Abstract
Intralocus sexual conflict occurs when a trait encoded by the same genetic locus in the two sexes has different optima in males and females. Such conflict is widespread across taxa, however, the shared phenotypic traits that mediate the conflict are largely unknown. We examined whether the sex hormone, testosterone (T), that controls sexual differentiation, contributes to sexually antagonistic fitness variation in the bank vole, Myodes glareolus. We compared (opposite-sex) sibling reproductive fitness in the bank vole after creating divergent selection lines for T. This study shows that selection for T was differentially associated with son versus daughter reproductive success, causing a negative correlation in fitness between full siblings. Our results demonstrate the presence of intralocus sexual conflict for fitness in this small mammal and that sexually antagonistic selection is acting on T. We also found a negative correlation in fitness between parents and their opposite-sex progeny (e.g. father–daughter), highlighting a dilemma for females, as the indirect genetic benefits of selecting reproductively successful males (high T) are lost with daughters. We discuss mechanisms that may mitigate this disparity between progeny quality.
Keywords: sex-limited epistasis, mammal, artificial selection, sexually antagonistic selection, sexual selection
1. Introduction
Conflict exists between the evolutionary interests of individuals of the two sexes [1–3]. When males and females differ in their optima for a morphological, physiological or behavioural trait that has a strong intersexual genetic correlation, the alleles of the underlying polymorphic genes are beneficial to one sex but detrimental to the other, and an intralocus sexual conflict is borne [4–8]. Intralocus conflict has the potential to generate sexually antagonistic selection affecting important evolutionary processes, such as sexual coevolution, adaptive evolution and speciation [4,9–11]. Since the seminal paper by Chippindale et al. [5], interest in intralocus conflict has surged with further empirical evidence gathered from both laboratory and natural populations; not only for Drosophila melanogaster [12–16] but also in other invertebrates and vertebrates [17–22]. However, it is less well-established that shared phenotypic traits mediate the conflict [4,15,23].
Shared phenotypic traits suggested or shown to underly intralocus sexual conflict include body size [15,17,22,24], development time [22,25], longevity [22], locomotory activity [23], immune defence [26–30], diet [31] and the hormone corticosterone [32]. We concentrate on steroid hormones as potential mediators of conflict owing to their importance in controlling sexual dimorphism in mammals [33]. The steroid hormone, testosterone (T), is essential for mammalian male reproductive behaviour [34], and directional selection for increased T in males might be linked to an associated T increase in females [35], as has been documented in some avian taxa [36,37]. If increased T level had negative effects on female fitness, this would result in a sexually antagonistic hormone expression profile, unless selection has decoupled male and female T levels [38].
In this paper, we used a small mammal, the bank vole Myodes (Clethrionomys) glareolus, to investigate intralocus sexual conflict for fitness and its underlying traits. Potential for intralocus conflict is high in the bank vole owing to several characteristics in its life history. First, the bank vole shows polygynandrous behaviour [39], different reproductive roles between the sexes [40], selection on male-mating and reproductive successes [41] and heritable male-mating success [42]. Second, the sex hormone T governs the expression of male bank vole social status, mate searching, mobility and reproductive success [41–44] is heritable (h2 = 0.32, [45]), and male and female T levels are considered to be genetically correlated [46]. Third, selection for T increases multiple mating in male bank voles, but decreases multiple mating in females [47]. Together these observations indicate that T is a candidate for mediating intralocus sexual conflict over reproduction, and here we set out to explicitly test whether this is the case.
We created artificial selection lines divergent for male T and measured relative adult fitness using genetic paternity analyses and competition trials. If the endocrine pathway was involved in mediating intralocus conflict in the bank vole, we predicted to find: (i) that offspring fitness would be sensitive to selection on parental circulating T levels via between-sex trait heritability, i.e. a negative correlation in fitness between parents and their opposite-sex progeny [48], (ii) a genotype by sex interaction [20], and (iii) a negative genetic correlation between fitness estimates in full-sibling brothers and sisters [16].
