Abstract
Theory suggests that individual personality is tightly linked to individual life histories and to environmental variation. The reactive–proactive axis, for example, is thought to reflect whether individuals prioritize productivity or survival, mutually exclusive options that can be caused by conflicts between foraging and anti-predation behaviour. Evidence for this trade-off hypothesis, however, is limited. Here, we tested experimentally whether exploration behaviour (EB), an assay of proactivity, could explain how great tits (Parus major) respond to changes in starvation and predation risk. Individuals were presented with two feeders, holding good or poor quality food, which interchanged between safe and dangerous positions 10 m apart, across two 24 h treatments. Starvation risk was assumed to be highest in the morning and lowest in the afternoon. The proportion of time spent feeding on good quality food (PTG) rather than poor quality food was repeatable within treatments, but individuals varied in how PTG changed with respect to predation- and starvation-risk across treatments. This individual plasticity variation in foraging behaviour was linked to EB, as predicted by the reactive–proactive axis, but only among individuals in dominant social classes. Our results support the trade-off hypothesis at the level of individuals in a wild population, and suggest that fine-scale temporal and spatial variation may play important roles in the evolution of personality.
Keywords: personality, plasticity, life-history variation, predation, starvation, trade-off
1. Introduction
The coexistence of discrete behavioural differences between individuals in populations has been relatively well studied and can often be explained by frequency-dependent selection acting on, for example, alternative mating strategies [1,2]. In contrast, understanding the adaptive significance of individual variation in continuous behavioural traits remains a major challenge. Recently, focus has centred on personality or behavioural syndromes within animal populations, that is, behavioural traits that are consistent over time, across situations or correlate with other behavioural traits [3–6]. How consistent behavioural differences arise is puzzling because of the intuitive expectation that natural selection should favour complete behavioural plasticity. Potential explanations for how behavioural consistency is maintained within populations are diverse and most are supported only by scant empirical evidence [7–10]. While costs associated with plasticity are thought to be a major proximate driver of behavioural consistency, links with individual state, life-history variation and the selective consequences of environmental heterogeneity and uncertainty are also thought to be pivotal [5–10]. Experimental evidence for any of these processes in natural populations at the level of individuals, however, remains scarce [7–10].
State dependency has long been a key concept in decision-making [11] and is central to many recent models developed to explain personality [12–15]. Some of these view variation in life-history strategy as the key state variable; for example, one model suggests that selection should favour consistent boldness and aggressiveness for individuals with low-residual reproductive value, but the opposite for those with high-residual value [14]. Another model suggests a life-history strategy that prioritizes growth or productivity, or that minimizes starvation risk, will be reflected by increased foraging activity and consistently predation-risk-prone behaviour across situations; on the other hand, a strategy that prioritizes survival leads to selection for consistently predation-risk-averse behaviour [7]. The reactive–proactive personality axis is common in many vertebrate groups and has been linked to this trade-off because, at one end of the continuum, reactive or responsive individuals tend to be slow but thorough-exploring, cautious and shy, compared with those at the other end of the continuum, where proactive routine-forming individuals tend to be fast but superficial-exploring, bold and predation-risk prone [16]. Existing evidence suggests that proactives tend to sustain high productivity but at a potential cost to survival, while reactives do the opposite (reviewed by Smith & Blumstein [17]). To meet the demands of their faster pace of life, proactives should, therefore, be relatively starvation-risk averse, but as a result may be more predation-risk prone than reactives. In other words, the constraints on behavioural plasticity caused by personality arise because they reflect differences in state, or in this case, consistent differences in how individuals manage the trade-off between foraging and anti-predation behaviour.
Evidence in support of the trade-off hypothesis is growing [18–20] but limited for several reasons. First, in many studies where links between personality and productivity have been inferred, the underlying mechanisms remain unclear because (i) the nature of the risks associated with the behavioural decisions is poorly characterized or unknown [21–23] and (ii) responses to experimental changes in these risks have not been examined [8]. Second, other studies have only considered how personality is linked to variation in predation risk [19,24–26]. This infers indirect links with the risk of starvation or poor self-maintenance, but the extent to which personality might simultaneously influence responses to starvation-risk variation directly is unclear. This is important because the absolute levels of starvation and predation risk may be independent. Third, some of the most influential experimental studies to date have compared differences between discrete subpopulations [18,27], which make it difficult to draw direct conclusions about individual behavioural consistency, or to control for how other factors simultaneously influence behavioural decisions. Experimental tests on how personality influences the way individuals respond to variation in predation and starvation risk are therefore needed.
Using a long-term study population of the great tit, Parus major (reviewed by Verhulst et al. [28] and McCleery et al. [29]), our aim was to examine whether the reactive–proactive axis predicts key foraging decisions made in the context of the trade-off between foraging and anti-predation behaviour. Studies in our population and elsewhere have shown that an assay of ‘exploration behaviour in a novel environment’ (EB) among wild great tits temporarily taken into captivity is a good proxy for the reactive–proactive axis. Not only is EB repeatable within individuals but it is also heritable, under selection [21,25,30–32], and correlates with a variety of different behaviours, including dispersal [33,34], promiscuity [35,36] and dominance [37,38]. Although EB has been linked to risk-taking and foraging behaviour [25,39], the significance of these effects under natural conditions has yet to be tested. Moreover, the importance of EB in the context of the trade-off between foraging and predation has not been examined.
