Abstract
Host density thresholds are a fundamental component of the population dynamics of pathogens, but empirical evidence and estimates are lacking. We studied host density thresholds in the dynamics of ectoparasitic sea lice (Lepeophtheirus salmonis) on salmon farms. Empirical examples include a 1994 epidemic in Atlantic Canada and a 2001 epidemic in Pacific Canada. A mathematical model suggests dynamics of lice are governed by a stable endemic equilibrium until the critical host density threshold drops owing to environmental change, or is exceeded by stocking, causing epidemics that require rapid harvest or treatment. Sensitivity analysis of the critical threshold suggests variation in dependence on biotic parameters and high sensitivity to temperature and salinity. We provide a method for estimating the critical threshold from parasite abundances at subcritical host densities and estimate the critical threshold and transmission coefficient for the two epidemics. Host density thresholds may be a fundamental component of disease dynamics in coastal seas where salmon farming occurs.
Keywords: sea lice, salmon, epidemic, critical threshold, aquaculture
1. Introduction
Host density thresholds are a foundation of epidemiological theory and practice [1,2], but compelling examples are rare [3]. Host density can influence parasite transmission because a pathogen is more likely to encounter a host if there are more host individuals in the vicinity, although there are exceptions [4,5]. This can create a critical host density threshold below which low parasite transmission rates lead to disease eradication and above which high parasite transmission leads to disease outbreaks or persistence in a host population [1,2]. Such thresholds underlie the epidemiological reasoning for vaccination, culling and herd immunity as components of population health policy and management [3]. In fisheries and aquaculture, although recent epidemics of infectious diseases have had adverse economic and conservation effects [6,7], the role of host density in explaining the sudden emergence of epidemics has not been carefully considered [8]. In this paper, we examine how density of farmed salmon can explain the emergence of salmon lice (Lepeophtheirus salmonis) epidemics in farmed salmon populations.
Sea lice are parasitic copepods that typically infest the external surfaces of marine and brackish-water fish. Lice are not unique to fish—Ho & Lin [9] note that no phylum of animals in the ocean is without copepod parasites—but fish lice tend to be larger than those on invertebrates. Pre-adult and adult fish lice consume mucus, epidermis and blood, causing morbidity and mortality of host fish at high infection intensity [10] as well as size-dependent sublethal physiological and behavioural changes [11–13]. The costs of lice in salmon aquaculture have ranged as high as 20 per cent of production [14,15]. Costello [7] estimates the direct costs of lice in modern salmon aquaculture at 4–10% of product value, depending on region. The costs of lice in the aquaculture of other marine fishes are probably similar in magnitude [9]. Pike & Wadsworth [10] give a comprehensive review of salmon louse biology, while Boxaspen [16] reviews recent developments in louse biology and genetics, and Costello [17] and Krkošek [18] review louse ecology. Ho & Lin [9] review lice that are important in Asian aquaculture, with detailed morphologies.
Sea lice are macroparasites [1,19] with a direct life cycle requiring no intermediate hosts. Lice eggs hatch into free-living, non-feeding larvae that drift in the ocean for several days while developing through non-infective naupliar stages to an infective stage that can attach to a passing host. The infective-stage larvae of some lice undergo a diel migration (up during day, down during night) opposite to that of potential hosts [20], suggesting that larvae might access a variety of ocean current directions and speeds by controlling their depth, a strategy familiar to biology from the larvae of some fishes. Coastal ocean circulation is thought to be a major factor in the distribution of infective-stage sea lice larvae [21,22]. The high dispersal potential of sea lice larvae indicates that populations of lice on salmon farms are likely connected within a region, such as a fjord, archipelago or large embayment. It is thus the regional density of farmed salmon that is important to the outbreak or eradication of sea lice epidemics on salmon farms.
Two observations motivate this paper. One is that sea lice are seldom a problem in areas with low production even when lice are present on local wild hosts [23]. The other is that lice are seldom a problem when sea-cage aquaculture is new to an area. MacKinnon [14, p. 5] writes
‘Why are you working on sea lice? Seals are more of a problem to us.’ This comment came from a salmon farmer who was helping me catch Atlantic salmon. The year was 1992, and we were surveying for sea lice at an Atlantic salmon farm in Passamaquoddy Bay, New Brunswick [Canada]. Because of the history of the sea lice problem in Norway, the UK and western Canada, we knew that eastern Canada and the north eastern United States would eventually have an epidemic. In 1992 it was difficult to convince salmon farmers of that likelihood, but by 1994 the factors that facilitate the transmission of infective stages had developed and sea lice soon became the major cause of fish mortality and economic loss to the aquaculture industry in eastern Canada and Maine.
MacKinnon's [14] use of the word epidemic conveys the suddenness with which lice can become problematic in salmon farming. The exponential nature of parasite population growth and the existence of a critical stocking level follow from elementary probability theory [24] and classical dynamical models [19]. Here, we offer a host–parasite model that explains epidemics of lice in two study areas and provides estimates of the transmission coefficient. We also give a method for threshold estimation from lice data at subcritical stocking levels, data and we analyse the sensitivity of the critical threshold to biotic and abiotic parameters.
