Abstract
We report for the first time the fabrication of a three-dimensional tissue structure containing, in a continuous layer, the morphological features of a lip: epidermal skin, vermillion, and oral mucosa. This tissue engineered muco-cutaneous (M/C) equivalent was manufactured using human oral and skin keratinocytes grown on an acellular, nonimmunogenic dermal equivalent (AlloDerm®) to produce a tissue equivalent with similar anatomic and handling properties as native human lips. Confirmation of the structural composition of the construct was performed using routine histology and immunohistochemistry by identification of epithelial markers that are differentially expressed in separate anatomic areas of the lips. These full-thickness human lip skin equivalents can be used in surgical lip reconstruction in individuals suffering from lip loss from cancer, congenital deformations, and injuries after accidents. We propose this technique can be used as a general basis for tissue engineering of M/C junctions in other parts of the body, such as anus and vagina.
Introduction
A difficult structure of the face to reconstruct after surgery or avulsion is the lips because they represent a composite tissue of mucosa, skin, and muscles1–3 Significant loss of lips is a functional and esthetic concern because the neuromuscular control of normal lip structures is required for activities of eating, drinking, talking, and social gestures. Avulsion or partial loss of the lips is a survivable injury but without functional lip reconstruction life for injured individuals is burdened by drooling, food spillage while eating, unintelligible speech, and social rejection. Functional reconstruction of the lips is so important that when greater than 50% of the lips are avulsed face transplantation under lifetime immunosuppression becomes an option.
Tissue engineering and regenerative medicine offer a unique opportunity to create composite tissue structures in the area of soft tissue reconstruction with tissue units with a muco-cutaneous (M/C) junction such as the lip. Several models of in vitro bioengineered human skin4,5 and oral mucosa6–8 have been developed to date but separately used in applications to repair, respectively, skin and oral mucosa. These models can be classified in different ways: by the anatomical structure they target (epidermis, dermis, or both), by the type of materials used (biological, synthetic), by the cellular component present in the composite (cellular or acellular), or by the technique (in vitro, in vivo).9–11 These bioengineered tissue equivalents are developed to address epidermal, partial thickness, and full thickness injuries and so the approach to use them is different.12 Several products are currently commercially available and have been extensively described.5 None of them, however, present a tissue composite with a M/C junction. For this reason, development of continuous human oral mucosa-lip-skin constructs or with other M/C junctions is necessary. The oral mucosa forms the inner aspect of the lip and extends and unites with the skin by a junction known as the vermillion border that unites the oral mucosa with the skin of the face.13,14
Here we report for the first time the fabrication of a M/C tissue engineered construct in vitro. The process consists of growing human oral and skin keratinocytes into a three-dimensional (3D) construct to form a M/C junction containing stratified oral mucosa and stratified skin on the same dermal component. This 3D lip construct contains all functional areas of the epithelium of the human lip and can be used as the basis for the fabrication of vascularized prelaminated flaps for full lip reconstruction or for repair of the lips when both the oral and epidermal areas require tissue replacement. In addition, this technique can be extended to other anatomic areas for the repair of tissues where a M/C junction is present, including the nares, eyelids, vagina, and others. Cancer patients are one group of patients who will also benefit since cancer surgery leaves complicated defects that can be repaired using this type of technology, and treatment of developmental defects in children.
The manufacture of the M/C constructs is based on a technique previously reported by Izumi et al15 where oral keratinocytes were used to prepare human oral mucosa engineered constructs termed ex vivo produced oral mucosal equivalents (EVPOME) consisting of a dermal component with AlloDerm®, (LifeCell, Branchburg, NJ) that is seeded with autologous human oral keratinocytes to form an overlying stratified parakeratinized or nonkeratinized epithelial layer.
Materials and Methods
Fabrication of 3D lip constructs
We used previously frozen oral and skin adult human keratinocytes to fabricate a composite EVPOME. Full thickness human skin (from a breast reduction) and oral mucosa explants (taken from the upper inner aspect of the lip) that came from two different individuals obtained from discarded material from surgeries performed at the University of Michigan Health System. The Institutional Review Board approved use of the mucosa and skin, and donors provided informed consent for research use. Preparation of the explants and extraction of keratinocytes was performed as previously reported by Marcelo16 for skin and by Izumi et al17 in the case of oral mucosa. Tissue samples were digested with a 0.04% trypsin solution (oral tissues) or 0.125% trypsin solution (skin tissues) overnight at room temperature (Sigma-Aldrich, St Louis, MO). Enzymatically dissociated keratinocytes were plated at a density of 7.0×106 cells per T-25 flask (Corning, Corning, NY). The cells were cultured without a feeder layer, fetal bovine serum, or bovine pituitary extracts in an animal product-free culture medium, EpiLife® M-EPI-500, (Cascade Biologics, Portland, OR) that was supplemented with EpiLife defined Growth Supplement (EDGS; Cascade Biologics). In addition, the medium was supplemented with 25 μg/mL of Gentamicin (Sigma-Aldrich) and 0.375 μg/mL of Amphothericin B (Gibco, Invitrogen Carlsbad, CA). Primary keratinocytes were harvested when 70%–80% confluent and were subsequently subcultured for use in the fabrication of EVPOMEs. In these techniques, both oral and skin keratinocytes are expanded in a serum-free defined low calcium medium (0.06 mM CaCl2).