We recorded the behaviour of free-ranging, tagged tits visiting feeders using passive integrated transponder (PIT) loggers. After assaying EB among wild birds temporarily taken into captivity, we monitored how the proportion of time birds spent feeding on a feeder with good quality food (proportion of time on good food, or PTG), rather than on a second feeder 10 m away with poor quality food, changed with respect to (i) experimentally manipulated variation in predation risk (feeders were either in a ‘safe’ or ‘dangerous’ position from predators) and (ii) natural variation in perceived starvation risk, which we assumed increased from the evening to the morning because birds are unable to feed for up to 12 h overnight [40]. First, we tested whether (i) great tits traded-off the need to feed against the risk of predation, expecting that PTG would be lower when the good quality feeder was in the dangerous position compared with when in the safe position, and that PTG would be higher in the morning when starvation risk is highest. We then used a mixed-model approach to test whether individuals (ii) differed consistently from one another with respect to PTG [3,41], and (iii) varied in their responsiveness to a change in predation or starvation risk. Finally, we tested the hypothesis that personality is linked to the trade-off by asking whether EB explains the behavioural responses observed to changes in feeder position and time of day. Our general expectation was that relatively fast-exploring birds should prioritize foraging at the expense of being predation-risk prone, but slow-exploring birds should prioritize minimizing predation risk but at the expense of being starvation-risk prone. Nevertheless, we expected this effect could be moderated by the well-known link between dominance and both EB [37] and age or sex [42]. In Wytham, competition among feeders containing poor quality food can be intense when in the safe position [38]; therefore, we expected the least-dominant individuals in the population, slow-exploring females and juveniles, could be forced to feed more in the dangerous position, when the poor quality food was in the safe position.
2. Methods
(a). Study population
The field experiment was conducted in the context of a long-term study of the great tit [29] at Wytham Woods, Oxford, UK (51°46′26.57″ N, 1°18′38.71″ W) between January and March 2009. Wytham Wood is a 338 ha mixed deciduous forest 6.5 km outside Oxford, UK. From 2006 to 2008, the entire breeding population and their offspring were individually tagged with both metal and PIT tags during the breeding season. Immigrants and older birds were also caught during winter and PIT tagged, which in 2009 occurred at each of the eight trial locations in the days before or after the trials, at which time birds were also taken into captivity for behavioural assays (§2c). Biometric data including age and sex were recorded for all birds. Great tits are social, form loose flocks in winter and have a generalist feeding habit, foraging primarily on invertebrates, seeds and beech mast in winter. Their main predator in winter, the sparrowhawk (Accipiter nisus), is also the main predator of adult songbirds generally across most of Eurasia [43], and can attack prey at speeds of up to 25 m s−1 [44]. Typically, they rely on ambush before their prey have had time to escape [45,46].
(b). Experimental setup
Two-day trials were carried out at eight different sites around Wytham. Twenty-four hours before the 2-day trial, a single bird feeder was placed at an experimental site with a mix of good quality peanuts (whole-nuts) and poor quality peanuts (granules), both supplied by Jacobi Jayne Ltd, in order to attract birds to the area and to acclimatize them to the food used during subsequent trials. Note that it was expected a priori that the whole-nuts would be perceived as good quality (see electronic supplementary material) which was supported by the analysis reported below. On the first day of the trial itself, which followed immediately after the acclimatization period, single feeders were placed in two different ‘feeder positions’ at around midday: the low predation risk or ‘safe’ position (2–3 m near dense undergrowth), and the ‘dangerous’ position in the open approximately 10 m from the safe feeder, away from any protective cover. One of the feeders contained whole-nuts and the other granules. Both feeders were left in place for a 24 h treatment, after which the position of the feeders was switched and left for a second 24 h treatment. Conditions were best for the birds during the treatment in which the whole-nut feeder was in the safe position and the granules in the dangerous position. The order of the two treatments was randomly allocated across sites, but ensuring best and worst conditions occurred on the first day at an equal number of sites.
An antenna was placed below the single, food-access points at each feeder. Visits by birds with PIT tags on leg rings were then detected and recorded by a data logger with a resolution of one-sixteenth of a second. This potentially gave samples for the morning and the afternoon periods (‘time of day’ fixed effect) in both the first and second 24 h treatments, i.e. a maximum of four samples per individual. In all analyses, we used PTG as our response variable, that is, the proportion of time during any one of these sample periods that an individual spent on the good quality (whole-nut) food, using the total time on the whole-nut and granule feeders as the denominator. PTG was assumed to be a concise estimate of the behavioural decisions made when presented with feeders of different starvation and predation risk. Many birds visited only one of the two feeders during each treatment; however, our test of the trade-off hypothesis does not depend on birds making informed choices by visiting both feeders within either treatment, because we explore how EB influences changes in the decisions made across treatments, although we note that the feeders were so close to one another and visible that birds were probably aware that both feeders were present.
(c). Exploration behaviour assays in captivity
To assay EB, birds were taken under licence from Natural England and brought to the John Krebs Field Station, Oxford, UK and housed individually on a natural light–dark cycle in 45 × 45 × 68 cm wire cages, and given food and water ad libitum. A total of 16 cages were available for housing, eight on each side of a large observation room to which the cages were connected by a trap door. Details of the EB assay are provided elsewhere [31] and further information is provided in the electronic supplementary material. All birds were released back at the capture site within 24 h of being caught.