2. Model description
We consider an area of sea-cage farming, such as a large fjord containing a number of farms that are connected by parasite dispersal. The number of farmed fish hosts in the area is denoted by F. Our model tracks the abundance of adult female lice, denoted by P, and free-living, infective-stage lice, called copepodites, denoted by L. Copepodites from lice in one farm can infect fish in another farm within the area, whereas lice from a different coastal area are unlikely to infect the fish in the focal area. P is thus the total number of lice on farm hosts in the focal area and L is the total number of copepodites that originated from lice on farm hosts. In addition to larvae from farms, there is a low background infection pressure due to copepodites L0 from lice on wild hosts. Owing to the migrations of wild salmon between freshwater and offshore marine waters, the wild host population is sympatric with farmed hosts for only a brief period in the wild host life cycle. The parasite population on wild hosts is therefore maintained by processes occurring primarily in offshore marine habitats, and L0 reflects the time-averaged immigration of lice from offshore waters into the farming region. The meanings of all variables, parameters and abbreviations are given in table 1. Details of the parametrizations are given in the electronic supplementary material, Parameter values and table S1.
Table 1.
Variables, parameters and abbreviations in the salmon louse model.
| symbol | definition (units) |
|---|---|
| F | number of farm fish in the area |
| P | total number of lice on farmed fish in the area |
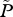 |
average abundance of lice per farmed fish, P/F |
| L | number of free-living copepodites from lice on farmed fish |
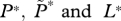 |
equilibrium values of 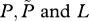 , respectively , respectively |
| L0 | number of free-living copepodites from lice on wild fish |
| μ | louse mortality rate not caused by treatment of harvest (one per day) |
| Tg | grow-out time (farmed fish harvest rate is 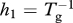 ) (days) ) (days) |
| h = h1 + h2 | louse mortality rate from harvest and treatment, respectively (one per day) |
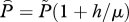 |
scaled louse abundance used in §5. |
| γ | mortality rate of free-living copepodites (one per day) |
| β | transmission coefficient (captures per copepodite per host per unit time) |
| λ | natality (copepodites produced per adult female louse per unit time) |
| ψ | settlement success (probability an attached copepodite survives to adult) |
| λ0, ψ0, μ0, γ0, β0, h0 | reference values for λ, ψ, μ, γ, β, h, respectively |
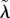 |
scaled parameter (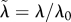 ), and similarly for ), and similarly for 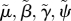
|
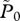 |
the limit of 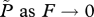 at reference values ( at reference values (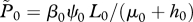 ) ) |
| Fx | critical stocking level, given by equation (3.2) |
| Fx0 | Fx evaluated at λ0, ψ0, μ0, β0, γ0, h0 |
| S | salinity in parts per thousand (‰) |
| θ | temperature in °C |
| aμ, aγ, aβ, aλ, aψ | salinity coefficients of μ, γ, β, λ, ψ (1/‰) |
| bμ, bγ, bβ, bλ, bψ | temperature coefficients of μ, γ, β, λ, ψ (1/°C) |
| gm, gstd | geometric mean and standard deviation |
| G | weight of market fish (kg) |
| Kx | critical production level (kT yr–1) |
As the stocking level F is controlled by farmers, P and L are the only dependent variables. An Anderson–May type host–parasite model [19] for lice on farmed salmon is
| 2.1a |
and
| 2.1b |
The background level of copepodites L0 is an exogenous variable and is assumed here to be constant, although constancy is not required by the model. The production rate of copepodites by lice on farm fish is referred to as natality, and is denoted by λ. Thus, λ is fecundity (eggs per louse per unit time) times an egg-to-copepodite survival factor and a sex ratio of 1/2. Copepodites die at rate γ and attach to host fish at rate βF. Once attached to a host, lice survive to the adult stage with probability ψ, then die of natural processes at rate μ. Lice also die as hosts are harvested and treated, and the rates of harvest and treatment can incorporate the fraction of lice killed during those operations. For example, if 95 per cent of the lice on a host are killed during harvest then h1 = 0.95/(grow-out time) and if 90 per cent of lice are killed by treatment then h2 = 0.9/(treatment interval). As the two rates occur together in the model, we use the notation h = h1 + h2.
Inclusion of the settlement success factor ψ is a departure from classical Anderson–May theory that makes the model more realistic without the complications of explicitly including intermediate parasite life stages [25]. In our equations, the parameters F, λ, ψ, β, μ, h, γ and L0 are not required to be constant, and later we consider their variation with temperature and salinity. The equations describe one farm, or a system of many farms that are connected in the sense that lice larvae from any one farm can infect hosts at any other farm. As hosts are concentrated at farms, the infection rate product βLF cannot be justified by a mean field assumption, but we show in the electronic supplementary material, Louse transmission, that the focal area can be complicated on a map without invalidating the model and that the spatial distributions of larvae and hosts are not required to be uniform or coincident with each other.
Equations (2.1a,b) simplify reality by lumping all attached stages of lice into the adult stage, and all larval stages into the copepodite stage. More advanced models can be made by using delay differential equations [19], or by adding more differential equations for the developmental stages of lice and larvae, as in the Erlang models of Frazer [26], or by modelling a network of farms with inter-farm transmission coefficients. As the mathematical complexity of such models tends to obscure the essential physics, we chose this simple Anderson–May model. An important feature of all Anderson–May models is that they explicitly include the infection process, i.e. the term βLF in equations (2.1a,b), whereas most sea lice models in the aquaculture literature are developmental models—they only model the development of lice after infection [26–29]. Most Anderson–May models also include host reproduction, a feature not needed here, as the number of hosts is under human control.