2. Material and methods
(a). Parental selection lines
A total of 200 males and 200 females were wild captured from Konnevesi, Central Finland (62°37′ N, 26°20′ E) in 2004. The animals were maintained in laboratory conditions described in Mills et al. [41] with food and water ad libitum. Retro-orbital blood samples were collected from all individual males over 48 h (see methods in Oksanen et al. [49]), centrifuged (12 000 r.p.m. for 5 min; Heraeus Biofuge) to separate plasma from the blood cells, and plasma was stored at −20°C. Plasma T was measured using a radioimmunoassay kit (TESTO-CTK, DiaSorin, Byk-Sangtec Diagonstica GmbH & Co., Dietzenbach, Germany) and both methods and the test for parallelism are described in Mills et al. [39]. Repeatability was calculated for male T values (n = 56) recorded in either duplicate (n = 43) or triplicate (n = 13) over a four-month period during the breeding season using analyses of variance [50]; repeatability = 0.637 (F ratio = 4.914). High plasma T (H) and low T (L) classes of male bank voles (64 and 62 individuals, respectively), based on upper and lower quartiles of median plasma T levels were established. High (H) and low T (L) females (64 and 62 individuals, respectively), whose brothers had high and low T values, respectively, were also established. Early in 2005, four crosses were performed: (i) selection for male T–HH (maternal/paternal), 34 matings; (ii) selection against male T–LL, 32 matings; and (iii) two controls—HL and LH, 30 and 30 matings, respectively. Each bank vole was only mated once. No differences in parental female size were found between groups (mean head size ± s.e.: HH = 13.82 ± 0.11; LL = 13.63 ± 0.15; HL = 13.66 ± 0.08; LH = 13.74 ± 0.09; two-way ANOVA, maternal: F1,47 = 0.275, p = 0.603; paternal: F1,47 = 1.478, p = 0.230; maternal × paternal: F1,47 = 0.054, p = 0.820).
(b). F1 offspring
Seventy-five litters were produced, 24, 13, 17 and 21 from HH, LL, HL and LH matings, respectively. However, only 53 litters were used for the experiment (13, 8, 14 and 18 from each cross, respectively), with the other 22 being single-sex litters. Pups were sexed, weighed and measured within 12 h of birth and progeny were reared in their natal litter until weaning (20 days of age), after which they were reared in individual cages. A one-way analysis of variance (ANOVA) was used to examine the effect of the number of F1 males in utero on the anogenital distance of F1 female pups measured at birth. Number of litters = 49, as birth measurements were not taken within 24 h for four F1 litters. We used a two-way ANOVA to examine the effects of paternal T line (fixed factor: H and L) and maternal T line (fixed factor: H and L) on mean F1 litter size at birth (number of litters = 49). We also tested for differences in mean F1 litter size at birth between paternal and maternal T lines with F1 progeny sex (fixed factor: male and female) using a three-way ANOVA (N = 98). Simple-effects analyses (pairwise options using command syntax in SPSS, v. 17, [51]) were used to break down the interaction term by analysing the effect of one independent variable (e.g. maternal T line) at individual levels of the other independent variable (e.g. paternal T line). In June 2005, T was measured from all F1 male offspring over 48 h.
One hundred and thirty-two F1 male and 128 F1 female offspring were randomly assigned, accordingly to their date of birth, to 33 and 32 test groups, respectively, each comprising four individuals, one from each cross (HH, LL, HL and LH). Multiple male and female offspring from one litter were assigned to different test groups.
(c). F1 adult fitness
(i). F1 males
Two intensive reproductive success trials were completed on F1 males from June to October 2005 to estimate their fitness [43]. (i) F1 male paternity was measured where one experimental F1 female in post-partum oestrus (having given birth in the previous 12 h) was presented consecutively with each of the four males per test group singly for 1 h. Four trials were carried out for each test group, alternating both the order in which the males were presented and the experimental cross of the F1 female (total of 132 trials). Pups were genotyped at six microsatellite loci and likelihood-based analysis of paternity was conducted with the software Cervus v. 2 [39,52,53]. Relative reproductive success per test group was determined. (ii) F1 male–male competition was also measured where males competed with another male for a female. A trial comprised two males from an F1 test group and one wild caught non-experimental female in post-partum oestrus in an arena (1 × 1 m) where observations were made until ejaculation occurred [41–44]. All males were assessed three times with the three other males from his test group (two males per trial, six trials in total per test group, total of 198 trials) and random unrelated, unfamiliar females. Males were ranked within each replicate group based on their copulation success per trial providing relative male–male competition per test group. Mean reproductive success of brothers based on both relative measures was calculated.