(d). Data analysis and predictions
In the focal winter, a total of 156 individually tagged great tits visited a feeder at least once during each experimental treatment at any site. Four of these were recorded at more than one site on multiple trials, giving a total of 164 individual trials. Individuals were included only if they were recorded in the morning and, or, in the afternoon, during both 24 h treatments (n = 131 individuals). We had five general objectives or predictions. First, we used linear-mixed models (LMMs) to estimate the individual component of variation in PTG, generating repeatability estimates by expressing the individual component of variation as a proportion of total sample variance [47]. Second, we tested whether individuals traded-off the risks of starvation and predation by asking whether PTG changed with respect to the following fixed effects in a LMM: (i) feeder position, (ii) time of day, and (iii) their interaction. Third, we tested whether there was significant variation in PTG plasticity across treatments, or more specifically whether individuals varied in the extent to which they traded-off starvation and predation when risk changed. This was carried out by testing the following so-called I × E random effects: individual × feeder position, individual × time of day or individual × feeder position × time of day as random effects in a LMM [41]; note that all E were categorical variables. Finally, we tested whether changes in PTG across the predation or starvation-risk categories were influenced by EB using fixed effect interactions (EB × time of day and EB × feeder position), or whether these effects were age- or sex-dependent (four possible three-way fixed effect interactions between EB, feeder position, age and sex). All four three-way interactions were included initially but only the final model in which all variables were significant is shown. We used LMMs with location and individual identity as random effects in all models, unless otherwise stated, with feeder position, age, sex and EB as fixed effects using GENSTAT v. 13 [48]. We followed standard protocol in REML analyses [49] where initially the random model is established using the REML deviance (or a REML-deviance-based Akaike Information Criterion (AIC) or Bayesian Information Criterion (BIC)), while including the full fixed model with all interactions. The fixed model is then simplified using F statistics with numbers, or F statistics with numbers of denominator degrees of freedom estimated using standard methods [50]. PTG was logit transformed according to the equation logit(100 × (time at whole-nut feeder)/(total time detected during treatment)), where zero values for the numerator and denominator were substituted with the smallest non-zero proportion. Errors approximated to a normal distribution. Further information is provided in the electronic supplementary material.
3. Results
(a). The starvation–predation trade-off
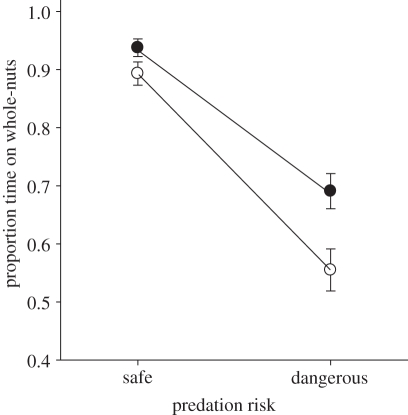
PTG was lower when the whole-nut feeder was placed in the dangerous position, and in the afternoon when the risk of starvation lowest (feeder position and time of day; table 1 and figure 1). Furthermore, PTG was especially low when the whole-nut feeder was in the dangerous position in the afternoon (feeder position × time of day, figure 1). Therefore, individuals traded-off their need to forage efficiently against the need to minimize predation risk; the way this happened was dependent on the relative size of these two risk factors.
Table 1.
Linear mixed model of PTG (the proportion of time individuals spent on the whole-nut rather than the granule feeder). ‘EB’, exploration behaviour; ‘I’, individual; ‘feeder’, feeder position; ‘time’, time of day. The I × time × feeder random effect just fell short of significance (log-likelihood = 2.7, p = 0.11). log-l, log-likelihood.
| random model | ||
|---|---|---|
| effect | log-l | component ± s.e. |
| I | 6.59** | −5.34 ± 1.32 |
| location | 24.41*** | 1.48 ± 0.93 |
| I × location | 4.62* | 4.60 ± 1.39 |
| I × feeder | 28.49*** | 5.62 ± 1.49 |
| I × time | 7.07* | −1.01 ± 0.71 |
| residual | — | 9.21 ± 1.18 |
| fixed model | ||
| effect | F (d.d.f.) | B ± s.e. |
| constant | — | 3.24 ± 0.62 |
| feeder | 71.27 (116.0)*** | 2.25 ± 0.65a |
| time | 4.68 (95.6)* | −1.81 ± 0.49a |
| age | 1.13 (55.9)n.s. | −0.83 ± 0.43a |
| sex | 1.18 (49)n.s. | −0.60 ± 0.59a |
| EB | 0.6 (38.1)n.s. | 0.61 ± 0.99 |
| sex × feeder | 0.68 (115.9)n.s. | 0.75 ± 0.83b |
| sex × time | 0.37 (97.1)n.s. | −0.30 ± 0.50c |
| EB × feeder | 0.02 (114.5)n.s. | 1.91 ± 1.39a |
| EB × time | 8.49 (94.9)** | −3.87 ± 0.83a |
| EB × sex | 0.01 (49.5)n.s. | −0.07 ± 1.29a |
| age × time | 4.01 (94.4)* | 1.04 ± 0.52d |
| feeder × time | 11.57 (143.6)*** | 1.94 ± 0.64e |
| EB × feeder × sex | 3.92 (114.0)* | −3.63 ± 1.83f |
| EB × time × sex | 13.66 (98.1)*** | 4.04 ± 1.09g |
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, n.s. > 0.05.
aDangerous, morning, adult or female set to 0.
bMales in safe position; all other levels set to 0.
cMale in afternoon; all other levels set to 0.
dJuvenile in afternoon; all other levels set to 0.
eAfternoon in safe position; all other levels set to 0.
fRefers to male in cover position; all others set to 0.
gMales in afternoon; all other levels set to 0.
Figure 1.