The primary oral and skin keratinocytes were cultured and grown in separate culture flasks and passaged to reach passage 2 and then frozen. Afterward, they were thawed and cultured to reach the necessary number of cells for seeding on the dermal allograft, at a density of 2.5×105 cells/cm2. This portion of the manufacturing protocol can vary in time and is mostly dependent on the need for oral keratinocyte amplification given that the oral explants are typically smaller. The number of keratinocytes necessary for fabrication of the 3D lip construct must be calculated because it is a function of the size of the surgical site grafting. The AlloDerm® was presoaked in 5 μg/cm2 human type IV collagen (Becton Dickinson Labware, Bedford, MA) for a minimum of 3 h before keratinocyte seeding. Once a sufficient number of both skin and oral keratinocytes had been grown in culture flasks, they are seeded in two separate compartments on the same piece of type IV collagen coated AlloDerm® to create an interface between the two keratinocyte populations (Fig. 1B). In the experimental protocol reported here, passage 3 cells were used for seeding in the 3D lip constructs.
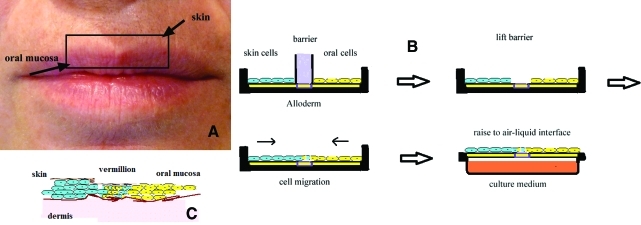
FIG. 1.
(A) This picture helps visualize the concept behind the construction of the three-dimensional (3D) lip tissue engineering construct. The inner aspect of the lip is made of oral mucosa and the outer aspect is made of epithelial cells. They form a muco-cutaneous junction at the vermillion. (B) Schematic of the system for the preparation of the 3D lip construct. The cells are seeded over an Alloderm® membrane temporarily divided (overnight) by a removable barrier made of polydimethylsiloxane (PDMS). After a few days in culture the whole construct is raised to the air/liquid interface to become a 3D organotypic construct. (C) Diagrammatic representation of the components of the muco-cutaneous junction of a lip. Oral and skin cells mingle in the vermillion. Color images available online at www.liebertonline.com/tec
The basic features of the procedure are represented in Figure 1. Figure 1A visualizes the concept behind the construction of the tissue engineering equivalent. The 3D lip construct consists of two areas separated by a removable barrier that can be made of varied thicknesses and manufactured using a biocompatible polymeric material, polydimethylsiloxane (PDMS; Sylgard 184 Dow Corning) (Fig. 1B). On one side skin keratinocytes were seeded and on the other side oral mucosa keratinocytes were seeded at the same concentration. It is convenient to resuspend the cells in low volumes of medium (125 μL) and carefully distribute them across the surface of the AlloDerm® to allow them to quickly settle for 30- to 45 min and then raise the medium volume. The intermediate area, the future vermillion border, was of a thickness equal to that of the removable barrier, 3 mm, remained without cells. The barriers were left in situ overnight and lifted the next day allowing the cells of the skin and oral areas, by migration, to come into contact to form the underlying basic lip structures (Figs. 1C and 2D, right).
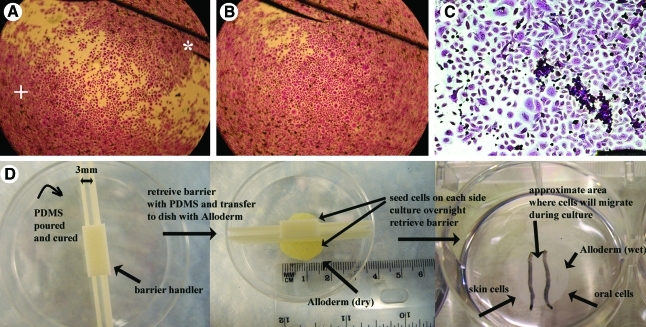
FIG. 2.
Before using Alloderm®, in preliminary experiments, oral (*) and skin keratinocytes (+) were seeded in Petri dishes with a PDMS barrier (black line in upper right quadrant) to determine whether they will mingle and form the equivalent of a vermillion. (A) Initial seeding, (B) oral and skin keratinocytes advancing fronts “mix” in the middle to form a “vermillion” after migrating in the center of the Petri dish, and (C) fixed and stained oral and skin keratinocytes after 6 days in culture showing confluence and “mixing” of two cell populations. We experimented with barriers of varied thicknesses and lengths to separate the areas where the cells would be seeded to adjust to the sizes of Petri dishes (D, left) or six-well cultures (D, right pictured without the barrier with the central area indicated with black lines). The process is explained in the sequence of image D. The plastic barrier is filled with PDMS in a separate container to allow curing of the PDMS and then used by placing it to adjust over the Alloderm® (tissue at the bottom in center D) and the container. The barrier shown in D works as a mold to pour the PDMS but several variations of this technique can be implemented to fit the needs of the researcher or to adjust to clinical applications. Color images available online at www.liebertonline.com/tec
The AlloDerm® composite with the seeded cells was then cultured and submerged for 4 days, during which time they migrated to form a continuous epithelial monolayer. After that period, the concentration of calcium in the culture medium was raised to 1.2 mM to enhance keratinocyte differentiation.18–20 After 4 more days of culture in submersion at 1.2 mM calcium concentration, the 3D lip constructs were raised to the air-liquid interface and maintained for 7 days to encourage stratification of the epithelial monolayer, for a total of 15 days. This resulted in a 3D construct with two stratified epithelial components, epidermal and oral. It should be noted that the number of days in this protocol are flexible and needs to be adjusted to the size of the AlloDerm® needed for reconstruction to allow enough time for cellular migration to form the vermillion.