(ii). F1 females
Bank vole litter size shows remarkably large phenotypic (2–10) and additive genetic variation [54,55], and is both an important female life-history trait as well as being used to measure female fitness [56]. In the laboratory, bank vole litter size at birth most frequently equates to litter size at weaning. F1 female reproductive success was determined from litter size in trials where all four females from one test group were placed in a competitive situation for one week with one non-experimental random male of proven fertility. The trials were carried out in a large cage (60 × 40 × 30 cm) with sawdust and hay for bedding, food and water ad libitum. Females were then monitored for births and litter size was measured. On hundred and fifty-nine trials were carried out on a total of 95 females from 53 litters.
We tested for differences in F1 adult fitness between F1 progeny sex (fixed factor: son and daughter) as a function of paternal and maternal T lines (fixed factors: H and L) using a three-way ANOVA (N = 106).
(d). F1 differential sex ratio allocation
Differential maternal investment was assessed from §2c F1 male reproductive success trials. Differential F2 litter sex ratio allocation (arcsine square-root transformed) was determined using a two-way ANOVA with F1 paternal and F1 maternal selection lines on F2 litter sex ratio. Only HH and LL lines were used, N = 34 litters.
3. Results
(a). Parental selection lines
The parental generation had a mean T ± s.e. of 11.85 ± 0.6 ng ml−1 and 3.11 ± 0.3 ng ml−1 for H and L classes of sires, respectively, and 10.87 ± 0.7 ng ml−1 and 3.62 ± 0.6 ng ml−1 for H and L classes of dams (mean T of their brothers), respectively. One generation of selection led to twofold higher values of F1 male T in the line selected for (T = 8.99 ± 1.1 ng ml−1) versus against T (T = 3.86 ± 1.0 ng ml−1) and intermediate values for the controls (HL: T = 4.07 ± 0.7 ng ml−1; LH: T = 5.90 ± 0.8 ng ml−1).
(b). F1 offspring
Offspring body size at birth was not significantly influenced by parental T line (two-way ANOVA, maternal: F1,45 = 1.564, p = 0.217; paternal: F1,45 = 0.001, p = 0.979; maternal × paternal: F1,45 = 3.805, p = 0.057). Furthermore, offspring body size at birth did not differ between F1 brothers and sisters as a function of parental T line (three-way ANOVA: maternal × paternal × progeny gender: F1,89 = 0.929, p = 0.338; mean litter weight (in grams) ± s.e.: F1 males—HH = 1.91 ± 0.04, LL = 1.86 ± 0.1, HL = 2.01 ± 0.1, LH = 1.93 ± 0.05; F1 females—HH = 1.84 ± 0.05, LL = 1.74 ± 0.1, HL = 2.00 ± 0.1, LH = 1.94 ± 0.04).
(c). F1 adult fitness
F1 male fitness was positively related to circulating T plasma levels in the four selection lines except HH (HH: F1,11 = 1, p = 0.339; r2 = 0.083; y = −0.035x + 0.986; HL: F1,12 = 4.790, p = 0.049; r2 = 0.285; y = 0.159x − 0.843; LH: F1,16 = 4.632, p = 0.047; r2 = 0.224; y = 0.110x − 0.682; LL: F1,6 = 70.579, p < 0.001; r2 = 0.922; y = 0.193x − 1.319). On the other hand, F1 female fitness did not show any relation to circulating T plasma levels of their brothers (all p > 0.05).
