Abstract
The evolution of different life-history strategies has been suggested as a major force constraining physiological mechanisms such as immunity. In some long-lived oviparous species, a prolonged persistence of maternal antibodies in offspring could thus be expected in order to protect them over their long growth period. Here, using an intergenerational vaccination design, we show that specific maternal antibodies can display an estimated half-life of 25 days post-hatching in the nestlings of a long-lived bird. This temporal persistence is much longer than previously known for birds and it suggests specific properties in the regulation of IgY immunoglobulin catabolism in such a species. We also show that maternal antibodies in the considered procellariiform species are functional as late as 20 days of age. Using a modelling approach, we highlight that the potential impact of such effects on population viability could be important, notably when using vaccination for conservation. These results have broad implications, from comparative immunology to evolutionary eco-epidemiology and conservation biology.
Keywords: Calonectris diomedea, Cory's shearwater, immuno-ecology, maternal effects, vaccination strategies, wildlife disease ecology
1. Introduction
The evolution of different paces of life has been suggested as a major force constraining physiological mechanisms [1]. In particular, species with a slow pace of life are expected to trade high adult survival rates with slow developmental rates [2]. As expected under this hypothesis, long-lived bird species display longer incubation periods [3] and longer nestling periods. Immune responses should exhibit the same trend, with a stronger allocation to responses favouring adult survival but requiring time to be fully effective in long-lived species [4]. However, the sophisticated response of the host immune system against parasites relies on different components involving various timing and allocation issues during their development [5]. In particular, the activation of acquired humoral immunity is a critical process to prevent the deleterious effects of exposure to a wide variety of parasites in vertebrates, but this response is not active at birth. Passive acquisition of immune compounds from the mother nevertheless occurs, notably via the egg yolk, placenta or colostrum [6] and can prevent negative effects of parasites [7,8]. The ability of mothers to transfer antibodies of their own acquired immunity to their young is thus probably an essential pathway to enhance offspring survival early in life [9,10]. Critical features of this transgenerational transfer of immunity could thus represent important life-history traits that have coevolved with other life-history traits and components of the immune system [4,9]. Although the evolutionary ecology of this transgenerational-induced response has recently attracted increasing attention [9–11], little is known about ecological and life-history factors affecting its variability among species [12], despite wide potential implications.
In birds, maternal antibodies accumulate in the oocyte during egg yolk formation and receptor-mediated absorption by the chick begins shortly before hatching [13]. These antibodies are usually considered to decay within a few days or weeks after hatching in classical model species, such as quails and chickens [14,15], although variability may exist among individuals and species, and the protection conferred by those antibodies can last after they reach undetectable levels [8,16]. The temporal persistence of those maternal antibodies has been shown to be positively related to the level of antibodies initially transferred in the egg yolk within a given species [17], but little is known about the potential role of other factors. In particular, within and among-species variability in the propensity to transfer immune protection has been largely overlooked in vertebrates [9,12], and investigations with non-model species in natural settings have the potential to lead to important findings. The few studies conducted so far with species other than classical avian models have consistently reported a relatively short temporal persistence of those antibodies [17–23] and some evidence of variability among species [17,23,24]. In particular, in species with a slow pace of life, one could expect maternal antibodies to persist for a prolonged time to provide hatchlings protection over the long rearing period, thus allowing for the slow development of a strong immune system. If maternal antibodies were to persist much longer in offspring of species with slow growth, this could have implications for the ecology and evolution of host–parasite interactions, but also with regards to allocation issues related to offspring growth.
Much interest has been developing on the relationship between immunocompetence and its costs during development. Different studies have demonstrated in birds that an immune challenge during development was responsible for a reduction in the growth rate [25,26], which is known to influence the fitness of nestlings [27]. However, those studies have mostly focused on innate immune mechanisms, and the potential role of the transfer of maternal antibodies as a mechanism favouring chick growth in the face of parasitic challenge has often been neglected (but see [28]). This is perhaps because experimentally assessing such a role requires a specific challenge of mothers before breeding and the monitoring of the dynamics of specific maternal antibodies in offspring via repeated sampling during the subsequent rearing period. Although such studies would greatly benefit from considering species with contrasting life histories, to date, data are mostly available for relatively fast-living species, precocial or altricial [14,16,21–23,29,30]. Some studies on long-lived species, such as the California condor (Gymnogyps californianus), suggest a longer maternal protection [24], but design limitations do not permit a clear interpretation of the dynamics of maternal antibody decay. Very little information is also available for other important groups of slow-developing birds displaying long rearing period, such as Procellariiformes. Using a transgenerational vaccination experimental design, we explored the temporal persistence and functionality of maternal antibodies in such species.
Procellariiform species are long-lived colonial seabirds that display a slow pace of life with among the longest chick-rearing periods in birds [31]. For instance, the Cory's shearwater (Calonectris diomedea) develops very slowly with chicks spending about 90 days at the nest. Despite such extreme and specific life histories, little information exists on the ecology of immunity in these species [32–34], notably on specific humoral immunity. Prior to egg laying, and using a vaccine to mimic a microparasitic infection, we experimentally manipulated exposure to Newcastle disease virus (NDV) in female Cory's shearwaters in order to explore properties of the maternal transfer of antibodies in this species, notably by monitoring the dynamics of specific anti-NDV antibody levels in chicks throughout the rearing period. Here, we report a long temporal persistence of maternal antibodies in nestlings of this long-lived colonial procellariiform species. Data gathered using a similar design in the black-legged kittiwake (Rissa tridactyla) and in the common quail (Coturnix coturnix) allow a comparison of the temporal persistence among species and highlight a previously unsuspected strong variability of this important trait that deserves more attention. We also show that maternal antibodies in the considered procellariiform species are functional as late as 20 days of age. A modelling approach exploring a realistic but hypothetical situation allowed us to illustrate that the potential impact of such effects on population viability could be important, notably if vaccination was used for conservation of populations threatened by pathogens affecting nestling survival. These results, which have potential implications not only in eco-epidemiology and wildlife conservation, but also in biomedicine, highlight how basic approaches in ecological immunology and the evolution of life histories can lead to findings that can have far-reaching implications, well beyond these already vast fields.