In a separate and preliminary experiment, a 3D construct was fabricated in which no migration period was allowed and where the cells in the two areas did not migrate because they were maintained in 1.2 mM calcium medium from the beginning of the experiment and the submersed incubation time was only 4 days. In this special case, the oral cells used came from the masticatory mucosa of the alveolar ridge which has a keratinization profile similar to that of skin.
Materials and components design
AlloDerm® membranes coated with type IV collagen were placed in a 2 cm diameter Transwell carrier system (shown at the bottom of Fig. 2D for illustration purposes). The barriers (also pictured in Fig. 2D) were made of acrylonitrile butadiene styrene (ABS) plastic (yellow) using fused deposition modeling with a Dimension Elite FDM 3D Printer (Stratasys, Eden Prairie, MN) available at the University of Michigan 3D Lab, a system that is used to print functional models of parts such as medical devices. The barriers work as a mold, are put inside a Petri dish and PDMS is poured and then cured (left image, Fig. 2D). After curing, the barriers with the PDMS are retrieved and can be used for the experiments as is. Another option is to retrieve the cured PDMS molded in the space between the plastic barriers and directly use it. Both options were successfully tested in our experiments but it was easier to handle the combined plastic barriers with the PDMS rather than the PDMS alone. The yellow plastic barriers were designed with a small handler on top to allow easier manipulation. In these experiments the PDMS provided a temporary hydrophobic membrane to separate cell populations seeded on each side of the barriers. The barrier is placed over the AlloDerm® (center image, Fig. 2D) exerting a slight pressure to secure the barrier and to prevent substantial transfer of the cell containing medium from one area to the other. It should be noted, however, that the barriers used cannot provide a complete seal to prevent liquid transfer because exerting too much pressure will compress the AlloDerm®. As can be seen in our results, liquid exchange between the two areas did not prevent the formation of the full construct. Given that the polycarbonate membrane of the Transwell carrier is too weak and thin to withstand any pressure without breakage, we found that the easiest way of seeding the cells on the AlloDerm® without cell mixing would be to use small six-well plates or similar sized Petri dishes (Fig. 2D right). The constructs and cells are then cultured, submersed, in these plates until the time they are transferred to the air-liquid interface carrier.
Preliminary evaluation of the formation of a cellular M/C junction
Before using the AlloDerm®, a preliminary experiment was designed where oral keratinocytes from the inner aspect of the upper lip and skin keratinocytes were seeded, without AlloDerm®, directly on regular cell culture treated Petri dishes divided in half by a PDMS barrier, as described earlier. After cell seeding and after removal of the barrier the next day, keratinocytes were culturedand submersed for a total of 6 days. At the end of this period the cells were fixed with 95% ethanol and stained with Coomassie Blue before light microscopy evaluation.
Keratinization profile of the seeded cells
The cells dissociated from the tissues and amplified for use in the 3D lip constructs were separately analyzed for epithelial markers to determine their keratinization profile. In addition, and for comparison, oral mucosa cells taken from the cheek were also studied for keratin expression because this area of the oral mucosa, the cheek, shows an in vivo keratinization profile similar to that of the inner aspect of the lip21,22 and, thus, can be used as an alternative source of the cells. The cheek cells were not used in the Alloderm® experiments.
After trypsinization and centrifugation, an aliquot of the cells (at passage 3) that were seeded on the constructs (and the cheek cells) were resuspended in EpiLife medium and cytospun at 750 rpm for 5 min in a ThermoShandon Cytopsin (ThermoSahndon, Pittsburg, PA using Shandon Cytoslide and ThermoShandon single cytology Funnel, from Fisher Scientific, Pittsburh, PA). Afterward the cells were fixed and maintained in 95% ethanol until immunohistochemical analysis was performed. The cytokeratins evaluated were K4 (mouse monoclonal, dilution 1:100; Abcam, Inc., Cambridge, MA); K10 (mouse monoclonal, dilution 1:200; Novus Biologicals, Littleton, CO); K13 (mouse monoclonal, dilution 1:100; Abcam, Inc.); and K19 (mouse monoclonal, dilution 1:100; Vector Laboratories, Burlingame, CA).
Evaluation of 3D lip manufactured constructs
Fifteen days after initial culture the 3D lip constructs were fixed in 10% phosphate buffered formalin and the morphological features evaluated by optical microscopy with routine tissue staining techniques (hematoxylin and eosin [H&E]). In addition, immunohistochemical markers were used to identify the epidermal and oral cell populations in the different areas of the M/C construct. Our initial focus was in identifying K10 positive and K4, K13, and K19 negative areas for the skin part and the opposite for the oral mucosa part.23,24
Results
Preliminary evaluation of the formation of a cellular M/C junction in vitro
Before attempting to manufacture the 3D lip construct we evaluated whether the cells would form the cellular equivalent of a M/C junction in vitro. We observed (Fig. 2A–C) that both cell strains, after 6 days of culture, formed an advancing front from the original seeding area and mingled and contacted at several points along the division where the barrier was located (which would be the equivalent of the vermillion in vivo).
Observation of cellular growth and migration during initial phase of 3D construct preparation
For the size of our constructs and concentration of cells used we found that a period of 3–5 days of preliminary culture in submersion would allow cell migration to form the interface or vermillion, but this is a parameter that needs to be adjusted to the specifics of the experimental setting.
