least developed strategy involves creating a scaffold with completely biological matrix components.17,18 This approach is superior to the other two with regard to producing large supplies from xenogenic sources, which can readily accommodate cellular ingrowth without cytotoxic degradation products. However, this strategy is restricted due to mechanical fragility of the scaffold, and the low potentials for creating complex tissue structures.19
In this work, we have developed a novel hybrid scaffold that is utilized for tissue engineering of membranes, particularly when resistance of the membrane is crucial (e.g., artificial heart valves and vascular grafts). This scaffold is made of an extra thin layer of metal mesh tightly enclosed by biological matrix components (Fig. 1). This approach retains all the advantages of using biological scaffolds while developing a strong ECM backbone composed of the mesh that can stand various types of loads after implantation inside the body. Additionally, such a mesh pattern assures structural integration of the formed tissue and allows cells and ECM components on both sides of the mesh to interact with each other. The formed tissue is expected to be biomechanically resilient against the physiological stresses inside the body, and, in particular, can be an alternative for heart valve leaflets on utilizing a proper elastic mesh.
FIG. 1.
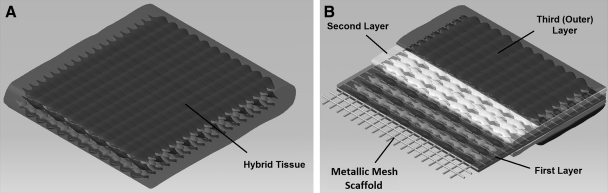
Schematic representation of a hybrid scaffold and the multiple tissue layers enclosing it; (A) tissue construct with a rectangular-shape metallic mesh as its core, (B) three layers of cells that mimic the heart valve tissue structure; the first layer consists of smooth muscle cells and myofibroblasts on both sides of the core, the second layer consists of fibroblast/myofibroblast cells that are cultured on top of the first layers, and the third layer consists of endothelial cells that act as the cover layer of the structure.
Materials and Methods
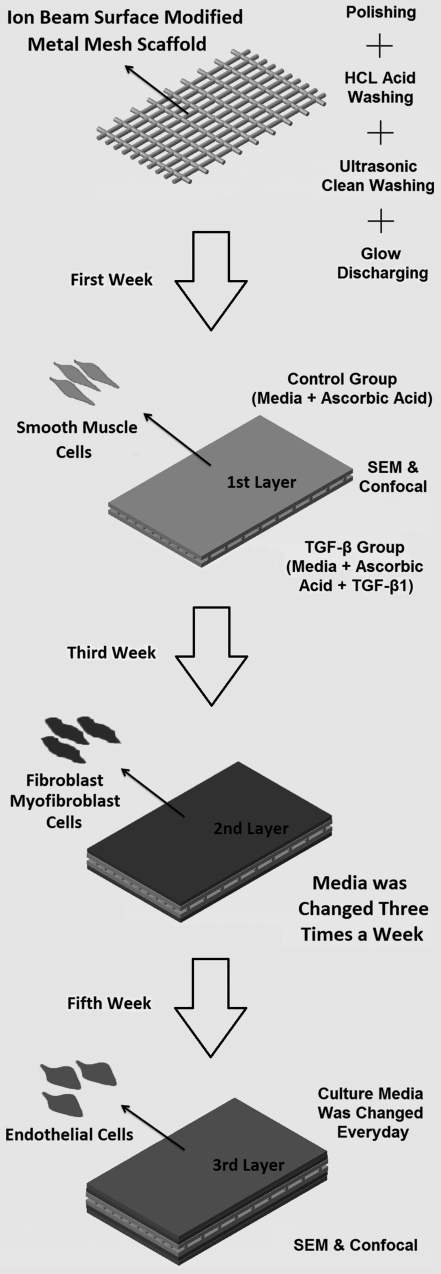
Flat mesh of T316 Stainless Steel woven from 0.0037″ round wires, targeting at 80 EPI×80 PPI* (TWP Inc., Berkeley, CA), was used as a test material. The mesh possesses an opening size of 0.0088″, which is almost 10 times larger than the dimension of the cells being used in this study. The base material was heated at 520°C for 5 min, followed by water quenching. The oxidized film was removed at multiple stages; by polishing the surface, using hydrochloric acid wash, ultrasonic cleaning wash in ethanol for 15 min, and glow discharging for 40 s. Finally, the mesh was cut into pieces with an area of 1 cm2 to be used for cell culture.