3. Threshold and stability
The most fundamental quantity in epidemiology may be the net reproductive value, R0, which is the expected number of adult female parasites produced by a single adult female [1,2]. In general, if R0 > 1, then on average an individual parasite will produce more than one adult female offspring and the parasite population will grow into an epidemic. Alternatively, if R0 < 1 then an epidemic will not occur and the parasite population will either die out or persist at some low endemic abundance. An expression for R0 can be read directly from equations (2.1a,b) as
| 3.1 |
The first factor in R0 is the lifetime natality of a louse, calculated as the expected lifetime of an adult female louse, (μ + h)−1, multiplied by the natality rate, λ. Thus, the first factor is the expected number of copepodites produced in the lifetime of an adult female louse. The second factor, βF/(βF + γ), is the probability that a copepodite will attach to a host fish rather than die. The product of the first two terms gives the total number of copepodites produced in the lifetime of an adult female louse that will survive and attach to a host fish. The quantity ψ is the probability that an attached copepodite survives to adulthood.
To calculate the critical stocking level, we set R0 to 1 and solve for F, giving
| 3.2 |
If the number of farmed fish exceeds this critical density then R0 exceeds 1, and a sea lice epidemic will occur. This can also be seen via an equilibrium analysis, for which we set the derivatives on the left-hand sides of equations (2.1a,b) to zero and solve the right-hand sides for the equilibrium values. A little algebra gives the equilibrium lice abundance 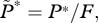 and the level of free-living copepodites L*, as
and the level of free-living copepodites L*, as
| 3.3a |
and
| 3.3b |
Equation (3.3a) shows that if the number of farm fish is very small (F → 0; in which case background infection pressure is the only source of larvae), equilibrium lice abundance is βψL0/(μ + h). On the other hand, as F → Fx, equilibrium lice abundance increases without bound unless the treatment interval or grow-out interval is decreased; hence the name ‘critical stocking level’. For use below, note that in equation (3.2), 0 < (μ + h)/(λψ − μ− h) ≪ 1 because λψ is large (10 to 100 times greater) relative to μ + h. That is, adult females produce many eggs and live many days, whereas harvesting and treatment happen much less frequently than once per day (electronic supplementary material, table S1). These inequalities, together with equation (3.2), show that when stocking level F is less than, or not much greater than Fx, the inequality βF/γ ≪ 1 is also satisfied. That last relation and a little algebra give a useful approximation for R0:
| 3.4 |
4. Sensitivity analysis
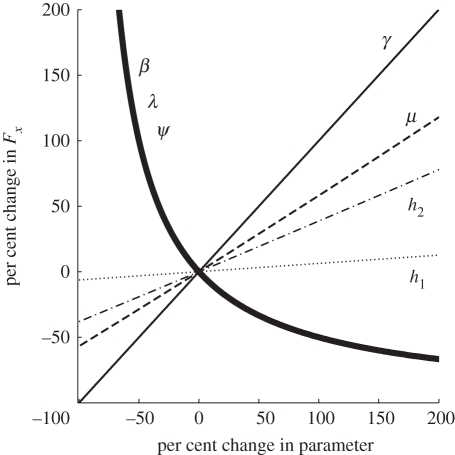
One would like to know the percentage change in critical stocking level that is likely to result from a given percentage change in each model parameter. Thus for louse natality λ, we want the ratio (ΔFx/Fx)/(Δλ/λ) in which the change ΔFx is solely due to the change Δλ. (If Δλ/λ is small compared with one, the sensitivity is approximately equal to (λ/Fx)(∂Fx/∂λ).) The sensitivity of critical stocking varied among model parameters (figure 1 and electronic supplementary material, table S2). Harvest rate and treatment rate are easily controlled in management, and the high sensitivity of Fx to transmission coefficient shows how important it is to site farms so as to minimize transmission.
Figure 1.
Sensitivity of critical stocking level to model parameters. The curve for λ gives the quantity [Fx(λ) − Fx(λ0)]/Fx(λ0) versus (λ − λ0)/λ0, and similarly for other parameters. Fx is almost equally sensitive to β, λ and ψ because λψ ≫ μ > h in the formula for Fx.
For sensitivity to temperature and salinity, recall that in the formula for Fx (equation (3.2)) the sum of parasite mortality and harvest rate is much smaller than the product of parasite natality and settlement success. Therefore, Fx ≈ γμ/(βλψ), and dividing by a reference critical stocking level Fx0 ≈ γ0μ0/(β0λ0ψ0) gives the notation fx = Fx/Fx0. It is shown in the electronic supplementary material, Sensitivity analysis, that
| 4.1 |
where S0 and θ0 are some reference salinity and temperature (say, 28‰ and 10°C). Equation (4.1) suggests high sensitivity of the threshold host abundance: for example, an increase of 5‰ salinity causes fx to decline by 89 per cent, while a temperature increase of 5°C causes it to decline by 45 per cent.
In view of the variability of environmental parameters (figure 2), the concept of a critical band may be more useful than the critical level. To see this, suppose the natural variation in environmental parameters is such that for some number Δ the inequality |Fx − Fx0| < Δ is satisfied more than 95 per cent of the time. The critical band is then Fx0 ± Δ. For stocking levels below the critical band, epidemic conditions are unlikely (i.e. only rarely will treatment be required), while for stocking levels above the critical band treatment will necessarily be frequent. As the range of environmental variability is greater for a year than for a month, and greater for a decade than for a year, the definition of critical band depends on the time interval of interest. For planning purposes the interval for estimation of a critical band should be greater than the grow-out time.
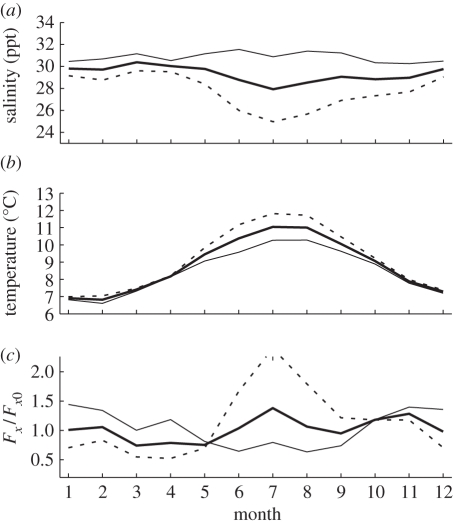
Figure 2.