2. Material and methods
(a). Model species and study populations
The Cory's shearwater has a long lifespan of over 30 years and a long annual breeding season (eight months). As do all Procellariiforms, Cory's shearwater lays one large single egg [35] and incubation is quite long (54 days in this species). The hatched chick takes about 90 days to complete its development and to fledge [36]. By contrast, incubation lasts 17 days in quails and 27 days in black-legged kittiwakes. Quail chicks leave the nest just after hatching, while kittiwake chicks fledge at about 35 days of age [37]. Fieldwork with Cory's shearwaters was conducted in Gran Canaria (15°47′ 18″ N; 27°50′41″ E, Canary Archipelago, Spain) in 2010. The experiment with kittiwakes took place in Northern Norway [21]. Quails were bred in Konnevesi Research Station (University of Jyväskylä, Finland; [29]).
(b). Vaccination design and quantification of NDV antibodies
In all three species, vaccines were used to mimic the exposure of mothers to microparasitic antigens prior to egg laying [38]. At the time of their first capture, females either received a subcutaneous injection with a killed NDV vaccine (Nobivac Paramyxo P201, Intervet, France) or a subcutaneous injection of saline solution. We checked that all females did not have anti-NDV antibodies prior to vaccination. When sampled at the time of egg-laying, all vaccinated females subsequently displayed detectable levels of anti-NDV antibodies while all control females remained negative throughout the study. Chicks were sampled repeatedly throughout the rearing period, starting at 1 day post-hatch in shearwaters and 5 days post-hatch in kittiwakes and quails (see figure 1 for the ages), to allow the determination of the dynamics of maternal antibodies in their plasma.
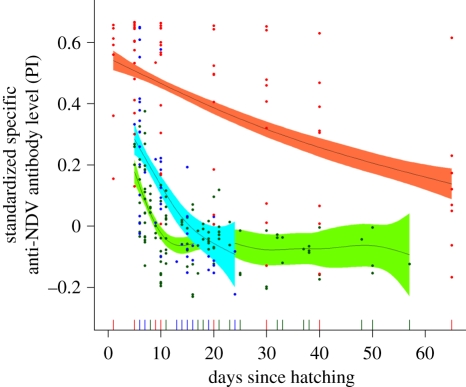
Figure 1.
Decay of specific anti-NDV antibody levels in chicks from mothers exposed to NDV vaccine for three different bird species, the quail (green), the black-legged kittiwake (blue) and the Cory's shearwater (red). The standardized percentage of inhibition (PI) is presented on the y-axis; 0 represents the negative threshold. The lines correspond to the mean for each species estimated using generalized additive mixed models (GAMM), and the coloured regions around the means represent the associated 95% CI of the slopes.
On each occasion, blood was collected from the ulnar vein (kittiwake, quail) or the tarsal vein (shearwater) using 1 ml syringes and stored at 4°C. Within a few hours, samples were centrifuged and plasma was stored at −20°C pending serological analyses. Measures of specific anti-NDV antibody levels in females and chicks were performed once for each sample using a competitive enzyme-linked immunosorbent assay (ELISA) test (Svanovir NDV-Ab, Svanova Biotech, Sweden) and are expressed as percentage of inhibition (PI). Analyses conducted on sub-samples allowed us to check the high repeatability of the measures. Antibody levels were standardized across species by subtracting the negativity threshold to the inhibition percentage (negativity threshold: mean of negative controls + 2 s.d.; shearwater: 0.31, kittiwake: 0.30 and quail: 0.22). In addition, in shearwaters, a subset of chicks was vaccinated when 20 days old (n = 11 chicks from control mothers, n = 5 chicks from vaccinated mothers) in order to investigate whether persistent anti-NDV maternal antibodies might block a response to vaccination, as previously shown for other species [21].
(c). Statistical analyses
We analysed the dynamics of anti-NDV antibody levels in chicks according to their treatment and the treatment of their mothers using generalized additive mixed models (GAMMs), using the library mgcv in R [39], based on penalized regression splines and generalized cross-validation to select the appropriate smoothing parameters. GAMMs combine the utilities of linear mixed models [40] and generalized additive models [41] so that random factors, fixed factors and nonlinear predictor variables can all be estimated in the same statistical model. To compare dynamics of decay of maternal antibodies accounting for their level at hatching, we also calculated the half-lives of these antibodies for each species. To do so, we determined the curve of exponential decrease in concentration using mixed models with chick nested within species as a random effect. We then calculated the half-life for each species using the equation:
Results present half-lives and associated 95% CI.
(d). Modelling effects on population viability
In order to illustrate the effect of the temporal persistence of maternal antibodies on population viability in such long-lived species and its potentially strong conservation implications, we built an age-structured matrix population model [42] that allowed comparison of contrasted scenarios with regard to the protective effect of maternally acquired antibodies. To consider a simple and demonstrative situation, we hypothesized that a vaccine could be available against a pathogen negatively affecting young offspring survival and circulating in a small population of a long-lived wild bird species. Such a situation is highly plausible if we consider a wild bird species threatened by a pathogen originating from domesticated animals [43]. The model enabled us to address the importance of the length of the protection offered by maternally acquired antibodies as a result of the vaccination. The model was parameterized for a small population of an endangered procellariiform species exposed to annual epidemics of a pathogenic microparasite greatly impairing newborn survival during the rearing period. For this modelling approach, we focused on the realistic case of the endangered Amsterdam albatross (Diomedea amsterdamensis) on Amsterdam Island, breeding close to a population of yellow-nosed albatross (Thalassarche chlororhynchos) which is exposed to recurrent epizootics during the breeding season, possibly owing to the avian cholera agent [44,45]. Realistic parameter values for survival and reproductive rates are available for this yellow-nosed albatross population [44]. Reproduction happens once a year and births are synchronous in the population. The annual interbreeding life cycle of individuals can be described by a Leslie matrix so that population at time t + 1 can be obtained from the equation:
with A designating the Leslie matrix, and Nt and Nt+1 describing the population, respectively, at time t and t + 1.