Surface modification
An ion beam surface modification method was used to get a biocompatible surface. The meshes were mechanically polished with wetted metallographic polishing high-grade silicon carbide (SiC) papers. Afterward, the meshes were acid washed and then degreased in an ultrasonic vibrobath before final rinsing with distilled water. Before cell culture, the samples were irradiated by He+ ion beam at energy of 150 keV with fluences of 1×1014 ions/cm2. The surface modification technique ensures the biocompatibility and enhanced cell attachment for the Stainless Steel meshes.20,21
Cell isolation and culture
A cell pattern similar to a natural heart valve leaflet was chosen for the experiment. Three different cell types were isolated and used for preliminary assay; human aortic smooth muscle cells and human aortic adventitial fibroblast/myofibroblast cells to fulfill the role of valvular interstitial cells (VICs), and human umbilical vascular endothelial cells to act as the valvular endothelial cells. The basal media for culturing cells contained (Dulbecco's Modified Eagle Medium; Gibco, Carlsbad, CA), 10% fetal bovine serum (HyClone, Rockford, IL), 1% penicillin/streptomycin (Gibco), and 1% L-glutamine (Gibco), with appropriate growth factors added to it for the enhancement of growth and proliferation. Cultured cells were fed every two to three days, and split 1 to 3 at confluence. Cells were used in passages 3 to 5 for the experiment.
Three-dimensional construct
Each mesh was coated with a mixture of bovine and rat tail collagen (Gibco) in a tissue culture hood with an aligned appearance. This mixture in optimal concentration ensures development of an interconnected pore network, which is essential for cell growth, nutrient supply, and removal of metabolic waste products. Briefly, liquid collagen mixture was neutralized using NaOH. Cell-seeded collagen constructs were prepared by first casting an acellular collagen solution and then adding a total of 3×105 cells for each cell type to it, before the collagen had set. After placing the Stainless Steel meshes appropriately among the solutions, the constructs were incubated at 37°C in a 5% CO2 humidified incubator for polymerization. This method ensures that collagen constructs have a uniform cell density (3×106 cells/cm2) after gel formation. The hybrid tissue constructs were cultured at 37°C with replacement of culture media every two days. To achieve a phenotype similar to the natural valve leaflets in-vivo, the cells in the next layers were plated over the constructs at time intervals of 2 weeks in a similar way described earlier. Media was also supplemented with ascorbic acid (Sigma-Aldrich Inc., St. Louis, MO) as an additive to promote matrix production. To increase the rate of ECM production, 10 ng/mL of TGF-β1 (R&D Systems Inc., Minneapolis, MN) was added to the collagen gels in each layer to increase the rate of ECM production. These cultures were later on compared with the control group with no TGF-β supplementation. Figure 2 shows a schematic diagram of the experimental methods for producing the hybrid multilayered tissue. Through the 3D cell-culture technique, all layers of the cells were seeded on the rectangular-shaped Stainless Steel meshes to produce ECM or connective tissue.
FIG. 2.
Different steps in the 3D cell culture method used to develop a hybrid tissue.
Confocal microscopy
After placing all three cell layers, the constructs were analyzed using a Zeiss LSM 510 Metaconfocal microscope. Antibodies against SM22, Phalloidin 488, Pecam were used for smooth muscle cells, fibroblasts/myofibroblasts, and endothelial cells, respectively. Cellular phenotypes were determined after 8 weeks. Samples were also counterstained with 4',6-diamidino-2-phenylindole (DAPI) to reveal cell nuclei.
Scanning electron microscopy
Different sections of the hybrid tissue with and without addition of TGF-β were used for scanning electron microscopy (SEM) analysis. Samples were dehydrated in ascending concentrations of ethanol (30–100%) followed by a critical point dried in CO2. Then, the samples were mounted on carbon stubs, and sputtered using a 4 nm coating of gold (VCR Group, San Francisco, CA). A low-voltage, high-resolution SEM (Quanta 3D FEG Electron Microscope, FEI, Hillsboro, OR) was used at 10 kV to capture images at various magnifications ranging from 10 to 1000 μ.
Results
In the present study, we have developed a tissue membrane in vitro using a core Stainless Steel mesh for the first time (Fig. 3). There are a total of three layers of cells at each side of the mesh where the two layers proximal to the mesh (smooth muscle cells) tightly enclose it in three dimensions. These three layers at each side are the mimic ventricularis, spongiosa, and fibrosa layers of a heart valve leaflet. All groups showed a shiny smooth surface and macroscopically, there were differences in the size of the membranes between the controls and the TGF-β conditioned constructs. The maximum overall thickness that the membrane reached was 1.2 mm (containing the mesh with the overall thickness of 0.188 mm), which was observed in the TGF-β group. The control groups showed less tissue formation and organization at all time points. Proliferation was also exceeded in the TGF-β group when compared with the control ones. Consistent with known pleiotropic actions of TGF-β, stimulated scaffolds revealed dramatically enhanced collagen production, indicating an increase in proliferation and apoptosis, respectively, and providing a potential mechanism for the continuing process of remodeling and maturation in vitro. Furthermore, due to the membrane-like nature of the formed tissues, the cells in deeper layers remained alive merely based on the diffusion of essential gasses and nutrients, and no evidence of neovascularization inside the tissue was observed, as we did not add any angiogenic growth factor into the scaffold. Here, we have described the three grown layers in greater detail.