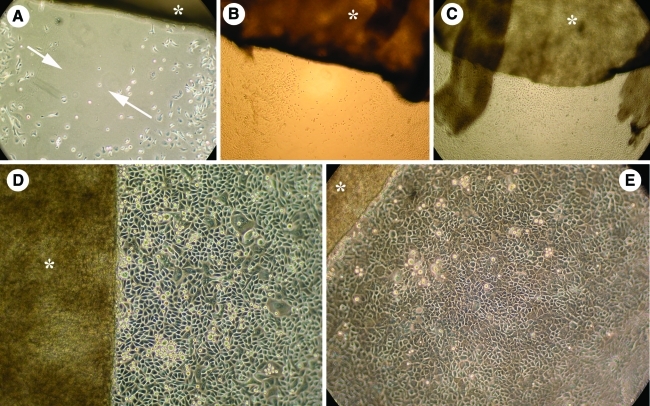
In the technique presented, where the AlloDerm® is smaller than the size of the six-well plate, we found there is enough space in the areas around the AlloDerm® to follow and observe keratinocyte expansion, migration, and differentiation during the time they are submersed in the six-well plates and before transfer to the air-liquid interface. This actually permits the person controlling the experiment to make the decision of when to increase the calcium concentration to 1.2 mM to start the differentiation process. This sequence is shown in Figure 3. We found that, effectively, both cell strains advanced at the bottom of the well plate and also on top of the AlloDerm®. Obvious phenotypic changes occurred when the calcium in the medium was raised to 1.2 mM, as observed when comparing Figure 3D, E where keratinocytes changed from a small, polygonal shape with a mosaic pattern to a larger more irregular shape with a densely packed and stratified distribution.
FIG. 3.
A technique to help control on which day the 3D lip construct should be raised to the air-liquid interface is shown in the sequence of pictures in this figure. In all pictures the Alloderm® (indicated with an *) with the cells is cultured in a six-well plate whose dimensions are bigger than the construct (see Fig. 2E). Two (A) and 3 (B) days after cell seeding the cells (skin on the left, oral on the right) can be seen growing and migrating on the bottom of the well, toward each other, in the direction indicated by the arrows. At 5 days (C) the gap has closed. This provides a good visual indication of cell migration on the Alloderm® itself and could be used as a predictor of the formation of the vermillion. The cells form a packed monolayer while cultured with low calcium medium (D), but clearly change to a differentiated cell phenotype after changing the culture medium to 1.2 mM to induce keratinocyte differentiation (E, picture taken 24 h after medium change). After 4 more days in culture in these conditions, the 3D lip construct is then raised to the air-liquid interface to allow epidermal stratification. Color images available online at www.liebertonline.com/tec
Structure of the 3D lip constructs
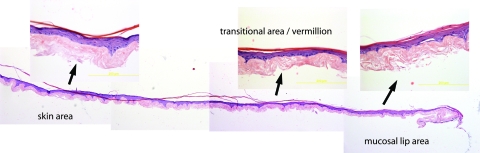
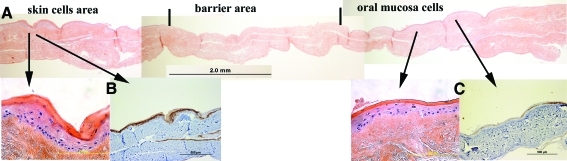
The morphology of the construct replicates a continuous 3D skin-mucosal artificial structure with well-defined mucosal lip (no granular layer) section (right part of Fig. 4) and a clearly identifiable skin section (left part of Fig. 4). The full 3D lip construct showed normal epidermal stratification several cells thick. We were not able to clearly identify the section corresponding to the vermillion or transitional area solely by observing the H&E sections, but this transition was easily identified with the cytokeratin markers.
FIG. 4.
The 3D construct shows the typical aspect of artificial epithelial replacements, both the oral and skin part, as observed in the magnified images. It should be noted however that the transitional area/vermillion cannot be identified solely using hematoxylin and eosin and it was necessary to identify its approximate location using the keratin markers k10 and k19 (see Fig. 6). Color images available online at www.liebertonline.com/tec
Keratinization profile of the cytospun cells
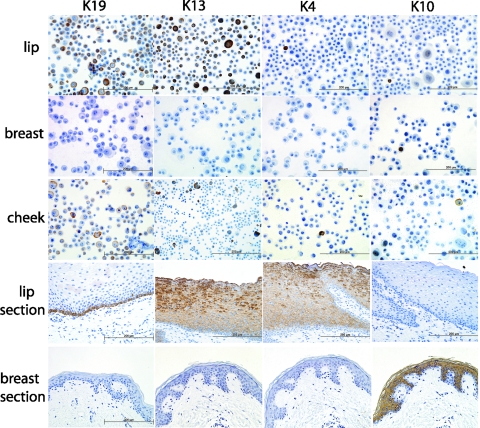
Cytospun cheek cells and, at the time of seeding on the Alloderm®, oral lip cells, did not express keratin 4 while most skin cells lacked keratin 10 (Fig. 5). This was in clear contrast to the expression of these cytokeratins in their respective native tissues, as seen observing the images in Figure 4. On the other hand, both cytospun oral cells showed strong expression of the basal cell marker keratin 19 and the suprabasal cell marker keratin 13. Broadly, these results indicated that some of the keratins expressed in vivo in oral tissues are lost or show reduced expression at the time of cell seeding when manufacturing the 3D constructs. This is summarized in Table 1 where a basic analysis of the keratin expression found in the 3D constructs, native tissues and cells is presented.
FIG. 5.
To assess changes in the keratinization profile of the seeded cells the presence of cytokeratins 19, 13, 4, and 10 was investigated. The cells shown are mucosal lip (first row), breast skin (second row), and mucosal cheek (third row). For reference, sections of labial mucosa and breast skin are also shown. Changes in the keratinization profile are most evident in the lack of keratin 4 in lip (compared to the tissue counterpart) and cheek cells and lack of keratin 10 expression in breast skin cells (compared to the breast skin tissue sections). Qualitatively, we found that cultured cells varied their keratinization profile with the passaging number and storage (freezing) (see Table 1 for additional information) but that keratin expression may recover during EVPOME reconstruction, as seen in Figure 6. Color images available online at www.liebertonline.com/tec
Table 1.