We assumed a density-dependent decrease of reproductive rate in order to keep the maximum population sizes below a certain threshold (fecundity = f0(1 − N/K) with f0 the maximal fecundity, K the carrying capacity and N the population size). Massive die-offs have been reported in the first weeks after hatching in yellow-nosed albatross chicks of this population, with mortalities up to 74 per cent, while adults remained mostly unaffected [45]. We considered in the model that a fraction of the sensitive breeding female population could be vaccinated each year against the disease-causing agent and thus transmit a temporary passive protection to their chicks. We assumed that a safe and efficient vaccine is available [46] and that protection given by vaccination is lifelong. Females are individually marked when vaccinated (e.g. using leg rings), thus sensitive and vaccinated females could be distinguished at any time, and a protocol relying on the vaccination of only sensitive females could be implemented.
Following vaccination, the lifelong protection of adult females is supposed to come with lifelong detectable levels of specific antibodies. The transmission of maternal antibodies by adult females is thus assumed to be recurrent over the rest of their lives. Maternal antibodies are assumed to be protective for chicks during a time equivalent to two half-lives after hatching. Assuming an exponential decay, when this time point is reached, chicks have antibody levels corresponding to a quarter of their initial level. We set that each year newborns from sensitive mothers suffered an additional mortality of 70 per cent owing to an annual epidemic of the parasite occurring when they are a few weeks of age, while offspring born to vaccinated mothers suffered no additional mortality. All surviving offspring and subsequent adults are considered not to transmit protective antibodies against the parasite until they may be vaccinated (this is either because they have not been exposed to the parasite, given that 100% of exposed chicks without maternal antibodies are assumed to die, or because they have lost their maternal protection owing to the natural decay of maternal antibodies). For simplicity, no further heterogeneities among individuals are considered. Also, the dynamics of pathogen circulation is not modelled as a function of the proportion of susceptible individuals, although maternal protection decreases the pulse of sensitive newborns and is thus likely to affect the persistence of the pathogen [46]. This other potential benefit of the vaccination design is not explored in the current paper. Projections of the long-term impact of the maternal effect on population viability are then evaluated by comparing scenarios with no (or short term) maternal protection of newborns to scenarios with a long-term maternal protection of nestlings on the breeding ground (outside the breeding season, no transmission occurs as the birds are out at sea). All evaluations begin with a population displaying the stable age structure of the population and are run with the software Scilab (the code is provided as the electronic supplementary material).
3. Results
(a). Temporal persistence of maternal antibodies
A total of 314 blood samples from females and chicks of various ages, obtained during a period of six months of fieldwork, allowed us to explore in detail the dynamics of maternal antibodies in nestlings of a natural population of Cory's shearwater. All chicks from control mothers (n = 37) were negative at hatching, while immunization of mothers (n = 19) resulted in detectable levels of anti-NDV antibody in early offspring life. Maternal antibodies decayed at a much slower rate in shearwaters than in the two other species (figure 1). While maternal antibodies waned before 15 days in kittiwake and quail chicks, most shearwater chicks still had detectable antibodies at 30 and 40 days of age. The half-life of maternal anti-NDV antibodies was indeed much higher in shearwaters (24.75 days (95% CI: 18.07–39.24)) than in kittiwakes (5.43 days (95% CI: 3.46–11.55)) or quails (5.25 days (95% CI: 3.58–9.90)).
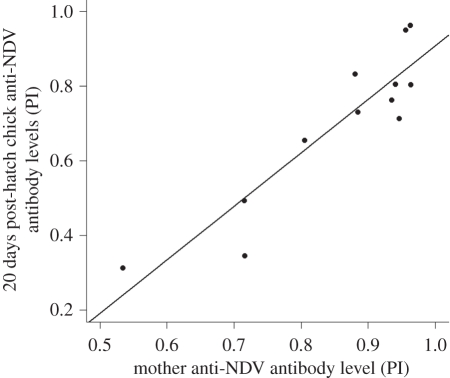
As in quails and kittiwakes [21,29], the antibody levels in females of Cory's shearwaters showed a positive correlation with chick levels soon after hatching (Pearson's correlation coefficient at 5 days of age: r5d = 0.81, n = 17 chicks from vaccinated mothers, p < 0.001). Importantly, this correlation between females and chicks antibody levels lasted throughout the rearing period in shearwaters despite decreasing numbers of chicks owing to natural mortality during rearing and the use of a sub-sample of chicks for testing a late blocking effect (figure 2; at 10 days of age, r10d = 0.95, n = 17; at 20 days of age, r20d = 0.91, n = 12; at 30 days of age, r30d = 0.91, n = 12, at 40 days of age, r40d = 0.91, n = 9, p < 0.001 and at 65 days of age, r65d = 0.72, n = 8, p = 0.042).
Figure 2.
Specific anti-NDV antibody levels in 20 days post-hatch Cory's shearwater chicks from vaccinated mothers as a function of their mother anti-NDV antibody level at the time of laying (r20d = 0.91, n = 12, p < 0.001).
(b). Late blocking effect of the maternal antibodies
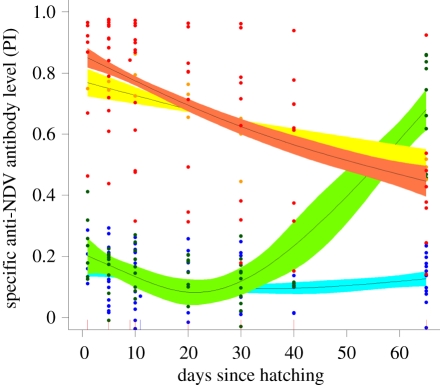
To investigate a potential blocking effect of maternal antibodies, we exposed a subgroup of nestlings to the NDV vaccine at 20 days of age and found that nestlings which had received maternal anti-NDV antibodies did not show an increase in antibody levels by 65 days, while nestlings from control mothers did (65 days mean level of nestlings from vaccinated mothers ± s.e.: 0.49 ± 0.03, n = 3; 65 days mean level of nestlings from non-vaccinated mothers: 0.70 ± 0.16, n = 9; figure 3). This blocking effect suggests that maternal antibodies are functional as late as 20 days after hatching in shearwaters. By contrast, quail chicks that received maternal antibodies mounted an immune response after vaccination at 20 days of age [29].