FIG. 3.
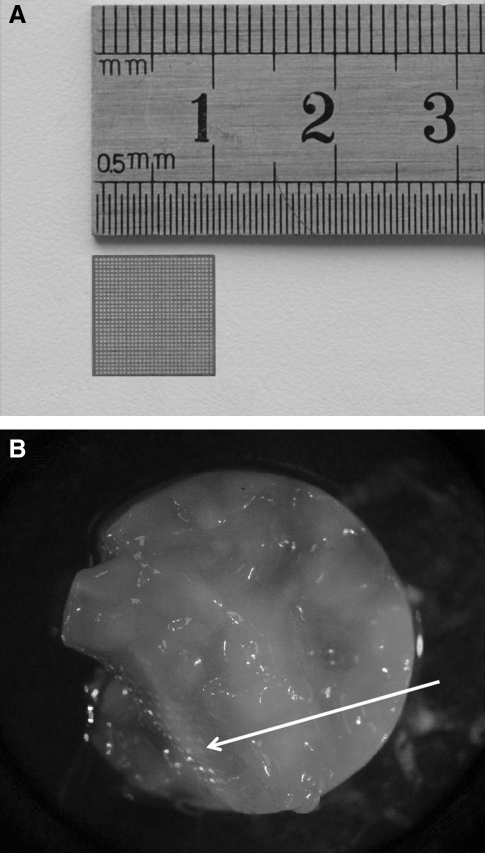
(A) Stainless Steel mesh with a surface area of about 1 cm2; (B) macroscopic view of the engineered hybrid tissue after 3 months of cell culture. White arrow shows the metallic mesh inside the tissue.
Smooth muscle cell layers
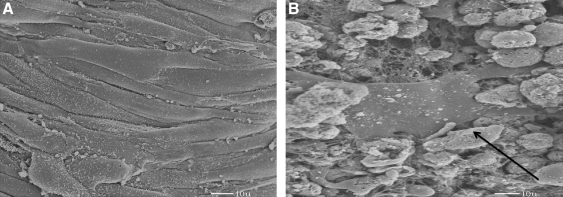
These layers are the ones covering the mesh. They were first arranged as a cluster of single cells when plated in aligned collagen matrix (Fig. 4), similar to the works done by other investigators.22,23 Later, they were arranged in small bundles of 5–20 cells within the first covering layer, started attaching to the wires' surfaces through the openings of the mesh, and were spread throughout the mesh making a tight network around it (Fig. 4A). Populated constructs compacted during the culture period, which confirmed expression of α-SMA, as its expression is correlated with gel compaction in this type of cell. The formed gel degreased rapidly before the next layer was cultured on it (Fig. 4B).
FIG. 4.
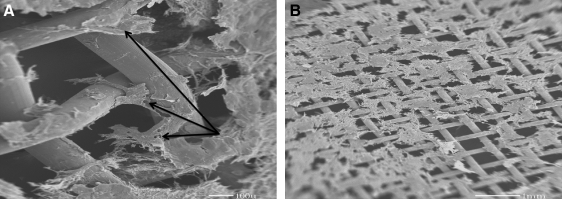
Scanning electron micrographs of the first layer on the mesh show that smooth muscle cells are attached over the mesh. The collagen gel degrades rapidly, and the cells are attached throughout the mesh either in bundles or as single cells; (A) The same image as (A); arrows indicate smooth muscle cells attached to mesh; (B) A larger view that shows the arrangement of first layer, and verifies that collagen gel has been degraded.
Fibroblast/Myofibroblast cell layers
These layers covered on top of the first layers, in parallel alignment with the underlying cell layer. After a short period of time, the collagen layers on the top and bottom of the culture altered in such a way that they no longer stayed aligned. There was a significant decrease in the degradation rate of collagen matrix in culture. They started maintaining the ECM, which was obvious during the microscopy phases, but increasing the number of layers required daily changing of the media due to high oxygen and nutrition consumption. The addition of TGF-β increased the number of cells with either fibroblasts or myofibroblasts within the second layer (Fig. 5).