Seasonal variation in (a) salinity, (b) temperature and (c) critical stocking level for the salmon farm system in Canada's Broughton Archipelago, estimated using equation (4.1). Data in (a) and (b) represent 3 years of monthly samples from the BC Salmon Farmers Association [30]. Dashed lines denote average over farms and years and samples at 0–1 m. Thin solid lines denote average over farms and years at 5 m depth. Thick solid lines denote average over farms and years and values at 0–1 and 5 m depth. As free-living larvae can control their depth, the thick solid lines in (a) and (b) may represent the salinity and temperature environment averaged over farms. Salinity takes its highest value early in spring when river discharge is lowest; as critical stocking level is more sensitive to salinity than to temperature, critical stocking level is also lowest during that interval.
5. Subcritical estimation
Here, we show how to determine the critical stocking level from parasite abundance data below critical stocking levels. First, we derive an approximate algorithm for determining critical stocking level; then we apply it to data generated by numerical simulation of the dynamical model (equations (2.1a,b)). We begin with the expression for equilibrium abundance (equation (3.3a) neglecting the small quantity βF/γ. The unknown factor βψL0 in equation (3.3a) is independent of stocking level, so we solve for it at stocking level F1 and at stocking level F2, and set the resulting two expressions equal to each other. The result is the approximate relation
| 5.1 |
in which we have replaced μ1 and μ2 by the reference louse mortality rate μ0 for added realism in the simulation below. We assume that there has been no treatment, so h1 is the harvest rate at time 1 and h2 is the harvest rate at time 2. To review: our assumptions are that βF/γ ≪ 1, that lice are in equilibrium with the level of hosts (static approximation), and that louse mortality rate is the same at both times. Solving for Fx gives
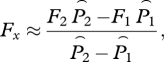 |
5.2 |
in which 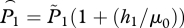 is the adjusted lice abundance at time 1, with a similar expression for time 2.
is the adjusted lice abundance at time 1, with a similar expression for time 2.
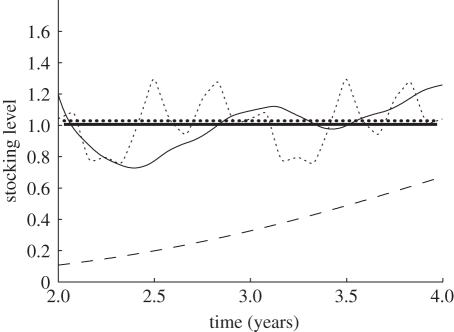
To test the method, we applied it to the subcritical intervals of the numerical simulation in electronic supplementary material, figure S1, with a 1 year interval between each time 1 and time 2, obtaining the result shown in figure 3 for the first interval (years 2–4; see electronic supplementary material, Numerical simulation, for details on the simulations). This is a fair test because the data in electronic supplementary material, figure S1 were computed using the full system of differential equations with seasonal variation in demographic parameters due to salinity and temperature (figure 2); i.e. none of the assumptions used to obtain the static formula is satisfied. Figure 3 shows that although the static approximation gives noisy point-wise estimates of critical stocking level, the median of the estimates is very near the median of the true critical stocking level. Results for the 9–11 year interval in electronic supplementary material, figure S1 were slightly better.
Figure 3.
Use of the static approximation to estimate critical stocking level from lice abundances at subcritical stocking levels. The abundance and stocking data were taken from years 1–4 of the numerical simulation shown in electronic supplementary material, figure S1. The dashed line is the actual stocking level. The thin dotted line is the true, time-varying critical stocking level, calculated using equation (3.2). The thick dotted line shows its median over the 2 year interval. The thin solid line is the critical stocking level estimated using relation (5.2) and plotted over time t2, with t1 a year earlier than time t2. The thick solid line is the median of those interval estimates. Although the interval estimates of critical stocking level are inaccurate, the two medians are nearly indistinguishable. Years 8–11 of electronic supplementary material, figure S1 give a similar result.
6. Epidemics in Canada
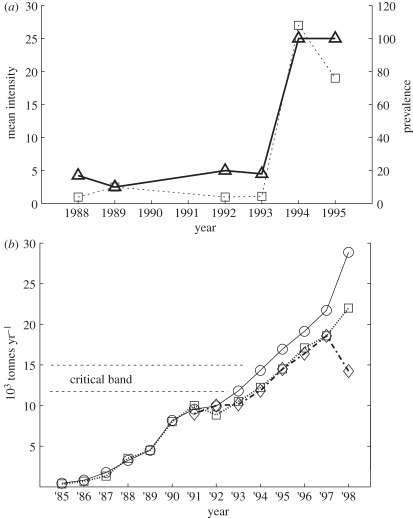
The largest concentration of sea-cage salmon in Atlantic Canada is in the Quoddy Region of the Bay of Fundy. Production began in the early 1980s and expanded rapidly after 1986, but the prevalence and intensity of sea lice were low prior to a sudden epidemic in the autumn of 1994 (figure 4). Many thousands of salmon suffered direct mortalities or extensive tissue damage [31]. The unexpected nature of the epidemic is inferred from the fact that in 1994 no drugs or pesticides were approved by Canada for use in the marine environment. In response to the epidemic, federal emergency registration of hydrogen peroxide and pyrethrin were approved, while cypermethrin was also used, but illegally [32].
Figure 4.