Figure 3.
Specific anti-NDV antibody levels in Cory's shearwater chicks for four treatment groups: chicks from control females, non-vaccinated (blue) and vaccinated when 20 days old (green); chicks from vaccinated females, non-vaccinated (red) and vaccinated when 20 days old (orange). GAMM are used to control for individual effect and nonlinear dynamics. Means and 95% CI of the slopes of the models are presented.
(c). Population projections
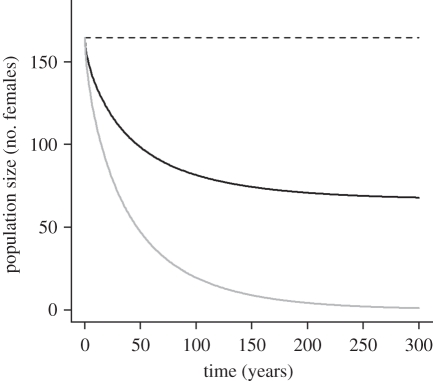
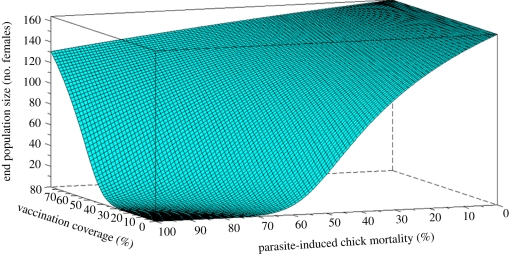
The long persistence of maternal protection suggested by the empirical results could prevent the local extinction of an endangered albatross population hypothetically facing a recurrent epidemic affecting newborns at the end of their fourth week of rearing (figure 4, green curve, corresponding to maintaining 40% of the females vaccinated). The effect of an assumed fast decay of maternal antibodies is no different from an absence of protection if newborns are facing an epidemic at the end of their fourth week of rearing. In the considered situation, it leads to a dramatic reduction of population size over a few decades and probable extinction (figure 4, red curve). By simulating different scenarios of vaccination coverage of females and parasite-induced chick mortality, we further show that when a transfer of temporally persistent maternal antibodies is accounted for, a realistic range of vaccination coverage can lead to important population rescue effects (figure 5).
Figure 4.
Dynamics of a hypothetical albatross population under scenarios of exposure to a disease agent deleterious to nestlings and against which an antibody-based vaccine would exist. Dashed curve: when there is no exposure to the disease agent in the population, the population is maintained stable by density-dependent reproduction. Grey curve: when there is no maternal protection or when maternal protection vanishes before an annual epidemic of the disease agent (half-life of maternal antibodies shorter than 10 days, including the 5 days reported for quail and kittiwake), the population is driven towards extinction on a short time scale even if adult females are vaccinated. Black curve: a vaccination coverage of 40% of the sensitive breeding females associated with a sufficiently long half-life of maternal antibodies in offspring (over 10 days, thus including the 24.75 days half-life reported for Cory's shearwater) can dramatically dampen the effects of the annual epidemic and prevent local extinction. Parameters: adult annual survival rate: 0.95; subadult annual survival rate: 0.87; juvenile annual survival rate: 0.7; parasite-induced chick mortality: 0.7; fecundity rate: 0.3 female/female. Initial population size corresponds to the equilibrium population size and age structure at carrying capacity.
Figure 5.
Effect of the vaccination coverage on the size of a hypothetical albatross population at the end of the evaluation (300 years) for various levels of parasite-induced chick mortality. Reasonable vaccination efforts have a strong rescue effect on the population: after a first year of high vaccination effort (60–70 females), the vaccination of only a few (generally 1 or 2) sensitive adult females is needed each year to maintain a vaccination coverage of 40% of the adult females. Parameters: adult annual survival rate, 0.95; subadult annual survival rate, 0.87; juvenile annual survival rate, 0.7; fecundity rate, 0.3 female/female; half-life of maternal antibodies = 25 days. Initial population size corresponds to the equilibrium population size and age structure at carrying capacity.
4. Discussion
The results obtained show a predicted but surprisingly long persistence of maternal antibodies in nestlings of a long-lived colonial procellariiform species, an order of birds with among the longest chick-rearing periods. By implementing a field experiment using an intergenerational vaccination design, we indeed found that specific maternal antibodies can persist more than 40 days in the plasma of Cory's shearwater nestlings, with an estimated half-life of 25 days post-hatching. Further, we provide evidence that this temporal persistence is much longer than previously known for birds by comparing the results with comparable data obtained from two shorter lived species (the quail and the kittiwake). We also show that maternal antibodies in Cory's shearwater are functional as late as 20 days of age and that the impact of such effects on population viability could be important, notably when using vaccination for conservation.
Life-history theories predict that investment in resource-demanding processes should depend on the individual pace of life [1]. In particular, this implies a stronger investment in acquired rather than innate immunity in long-lived species [4]. This is because innate immunity is associated with a highly costly response, while acquired responses in vertebrates include memory mechanisms that reduce the costs of multiple encounters of the same parasite, an event more likely to happen in long-lived species. Although evidence is scarce, the negative correlation between the response to injection of phytohaemagglutinin (a test commonly used to assess innate cellular immune responsiveness) and survival in adult birds [47], and the absence of decay in acquired immunity with age in a long-lived procellariiform species [34] tend to support this hypothesis. The results we report here further show that the transfer and the temporal persistence of maternal antibodies in offspring are key maternal effects that may relate tightly with the evolution of other life-history characteristics.