FIG. 5.
Scanning electron microscopy images taken after culturing the second layer of cells containing fibroblasts/myofibroblasts; (A) formation of ECM and a confluent layer around the construct are visible, (B) black arrow indicates a single fibroblast cell inside view of the tissue. ECM, extracellular matrix.
Endothelial cell layer
Seeding the third layer completely concealed the mesh and formed a smooth, confluent surface around the construct (Fig. 3B). The endothelial layer seemed to be strongly attached to the inner two layers and participated in remodeling activities of the membrane. This stable lining layer potentially provides a surface with reduced thrombogenicity when implanted in-vivo. The biologic part of the membranes showed some level of shrinkage after the addition of each layer, mainly due to utilizing collagen as the biologic scaffold. The shape of hybrid tissues was monitored every week. It was found that the tissue was maximally organized, and the shrinkage terminated after the eight week. The mesh acts as a backbone that minimizes further shrinkage of the membrane by tightly holding each segment of the tissue together. No further changes in dimension of the tissue construct were observed thereafter.
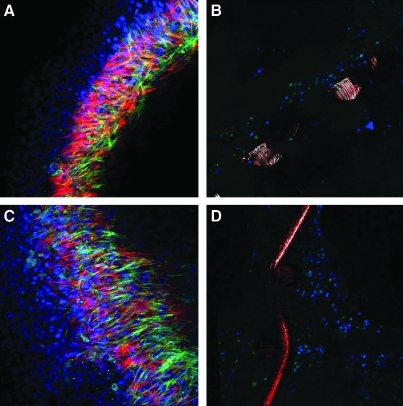
Figure 6 shows the result of confocal microscopy from top and side views at the end of the eighth week. At this stage, the deeper layers had a denser cellularity compared with the superficial layers, which were less packed. The control group showed tissue formation mainly at the surface of the mesh, which was less dense compared with the group with TGF-β supplement. Additionally, the group with TGF-β supplement showed an increase in proliferation and more ingrowths into the outer portions of the tissue. Figure 7 shows the result of SEM at the end of eighth week with all three layers present. It can be observed that adding the second and the third layers improves production of the ECM (mainly collagen and glycosaminoglycans) that covers the mesh, forming a confluent smooth surface with endothelial cell lining in both experimental groups.
FIG. 6.
Compares the images of confocal microscopy with and without addition of TGF-β. Greater extracellular matrix deposition is observed when TGF-β is added compared with the control groups. DAPI staining of nuclei in the construct shows that the number of cells at the surface of the mesh increased progressively in TGF-β groups, eventually formed a thicker tissue around the mesh; (A) control group from top view, (B) control group from side view; (C) TGF-β group from top view; and (D) TGF-β group from side view. Nuclei, fibroblasts, smooth muscle cells, and endothelial cells were stained as blue, green, red, and magenta, respectively. TGF-β, transforming growth factor.
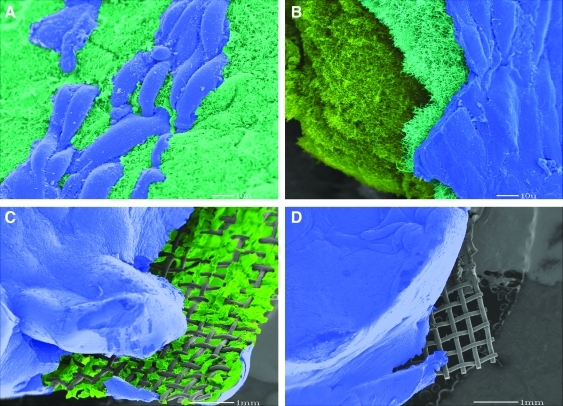
FIG. 7.
Result of scanning electron microscopy after 8 weeks with three layers of cells shows the formed tissue tightly enclosing the Stainless Steel mesh; (A) endothelial surface layer (blue) starts covering the construct right after culturing around it; (B) after 8 weeks, the tissue shows three different cell layers in sequence; surface endothelial layer (blue), middle fibroblast/myofibroblast (emerald green), and base layer of smooth muscle (bright green); (C) and (D) show that the mesh is tightly integrated within the tissue construct, and the cells are penetrating through the mesh opening holes. The tissue is intentionally sliced before microscopy to visualize the layers and the mesh.