Change in the proportion of time spent on the good quality, whole-nut feeder (PTG) with respect to predation risk (safe and dangerous) and starvation risk (filled circles represent morning, high risk; open circles represent afternoon, low risk) treatments.
(b). Repeatability of poor quality food
PTG showed low–moderate repeatability across the morning and afternoon periods when the whole-nut feeder was in the safe position (r = 0.17; individual variance component ± s.e. = 1.234 ± 0.594, log-likelihood test = 4.89, p < 0.027) and moderately high repeatability across periods when in the dangerous position (r = 0.45; individual variance component ± s.e. = 10.66 ± 2.15, log-likelihood test = 29.78 p < 0.001). However, PTG was not repeatable across morning and afternoon periods over both treatments (r = 0.05; individual variance component ± s.e. was 0.82 ± 0.66, log-likelihood test = 1.70, p < 0.19). Therefore, consistent individual differences in PTG were detected within treatments but this consistency disappeared across treatments, pointing to substantial behavioural plasticity.
(c). Individual variation in foraging plasticity
Our analysis suggested significant individual plasticity variation in PTG across safe and dangerous positions (individual × feeder position, table 1). While most individuals avoided the whole-nut feeder when this was in the dangerous position, some showed no change and others increased their use of whole-nuts (see electronic supplementary material; figure 1). There was also significant plasticity variation across morning and afternoon periods (individual × time of day, table 1), which suggested that while PTG was higher in the morning among the majority of individuals when presumably hungry, for some birds the opposite was true (figure 2). This temporal plasticity variation appeared to be most pronounced when the whole-nut feeder was in the dangerous position (figure 2b) and was less evident when in the safe (figure 2a; individual × time of day × feeder position, p = 0.11, log-likelihood = 2.70; model otherwise the same as in table 1). Therefore, individuals varied in the extent to which the proportion of time they spent feeding on the whole-nut feeder changed simultaneously along two different risk gradients.
Figure 2.
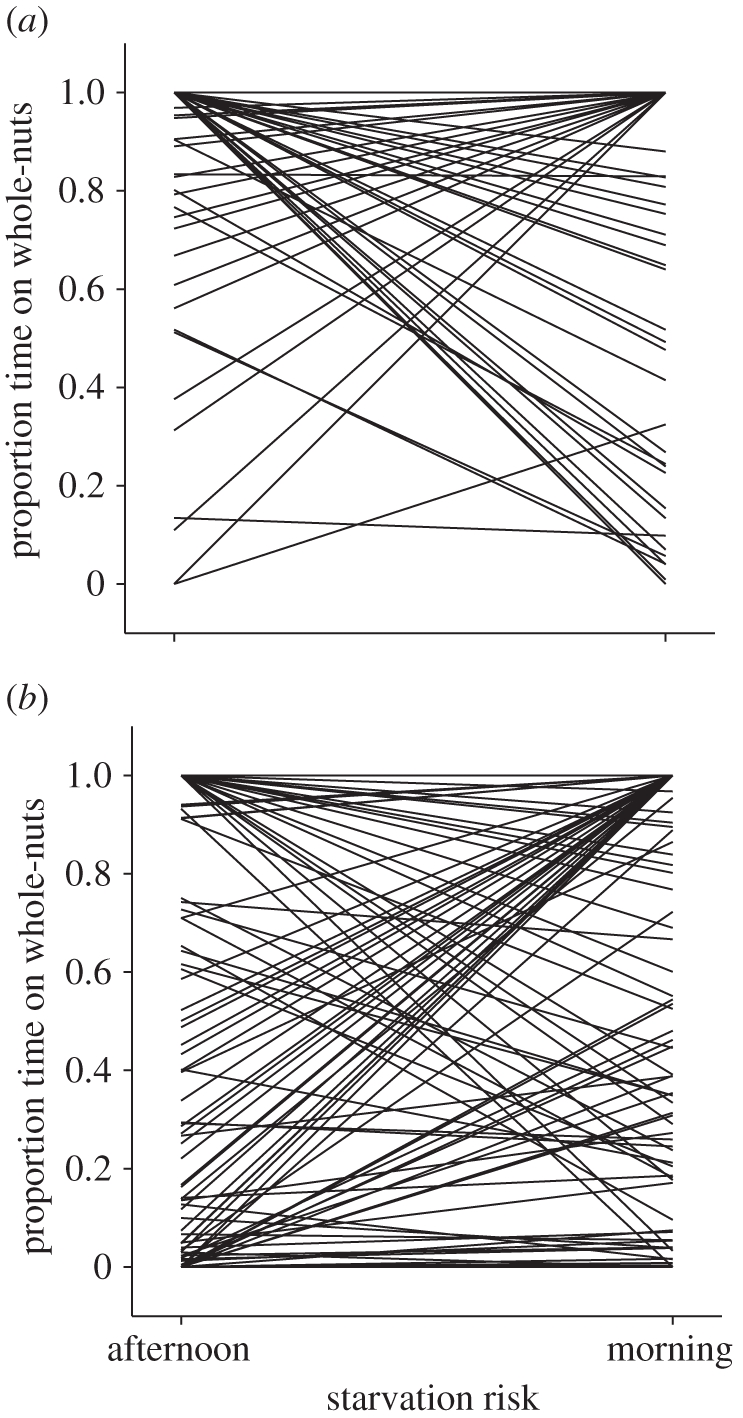
Reaction norm plots showing individual plasticity in the proportion of time spent on the good quality, whole-nut feeder (PTG) with respect to time of day when the whole-nut feeder was placed in the (a) safe (n = 144 birds) and (b) dangerous (n = 133 birds) positions, and the low quality granule feeder was placed in the opposing positions. Each line is drawn from raw data for separate individuals (n = 2 per individual); some birds that spent all time on one feeder type are obscured.