Short Summary of the Keratinization Profile Observed in Cells, Oral and Skin Specimens and Three-Dimensional Muco-Cutaneous Lip Constructs
| Notes | K4 | K10 | K13 | K19 | |
|---|---|---|---|---|---|
| Cytospun oral lip cells | From previously frozen lip cells and seeded on 3D lip construct | Negative (1 or 2 cells positive) | Negative | Present in around 10% of cells | Present in around 70%–80% of cells, particularly big size cells |
| Cytospun skin cells | From previously frozen breast skin cells and seeded on 3D lip construct | Negative | Present in around 2% of cells, mostly big size cells | Negative | Negative |
| Oral cheek cells | Never frozen cells | Negative (1 or 2 cells positive) | Negative (1 or 2 cells positive) | Present in 15% of the cells, mostly big size cells | Present in around 40% of cells, particularly big size |
| Skin | Breast skin section | Negative | Strong positive suprabasally | Negative | Negative |
| Labial mucosa | Native oral lip tissue section | Strong positive suprabasally | Negative | Strong positive suprabasally | Positive only in basal layer |
| 3D lip constructs | Fabricated with deposited lip and skin cells | Negative in all areas of the construct | Strong suprabasal presence on the skin side, expression vanishing toward the oral side | Dim staining in the oral part, negative in the rest | Positive in basal cells oral part, negative in rest |
Observations were made for cytokeratins 4, 10, 13, and 19. Compared to native tissue K4 disappears in oral mucosa cells from lip and cheek and was not observed in the 3D lip constructs. It was also noticed that previously frozen breast skin cells lost most of their keratin 10 expression. In contrast, keratin 13 and keratin 19 were observed in both cheek and lip cells but the percentage of cells expressing both markers was much higher in lip cells. Interestingly keratin 19 was present in a high percentage of the cytospun oral lip cells. Both keratin 19 and 13 were observed in the oral part of the 3D lip construct. In essence, the keratinization profile may change with the typical processing during cell culture but may recover during the time the cells are cultured on top of the AlloDerm®.
3D, three-dimensional.
Keratinization profile of the 3D lip constructs
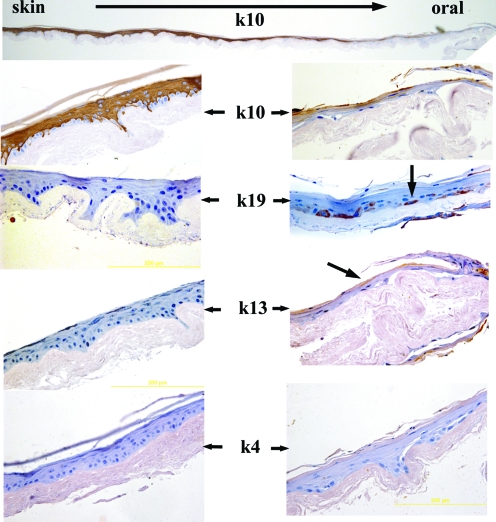
The keratinization of the constructs is clearly seen in the magnified images of Figure 6. The top image shows a full construct where strong presence of keratin 10 was suprabasally observed but gradually decreases and finally disappeared in the oral portion of the constructs. Conversely, in the oral portion, very clear presence of keratin 19 and light presence of keratin 13 was noticed. Keratin 4, which is strongly present in oral labial mucosa, was not observed in any area of the constructs probably because this depends on the thickness of the epidermal layer formed above the Alloderm. The area where keratin 10 started to disappear and keratin19 was present represents the vermillion which in native tissue is devoid of hair and glands and lacks a cornified layer.
FIG. 6.
Immunohistochemical analysis of the 3D skin lip construct for keratins K13, K19, K4, and K10. In the top image, keratin 10 is seen decreasing (in the direction of the arrow) from a strong expression in the skin area, disappearing toward the oral part. A magnified view is presented in the second row. Keratin 19 (third row) is clearly seen (arrow) in basal cells of the oral part of the lip construct and is absent in the skin part. Both K13 and K4 were also negative in the skin areas of the construct. K13 and K4, which are strongly present in the native tissue, as shown in Figure 5, are only lightly present (K13) or absent (K4) in the construct. At this stage in the development of the in vitro lip substitutes it appears that the best markers to identify both the epithelial and oral areas are K10 and K19, respectively. Color images available online at www.liebertonline.com/tec
Structure of a 3D lip construct without cell migration
When the time during which the cultures were submersed was reduced to 4 days using only 1.2 mM calcium medium (instead of using 4 days submersion in low calcium plus 4 days submersion in 1.2 mM calcium) it was observed, as expected, that the cells did not migrate after raising the construct to the air-liquid interface and the intermediate area did not show any cell ingrowth (Fig. 7). Alveolar ridge cells formed a keratinized construct, similar to the one seeded with skin cells, with strong presence of cytokeratin 10.
FIG. 7.