Discussion
The advantages of using completely biological matrix components are biocompatibility, cell-controlled degradability, and intrinsic cellular interaction. Although biological scaffolds appear to be the best choice for engineering tissue membranes so far, none has fulfilled all the requirements of an optimal scaffold; in particular, they lack structural resistance and desired mechanical property. In this study, we have devised a hybrid concept for tissue membranes that potentially overcomes the mechanical vulnerability limitations of biological ECMs while maintaining the biocompatibility and nontoxicity of them. This new technology provides an opportunity to develop membranes that are strong enough to resist the forces that exist inside the body (e.g., the fluid forces that are exerted on artificial heart valves inside the heart or the radial stresses available in a growing arterial aneurism) while possessing biocompatible surfaces.
Heart valve leaflets experience a variety of cyclic mechanical stresses inside the body, which are dynamic and complex, including (1) shear stress due to blood flow when the valve is open, (2) flexural stress due to cyclic opening and closure, and (3) tensile stress when the valve is closed. In natural valves, the principal determinant of the valve durability is the valvular ECM composition, which acts as the leaflet's backbone withstanding the stresses.24 Forces acting on the leaflets at the macroscopic level are translated into specific biomechanical responses at the tissue level, which will be transduced into VICs that are responsible for dynamic composition of the leaflet tissue. When the leaflets are exposed to higher levels of stress, higher stiffness is induced by VICs through increasing the α-smooth muscle actin (α-SMA) contents and additional collagen biosynthesis in the leaflet.25 Such an adapting mechanism does not currently exist in the bioprosthetic heart valve, which are glutaraldehyde fixed tissues.26,27 As a result, these valves lack dynamic control of tissue strength and are vulnerable when exposed to loads exerted on them in cardiac cycles.
Another application of hybrid tissue is in the development of vascular grafts. Investigators have tried using synthetic materials such as expanded polytetrafluoroethylene and Dacron (polyethylene terephthalate fiber) to bypass stenotic arteries in peripheral vascular disease28,29 and to replace an aneurismal segment.30 It has been shown that replacement of vessels with purely synthetic polymeric conduits often leads to an early failure of such grafts mainly due to the formation of thrombosis; this is particularly true in the smaller-diameter vasculature (i.e., less than 6 mm) and in the regions of low blood flow.31–33 These materials lack growth potential, and the long-term results have revealed several cases of stenosis, aneurismal formation, thromboembolization, calcium deposition, and infection, mainly due to material failure.34 The ineffectiveness of these materials is further noticeable in situations where a mechanical device needs to be anastomosed to the natural vasculature or to the heart; for example, ventricular assist devices. Vascular grafts made of hybrid tissues with metal scaffolds as the core may provide better solutions for the difficulty in anastomoses that arises from connection of a nonbiologic device to a biologic structure (e.g., vasculature, heart chambers, or loops of intestines). The scaffolds can either be sutured at the edges of the membranes that do not have a metal mesh core (Fig. 3) or by considering larger holes at the edge of the mesh just for suturing purposes.
Challenges in development of hybrid tissues
One of the main issues regarding the use of a metal scaffold is cell-metal interaction.35,36 Surface properties such as roughness, hydrophilicity, chemical composition, texture, charge, morphology, surface energy, and surface chemistry considerably influence the cellular responses to the metallic substrates. Studies have shown that cell attachment increases when the oxygen content at the surface of Stainless Steel is low. However, there has been little discussion regarding the effects of surface roughness.37,38 The cell attachment was found to increase due to surface energy enhancement, and change in surface roughness (decrease or increase depending on the cell type).37,39,40 In the current study, we used high-grade SiC polishing papers to make the surface smoother, which considers more favorable for attachment of smooth muscle cells (unlike the bone implantations). Surface energy has been identified as the main factor in the modification of the wettability characteristics of the metal and, therefore, a predominant mechanism governing the cell proliferation.41,42 We also found that glow discharging, which increases the surface energy, can enhance cell binding and proliferation.
Another strategy to improve the biocompatibility of Stainless Steel meshes is ion beam surface modification. It has been previously reported that ion beam surface modification of polymeric and metallic substrates can enhance cell attachment and growth,43,44 increase biocompatibility,45,46 prevent calcification,47 enhance adhesive strength between polymers and metals,48 create active binding sites for protein,49 control surface wettability,46,50 and even inhibit platelet adhesion.21 To achieve the best surface modification, we performed He+ ion beam irradiation of Stainless Steel meshes at an energy level of 150 keV with fluences of 1×1014 ions/cm2. This technique significantly improved cell attachment compared with the meshes without ion beam modification.
Limitations
In this work, we have used Stainless Steel meshes as test materials. However, these meshes may not provide adequate recoil required for dynamic tissue membranes such as heart valve leaflets. Further studies with superelastic Nitinol and Titanium are underway.