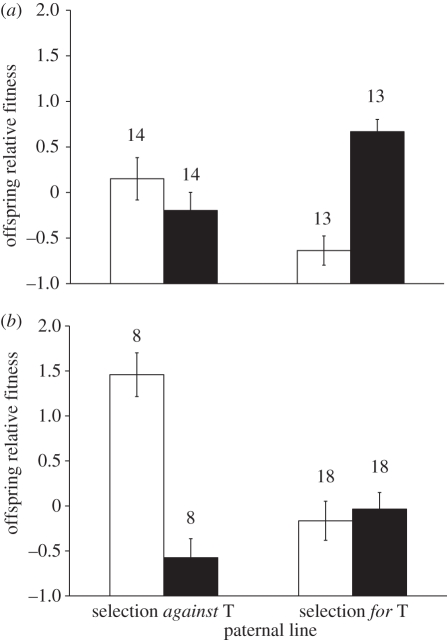
Selection on T in both paternal and maternal lines affected the adult fitness of both sons and daughters, but in opposite directions (table 1). Paternal T was positively related to son fitness (post hoc tests between H and L paternal lines: F1,103 = 6.11, p = 0.015; black bars in figure 1a,b), but negatively related to the relative fitness of opposite-sex progeny (daughters; F1,103 = 17.77, p < 0.001; white bars in figure 1a,b). Maternal selection for T was negatively related to daughter fitness (F1,103 = 5.40, p = 0.022; figure 1) and showed a trend for a positive relation to son fitness (F1,103 = 2.99, p = 0.087). There was a non-significant trend for an interaction between paternal and maternal line on offspring fitness (table 1) caused by a non-significant tendency for a higher fitness of offspring from L paternal lines compared with H paternal lines when both crossed with L maternal lines (post hoc tests: F1,102 = 3.26, p = 0.074; all other p > 0.1).
Table 1.
The effects of divergent maternal and paternal selection lines for testosterone on F1 progeny fitness as a function of gender analysed using a three-way ANOVA. (d.f., degrees of freedom; F, test statistic: p, probability; significant values are highlighted in bold text.)
| factor | d.f. | F | p |
|---|---|---|---|
| maternal line | 1,98 | 1.353 | 0.248 |
| paternal line | 1,98 | 2.808 | 0.097 |
| progeny gender | 1,98 | 2.413 | 0.124 |
| maternal line × paternal line | 1,98 | 3.688 | 0.058 |
| maternal line × progeny gender | 1,98 | 22.363 | <0.001 |
| paternal line × progeny gender | 1,98 | 39.790 | <0.001 |
| maternal line × paternal line × progeny gender | 2,98 | 0.719 | 0.399 |
Figure 1.
Mean (±s.e.) relative adult fitness of F1 sons (black bars) and daughters (white bars) from mixed-sex litters as a function of paternal lines and maternal lines selected (a) for T and (b) against T. Sample size (n) refers to the number of mixed-sex litters (means taken within a litter). Error bars indicate 1 standard error.
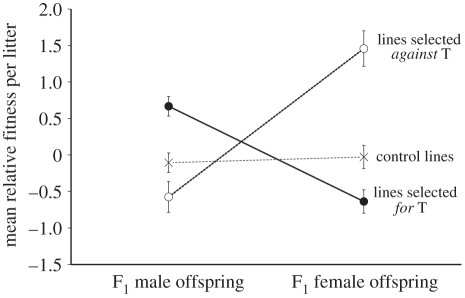
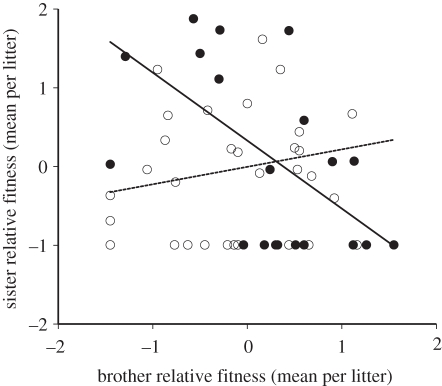
Relative adult fitness was reversed between F1 brothers and sisters between selection lines (two-way ANOVA: selection line: F2,100 = 2.987, p = 0.055; progeny gender: F1,100 = 2.516, p = 0.116; selection line × progeny gender: F2,100 = 24.930, p < 0.001; figure 2). Relative fitness differed between siblings in lines selected for male T (post hoc tests: F1,104 = 19.20, p < 0.001) with more reproductively fit sons produced compared with daughters (figure 2). Relative fitness differed between siblings in lines selected against male T (F1,104 = 28.68, p < 0.001) with more reproductively fit daughters produced compared with sons (figure 2). No fitness differences were found in controls (F1,104 = 0.17, p = 0.679). The fitness of adult F1 full-sibling brothers and sisters was negatively correlated across selection lines (Pearson's, HH and LL: r = −0.576, n = 21, p = 0.006; figure 3), but not across control lines (Pearson's HL and LH: r = 0.187, n = 32, p = 0.306; figure 3).