Procellariiformes are particular in many aspects, notably with regards to the precocity of chick development [35], and key perspectives of our results are whether the high temporal persistence of maternal antibodies we found in the Cory's shearwater (i) is a feature common to other Procellariiformes and (ii) is also found in other long-lived bird species with a long chick growing period at the nest. In addition to California condors [24], candidate species to be considered are notably griffon vultures (Gyps fulvus), king penguins (Aptenodytes patagonicus) and parrots. Complementary data on various species would allow a more formal comparative approach to test whether the species half-life of maternal antibodies is positively related to the length of the chick-rearing period when accounting for phylogenetic constraints and other ecological characteristics, such as general exposure to parasites or colonial breeding habits [9–11,48]. In any case, obtaining such data would allow further investigations of the implications and underlying causes of the variability of this neglected trait. It should also be noted that any data obtained to assess the persistence of maternal antibodies would also provide an efficient approach to explore the inter-species variability in immunoglobulin persistence in adults of oviparous species [15]. This would, however, be more complicated for mammals as immunoglobulins can also be transferred after birth via the colostrum and the milk [6].
Events occurring early in life can have long-lasting implications [27], and thus the discovery of a very slow decay of maternal antibodies in a long-lived species has implications with regards to the interpretation of the optimization of life histories and potential phylologenetic effects on the dynamics of host–parasite interactions. The very long persistence of immunoglobulins in the Cory's shearwater could thus be an adaptation favouring chick survival in early life and allowing the development, and potentially the shaping, of the humoral immune repertoire in procellariiform species.
Humoral immunity based on antibodies does not provide efficient protection against all parasites, but it clearly provides a powerful mechanism of protection against many [5]. The maternal transfer of antibodies could thus have strong effects on the dynamics of infectious diseases. Despite being temporary, the protection potentially provided by specific maternal antibodies may indeed dramatically change the susceptibility landscape of parasites. It is effective at a time thought to favour the spread of disease agents, as temporal aggregation of breeding individuals increases contact rates [49], while reproduction results in the production of a pulse of naive individuals [46]. This could be especially the case for long-lived colonial birds such as Procellariiformes, which can breed in aggregates of thousands of pairs. In addition to protective effects against pathogens, maternal antibodies have been suggested to be responsible for an educational effect by acting on the ongoing process of lymphocytes maturation to select clones that display a higher reaction to selected parasites [50]. Maternal antibodies could also allow the occurrence of a ‘natural vaccination’ by attenuating the effects of an infection by a parasite, thus producing optimal conditions for the immunization of the newborn [51], a mechanism that might be particularly valuable in long-lived species. Our results thus highlight that the maternal transfer of antibodies may well be a key example of an adaptive transgenerational-induced response with far-reaching implications [52,53].
The only study that investigated the relationship between the transfer of maternal antibodies and the pace of life suggested that slow-living species deposit less immunoglobulin Y (IgY) in the yolk of their eggs [12]. However, this study is based on the quantification of levels of antibodies in the egg yolk and did not consider the persistence of the transferred antibodies in the newborn, nor accounted for the level of circulating antibodies in the mother plasma at the time of egg laying. Moreover, species may vary in their propensity for taking up antibodies from the egg yolk (see below). Finally, the innate immune response of chicks [47] and levels of natural antibodies [54], which are important components of innate humoral immunity [55], have been found to be positively correlated with the length of the incubation period. Together with persistent levels of maternal antibodies, higher levels of both cellular and innate humoral immunity after hatching could thus be part of a strategy allowing for the slow development of a fully functional acquired immune system in long-lived species.
Understanding the factors affecting the persistence of immunoglobulins is important to gain further insights into the evolution of the vertebrate immune system, but also into the dynamics of immunity in natural populations and its consequences on the ecology of host–parasite interactions [56]. Infectious diseases are thought to be a major threat for endangered species [43] and the results we obtained could thus have relatively direct implications for the conservation of some species. For instance, if the current finding about maternal antibody persistence extends to other Procellariiformes, our modelling results suggest that the use of efficient vaccines on breeding females of species such as albatrosses may be a possible way of protecting populations against the risk of extinction, owing to pathogens affecting offspring during the rearing period in the colony. The modelling results highlight that despite a strong classical dependence of the rate of population change on adult survival in long-lived species, manipulating the levels of protection of endangered long-lived Procellariiformes could dramatically change population projections in the case of a highly virulent nestling pathogen, in particular, when disease agents are possibly locally novel pathogens introduced through human activities. Of course, such a conservation application would require more specific research work, notably in terms of detailed understanding of the host–pathogen system involved, and would need to account for ethical and practical issues regarding the use of vaccines in wild populations and especially in endangered species. Further modelling work could also be valuable to optimize potential vaccination coverage and management interventions [57]. Vaccination has been considered a relevant part of conservation programmes of endangered species [58] and our results suggest that considering the prolonged persistence of some protective antibodies in nestlings of long-lived colonial species could greatly increase the potential usefulness of specific vaccines as management tools.
Functionally, the strong temporal persistence of maternal antibodies could be a by-product of the evolution of a decreased catabolism of proteins in these species with a slow pace of life. IgY, the avian equivalent of the mammalian IgG, are protected from the normal catabolic pathway by an intracellular recycling mechanism that relies on a receptor, FcRY [59]. Interestingly, this receptor is also responsible for the uptake of antibodies from the egg yolk in the hatchling [60]. An increase in the expression of the FcRY or in the strength of the FcRY–IgY interaction could thus not only contribute to greater half-lives of IgY but would also help hatchlings achieve high levels of circulating IgY despite a possible lesser investment of females in egg yolk deposition of IgY in longer lived birds [12]. Evidence for the possibility of such mechanisms comes from studies of the mammalian counterpart of FcRY, the neonatal Fc receptor (FcRn), which is implicated in both the transfer of antibodies from mother to young and the recycling of IgG (see [61] for a review). Both the level of expression of FcRn [62] and the affinity of the receptor for IgG [63] are important factors governing the half-lives and the serum levels of antibodies in mammals. In parallel to comparative genomic approaches, investigations on the functionality and density of FcRY receptors in Procellariiformes and other bird species could thus yield important complementary findings. Understanding mechanisms underlying the prolonged half-life of IgY might also have direct relevance for the development and the engineering of more effective therapeutic antibodies [63], a fast developing and important way of treatment of autoimmune and inflammatory diseases.