(d). State, exploration behaviour and foraging plasticity
PTG was not directly age- or sex-dependent (main effects in table 1). Similarly, there was no overall variation between the sexes in how they responded to time of day or feeder position (two-way interactions in table 1). There was a marginally significant difference between adults and juveniles in their response over time of day (age × time of day, table 1), but the effect was negligible (back-transformed logits from morning to afternoon were, for males 0.99 and 0.96, and for first winter birds, 0.97 and 0.97). Therefore, individual variation in PTG when conditions changed could not be explained by age or sex.
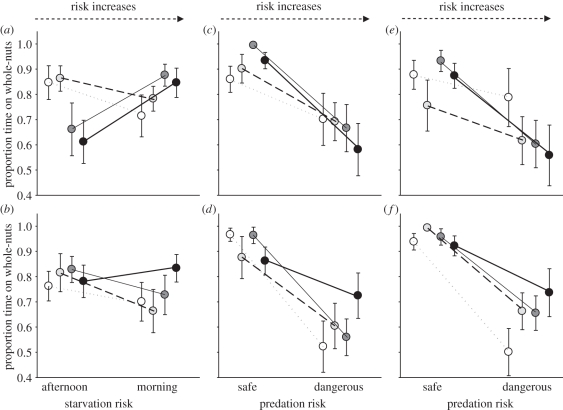
PTG plasticity with respect to a change in time of day was linked to EB (time of day × EB, table 1). This suggested that while slow-exploring individuals showed little or no change, fast explorers reduced their PTG in the afternoon, an effect that appeared to be only present in females (EB × time of day × sex, F1,110 = 13.7, p < 0.001, figure 3a,b).
Figure 3.
Exploration behaviour (EB: white circles, 0.12; light grey circles, 0.55; dark grey circles, 0.98; black circles, 1.40) and plasticity in the proportion of time spent on the good quality, whole-nut feeder (PTG) rather than on the granule feeder, with respect to a change in starvation risk for (a) females, (b) males and a change in predation risk for (c) females, (d) males, (e) juveniles and (f) adults. EB is split into four categories of equal width; values shown are the mid-range value for four equal intervals from −0.1 to 1.6. Data are raw means of individual values averaged across feeder position (a,b) or time of day (c–f) treatments. Sample sizes for individual points varied (mean ± s.d. = 16.12 ± 3.86, range 10–26 observations).
PTG plasticity with respect to feeder position was not related to EB across all individuals (feeder position × EB, table 1) but was related to EB within sexes (EB × feeder position × sex, table 1; figure 3c,d). Among females, PTG varied relatively little across feeder positions for slow-exploring individuals, while the opposite was true for fast females. Among males, the opposite pattern was observed. Finally, there was a tendency for PTG plasticity with respect to feeder position to vary with age (EB × feeder position × age, F1,110 = 3.7, p = 0.057 when dropped before the final model was selected). This suggested that among first winter birds, fast individuals showed greatest plasticity in PTG with respect to predation-risk variation but among adults, slow birds showed the greatest plasticity (figure 3e, f).
4. Discussion
Great tits traded-off the risks of starvation and predation during this study because they prioritized feeding on good quality food—that is, they became more starvation-risk averse—when starvation risk was highest in the morning or predation risk was lowest near cover. Our analyses demonstrated significant individual plasticity variation (individual × environment or I × E effects) simultaneously across two risk gradients. Although this variation was not directly related to sex or age, it was linked to our measure of personality, EB, in a sex- or age-dependent manner.
(a). Individual consistency and plasticity variation
Most empirical research on phenotypic plasticity has been at the level of changes within populations over time, or differences between genotypes in different locations or subpopulations, often focusing on non-labile traits under experimental conditions [51]. Here, we show that although PTG differed consistently between individuals within treatments, individual great tits varied substantially in the extent to which they traded-off the risks of starvation and predation when two different types of risk changed. There was relatively little variation between individuals when the whole-nut feeder was in the safe and the granule feeder was in the dangerous position, as most individuals behaved in a starvation-risk-averse manner because there was little cost involved with doing so. Repeatability was low in this treatment because the majority of individuals behaved similarly and therefore we do not emphasize the lack of consistency across treatments. Instead the plasticity variation or I × E is notable for two reasons. First, it explained a significant amount of variation over and above the individual random effects. Detecting significant I × E is often difficult [3,52,53] even when reaction norm plots visually suggest substantial plasticity variation [54]. Second, our results suggest plasticity variation along two independent ‘gradients’, linked to spatial and temporal variation in the risks of predation and starvation, respectively. Plasticity variation along even a single axis emphasizes the limitations of studying variation using average effects, but this demonstration of two simultaneous forms of plasticity variation further emphasizes the limitations of looking at mean effects alone across a heterogeneous environment [5,41]. Finally, most previous attempts to quantify plasticity variation using a mixed-model approach have been on life-history and morphological traits [55,56]. A recent review identified 14 studies that examined I × E in a range of behaviours [3], half of these using a mixed-model approach and two in the context of predation risk [57]. Our current study, however, represents the first clear demonstration of significant individual variation in behavioural plasticity in the context of the trade-off between foraging and anti-predation behaviour.