Figure 2.
Mean (±s.e.) relative adult fitness between F1 brothers and sisters from mixed-sex litters. Filled circles, lines selected for male T (HH), n = 13. Open circles, lines selected against male T (LL), n = 8. Cross symbols, control lines (HL and LH), n = 32. Sample size (n) refers to the number of mixed-sex litters (means taken within a litter). Error bars indicate 1 standard error.
Figure 3.
Intersexual regression of adult F1 fitness between brothers and sisters (litter means); selection lines and control lines are shown separately. Filled circles, lines selected for and against male T (simple linear regression, HH and LL: F1,19 = 9.447, p = 0.006, r2 = 0.33; y = −0.863x + 0.329). Open circles, control lines (simple linear regression, HL and LH: F1,30 = 1.085, p = 0.306, r2 = 0.035; y = 0.221x − 0.004).
(d). F1 differential sex ratio allocation
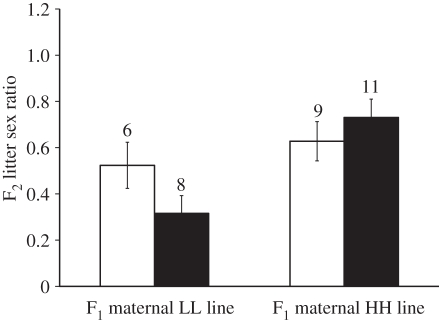
We found evidence for cryptic sex ratio bias in F2 litters as a function of F1 maternal line, but not as a function of F1 paternal line or an interaction between F1 parental lines (two-way ANOVA on F2 sex ratio; F1 maternal: F1,30 = 9.268, p = 0.005; F1 paternal: F1,30 = 0.329, p = 0.571; F1 maternal × F1 paternal: F1,30 = 3.055, p = 0.091). Litter sex ratios of F1 females from LL and HH lines differed significantly from a 1 : 1 sex ratio with the former producing female-biased F2 litters, whereas F1 females from HH lines produced male-biased F2 litters (LL: χ12 = 4.805, p < 0.05; HH: χ12 = 7.22, p < 0.01; figure 4).
Figure 4.
Differential sex ratio allocation of F2 litters between HH (white bars) and LL F1 parental lines (black bars).
4. Discussion
By creating artificial selection lines divergent for male T and measuring subsequent reproductive fitness of F1 full siblings, we demonstrated several important elements of intralocus sexual conflict in the small mammal M. glareolus. First, we show that inheritance patterns of paternal T reveal a negative correlation on the fitness of opposite-sex progeny (father–daughter, mother–son; table 1 and figure 1). Second, we show a selection line by sex interaction between reproductive fitness estimates in male and female bank voles (figure 2). Third, the fitness of full siblings is negatively correlated (figure 3). Accordingly, our study indicates that sexually antagonistic selection is probably acting on the steroid hormone T, which has importance implications for sexual selection.