To determine the structure of a full construct without formation of the vermillion, a preliminary experiment was performed in which the cells were not allowed to migrate and come into contact, being kept separated over the Alloderm® when submersed and using 1.2 mM calcium medium to prevent cell migration. In this case, alveolar ridge cells were used in the oral area. In (A) a panoramic view of the full 3D lip construct is shown, with skin seeded cells on the left and alveolar ridge cells on the right. Cells did not migrate toward the center and the barrier area remained without cells. A magnified view of the structure and keratin 10 expression of both epithelial and oral areas is shown in (B) and (C) respectively. This experiment illustrates the possibilities of the technique in the formation of 3D muco-cutaneous constructs, where varied cell types can be simultaneously seeded, or not, in the different compartments formed by the barrier. Color images available online at www.liebertonline.com/tec
Discussion
With the broad objective of developing a technique for the construction of M/C junctions, we have shown that in vitro reconstruction of the skin and mucosa of the lips is feasible using a simple tissue engineering method combined with basic epithelial cell culture techniques. We combined a commercial and widely used clinical dermal substitute (AlloDerm®) with a known procedure that allows the differentiation, in vitro, of keratinocytes from donor skin and oral mucosa. By temporarily using a biocompatible polymeric barrier to separate the cell population during the time required to ensure cell seeding over the dermal substitute, we allowed the formation of a full M/C 3D structure containing all three regional lip areas. This construct can then be used as the basis for the fabrication of a vascularized prelaminated flap for the repair of the lips or for epithelial repair of areas affecting both skin and lips. In our experiments, the size of the 3D lip constructs were the equivalent of 1/3 of the linear dimension of a upper human lip (Fig. 1A), but the technique can be expanded to a full lip or even to manufacture both upper and lower lips.
Functional skin equivalents have been reported and previously used in clinical applications for treatment of burns and chronic wounds.13 In addition ex vivo produced oral mucosal equivalents have been fabricated25 and grafted into humans.26 However, they have been separately implemented and the oral constructs have only been used for intraoral grafting procedures. The keratinization profiles of skin and oral epithelia have been extensively documented previously but mostly in separate accounts.22–23,27–29 However, evaluation of the differentiation profile of the human lips had been reported in a handful of occasions, particularly by Binnie14 and Barrett.30 Both groups showed clear differences in the differentiation profile of the stratified squamous epithelium of the lips and separated them by areas: orthokeratinized epidermis, vermilion, intermediate zone (which is absent in juvenile lips) and nonkeratinized, or sometimes parakeratinized, labial (buccal) oral mucosa. The studies presented by these authors were based on the analysis of whole tissue sections from human tissues, whereas in our case we fabricated tissue equivalents in vitro.
Given that our focus is lip reconstruction as a model for a more generalized tissue engineered M/C junction, an important part of our work was focused on the analysis of the keratinization of the oral part of the 3D construct, to determine whether it maintained, in vitro, a similar keratinization profile as that of the buccal mucosa, which composed the inner aspect of the lips. The buccal mucosa is formed by the inner aspect of the lip, the cheek, and the retromolar trigone. In contrast, the gingival and hard palate (masticatory mucosa) show keratinization profiles similar to that of skin.23 For this reason, except in one preliminary experiment, we always used keratinocytes obtained from oral tissues from the inner aspect of the upper lip (labial) as the cells used in the constructs. While in clinical applications it may be possible (but not always) to obtain biopsies from facial skin of the affected area; this was not possible in our work and we used keratinocytes derived from breast reductions. The keratinization profiles of the skin part of the constructs (Figs. 5–7) indicated that, in vitro, the skin part of these M/C junction constructs could probably be grown out of disparate skin areas.
For the above reasons, we focused our immunohistochemical analysis on the most prominent differences observed in epithelial markers present or absent in different areas of the construct. Given that cells may change their keratinization profile during the time they are frozen or cultured for expansion and then trypsinized, we also performed an additional analysis of the keratinization profile of the cells that were seeded onto the Alloderm®. We looked for epithelial markers that are known to be selectively expressed on each area and selected keratin 10 as positive indicator of skin cells and the skin portion of the construct and keratins K4, K13, and K19 for the oral keratinocytes and oral mucosa portion of the construct. K10 is a marker of normal skin differentiation and is suprabasally expressed on epithelial tissues as are K4 and K13 on buccal mucosa, while K19 is expressed by basal cells in nonkeratinized oral mucosa. Keratin 13 expression is normally limited to nonkeratinizing squamous epithelia and expression in skin is, together with the expression of keratins 4 and 19, uncommon and assumed to be a sign of re-induction of an embryonic type of differentiation.31,32 All of them are cytoplasmatic markers.
Interestingly, as shown in Figure 5, we found that, at the time of seeding, very few cytospun skin cells showed presence of keratin 10 and cytospun oral cells did not show the presence of keratin 4. However, there was a full recovery of K10 expression on the skin portion during incubation of the cells on the Alloderm®, indicating a healthy normal differentiation process. K19 and K13 expression was strongly maintained in the cytospun cells and noticed in the 3D lip constructs. We did not observe recovery of cytokeratin 4 in the constructs perhaps due to a limited epidermal cell thickness in the oral portion. The differentiation of the keratinocytes during the period they were cultured at the air-liquid interface (7 days) permits the formation of an epidermis two to three cells thick and keratin 4 in oral mucosa is seen above the third or fourth cell layer (Fig. 5). Additional confirmation that the 3D construct has the features of a lip was found when keratin 10 was not found in the oral section of the construct and keratin 19 was only expressed in the basal layer of the oral section of the construct as seen in Figure 6. These temporary and/or reversible changes in the keratinization profiles of natural and bioengineered oral mucosa constructs have also been previously reported 33 and also have been shown in mice.34
While our focus was in the fabrication of a construct incorporating labial mucosa cells, a preliminary experiment using cells taken from the alveolar ridge area of the mouth, which is keratinized and similar to skin, indicated that it is also possible to make tissue engineered M/C junctions with skin and keratinized mucosa (Fig. 7). This option opens up the possibility of creating composite tissues to repair any two (or even more) different areas of mucosas, keratinized (masticatory) and nonkeratinized and other combinations of epithelial tissues that have not been attempted so far. In fact, this will only require a redesign of the barriers.