Using an improper mesh may result in aberrant remodeling of the tissue. We are currently working with thinner meshes (∼25 micron thickness) with a larger open area percentage to reduce the chance of abberant remodeling. Additionally, we have not yet investigated the performance of these constructs in dynamic environments that mimic blood circulation. However, we expect that all the layers stay together without separation, and the thin metal mesh, due to its elasticity, performs passively when there is a flow.
Conclusions
This study focused on the developement of a novel hybrid tissue membrane comprising a metal mesh scaffold. The metal mesh scaffold overcomes the disadvantages associated with current polymeric, decellularized, and biological scaffolds by reinforcing the cultured tissue engineered membrane in vitro and in vivo after implantation. This developed hybrid tissue structure posseses a biological surface similar to a natural membrane while enclosing a thin metal mesh core that replicates the strong natural ECM backbone of the natual membranes inside the body. This technology is supposed to have applications for the development of membrane-like tissue constructs such as heart valves and vascular grafts, which are exposed to a variety of dynamic loads.
Acknowledgments
The authors would like to thank Prof. Richard Goodwin and Prof. Thomas Borg from University of South Carolina for their insightful comments and advice about this project, and Ms. Lorain Junor for her technical support during the project. This research was partially supported by a seed grant from The Edwards Lifesciences Center for Advanced Cardiovascular Technology and the grant number UL1 RR031985 from the National Center for Research Resources (NCRR) through the Institute for Clinical and Translational Science (ICTS) at UC Irvine.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hoerstrup S.P. Sodian R. Daebritz S. Wang J. Bacha E.A. Martin D.P. Moran A.M. Guleserian K.J. Sperling J.S. Kaushal S. Vacanti J.P. Schoen F.J. Mayer J.E., Jr Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102:44. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- 2.Sodian R. Hoerstrup S.P. Sperling J.S. Daebritz S. Martin D.P. Moran A.M. Kim B.S. Schoen F.J. Vacanti J.P. Mayer J.E., Jr Early in vivo experience with tissue-engineered trileafet heart valves. Circulation. 2000;102(19 Suppl 3):iii22. doi: 10.1161/01.cir.102.suppl_3.iii-22. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland F.W. Perry T.E. Yu Y. Sherwood M.C. Rabkin E. Masuda Y. Garcia A. McLellan D.L. Engelmayr G.C. Sacks M.S. Schoen F.J. Mayer J.E., Jr From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- 4.Shinoka T. Breuer C.K. Tanel R.E. Zund G. Miura T. Ma P.X. Langer R. Vacanti J.P. Mayer J.E. Tissue engineering heart valves: valve leafet replacement study in a lamb model. Ann Thorac Surg. 1995;60:13. doi: 10.1016/0003-4975(95)00733-4. [DOI] [PubMed] [Google Scholar]
- 5.Shinoka T. Ma P.X. Shum-Tim D. Breuer C.K. Cusick R.A. Zund G. Langer R. Vacanti J.P. Mayer J.E. Tissue-engineered heart valves: autologous valve leafet replacement study in a lamb model. Circulation. 1996;94(Suppl):ii164. [PubMed] [Google Scholar]
- 6.Sales V.L. Engelmayr G.C. Johnson J.A. Gao J. Wang Y. Sacks M.S. Mayer J.E., Jr Protein precoating of elastomeric tissue-engineering scaffolds increased cellularity, enhanced extracellular matrix protein production, and differentially regulated the phenotypes of circulating endothelial progenitor cells. Circulation. 2007;116:55. doi: 10.1161/CIRCULATIONAHA.106.6806637. [DOI] [PubMed] [Google Scholar]
- 7.Kim B.S. Nikolovski J. Bonadio J. Smiley E. Mooney D.J. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Exp Cell Res. 1995;15:318. doi: 10.1006/excr.1999.4595. [DOI] [PubMed] [Google Scholar]
- 8.Rowley J.A. Madlambayan G. Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 9.Hutmacher D.W. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 10.Hutmacher D.W. Goh J.C. Teoh S.H. An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med Singapore. 2001;30:183. [PubMed] [Google Scholar]
- 11.Chen G. Ushida T. Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002;2:67. [Google Scholar]