Could the negative correlation in fitness between F1 full siblings be owing to mechanisms other than intralocus conflict? Differential non-genetic investment by mothers may produce such a result. However, bank vole offspring body size at birth did not differ significantly with sex as a function of selection line (this study) and contrary to other species [57,58], female bank voles have previously been found not to adjust their maternal effort before or after birth according to male quality [42]. However, we cannot rule out the possibility that non-genetic maternal effects may bias the present results. Negative effects of male hormones in utero on female fitness may also produce negative correlations in fitness between siblings. However, the number of F1 brothers in utero did not affect either their sister's fitness (ANOVA, number of males in utero: HH–F4,8 = 0.861, p = 0.526; HL–F2,8 = 1.316, p = 0.321; LH–F3,14 = 1.914, p = 0.174; LL–F2,4 = 0.968, p = 0.454), or their anogenital distance (ANOVA: HH–F4,8 = 0.593, p = 0.678; HL–F2,8 = 1.465, p = 0.287; LH–F3,13 = 0.656, p = 0.594; LL–F2,4 = 3.833, p = 0.118), a measure of male feminization [59]. The number of F1 brothers in utero did not affect their brother's fitness (ANOVA, HH–F4,8 = 1.820, p = 0.218; HL–F2,8 = 3.982, p = 0.063; LH–F3,14 = 1.441, p = 0.273; LL–F2,4 = 1.319, p = 0.363) or anogenital distance either (ANOVA, HH–F4,8 = 1.588, p = 0.268; HL–F2,8 = 0.077, p = 0.926; LH–F3,14 = 0.553, p = 0.654; LL–F2,4 = 0.016, p = 0.984). Therefore, the negative correlation in the estimate of fitness between sons and daughters (figure 3) and the selection group by sex interaction (figure 2) suggest the presence of intralocus conflict in the bank vole. Kruuk et al. [60] suggested that although negative cross-sex genetic correlations for morphological traits would seem unlikely, alleles associated with enhanced male reproductive success but reduced female fecundity would be plausible, owing to the allelic effects on hormone levels, such as T. Our data support this hypothesis showing that reproductive fitness was sensitive to selection on T levels in both bank vole sexes, but in opposite directions.
The specific traits that are targets of intralocus sexual conflict are largely unknown [30], and here we show that for bank vole fitness, sexually antagonistic selection is acting on circulating male T levels, a result also found to maintain the strategy of multiple mating in the bank vole [47]. Directional selection for increased T production in males is clearly linked to changes in females, hence a genetic correlation between female and male T expression levels is likely to be strong, as has been documented for some avian taxa [36,37]. As suggested by Mank [38], it is therefore unlikely that female bank voles lack androgen receptors entirely, else there would be fewer negative consequences of increased T levels for females. Female bank voles are likely to have had higher and lower T expression that may have decreased and increased their fecundity, respectively, possibly owing to resource re-allocation or non-optimal hormone levels. Other proximate explanations include genomic imprinting [61,62], a common process when multiple males sire the offspring of one female [63], or paternal alleles may have had sexually antagonistic effects on offspring condition, i.e. the efficiency of acquiring and converting resources into fitness [64]. Alternatively, our selection lines might have differed in other traits, such that a correlated trait, and not T, caused the observed effects, but we are unable to test this hypothesis with our data.
This study highlights a negative genetic correlation between selection for T in sires which determines reproductive success [39] and their daughter's fitness (figure 1). Other studies, although rare, have also shown that the heritability of fitness is negatively correlated between parents and their opposite-sex progeny (e.g. father–daughter or mother–son) [14,19,20,26]. As previously highlighted by Fedorka & Mousseau [19], intralocus sexual conflict has important fitness consequences for females, as the indirect genetic benefits of selecting reproductively successful males as mating partners (dominant bank vole males with high T levels) may be lost when a daughter is produced. What, therefore, are the consequences of this between-sex component of trait heritability for sexual selection? Male bank voles advertise their quality by dominance dependent on T [43,65] and both male–male competition, and female choice for dominant males causes strong selection for higher T levels [39]. Directional selection for increased T in males is therefore high [39,43], but a correlated [45] and antagonistic increase would be manifest in females (this study). Moreover, a recent multivariate quantitative genetic study by Schroderus et al. [45] highlighted that selection on male T would also be constrained by the antagonistic effect on immunity in males as well as in females. Such opposing selection mechanisms are likely to be powerful forces promoting the maintenance of genetic variation not only on T, but also on secondary sexual characteristics mediated by T and on bank vole fitness itself.