(a) Mean intensity (squares; average lice per infected host; left scale) and prevalence (triangles; right scale) of L. salmonis on farmed salmon in the Quoddy Region [31]. (b) New Brunswick farmed salmon production. (Nearly, all production is from the Quoddy.) The band shown here is an estimated critical production band for husbandry without treatment of fish for lice. The corresponding critical stocking band can be obtained by multiplying the production band by grow-out time (1.5–2 years). The drop of production in 1998 is due to an epidemic of infectious salmon anaemia that required eradication of fish on 20 farms. Production estimates are from the New Brunswick Department of Agriculture and Aquaculture (circles), Statistics Canada (diamonds) and the New Brunswick Salmon Growers Association (squares).
The 22 sites studied by Hogans [31] were self-selected by operators who requested help with lice problems. Although the epidemic was most severe in two areas (Lime Kiln Bay and Back Bay) the abundance of L. salmonis increased significantly at other sites in the Quoddy. Two years after the epidemic, production resumed its expansion (figure 4b), but control of sea lice outbreaks is an ongoing challenge for industry, requiring continuous monitoring of the efficacy of chemical treatment [33]. The critical band shown in figure 4b was estimated as the interval between 1993 and 1995 production.
In the Broughton Archipelago region of Pacific Canada, farming of Atlantic salmon (Salmo salar) began in the 1980s, but sea lice were seldom a problem that required treatment until epidemics began in 2000–2002 [34]. In spring of 2001, pink salmon fry migrating past farms there experienced an unprecedented epidemic of sea lice [35], and their return in autumn 2002 was anomalously low [36]. Significant declines in productivity of local stocks of pink salmon and coho salmon were evident during 4 years of epidemics that followed [37,38]. Lice data during subcritical stocking in the area are not available because monitoring of lice began after the epidemics emerged. However, assuming that the critical stocking threshold was exceeded in 2000–2002, the critical band is estimable (figure 5).
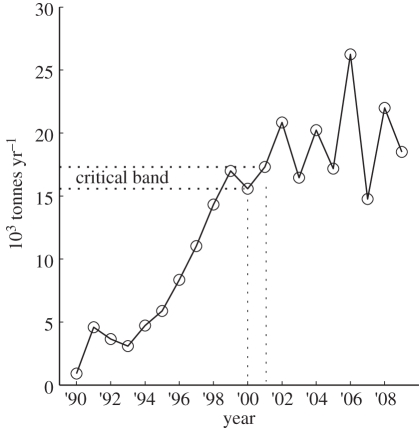
Figure 5.
Farmed salmon production in the Broughton Archipelago of Pacific Canada from Marty et al. [34] and Pearsall [30]. The first known epidemic of sea lice on out-migrating juvenile pink salmon occurred in the spring of 2001 suggesting a critical band between 2000 production of 15.6 kT yr–1 and 2001 production of 17.3 kT yr–1.
7. Transmission coefficient
From estimates of critical production band in the Broughton Archipelago and Quoddy Region, we can estimate the transmission coefficient β for each region (see electronic supplementary material, Estimating the transmission coefficient, for details). As both epidemics took place before fish were treated for lice, we use the no-treatment approximation for critical stocking level Fx ≈ γμ/(βψλ). To estimate the critical stocking level in host numbers, we use Fx ≈ KxTg/G in which Kx is critical production level, Tg is the grow-out time and G is the weight of a market fish. Substituting the second of these relations into the first and solving for transmission coefficient gives
| 7.1 |
Using the model parameter values (electronic supplementary material, table S1), the critical production for the Quoddy Region from figure 4b, and critical production for the Broughton Archipelago from figure 4, we estimate (in units of infections per host per larva per day) β = (5.9 × 10−10)/1.3 d−1 for the Quoddy and β = (4.8 × 10−10)/1.3 d−1 for the Broughton Archipelago. The higher value of the Quoddy transmission coefficient compared with that of the Broughton may be the result of a smaller average inter-farm distance in the Quoddy and higher rates of hydrodynamic mixing, but such questions are beyond the scope of this paper.
8. Discussion
Although critical host density thresholds are a fundamental property of disease dynamics, empirical examples are rare [3]. The data for sea lice on salmon farms in the Quoddy region of Atlantic Canada provide a rare and compelling example of the effect. There, lice remained at a low and relatively stable abundance that did not require treatment until a sudden change in dynamics occurred following a gradual multi-year increase in production. A similar change in dynamics is implied by the data from the Broughton Archipelago region of Pacific Canada, but sea lice data from subcritical periods preceding epidemics are lacking to confirm the effect. Similar effects of high regional abundances of hosts have been associated with louse infestations in Norway [39]. These changes in the dynamics of lice are exactly as predicted by the model. Further analysis of the model gave critical host density thresholds as well as estimates of the transmission coefficients.
Our analysis of the critical stocking level indicated high sensitivity to temperature and salinity in accordance with general observations. Berland [40] notes that lice are not a problem for salmon farmers in the Baltic, which has relatively low salinity, and Costello [7] notes that lice are seldom a problem in Australia and Finland where most production is from brackish waters (although there are relatively few farms in these regions). Stien et al. [29] mention a clear drop in infection rates over the winter in both Norway and New Brunswick, Canada, where winter ocean temperatures can be close to freezing, as well as the lack of an obvious temperature effect on the west coast of Scotland where winter temperatures seldom drop below 7°C [41]. The sensitivity of thresholds to abiotic factors, as well as regional variation in the connectivity and density of salmon farms suggests there will be substantial variation in threshold values. Epidemics may thus still occur in low-producing countries, such as Ireland, if the conditions of abiotic factors and farm abundances in a focal loch or embayment are sufficient.