(b). Exploration behaviour and the starvation–predation trade-off
EB is influenced by both additive genetic and environmental sources of variation in the Wytham population [31,58], where it also predicts dispersal, competitive ability, promiscuity and reproductive fitness [31,34,36,38]. Here, we show that EB also predicts how individuals managed the key trade-off between foraging and anti-predation behaviour, adding to the idea that it represents an important personality axis of variation [4]. Our results show that EB correlated positively with a tendency to prioritize feeding on high quality food when starvation risk was naturally highest in the morning (for females), and to do the same when predation risk was experimentally increased (for males and adults), supporting the hypothesis that personality reflects life-history variation [4,7,10,14,59]. In addition to being one of the few experimental tests of the hypothesis [8], this represents perhaps an advance on previous findings for several reasons. First, previous studies have suggested that personality variation probably arises because of variation in risk or other environmental factors across a relatively large spatial scale—across populations or in spatially isolated niches within populations [26,60]. In contrast, our predation-risk categories were only 10 m apart within locations, suggesting that personality variation can be linked to fine-scale environmental variation within a population. Second, although some personality traits have previously been linked to foraging behaviour [61,62], here we show that personality predicts responses to changes in the risk of starvation specifically, which was the strongest effect involving EB. Slow females showed relatively little change in PTG during the day but fast females showed highest starvation risk aversion in the morning when starvation risk is generally thought to be highest.
Against predictions of the trade-off hypothesis, however, slow females and slow juveniles were less responsive than fast females and fast juveniles, respectively, to a change in predation risk. There was a tendency for slow females and juveniles to have relatively lower access to the poor quality, granule feeder when in the low predation-risk position, or relatively higher dependency on the granule feeder when in the high predation-risk situation. This unexpected tendency to be more, not less, predation-risk prone is likely explained by differences in competitive ability at the low quality feeder when near cover. Previous research shows that competition is high at poor quality, granule feeders when near cover [38]. Given the positive correlation between dominance and EB in our population [37,38], and the tendency for females and juveniles to be sub-dominant in this species generally [42], slow females and juveniles were likely to be the least-dominant individuals, explaining their relatively high usage of the good quality feeder in the dangerous position but their lower usage of the poor quality feeder in the safe position. Also against predictions of the productivity hypothesis, EB did not predict how males responded to a change in starvation risk, reasons for which are not readily surmised. Therefore, we conclude that support for the trade-off hypothesis in our population is strong but nevertheless dependent on an individual's state (age and sex) and the specific risk-gradient.
(c). General conclusions
Two broad conclusions can be drawn from our field experiment. First, foraging behaviour in the context of the trade-off between foraging and anti-predation behaviour is influenced by multiple sources of individual variation: consistent differences within environments, plasticity variation across environments and state-dependent links with a personality axis of variation. They support the assertion that mean effects alone are unlikely to explain behavioural decisions made [7,18,19,63] and add to the growing evidence that simple personality traits predict constraints on behavioural variation generally. Nevertheless, few studies have yet to establish whether such constraints have a genetic basis in natural populations [64,65]. Second, our results support general theoretical expectations about the evolution of personality because EB was linked to important axes of life-history and environmental variation [7,8,10]. Furthermore, uncertainty is an important element of many theoretical models [7,8,10,13,15] and in our study is reflected in the mismatch between the perceived and actual risks associated with the fine-scale spatio-temporal gradients we consider. While selection estimates for personality traits and behavioural syndromes are still relatively scarce [17], elsewhere the absence of annual survival selection in EB in our population is reported [31]. This lack of survival selection could be explained by fine-scale fluctuating selection inferred by the fine scale of the risks we examine, though we note that it could also be explained by generally high levels of plasticity, a possibility that is supported by the strongly context-dependent link between personality and the trade-off. Finally, we note that the possibility of such an evolutionary mechanism does not preclude a role for frequency-dependent selection, because the costs and benefits for different personality types that arise owing to either (i) the risks of starvation or predation or (ii) competition are likely to be frequency-dependent.
Acknowledgements
We thank J. Morand-Ferron, S. Dall and two anonymous referees for helpful comments on the manuscript, D. Wilson for taking care of birds in captivity, C. O'Luanaigh for logistical support, and A. Gosler and a variety of members of the EGI for help catching birds in Wytham Wood. We thank B. Sheldon for support in a variety of ways. Birds were held in captivity under licence to Natural England. Funded by BBSRC studentship to E.C. and Royal Society grant to J.Q.