Why have not bank voles evolved sex-specific gene expression to resolve this sexual conflict for fitness? First, most genes are, to some degree, pleiotropic [66–68] and such constraints may hinder the resolution of sexual antagonism [69]. Owing to the multiple functions of T and thus complex selective pressures, we predict that pleiotropic constraints are likely to be strong on T as highlighted by Schroderus et al. [45]. Second, female bank voles are known to mate polyandrously, producing litters with multiple sires [39], a strategy that may alone mitigate the disparity between progeny quality [70]. However, when sire genotypes have differential fitness effects on sons versus daughters, female side-blotched lizards, Uta stansburiana, are capable of altering progeny sex ratio accordingly [17,26,71]. We also found evidence for cryptic sex ratio bias in bank voles as the litters of high-quality females (LL: high fecundity) were biased towards daughters and low-quality females (HH: low fecundity) towards sons (figure 4). This raises the possibility of whether females could actively bias their litter sex ratio to increase their fitness either through daughters or sons as a function of their genotype, or whether differential litter sex ratios between maternal lines are a physiological response to female circulating T levels.
The bank vole is naturally exposed to considerable sources of spatial and temporal environmental variation creating a variable selective environment that may partly resolve the negative correlation in fitness between siblings. Bank vole population densities fluctuate with distinct density cycles in northern Fennoscandia [72] and a genotype-by-environment interaction has been found on male bank vole reproductive success [41], thus under changing environmental conditions the correlation between sibling fitness may vary. Bank voles also show a phenotypic and genetic trade-off between immune response and T [43–45] and measures of immunity differ across density cycles [73]. Therefore, selection on different immune-related traits is likely to differ between peak and crash years with concomitant effects of fitness and its correlation between the sexes. Recent theoretical and empirical data also highlights that genetic variation in sexually antagonistic traits can be maintained via negative frequency-dependent selection [74]. Variable selection environments may select for different traits in one or both of the sexes, potentially altering the correlation in fitness between them, and may thus play a role in maintaining the negative correlation in fitness between bank vole siblings.
Acknowledgements
The use of animals adhered to ethical guidelines for animal research in Finland.
We would like to thank Aili-Maria Pihlajamäki and Riikka Närä for their invaluable help with the study species, Ricardo Beldade, Dan Malone, Mikael Mokkonen and two anonymous referees for useful comments. The study was financially supported by the Academy of Finland (grant nos. 103508 and 108566 to S.C.M.; 115961, 119200, 140767 and 218107 to E.K.; and 118603, 109165 and 204284 to T.M.) and the Centre of Excellence in Evolutionary Research of the Academy of Finland.
References
- 1.Dawkins R. 1976. The selfish gene. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Parker G. A. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. A.), pp. 123–166 New York, NY: Academic Press [Google Scholar]
- 3.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell B.), pp. 139–179 Chicago, IL: Aldine Press [Google Scholar]
- 4.Bonduriansky R., Chenoweth S. F. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288 10.1016/j.tree.2008.12.005 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 5.Chippindale A. K., Gibson J. R., Rice W. R. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675 10.1073/pnas.041378098 (doi:10.1073/pnas.041378098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 10.2307/2407393 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 7.Rice W. R. 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38, 735–742 10.2307/2408385 (doi:10.2307/2408385) [DOI] [PubMed] [Google Scholar]