In conclusion, using an intergenerational vaccination design, we showed in a long-lived species that specific maternal antibodies can persist in offspring much longer than previously known for birds. Using a modelling approach, we show that this could importantly affect the dynamics of host–parasite interactions, and can have strong conservation implications. The results also raise important questions about the underlying mechanisms involved in the temporal persistence of antibodies in species with contrasting life histories. Overall, our study underlines how current interest in ecological immunology, provided that it is based on sound comparative and experimental approaches, has the potential to lead to further important new findings at the interface between fields such as evolutionary ecology, biomedicine, conservation biology and eco-epidemiology.
Acknowledgements
Permits for the experiments were granted, respectively, by the Consejería de Medio Ambiente del Cabildo de Gran Canaria for shearwaters and the Norwegian Animal Research Authority for kittiwakes. The experiment with quails conformed to legal requirements in Finland. The intergenerational vaccination design was approved by the Ethical committee of the French Polar Institute.
We thank the Ministerio de Ciencia e Innovación and Fondos FEDER (CGL2009-11278/BOS), the ANR (project ANR—2011, EVEMATA) and the French Polar Institute (IPEV programme no. 0333) for funding and Pascual Calabuig, Loly Estévez, José Manuel de los Reyes, Rosana Arizmendi, Néstor Perez, Antonio Vulcano, Heli Siitari, Karen McCoy, Muriel Dietrich, Sylvain Gandon and Torkild Tveraa for help at various stages of the work. E.L. was supported by a postdoctoral grant from Spanish Ministry of Science. We also thank Cabildo de Gran Canaria for permission and support and Robert E. Ricklefs and three referees for their comments on earlier versions of the manuscript.
References
- 1.Ricklefs R. E., Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.16/S0169-5347(02)02578-8 (doi:10.16/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 2.Stearns S. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Martin T. E. 2002. A new view of avian life-history evolution tested on an incubation paradox. Proc. R. Soc. Lond. B 269, 309–316 10.1098/rspb.2001.1879 (doi:10.1098/rspb.2001.1879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K. A. 2006. Linking immune defenses and life history at the levels of the individual and the species. Integr. Comp. Biol. 46, 1000–1015 10.1093/icb/icl049 (doi:10.1093/icb/icl049) [DOI] [PubMed] [Google Scholar]
- 5.Frank S. A. 2002. Immunology and evolution of infectious disease. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 6.Brambell F. W. R. 1970. The transmission of passive immunity from mother to young. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 7.Carlier Y., Truyens C. 1995. Influence of maternal infection on offspring resistance towards parasites. Parasitol. Today 11, 94–99 10.1016/0169-4758(95)80165-0 (doi:10.1016/0169-4758(95)80165-0) [DOI] [PubMed] [Google Scholar]
- 8.Al-Natour M. Q., Ward A., Saif Y. M., Stewart-Brown B., Keck L. D. 2004. Effect of different levels of maternally derived antibodies on protection against Infectious Bursal Disease Virus. Avian Dis. 48, 177–182 10.1637/5319 (doi:10.1637/5319) [DOI] [PubMed] [Google Scholar]
- 9.Boulinier T., Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288 10.1016/j.tree.2007.12.006 (doi:10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 10.Grindstaff J. L., Brodie E. D., III, Ketterson E. D. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319 10.1098/rspb.2003.2485 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasselquist D., Nilsson J. A. 2009. Maternal transfer of antibodies in vertebrates: transgenerational effects on offspring immunity. Phil. Trans. R. Soc. B 364, 51–60 10.1098/rstb.2008.0137 (doi:10.1098/rstb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Addison B., Klasing K. C., Robinson W. D., Austin S. H., Ricklefs R. E. 2009. Ecological and life-history factors influencing the evolution of maternal antibody allocation: a phylogenetic comparison. Proc. R. Soc. B 1296, 3979–3987 10.1098/rspb.2009.1296 (doi:10.1098/rspb.2009.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowalczyk K., Daiss J., Halpern J., Roth T. F. 1985. Quantitation of maternal-fetal IgG transport in the chicken. Immunology 54, 755–762 [PMC free article] [PubMed] [Google Scholar]
- 14.Apanius V. 1998. Ontogeny of immune function. In Avian growth and development: evolution within the altricial-precocial spectrum (eds Starck J. M., Ricklefs R. E.), pp. 203–222 Oxford, UK: Oxford University Press [Google Scholar]
- 15.Davison F., Kaspers B., Schat K. A. 2008. Avian immunology. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 16.Nemeth N. M., Oesterle P. T., Bowen R. A. 2008. Passive immunity to West Nile Virus provides limited protection in a common passerine species. Am. J. Trop. Med. Hyg. 79, 283–290 [PubMed] [Google Scholar]
- 17.Grindstaff J. L. 2010. Initial levels of maternally derived antibodies predict persistence time in offspring circulation. J. Ornithol. 151, 423–428 10.1007/s10336-009-0472-5 (doi:10.1007/s10336-009-0472-5) [DOI] [Google Scholar]