References
- 1.Sinervo B., Lively C. M. 1996. The rock–paper–scissors game and the evolution of alternative male strategies. Nature 380, 240–243 10.1038/380240a0 (doi:10.1038/380240a0) [DOI] [Google Scholar]
- 2.Shuster S. M., Wade M. J. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Dingemanse N. J., Kazem A. J. N., Reale D., Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 4.Reale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 5.Sih A., Bell A. M., Johnson J. C., Ziemba R. E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 6.Wilson D. S., Clark A. B., Coleman K., Dearstyne T. 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446 10.1016/0169-5347(94)90134-1 (doi:10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 7.Biro P. A., Stamps J. A. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 8.Dingemanse N. J., Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sih A., Bell A. M. 2008. Insights for behavioral ecology from behavioral syndromes. Adv. Study Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf M., Weissing F. J. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houston A. I., McNamara J. 1999. Models of adaptive behavior. Cambridge, UK: Cambridge University Press [Google Scholar]
- 12.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 13.McElreath R., Strimling P. 2006. How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim. Behav. 72, 1135–1139 10.1016/j.anbehav.2006.04.001 (doi:10.1016/j.anbehav.2006.04.001) [DOI] [Google Scholar]
- 14.Wolf M., van Doorn G. S., Leimar O., Weissing F. J. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 15.Wolf M., van Doorn G. S., Weissing F. J. 2008. Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830 10.1073/pnas.0805473105 (doi:10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koolhaas J. M., Korte S. M., de Boer S. F., van der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 17.Smith B. R., Blumstein D. T. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- 18.Biro P. A., Post J. R. 2008. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922 10.1073/pnas.0708159105 (doi:10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sih A., Kats L. B., Maurer E. F. 2003. Behavioural correlations across situations and the evolution of antipredator behaviour in a sunfish–salamander system. Anim. Behav. 65, 29–44 10.1006/anbe.2002.2025 (doi:10.1006/anbe.2002.2025) [DOI] [Google Scholar]
- 20.Wilson D. S. 1998. Adaptive individual differences within single populations. Phil. Trans. R. Soc. Lond. B 353, 199–205 10.1098/rstb.1998.0202 (doi:10.1098/rstb.1998.0202) [DOI] [Google Scholar]
- 21.Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Réale D., Berteaux D., McAdam A. G., Boutin S. 2003. Lifetime selection on heritable life-history traits in a natural population of red squirrels. Evolution 57, 2416–2423 [DOI] [PubMed] [Google Scholar]
- 23.Réale D., Festa-Bianchet M. 2003. Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 65, 463–470 10.1006/anbe.2003.2100 (doi:10.1006/anbe.2003.2100) [DOI] [PubMed] [Google Scholar]
- 24.Bell A. M., Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 25.van Oers K., Drent P. J., de Goede P., van Noordwijk A. J. 2004. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. Lond. B. 271, 65–73 10.1098/rspb.2003.2518 (doi:10.1098/rspb.2003.2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson D. S., Coleman K., Clark A. B., Biederman L. 1993. Shy bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260 10.1037/0735-7036.107.3.250 (doi:10.1037/0735-7036.107.3.250) [DOI] [Google Scholar]
- 27.Biro P. A., Abrahams M. V., Post J. R. 2007. Direct manipulation of behaviour reveals a mechanism for variation in growth and mortality among prey populations. Anim. Behav. 73, 891–896 10.1016/j.anbehav.2006.10.019 (doi:10.1016/j.anbehav.2006.10.019) [DOI] [Google Scholar]
- 28.Verhulst S., Perrins C. M., Riddington R. 1997. Natal dispersal of great tits in a patchy environment. Ecology 78, 864–872 10.1890/0012-9658(1997)078[0864:NDOGTI]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[0864:NDOGTI]2.0.CO;2) [DOI] [Google Scholar]
- 29.McCleery R. H., Pettifor R. A., Armbruster P., Meyer K., Sheldon B. C., Perrins C. M. 2004. Components of variance underlying fitness in a natural population of the great tit. Am. Nat. 164, E162–E172 10.1086/422660 (doi:10.1086/422660) [DOI] [PubMed] [Google Scholar]
- 30.Dingemanse N. J., Both C., Drent P. J., van Oers K., van Noordwijk A. J. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938 10.1006/anbe.2002.2006 (doi:10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- 31.Quinn J. L., Patrick S. C., Bouwhuis S., Wilkin T. D., Sheldon B. C. 2009. Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215 10.1111/j.1365-2656.2009.01585.x (doi:10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
- 32.van Oers K., de Jong G., Drent P. J., van Noordwijk A. J. 2004. A genetic analysis of avian personality traits: correlated, response to artificial selection. Behav. Genet. 34, 611–619 10.1007/s10519-004-5588-z (doi:10.1007/s10519-004-5588-z) [DOI] [PubMed] [Google Scholar]
- 33.Dingemanse N. J., Both C., van Noordwijk A. J., Rutten A. L., Drent P. J. 2003. Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 10.1098/rspb.2002.2300 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn J. L., Cole E. F., Patrick S. C., Sheldon B. C. 2011. Scale and state dependence of the relationship between personality and dispersal in a great tit population. J. Anim. Ecol. 80, 918–928 10.1111/j.1365-2656.2011.01835.x (doi:10.1111/j.1365-2656.2011.01835.x) [DOI] [PubMed] [Google Scholar]
- 35.Van Oers K., Drent P. J., Dingemanse N. J., Kempenaers B. 2008. Personality is associated with extrapair paternity in great tits, Parus major. Anim. Behav. 76, 555–563 10.1016/j.anbehav.2008.03.011 (doi:10.1016/j.anbehav.2008.03.011) [DOI] [Google Scholar]