A strategy to increase production, while avoiding epidemics, is based on the sensitivities shown in figure 1. Only harvest rate and treatment rate are easily controlled, but the high sensitivity of Fx to transmission rate shows how important it is to site farms so as to minimize transmission both within farms and among farms. Although Fx appears relatively insensitive to harvest rate, a reduction in grow-out time is the sea lice control strategy used in the marine culture of coho salmon (Oncorhynchus kisutch) in Japan with apparently good effect [42], perhaps because a reduction in grow-out time reduces the average size of hosts. The model also illuminates how epidemics can be predicted if the dependence of biological parameters on salinity and temperature is known. Notably, only the derivatives of these parameters with respect to temperature and salinity are needed to estimate the relative change in critical stocking level Fx. If changes in temperature and salinity could be forecasted, farmers could pre-emptively harvest or treat. Also, locating farms in low-saline conditions may raise threshold values and prevent epidemics.
To our knowledge, this is the first analysis of sea lice epidemics that gives the functional dependence on stocking density, biological variables and transmission coefficient. A natural extension of the model would represent the farm system as a network of meta-populations with two equations similar to equations (2.1a,b) for each farm, the equations being coupled with inter-farm transmission coefficients possibly derived from hydrodynamic models [43]. The model could also be improved by incorporating age classes of farmed fish and by more realistic transmission between wild and farmed fish. Here for simplicity, we have assumed that infection pressure from wild fish was constant, ignoring the brief but intense inoculum of lice that farmed salmon receive from in-migrating adult wild salmon, and for similar reasons, we have ignored the increase in host density as juvenile wild salmon migrate seaward past farms. The time behaviour of the model could be improved by explicitly modelling more life stages of the louse [26,29] instead of using a settlement success parameter, which could also allow an analysis of various types of therapeutants that target specific development stages.
Despite its simplicities, the model captures important features of sea lice dynamics on farmed fish in a way that makes the origins of epidemics clear while showing how they can be avoided. One strategy for avoiding them is to keep stocking levels below the no-treatment critical band, while holding treatment in reserve for unaccustomed environmental variation. A benefit of that strategy is that it does not promote resistance to medication. For the Broughton Archipelago, the Pacific Salmon Forum (PSF) recommends limiting Broughton Archipelago farmed salmon production to less than 18.5 kT yr–1 ([44], p. 13), just above our estimate of the critical production band from the first sea lice epidemic in that area. We speculate that a third benefit of such a strategy may be to prevent epidemics of other parasites for which treatments are not yet available. We base this speculation on the fact that the two areas studied above both experienced microparasite epidemics at stocking levels similar to those that precipitated the lice epidemics. An epidemic of infectious salmon anaemia struck the Quoddy in 1998, at production levels not much greater than the critical production band for sea lice epidemics. It seems clear that after the lice epidemic in the Quoddy the critical stocking level for lice was increased by regular treatment, along with the actual stocking level, but as the treatment was specific to lice it did not protect fish from other pathogens.
Our model and analysis suggests the concept of a critical stocking band: below that band, epidemics are infrequent, and above the band they are almost inevitable. The model explains the general pattern of abrupt emergence of sea lice epidemics in two regions of Canada, and suggests that these dynamics are likely a fundamental property of sea lice dynamics in salmon farming regions. Analysis of the model led to (i) formulae for predicting the change in critical stocking level due to changes in temperature and salinity and (ii) a method for predicting critical stocking level from lice abundances at subcritical stocking levels. Thus, if good records are kept while an aquaculture industry expands in a particular region, the critical stocking level can be estimated without ever experiencing an epidemic. These results may be broadly applicable to systems where farmed and wild host populations share native parasites.
Acknowledgements
Earlier versions of this paper were presented by one of us (L.N.F.) at Sea Lice 2008, at Puerto Varas, Chile 31 March–1 April 2008, and at Sea Lice 2010 in Victoria BC; L.N.F. and M.K. thank all organizers and especially our gracious Chilean hosts. We also thank I. Milewski, B. Chang and F. Whoriskey for help with New Brunswick farm practices. This work was funded by the University of Hawaii at Mānoa and the University of Otago.