- 12.Gunatillake P.A. Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 13.Bader A. Schilling T. Teebken O.E. Brandes G. Herden T. Steinhoff G. Haverich A. Tissue engineering of heart valves—human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg. 1998;14:279. doi: 10.1016/s1010-7940(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien M.F. Goldstein S. Walsh S. Black K.S. Elkins R. Clarke D. The synergraft valve: a new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical implantation. Semin Thorac Cardiovasc Surg. 1999;11:194. [PubMed] [Google Scholar]
- 15.Sievers H.H. Stierle U. Schmidtke C. Bechtel M. Decellularized pulmonary homograft (synergraft) for reconstruction of the right ventricular outflow tract: first clinical experience. Z Kardiol. 2003;92:53. doi: 10.1007/s00392-003-0883-x. [DOI] [PubMed] [Google Scholar]
- 16.Stock U.A. Schenke-Layland K. Performance of decellularized xenogeneic tissue in heart valve replacement. Biomaterials. 2006;27:1. doi: 10.1016/j.biomaterials.2005.05.100. [DOI] [PubMed] [Google Scholar]
- 17.Robinson P.S. Johnson S.L. Evans M.C. Barocas V.H. Tranquillo R.T. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A. 2008;14:83. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 18.Neidert M.R. Tranquillo R.T. Tissue-engineered valves with commissural alignment. Tissue Eng. 2006;12:891. doi: 10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- 19.Seliktar D. Nerem R.M. Galis Z.S. The role of matrix metalloproteinase-2 in the remodeling of cell-seeded vascular constructs subjected to cyclic strain. Ann Biomed Eng. 2001;29:923. doi: 10.1114/1.1415522. [DOI] [PubMed] [Google Scholar]
- 20.Wang X. Lei M.K. Zhang J.S. Surface modification of 316l stainless steel with high-intensity pulsed ion beams. Surf Coat Technol. 2007;201:5884. [Google Scholar]
- 21.Sugita Y. Suzuki Y. Someya K. Ogawa A. Furuhata H. Miyoshi S. Motomura T. Miyamoto H. Igo S. Nosé Y. Experimental evaluation of a new antithrombogenic stent using ion beam surface modification. Artif Organs. 2009;33:456. doi: 10.1111/j.1525-1594.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- 22.Bairati A. DeBiasi S. Presence of a smooth muscle system in aortic valve leaflets. Anat Embryol. 1981;161:329. doi: 10.1007/BF00301830. [DOI] [PubMed] [Google Scholar]
- 23.Cimini M. Rogers K.A. Boughner D.R. Smoothelin-positive cells in human and porcine semilunar valves. Histochem Cell Biol. 2003;120:307. doi: 10.1007/s00418-003-0570-z. [DOI] [PubMed] [Google Scholar]
- 24.Schoen F.J. Future directions in tissue heart valves: impact of recent insights from biology and pathology. J Heart Valve Dis. 1999;8:350. [PubMed] [Google Scholar]
- 25.Merryman W.D. Youn I. Lukoff H.D. Krueger P.M. Guilak F. Hopkins R.A. Sacks M.S. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290:224. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 26.Schoen F. Levy R. Pathology of substitute heart valves. J Card Surg. 1994;9:222. doi: 10.1111/j.1540-8191.1994.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoganathan A. The Biomedical Engineering Handbook. Boca Raton: CRC Press; 1995. Cardiac Valve Prostheses. [Google Scholar]
- 28.Bauermeister G. Endovascular stent-grafting in the treatment of superficial femoral artery occlusive disease. J Endovasc Ther. 2001;8:315. doi: 10.1177/152660280100800312. [DOI] [PubMed] [Google Scholar]
- 29.Willigendael E.M. Teijink J.A.W. Bartelink M.L. Peters R.J.G. Büller H.R. Prins M.H. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J Vasc Surg. 2005;42:67. doi: 10.1016/j.jvs.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Sporn E. Kubin K. Hoblaj T. Ploner M. Böhmig H.J. Nanobashvili J. Huk I. Prager M.R. Polterauer P. Dacron grafts dilate more than stretch ptfe grafts after abdominal aortic aneurysm repair–long-term results of a prospective randomized trial. Eur Surg. 2008;40:66. [Google Scholar]
- 31.Greisler H.P. Interactions at the blood/material interface. Ann Vasc Surg. 1990;4:98. doi: 10.1007/BF02042699. [DOI] [PubMed] [Google Scholar]
- 32.Whittemore A.D. Kent K.C. Donaldson M.C. Couch N.P. Mannick J.A. What is the proper role of polytetrafluoroethylene grafts in infrainguinal reconstruction? J Vasc Surg. 1989;10:299. doi: 10.1067/mva.1989.14116. [DOI] [PubMed] [Google Scholar]