- 18.Graczyk T. K., Cranfield M. R., Shaw M. L., Craig L. E. 1994. Maternal antibodies against Plasmodium spp. in African black footed penguin (Sphenicus demersus) chicks. J. Wildl. Dis. 30, 365–371 [DOI] [PubMed] [Google Scholar]
- 19.Hahn D. C., Nemeth N., Edwards E., Bright P., Komar N. 2006. Passive West Nile Virus antibody transfer from maternal eastern screech owls (Megascops asio) to progeny. Avian Dis. 50, 454–455 10.1637/7509-012606R1.1 (doi:10.1637/7509-012606R1.1) [DOI] [PubMed] [Google Scholar]
- 20.Liu S. S., Higgins D. A. 1990. Yolk-sac transmission and post-hatching ontogeny of serum immunoglobulins in the duck (Anas platyrhynchos). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 97, 637–644 10.1016/0305-0491(90)90100-8 (doi:10.1016/0305-0491(90)90100-8) [DOI] [PubMed] [Google Scholar]
- 21.Staszewski V., Gasparini J., McCoy K. D., Tveraa T., Boulinier T. 2007. Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. J. Anim. Ecol. 76, 1215–1223 10.1111/j.1365-2656.2007.01293.x (doi:10.1111/j.1365-2656.2007.01293.x) [DOI] [PubMed] [Google Scholar]
- 22.Gibbs S. E. J., Hoffman D. M., Stark L. M., Marlenee N. L., Blitvich B. J., Beaty B. J., Stallknecht D. E. 2005. Persistence of antibodies to West Nile Virus in naturally infected rock pigeons (Columba livia). Clin. Diagn. Lab. Immunol. 12, 665–667 10.1128/CDLI.12.5.665-667.2005 (doi:10.1128/CDLI.12.5.665-667.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King M. O., Owen J. P., Schwabl H. G. 2010. Are maternal antibodies really that important? Patterns in the immunologic development of altricial passerine house sparrows (Passer domesticus). PLoS ONE 5, e9639. 10.1371/journal.pone.0009639 (doi:10.1371/journal.pone.0009639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang G.-J. J., Davis B. S., Stringfield C., Lutz C. 2007. Prospective immunization of the endangered California condors (Gymnogyps californianus) protect this species from lethal West Nile Virus infection. Vaccine 25, 2325–2330 10.1016/j.vaccine.2006.11.056 (doi:10.1016/j.vaccine.2006.11.056) [DOI] [PubMed] [Google Scholar]
- 25.Soler J. J., de Neve L., Perez-Contreras T., Soler M., Sorci G. 2003. Trade-off between immunocompetence and growth in magpies: an experimental study. Proc. R. Soc. Lond. B 270, 241–248 10.1098/rspb.2002.2217 (doi:10.1098/rspb.2002.2217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brommer J. E. 2004. Immunocompetence and its costs during development: an experimental study in blue tit nestlings. Proc. R. Soc. Lond. B 271, S110–S113 10.1098/rsbl.2003.0103 (doi:10.1098/rsbl.2003.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 28.Grindstaff J. L. 2008. Maternal antibodies reduce costs of an immune response during development. J. Exp. Biol. 211, 654–660 10.1242/jeb.012344 (doi:10.1242/jeb.012344) [DOI] [PubMed] [Google Scholar]
- 29.Staszewski V., Siitari H. 2010. Antibody injection in the egg yolk: maternal antibodies affect humoral immune response of the offspring. Funct. Ecol. 24, 1333–1341 10.1111/j.1365-2435.2010.01745.x (doi:10.1111/j.1365-2435.2010.01745.x) [DOI] [Google Scholar]
- 30.Nemeth N. M., Bowen R. A. 2007. Dynamics of passive immunity to West Nile Virus in domestic chickens (Gallus gallus domesticus). Am. J. Trop. Med. Hyg. 76, 310–317 [PubMed] [Google Scholar]
- 31.Starck J. M., Ricklefs R. E. 1998. Avian growth and development: evolution within the altricial-precocial spectrum. Oxford, UK: Oxford University Press [Google Scholar]
- 32.Mauck R. A., Matson K. D., Philipsborn J., Ricklefs R. E. 2005. Increase in the constitutive innate humoral immune system in Leach's storm-petrel chicks is negatively correlated with growth rate. Funct. Ecol. 19, 1048–1052 10.1111/j.1365-2435.2005.01060.x (doi:10.1111/j.1365-2435.2005.01060.x) [DOI] [Google Scholar]
- 33.Forero M. G., González-Solis J., Igual J. M., Hobson K. A., Ruíz X., Viscor G. 2006. Ecological and physiological variance in T-cell mediated immune response in Cory's shearwaters. Condor 108, 865–876 10.1650/0010-5422(2006)108[865:EAPVIT]2.0.CO;2 (doi:10.1650/0010-5422(2006)108[865:EAPVIT]2.0.CO;2) [DOI] [Google Scholar]
- 34.Lecomte V. J., et al. 2010. Patterns of aging in the long-lived wandering albatross. Proc. Natl Acad. Sci. USA 107, 6370–6375 10.1073/pnas.0911181107 (doi:10.1073/pnas.0911181107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warham J. 1983. The composition of petrel eggs. Condor 85, 194–199 10.2307/1367254 (doi:10.2307/1367254) [DOI] [Google Scholar]
- 36.Giudici A., Navarro J., Candelaria J., González-Solis J. 2010. Physiological ecology of breeders and sabbaticals in a pelagic seabird. J. Exp. Mar. Biol. Ecol. 389, 13–17 10.1016/j.jembe.2010.04.002 (doi:10.1016/j.jembe.2010.04.002) [DOI] [Google Scholar]
- 37.Mulard H., Danchin E. 2008. The role of parent-offspring interactions during and after fledging in the black-legged kittiwake. Behav. Process. 79, 1–6 10.1016/j.beproc.2008.03.005 (doi:10.1016/j.beproc.2008.03.005) [DOI] [PubMed] [Google Scholar]
- 38.Staszewski V., Boulinier T. 2004. Vaccination: a way to address questions in behavioral and population ecology? Trends Parasitol. 20, 17–22 10.1016/j.pt.2003.11.005 (doi:10.1016/j.pt.2003.11.005) [DOI] [PubMed] [Google Scholar]