References
- 1.Grenfell B. T., Dobson A. P. 1995. Ecology of infectious diseases in natural populations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Anderson R. M., May R. M. 1991. Infectious diseases of humans. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Lloyd-Smith J. O., Cross P. C., Briggs C. J., Daugherty M., Getz W. M., Latto J., Sanchez M. S., Smith A. B., Swei A. 2005. Should we expect population thresholds for wildlife disease? Trends. Ecol. Evol. 20, 511–519 10.1016/j.tree.2005.07.004 (doi:10.1016/j.tree.2005.07.004) [DOI] [PubMed] [Google Scholar]
- 4.McCallum H., Barlow N., Hone J. 2001. How should pathogen transmission be modelled? Trends Ecol. Evol. 16, 295–300 10.1016/S0169-5347(01)02144-9 (doi:10.1016/S0169-5347(01)02144-9) [DOI] [PubMed] [Google Scholar]
- 5.Smith M. J., Telfer S., Kallio E. R., Burthe S., Cook A. R., Lambin X., Begon M. 2009. Host-pathogen time series data in wildlife support a transmission function between density and frequency dependence. Proc. Natl Acad. Sci. USA 106, 7905–7909 10.1073/pnas.0809145106 (doi:10.1073/pnas.0809145106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krkošek M., Ford J. S., Morton A., Lele S., Myers R. A., Lewis M. A. 2007. Declining wild salmon populations in relation to parasites from farm salmon. Science 318, 1772–1775 10.1126/science.1148744 (doi:10.1126/science.1148744) [DOI] [PubMed] [Google Scholar]
- 7.Costello M. J. 2009. The global economic cost of sea lice to the salmonid farming industry. J. Fish Dis. 32, 115–118 10.1111/j.1365-2761.2008.01011.x (doi:10.1111/j.1365-2761.2008.01011.x) [DOI] [PubMed] [Google Scholar]
- 8.Krkošek M. 2010. Host density thresholds and disease control for fisheries and aquaculture. Aquac. Env. Interac. 1, 21–32 10.3354/aei0004 (doi:10.3354/aei0004) [DOI] [Google Scholar]
- 9.Ho J. S., Lin C. L. 2004. Sea lice of Taiwan. Keelung, Taiwan: The Sueichan Press [Google Scholar]
- 10.Pike A. W., Wadsworth S. L. 2000. Sealice on salmonids: their biology and control. Adv. Parasit. 44, 233–337 10.1016/S0065-308X(08)60233-X (doi:10.1016/S0065-308X(08)60233-X) [DOI] [PubMed] [Google Scholar]
- 11.Nendick L., Sackville M., Tang S., Brauner C. J., Farrell A. P. 2011. Sea lice infection of juvenile pink salmon (Oncorhynchus gorbuscha): effects on swimming performance and postexercise ion balance. 68, 241–249 [Google Scholar]
- 12.Sackville M., Tang S., Nendick L., Farrell A. P., Brauner C. J. 2011. Pink salmon (Oncorhynchus gorbuscha) osmoregulatory development plays a key role in sea louse (Lepeophtheirus salmonis) tolerance. 68, 1087–1096 [Google Scholar]
- 13.Krkošek M., et al. 2011. Fish farms, parasites, and predators: implications for salmon population dynamics. Ecol. Appl. 21, 897–914 10.1890/09-1861.1 (doi:10.1890/09-1861.1) [DOI] [PubMed] [Google Scholar]
- 14.MacKinnon B. M. 1997. Sea lice: a review. World Aquacult. 28, 5–10 [Google Scholar]
- 15.Johnson S. C., Treasurer J. W., Bravo S., Nagasawa K., Kabata Z. 2004. A review of the impact of parasitic copepods on marine aquaculture. Zool. Stud. 43, 229–243 [Google Scholar]
- 16.Boxaspen K. 2006. A review of the biology and genetics of sea lice. ICES J. Mar. Sci. 63, 1304–1316 10.1016/j.icesjms.2006.04.017 (doi:10.1016/j.icesjms.2006.04.017) [DOI] [Google Scholar]
- 17.Costello M. J. 2006. Ecology of sea lice parasitic on farmed and wild fish. Trends. Parasitol. 22, 475–483 10.1016/j.pt.2006.08.006 (doi:10.1016/j.pt.2006.08.006) [DOI] [PubMed] [Google Scholar]
- 18.Krkošek M. 2010. Sea lice and salmon in Pacific Canada: ecology and policy. Front. Ecol. Env. 86, 201–209 10.1890/080097 (doi:10.1890/080097) [DOI] [Google Scholar]
- 19.Anderson R. M., May R. M. 1978. Regulation and stability of host–parasite population interactions. I. Regulatory processes. J. Anim. Ecol. 47, 219–247 10.2307/3933 (doi:10.2307/3933) [DOI] [Google Scholar]
- 20.Heuch P. A., Parsons A., Boxaspen K. 1995. Diel vertical migration: a possible host-finding mechanism in salmon louse (Lepeophtheirus salmonis) copepodids. Can. J. Fish. Aquat. Sci. 52, 681–689 10.1139/f95-069 (doi:10.1139/f95-069) [DOI] [Google Scholar]
- 21.Murray A. G., Gillibrand P. A. 2006. Modelling salmon lice dispersal in Loch Torridon, Scotland. Mar. Poll. Bull. 53, 128–135 10.1016/j.marpolbul.2005.09.013 (doi:10.1016/j.marpolbul.2005.09.013) [DOI] [PubMed] [Google Scholar]
- 22.Amundrud T. L., Murray A. G. 2009. Modelling sea lice dispersion under varying environmental forcing in a Scottish sea loch. J. Fish Dis. 32, 27–44 10.1111/j.1365-2761.2008.00980.x (doi:10.1111/j.1365-2761.2008.00980.x) [DOI] [PubMed] [Google Scholar]
- 23.Costello M. J. 2009. How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc. R. Soc. B 276, 3385–3394 10.1098/rspb.2009.0771 (doi:10.1098/rspb.2009.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazer L. N. 2009. Sea-cage aquaculture, sea lice, and declines of wild fish. Cons. Biol. 23, 599–607 10.1111/j.1523-1739.2008.01128.x (doi:10.1111/j.1523-1739.2008.01128.x) [DOI] [PubMed] [Google Scholar]