The technique presented in this manuscript was developed to adjust to the size of currently available commercial materials to grow tissue engineering constructs at the air-liquid interface. For this reason the total dimensions of the 3D constructs represent a relatively small area of a human lip, as suggested in Figure 1. Extension to bigger experimental models will not represent a problem. A great advantage of the model presented is that the construct is fully functional and all materials used are already approved for clinical use.
A final aspect to discuss is the fact that Alloderm® is a skin-derived substrate and has been repeatedly shown to reproduce epidermal tissue structures that resemble keratinized skin and oral mucosa. Our results indicate that, in vitro, Alloderm® also permits the differentiation and stratification of nonkeratinized, buccal mucosa. The development of full autologous mucosa equivalents with the inclusion of fibroblasts was only recently reported.35 Other dermal substitutes for development of nonkeratinized oral mucosa constructs have been reported using collagen-based membranes.36
The testing of these 3D constructs in vivo could initially be done by grafting the constructs into athymic (nude) rats to allow integration with the underlying striated muscular bed as previously done with mice25 to evaluate vascular ingrowth. It has been shown in clinical trials that use of the Alloderm® as a dermal substitute without the presence of fibroblasts in keratinized oral mucosa repair is successful on transplantation. The dermal component improves the handling, intraoral placement, and stabilization of the graft at the surgical site with limited scarring26 so we expect that our new lip constructs will reproduce these results in vivo.
An important advantage of the technique presented is the fact that the 3D lip constructs are manufactured in serum-free medium modified to contain totally defined animal-free supplements and without the use of feeder layers.17 This culturing approach will allow grafting the 3D lip constructs, back into autogenous human recipients with minimal risk of cellular cross-contamination or immunological rejection.37 The approach presented in tissue engineering 3D lips will have several advantages for the reconstruction of craniofacial soft tissue injuries. In particular, the source of the cells to develop the 3D skin lips constructs will come from small punch biopsies from the oral mucosa and skin of the patient, thus making the construct autochthonous.
In summary, our method is proposed as a general basis for tissue engineering of lips and other M/C junctions. Like any other first-time techniques, the one described here will require refinements and adjustments, particularly with the creation of larger lip or other M/C constructs. Sealing of the polymeric barrier can also be improved, particularly to decrease the pressure exerted on the AlloDerm during the initial preparation. Selection of PDMS was based on its extensive use in medical systems, biocompatibility, ease of manipulation and preparation, and lack of toxicity. Finding alternative biomaterials to be used as temporary, self-standing membranes would be an interesting option that we are currently exploring.
Acknowledgments
We thank Paula Arrowsmith from the University of Michigan Unit of Laboratory Animal Pathology for services provided with tissue sections. Funding for this work was provided by NIH DE13417 grant.
Disclosure Statement
No competing financial interests exist.
References
- 1.Vecchione T.R. Reconstruction of the oral mucocutanous junction. Plast Reconstr Surg. 1979;63:430. doi: 10.1097/00006534-197903000-00034. [DOI] [PubMed] [Google Scholar]
- 2.Coppit G.L. Lin D.T. Burkey B.B. Current concepts in lip reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2004;12:281e7. doi: 10.1097/01.moo.0000130574.03032.e2. [DOI] [PubMed] [Google Scholar]
- 3.Robotti E. Righi B. Carminati M. Ortelli L. Bonfirraro P.P. Devalle L. Bocchiotti M.A. Oral commissure reconstruction with orbicularis oris elastic musculomucosal flaps. J Plast Reconstr Aesthet Surg. 2010;63:431. doi: 10.1016/j.bjps.2008.11.082. [DOI] [PubMed] [Google Scholar]
- 4.Jones I. Currie L. Martin R. A guide to biological skin substitutes. Br J Plast Surg. 2002;55:185. doi: 10.1054/bjps.2002.3800. [DOI] [PubMed] [Google Scholar]
- 5.Shevchenko R.V. James S.L. James S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J.H. Cho K.H. Lee D.Y. Kwon O.S. Sung M.W. Kim K.H. Eun H.C. Human oral buccal mucosa reconstructed on dermal substrates: a model for oral epithelial differentiation. Arch Dermatol Res. 1997;289:677. doi: 10.1007/s004030050261. [DOI] [PubMed] [Google Scholar]
- 7.Moharamzadeh K. Brook I.M. Van Noort R. Scutt A.M. Thornhill M.H. Tissue-engineered oral mucosa: a review of the scientific literature. J Dent Res. 2007;86:115. doi: 10.1177/154405910708600203. [DOI] [PubMed] [Google Scholar]
- 8.Ophof R. Maltha J.C. Kuijpers-Jagtman A.M. Von den Hoff J.W. Implantation of tissue-engineered mucosal substitutes in the dog palate. Eur J Orthod. 2008;30:1. doi: 10.1093/ejo/cjm082. [DOI] [PubMed] [Google Scholar]
- 9.Clark R. A. Ghosh K. Tonnesen M.G. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018. doi: 10.1038/sj.jid.5700715. [DOI] [PubMed] [Google Scholar]