- 36.Patrick S. C., Chapman J. R., Dugdale H. L., Quinn J. L., Sheldon B. C. 2012. Promiscuity, paternity and personality in the great tit. Proc. R. Soc. B 279, 1724–1730 10.1098/rspb.2011.1820 (doi:10.1098/rspb.2011.1820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dingemanse N. J., de Goede P. 2004. The relation between dominance and exploratory behavior is context-dependent in wild great tits. Behav. Ecol. 15, 1023–1030 10.1093/beheco/arh115 (doi:10.1093/beheco/arh115) [DOI] [Google Scholar]
- 38.Cole E. F., Quinn J. L. 2012. Personality and problem-solving performance explain competitive ability in the wild. Proc. R. Soc. B 279, 1168–1175 10.1098/rspb.2011.1539 (doi:10.1098/rspb.2011.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verbeek M. E. M., Drent P. J., Wiepkema P. R. 1994. Consistent individual-differences in early exploratory-behavior of male great tits. Anim. Behav. 48, 1113–1121 10.1006/anbe.1994.1344 (doi:10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 40.Macleod R., Gosler A. G., Cresswell W. 2005. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 74, 956–964 10.1111/j.1365-2656.2005.00993.x (doi:10.1111/j.1365-2656.2005.00993.x) [DOI] [Google Scholar]
- 41.Nussey D. H., Wilson A. J., Brommer J. E. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 10.1111/j.1420-9101.2007.01300.x (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 42.Gosler A., Carruthers T. 1999. Body reserves and social dominance in the great tit Parus major in relation to winter weather in southwest Ireland. J. Avian Biol. 30, 447–459 10.2307/3677017 (doi:10.2307/3677017) [DOI] [Google Scholar]
- 43.Newton I. 1986. The sparrowhawk. Calton, UK: Poyser [Google Scholar]
- 44.Hilton G. M., Cresswell W., Ruxton G. D. 1999. Intra-flock variation in the speed of response on attack by an avian predator. Behav. Ecol. 10, 391–395 10.1093/beheco/10.4.391 (doi:10.1093/beheco/10.4.391) [DOI] [Google Scholar]
- 45.Cresswell W. 1996. Surprise as a winter hunting strategy in sparrowhawks Accipiter nisus, peregrines Falco peregrinus and merlins F. columbarius. Ibis 138, 684–692 [Google Scholar]
- 46.Cresswell W., Lind J., Quinn J. L. 2010. Predator hunting success and prey vulnerability: quantifying the spatial scale over which lethal and non-lethal effects of predation occur. J. Anim. Ecol. 79, 556–562 10.1111/j.1365-2656.2010.01671.x (doi:10.1111/j.1365-2656.2010.01671.x) [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa S., Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956 [DOI] [PubMed] [Google Scholar]
- 48.VSN International 2010. Genstat, 13th edn. Oxford, UK: VSN International Ltd [Google Scholar]
- 49.Payne R., Welham S., Harding S. 2010. A guide to REML in GenStat. Hertfordshire, UK: VSN International [Google Scholar]
- 50.Kenward M. G., Roger J. H. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53, 983–997 10.2307/2533558 (doi:10.2307/2533558) [DOI] [PubMed] [Google Scholar]
- 51.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: John Hopkins University Press [Google Scholar]
- 52.Reed T. E., Wanless S., Harris M. P., Frederiksen M., Kruuk L. E. B., Cunningham E. J. A. 2006. Responding to environmental change: plastic responses vary little in a synchronous breeder. Proc. R. Soc. B 273, 2713–2719 10.1098/rspb.2006.3631 (doi:10.1098/rspb.2006.3631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dingemanse N. J., Bouwman K. M., van De Pol M., van Overveld T., Patrick S. C., Matthysen E., Quinn J. L. 2011. Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J. Anim. Ecol. 10.1111/j.1365-2656.2011.01877.x (doi:10.1111/j.1365-2656.2011.01877.x) [DOI] [PubMed] [Google Scholar]
- 54.van de Pol M. V., Wright J. 2009. A simple method for distinguishing within-versus between-subject effects using mixed models. Anim. Behav. 77, 753–758 10.1016/j.anbehav.2008.11.006 (doi:10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 55.Husby A., Nussey D. H., Visser M. E., Wilson A. J., Sheldon B. C., Kruuk L. E. B. 2010. Contrasting patterns of phenotypic plasticity in reproductive traits in two great tit (Parus Major) populations. Evolution 64, 2221–2237 [DOI] [PubMed] [Google Scholar]
- 56.Nussey D. H., Clutton-Brock T. H., Albon S. D., Pemberton J., Kruuk L. E. B. 2005. Constraints on plastic responses to climate variation in red deer. Biol. Lett. 1, 457–460 10.1098/rsbl.2005.0352 (doi:10.1098/rsbl.2005.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinn J. L., Cresswell W. 2005. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour 142, 1377–1402 10.1163/156853905774539391 (doi:10.1163/156853905774539391) [DOI] [Google Scholar]
- 58.Dunn J. C., Cole E. F., Quinn J. L. 2011. Personality and parasites: sex-dependent associations between avian malaria infection and multiple behavioural traits. Behav. Ecol. Sociobiol. 65, 1459–1471 10.1007/s00265-011-1156-8 (doi:10.1007/s00265-011-1156-8) [DOI] [Google Scholar]
- 59.Reale D., Garant D., Humphries M. M., Bergeron P., Careau V., Montiglio P. O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063 10.1098/rstb.2010.0208 (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duckworth R. A., Badyaev A. V. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Overveld T., Matthysen E. 2010. Personality predicts spatial responses to food manipulations in free-ranging great tits (Parus major). Biol. Lett. 6, 187–190 10.1098/rsbl.2009.0764 (doi:10.1098/rsbl.2009.0764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herborn K. A., Macleod R., Miles W. T. S., Schofield A. N. B., Alexander L., Arnold K. E. 2010. Personality in captivity reflects personality in the wild. Anim. Behav. 79, 835–843 10.1016/j.anbehav.2009.12.026 (doi:10.1016/j.anbehav.2009.12.026) [DOI] [Google Scholar]
- 63.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 64.Bell A. M. 2005. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- 65.Duckworth R. A., Kruuk L. E. B. 2009. Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977 10.1111/j.1558-5646.2009.00625.x (doi:10.1111/j.1558-5646.2009.00625.x) [DOI] [PubMed] [Google Scholar]