- 33.Faries P.L. Logerfo F.W. Arora S. Hook S. Pulling M.C. Akbari C.M. Campbell D.R. Pomposelli F.B., Jr A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080. doi: 10.1067/mva.2000.111279. [DOI] [PubMed] [Google Scholar]
- 34.Kirklin J.W. Barratt-Boyes B.G. Ventricular septal defect with pulmonary stenosis or atresia. In: Kouchoukos N.T., editor; Blackstone E.H., editor; Doty D.B., editor; Hanley F.L., editor; Karp R.B., editor. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results and indicator. 3rd. Churchill; Livingstone, PA: 2003. pp. 946–1074. [Google Scholar]
- 35.Gaudenzi S. Furfaro M.G. Pozzi D. Silvestri I. Congiu Castellano A. Cell-metal interaction studied by cytotoxic and ft-ir spectroscopic methods. Environ Toxicol Pharmacol. 2003;14:51. doi: 10.1016/S1382-6689(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 36.Fermoso F.G. Bartacek J. Jansen S. Lens P.N. Metal supplementation to uasb bioreactors: from cell-metal interactions to full-scale application. Sci Total Environ. 2009;407:3652. doi: 10.1016/j.scitotenv.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Braunovic M. Konchits M.M. Myshkin N.K. Electrical contact: fundamentals, applications and technology. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 38.Oshida Y. Sachdeva R. Miyazaki S. Daly J. Effects of shot-peening on surface contact angles of biomaterials. J Mater Sci Mater Med. 1993;4:447. [Google Scholar]
- 39.Boyan B.D. Hummert T.W. Dean D.D. Schwartz Z. Role of material surfaces in regulating bone and cartilage cell responses. Biomaterials. 1996;17:137. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 40.Kieswener K. Schwartz Z. Hummert T.W. Cochran D.L. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like mg-63 cells. J Biomed Mater Res. 1996;32:55. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence J. Li L. On the mechanisms of wetting characteristics modification for selected metallic materials by means of high power diode laser radiation. J Laser Appl. 2002;14:107. [Google Scholar]
- 42.Schakenraad J.M. Busscher H.J. Wildevuur C.R.H. Arends J. Influence of substratum surface free energy on growth and spreading of human fibroblasts in the presence and absence of serum proteins. J Biomed Mater Res. 1986;20:773. doi: 10.1002/jbm.820200609. [DOI] [PubMed] [Google Scholar]
- 43.Pignataro B. Conte E. Scandurra A. Marletta G. Improved cell adhesion to ion beam-irradiated polymer surfaces. Biomaterials. 1997;18:1461. doi: 10.1016/s0142-9612(97)00090-2. [DOI] [PubMed] [Google Scholar]
- 44.Maritz M.F. Pham M.T. Matz W. Reuther H. Steiner G. Richter E. Ion beam treatment of titanium surfaces for enhancing deposition of hydroxyapatite from solution. Biomol Eng. 2002;19:269. doi: 10.1016/s1389-0344(02)00039-4. [DOI] [PubMed] [Google Scholar]
- 45.Leiao E. Barbosa M.A. De Groot K. In vitro testing of surfacemodified biomaterials. J Mater Sci Mater Med. 1998;9:543. doi: 10.1023/a:1008896106994. [DOI] [PubMed] [Google Scholar]
- 46.Shenton M.J. Bradley J.W. van den Berg J.A. Armour D.G. Stevens G.C. Ultralow energy ion beam surface modification of low density polyethylene. J Phys Chem B. 2005;109:22085. doi: 10.1021/jp054758m. [DOI] [PubMed] [Google Scholar]
- 47.Kondyurin A. Pecheva E. Pramatarova L. Calcium phosphate formation on plasma immersion ion implanted low density polyethylene and polytetrafluorethylene surfaces. J Mater Sci Mater Med. 2008;19:1145. doi: 10.1007/s10856-007-3231-2. [DOI] [PubMed] [Google Scholar]
- 48.Seo Y. Kim S. Kim H. Kim J. Nonwetting process for achieving surface functionalization of chemically stable poly(tetrafluoroethylene) Langmuir. 2005;21:3432. doi: 10.1021/la047690v. [DOI] [PubMed] [Google Scholar]
- 49.Gan B.K. Kondyurin A. Bilek M.M.M. Comparison of protein surface attachment on untreated and plasma immersion ion implantation treated polystyrene: Protein island and carpet. Langmuir. 2007;23:2741. doi: 10.1021/la062722v. [DOI] [PubMed] [Google Scholar]
- 50.Walachova K. Svorcik V. Bacakova L. Hnatowicz V. Colonization of ion-modified polyethylene with vascular smooth muscle cells in vitro. Biomaterials. 2002;23:2989. doi: 10.1016/s0142-9612(02)00029-7. [DOI] [PubMed] [Google Scholar]