- 10.MacNeil S. Progress and opportunities for tissue engineered skin. Nature. 2007;445:874. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 11.Supp D.M. Boyce S.T. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23:403. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Papini R. Management of burn injuries of various depths. Br Med J. 2004;329:158. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zugerman C. The lips: anatomy and differential diagnosis. Cutis. 1986;38:116. [PubMed] [Google Scholar]
- 14.Binnie W.H. Lehner T. Histology of the muco-cutaneous junction at the corner of the human mouth. Arch Oral Biol. 1970;15:777. doi: 10.1016/0003-9969(70)90041-5. [DOI] [PubMed] [Google Scholar]
- 15.Izumi K. Song J. Feinberg S.E. Development of a tissue engineered human oral mucosa: from the bench to the bed side. Cells Tissues Organs. 2004;176:134. doi: 10.1159/000075034. [DOI] [PubMed] [Google Scholar]
- 16.Marcelo C.L. Kim Y.G. Kane J.L. Voorhees J.J. Stratification, specialization and proliferation of primary keratinocyte cultures. J Cell Biol. 1978;79:356. doi: 10.1083/jcb.79.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi K. Terashi H. Marcelo C.L. Feinberg S.E. Development and characterization of a tissue-engineered human oral mucosa equivalent produced in a serum-free culture system. J Dental Res. 2000;79:798. doi: 10.1177/00220345000790030301. [DOI] [PubMed] [Google Scholar]
- 18.Bikle D.D. Ng D. Tu C.-L. Oda Y. Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 19.Milstone L.M. Calcium modulates growth of human keratinocytes in confluent cultures. Epithelia. 1987;1:129. [Google Scholar]
- 20.Boyce S.T. Ham R.G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 21.Clausen H. Moe D. Buschard K. Dabelsteen E. Keratin proteins in human oral mucosa. J Oral Pathol. 1986;15:36. doi: 10.1111/j.1600-0714.1986.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 22.Heilman E. Histology of the mucocutaneous junctions and the oral cavity. Clin Dermatol. 1987;5:10. doi: 10.1016/0738-081x(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 23.Presland R.B. Dale B.A. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 24.Barretta W. Morgana M. Nwaezea G. Kramerb G. Berkovitz B.K.B. The differentiation profile of the epithelium of the human lip. Arch Oral Biol. 2005;50:431. doi: 10.1016/j.archoralbio.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Izumi K. Feinberg S.E. Teraski H. Marcelo C.L. Evaluation of transplanted tissue-engineered oral mucosa equivalents in severe combined immunodeficient mice. Tissue Eng. 2003;9:163. doi: 10.1089/107632703762687645. [DOI] [PubMed] [Google Scholar]
- 26.Izumi K. Feinberg S.E. Iida A. Yoshizawa M. Intraoral grafting of an ex-vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003;32:188. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 27.Morgan P.R. Leigh I.M. Purkis P.E. Gardner I.D. van Muijen G.N.P. Lane E.B. Site variation in keratin expression in human oral epithelia - an immunocytochemical study of individual keratins. Epithelia. 1987;1:31. [Google Scholar]
- 28.Sawaf M.H. Ouhayoun J.P. Forest N. Cytokeratin profiles in oral epithelia: a review and a new classification. J Biol Buccale. 1991;19:187. [PubMed] [Google Scholar]
- 29.Oubayoun J.E. Gosselin F. Forest N. Winter S. Werner W. Ouhayoun F. Cytokeratin patterns of human oral epithelia: differences in cytokeratin synthesis in gingival epithelium and the adjacent alveolar mucosa. Differentiation. 1985;30:123. doi: 10.1111/j.1432-0436.1985.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 30.Barrett A.W. Morgan M. Nwaeze G. Kramer G. Berkovitz B.K. The differentiation profile of the epithelium of the human lip. Arch Oral Biol. 2005;50:431. doi: 10.1016/j.archoralbio.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Korge B. Stadler R. Mischke D. Effect of retinoids on hyperproliferation-associated keratins K6 and K16 in cultured human keratinocytes: a quantitative analysis. J Invest Dermatol. 1990;95:450. doi: 10.1111/1523-1747.ep12555613. [DOI] [PubMed] [Google Scholar]
- 32.Steijlen P.M. Happle R. van Muijen G.N. van de Kerkhof P.C. Topical treatment with 13-cis-retinoic acid improves Darier's disease and induces the expression of a unique keratin pattern. Dermatology. 1991;182:178. doi: 10.1159/000247778. [DOI] [PubMed] [Google Scholar]
- 33.Garzon I. Alaminos M. Campos A. In vitro cytokeratin expression profiling of human oral mucosa substitutes developed by tissue engineering. Int J Artif Organs. 2009;32:711. doi: 10.1177/039139880903201002. [DOI] [PubMed] [Google Scholar]
- 34.Riau A. Barathiv K. Veluchamy A. Beuerman R.W. Mucocutaneous junction of eyelid and lip: a study of the transition zone using epithelial cell markers. Curr Eye Res. 2008;33:912. doi: 10.1080/02713680802485147. [DOI] [PubMed] [Google Scholar]
- 35.Pena I. Junquera L.M. Meana A. Garcia E. Garcia V. De Vicente J.C. In vitro engineering of complete autologous oral mucosa equivalents: characterization of a novel scaffold. J Periodontal Res. 2010;45:375. doi: 10.1111/j.1600-0765.2009.01248.x. [DOI] [PubMed] [Google Scholar]
- 36.Kinikoglu B. Auxenfans C. Pierrillas P. Justin V. Breton P. Burillon C. Hasirci V. Damour O. Reconstruction of a full-thickness collagen-based human oral mucosal equivalent. Biomaterials. 2009;30:6418. doi: 10.1016/j.biomaterials.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Izumi K. Neiva R.F. Feinberg S.E. Intraoral grafting of tissue-engineered human oral mucosa. Oral Craniofac Tissue Eng. 2011;1:103. doi: 10.11607/jomi.te11. [DOI] [PMC free article] [PubMed] [Google Scholar]