- 25.Rosa R., Pugliese A. 2002. Aggregation, stability and oscillations in different models for host–macroparasite interactions. Theor. Pop. Biol. 61, 319–334 10.1006/tpbi.2002.1575 (doi:10.1006/tpbi.2002.1575) [DOI] [PubMed] [Google Scholar]
- 26.Frazer L. N. 2008. Sea lice infection models for fishes. J. Math. Biol. 57, 595–611 10.1007/s00285-008-0181-3 (doi:10.1007/s00285-008-0181-3) [DOI] [PubMed] [Google Scholar]
- 27.Tucker C. S., Norman R., Shinn A. P., Bron J. E., Sommerville C., Wootten R. 2002. A single cohort time delay model of the life-cycle of the salmon louse Lepeophtheirus salmonis on Atlantic salmon Salmo salar. Fish. Path. 37, 107–118 10.3147/jsfp.37.107 (doi:10.3147/jsfp.37.107) [DOI] [Google Scholar]
- 28.Revie C. W., Robbins C., Gettinby G., Kelly L., Treasurer J. W. 2005. A mathematical model of the growth of sea lice, Lepeophtheirus salmonis, populations on farmed Atlantic salmon, Salmo salar L., in Scotland and its use in the assessment of treatment strategies. J. Fish. Dis. 28, 603–613 10.1111/j.1365-2761.2005.00665.x (doi:10.1111/j.1365-2761.2005.00665.x) [DOI] [PubMed] [Google Scholar]
- 29.Stien A., Bjorn P. A., Heuch P. A., Elston D. A. 2005. Population dynamics of salmon lice Lepeophtheirus salmonis on Atlantic salmon and sea trout. Mar. Ecol. Prog. Ser. 290, 263–275 10.3354/meps290263 (doi:10.3354/meps290263) [DOI] [Google Scholar]
- 30.Pearsall I. A. 2008. Broughton Archipelago: a state of knowledge. Pacific Salmon Forum: http://www.pacificsalmonforum.ca/reports/
- 31.Hogans W. E. 1995. Infection dynamics of sea lice, Lepeophtheirus salmonis (Copepodae: Caligidae) parasitic on Atlantic salmon (Salmo salar) cultured in marine waters of the lower Bay of Fundy. Canadian Technical Report of Fisheries and Aquatic Sciences No. 2067 Fisheries and Oceans Canada, Ottawa [Google Scholar]
- 32.Harvey J., Milewski I. 2007. Salmon aquaculture in the Bay of Fundy. Canada: Conservation Council of New Brunswick Fredericton [Google Scholar]
- 33.Westcott J. D., Stryhn H., Burka J. F., Hammell K. L. 2008. Optimization and field use of a bioassay to monitor sea lice Lepeophtheirus salmonis sensitivity to emamectin benzoate. Dis. Aquat. Org. 79, 119–131 10.3354/dao01887 (doi:10.3354/dao01887) [DOI] [PubMed] [Google Scholar]
- 34.Marty G., Saksida S., Quinn T. J. 2010. Relationship of farm salmon, sea lice, and wild salmon populations. Proc. Natl Acad. Sci. USA 107, 22 599–22 604 10.1073/pnas.1009573108 (doi:10.1073/pnas.1009573108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morton A. B., Williams R. 2003. First report of a sea louse, Lepeophtheirus salmonis, infestation on juvenile pink salmon, Oncorhynchus gorbuscha, in nearshore habitat. Can. Field-Nat. 117, 634–641 [Google Scholar]
- 36.PFRCC 2002. (Pacific Fisheries Resource Conservation Council) 2002 Advisory: the protection of Broughton Archipelago pink salmon stocks. In Report to the Minister of Fisheries and Oceans and B.C. Minister of Agriculture, Food, and Fisheries. PFRCC, Vancouver [Google Scholar]
- 37.Connors B., Krkošek M., Ford J. S., Dill L. M. 2010. Coho salmon productivity in relation to salmon lice from infected prey and salmon farms. J. Appl. Ecol. 47, 1372–1377 10.1111/j.1365-2664.2010.01889.x (doi:10.1111/j.1365-2664.2010.01889.x) [DOI] [Google Scholar]
- 38.Krkošek M., Hilborn R. 2011. Sea lice (Lepeophtheirus salmonis) infestations and the productivity of pink salmon (Oncorhynchus gorbuscha) in the Broughton Archipelago, British Columbia, Canada. Can. J. Fish. Aquat. Sci. 68, 17–29 10.1139/F10-137 (doi:10.1139/F10-137) [DOI] [Google Scholar]
- 39.Bjorn P. A., et al. 2010. Is the aquaculture production in the Hardangerfjord system beyond sustainable frames?. Presented at Sea Lice 2010: The 8th Int. Sea Lice Conf., Victoria, Canada [Google Scholar]
- 40.Berland B. 1993. Salmon lice on wild salmon (Salmo salar, L.) in western Norway. In Pathogens of wild and farmed fish: sea lice (eds Boxshall G. A., Defaye D.), pp. 179–187 Chichester, UK: Ellis Horwood [Google Scholar]
- 41.Tucker C. S., Sommerville C., Wootten R. 2000. The effect of temperature and salinity on the settlement and survival of copepodids of Lepeophtheirus salmonis (Kroyer, 1837) on Atlantic salmon, Salmo salar L. J. Fish Dis. 23, 309–320 10.1046/j.1365-2761.2000.00219.x (doi:10.1046/j.1365-2761.2000.00219.x) [DOI] [Google Scholar]
- 42.Nagasawa K. 2004. Sea lice, Lepeophtheirus salmonis and Caligus orientalis (Copepoda: Caligidae), of wild and farmed fish in sea and brackish waters of Japan and adjacent regions: a review. Zool. Stud. 43, 173–178 [Google Scholar]
- 43.Foreman M. G. G., Czajko P., Stucchi D. J., Guo M. 2009. A finite volume model simulation for the Broughton Archipelago, Canada. Ocean. Mod. 30, 29–47 10.1016/j.ocemod.2009.05.009 (doi:10.1016/j.ocemod.2009.05.009) [DOI] [Google Scholar]
- 44.Pacific Salmon Forum 2009. Final report and recommendations to the Government of British Columbia. Victoria, Canada: Pacific Salmon Forum [Google Scholar]