- 39.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 40.Pinheiro J. C., Bates D. M. 2000. Mixed effect models in S and S plus. New York, NY: Springer [Google Scholar]
- 41.Hastie T., Tibshirani R. 1990. Generalized additive models. London, UK: Champman & Hall/CRC; [DOI] [PubMed] [Google Scholar]
- 42.Caswell H. 2001. Matrix population models. Construction, analysis and interpretation. 2nd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 43.Daszak P., Cunningham A. A., Hyatt A. D. 2000. Wildlife ecology—emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449 10.1126/science.287.5452.443 (doi:10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 44.Rolland V., Barbraud C., Weimerskirch H. 2009. Assessing the impact of fisheries, climate and disease on the dynamics of the Indian yellow-nosed albatross. Biol. Conserv. 142, 1084–1095 10.1016/j.biocon.2008.12.030 (doi:10.1016/j.biocon.2008.12.030) [DOI] [Google Scholar]
- 45.Weimerskirch H. 2004. Diseases threaten Southern Ocean albatrosses. Polar Biol. 27, 374–379 10.1007/s00300-004-0600-x (doi:10.1007/s00300-004-0600-x) [DOI] [Google Scholar]
- 46.Keeling M. J., Rohani P. 2008. Modeling infectious diseases in humans and animals. Princeton, NJ: Princeton University Press [Google Scholar]
- 47.Tella J. L., Scheuerlein A., Ricklefs R. E. 2002. Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proc. R. Soc. Lond. B 269, 1059–1066 10.1098/rspb.2001.1951 (doi:10.1098/rspb.2001.1951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasparini J., McCoy K. D., Haussy C., Tveraa T., Boulinier T. 2001. Induced maternal response to the Lyme disease spirochaete Borrelia burgdorferi sensu lato in a colonial seabird, the kittiwake Rissa tridactyla. Proc. R. Soc. Lond. B 268, 647–650 10.1098/rspb.2000.1411 (doi:10.1098/rspb.2000.1411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loehle C. 1995. Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335 10.2307/1941192 (doi:10.2307/1941192) [DOI] [Google Scholar]
- 50.Fink K., Zellweger R., Weber J., Manjarrez-Orduno N., Holdener M., Senn B. M., Hengartner H., Zinkernagel R. M., Macpherson A. J. 2008. Long-term maternal imprinting of the specific B cell repertoire by maternal antibodies. Eur. J. Immunol. 38, 90–101 10.1002/eji.200737872 (doi:10.1002/eji.200737872) [DOI] [PubMed] [Google Scholar]
- 51.Zinkernagel R. M. 2003. On natural and artificial vaccinations. Annu. Rev. Immunol. 21, 515–546 10.1146/annurev.immunol.21.120601.141045 (doi:10.1146/annurev.immunol.21.120601.141045) [DOI] [PubMed] [Google Scholar]
- 52.Mousseau T. A., Fox C. W. 1998. Maternal effects as adaptations. Oxford, UK: Oxford University Press [Google Scholar]
- 53.Frost S. D. W. 1999. The immune system as an inducible defense. In The ecology and evolution of inducible defenses (eds Tollrian R., Harvell C. D.), pp. 104–126 Princeton, NJ: Princeton University Press [Google Scholar]
- 54.Lee K. A., Wikelski M., Robinson W. D., Robinson T. R., Klasing K. C. 2008. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363 10.1111/j.1365-2656.2007.01347.x (doi:10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- 55.Ochsenbein A. F., Zinkernagel R. M. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21, 624–630 10.1016/S0167-5699(00)0174-0 (doi:10.1016/S0167-5699(00)0174-0) [DOI] [PubMed] [Google Scholar]
- 56.Graham A. L., Hayward A. D., Watt K. A., Pilkington J. G., Pemberton J. M., Nussey D. H. 2010. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330, 662–665 10.1126/science.1194878 (doi:10.1126/science.1194878) [DOI] [PubMed] [Google Scholar]
- 57.Haydon D. T., et al. 2006. Low-coverage vaccination strategies for the conservation of endangered species. Nature 443, 692–695 10.1038/nature05177 (doi:10.1038/nature05177) [DOI] [PubMed] [Google Scholar]
- 58.Boyce W. M., Vickers W., Morrison S. A., Sillett T. S., Caldwell L., Wheeler S. S., Barker C. M., Cummings R., Reisen W. K. 2011. Surveillance for West Nile Virus and vaccination of free-ranging Island Scrub Jays (Aphelocoma insularis) on Santa Cruz island, California. Vector Borne Zoonotic Dis. 11, 1063–1068 10.1089/vbz.2010.0171 (doi:10.1089/vbz.2010.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tesar D. B., Cheung E. J., Bjorkman P. J. 2008. The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Mol. Biol. Cell 19, 1587–1593 10.1091/mbc.E07-09-0972 (doi:10.1091/mbc.E07-09-0972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West A. P., Herr A. B., Bjorkman P. J. 2004. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity 20, 601–610 10.1016/S1074-7613(04)00113-X (doi:10.1016/S1074-7613(04)00113-X) [DOI] [PubMed] [Google Scholar]
- 61.Roopenian D. C., Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 10.1038/nri2155 (doi:10.1038/nri2155) [DOI] [PubMed] [Google Scholar]
- 62.Cervenak J., Bender B., Schneider Z., Magna M., Carstea B. V., Liliom K., Erdei A., Bosze Z., Kacskovics I. 2011. Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J. Immunol. 186, 959–968 10.4049/jimmunol.1000353 (doi:10.4049/jimmunol.1000353) [DOI] [PubMed] [Google Scholar]
- 63.Zalevsky J., Chamberlain A. K., Horton H. M., Karki S., Leung I. W. L., Sproule T. J., Lazar G. A., Roopenian D. C., Desjarlais J. R. 2010. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 28, 157–159 10.1038/nbt.1601 (doi:10.1038/nbt.1601) [DOI] [PMC free article] [PubMed] [Google Scholar]