- 8.Westneat D. F., Sih A. 2009. Sexual conflict as a partitioning of selection. Biol. Lett. 5, 675–677 10.1098/rsbl.2009.0195 (doi:10.1098/rsbl.2009.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lessells C. M. 2006. The evolutionary outcome of sexual conflict. Phil. Trans. R. Soc. B 361, 301–317 10.1098/rstb.2005.1795 (doi:10.1098/rstb.2005.1795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice W. R. 1992. Sexually antagonistic genes: experimental evidence. Science 256, 1436–1439 10.1126/science.1604317 (doi:10.1126/science.1604317) [DOI] [PubMed] [Google Scholar]
- 11.Rice W. R., Chippindale A. K. 2002. The evolution of hybrid infertility: perpetual coevolution between gender-specific and sexually antagonistic genes. Genetica 116, 179–188 10.1023/A:1021205130926 (doi:10.1023/A:1021205130926) [DOI] [PubMed] [Google Scholar]
- 12.Gibson J. R., Chippindale A. K., Rice W. R. 2002. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. R. Soc. Lond. B 269, 499–505 10.1098/rspb.2001.1863 (doi:10.1098/rspb.2001.1863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow E. H., Stewart A. D., Rice W. R. 2008. Assessing the extent of genome-wide intralocus sexual conflict via experimentally enforced gender-limited selection. J. Evol. Biol. 21, 1046–1054 10.1111/j.1420-9101.2008.01542.x (doi:10.1111/j.1420-9101.2008.01542.x) [DOI] [PubMed] [Google Scholar]
- 14.Pischedda A., Chippindale A. K. 2006. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4, 2099–2103 10.1371/journal.pbio.0040356 (doi:10.1371/journal.pbio.0040356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad N. G., Bedhomme S., Day T., Chippindale A. K. 2007. An evolutionary cost of separate genders revealed by male-limited evolution. Am. Nat. 169, 29–37 10.1086/509941 (doi:10.1086/509941) [DOI] [PubMed] [Google Scholar]
- 16.Rice W. R., Chippindale A. K. 2001. Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693 10.1046/j.1420-9101.2001.00319.x (doi:10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 17.Calsbeek R., Sinervo B. 2004. Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. J. Evol. Biol. 17, 464–470 10.1046/j.1420-9101.2003.00665.x (doi:10.1046/j.1420-9101.2003.00665.x) [DOI] [PubMed] [Google Scholar]
- 18.Camperio-Ciani A., Corna F., Capiluppi C. 2004. Evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity. Proc. R. Soc. Lond. B 271, 2217–2221 10.1098/rspb.2004.2872 (doi:10.1098/rspb.2004.2872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorka K. M., Mousseau T. A. 2004. Female mating bias results in conflicting sex-specific offspring fitness. Nature 429, 65–67 10.1038/nature02492 (doi:10.1038/nature02492) [DOI] [PubMed] [Google Scholar]
- 20.Foerster K., Coulson T., Sheldon B. C., Pemberton J. M., Clutton-Brock T. H., Kruuk L. E. B. 2007. Sexually antagonistic genetic variation for fitness in red deer. Nature 447, 1107–1109 10.1038/nature05912 (doi:10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- 21.Pai A., Yan G. 2002. Polyandry produces sexy sons at the cost of daughters in red flour beetles. Proc. R. Soc. Lond. B 269, 361–368 10.1098/rspb.2001.1893 (doi:10.1098/rspb.2001.1893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis Z., Wedell N., Hunt J. 2011. Evidence for strong intralocus sexual conflict in the Indian meal moth, Plodia interpunctella. Evolution 65, 2085–2097 10.1111/j.1558-5646.2011.01267.x (doi:10.1111/j.1558-5646.2011.01267.x) [DOI] [PubMed] [Google Scholar]
- 23.Long T. A. F., Rice W. R. 2007. Adult locomotory activity mediates intralocus sexual conflict in a laboratory-adapted population of Drosophila melanogaster. Proc. R. Soc. B 274, 3105–3112 10.1098/rspb.2007.1140 (doi:10.1098/rspb.2007.1140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badyaev A. V. 2002. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 17, 369–378 10.1016/S0169-5347(02)02569-7 (doi:10.1016/S0169-5347(02)02569-7) [DOI] [Google Scholar]
- 25.Abbott J., Svensson E. I. 2005. Phenotypic and genetic variation in emergence and development time of a trimorphic damselfly. J. Evol. Biol. 18, 1464–1470 10.1111/j.1420-9101.2005.01019.x (doi:10.1111/j.1420-9101.2005.01019.x) [DOI] [PubMed] [Google Scholar]
- 26.Calsbeek R., Bonneaud C. 2008. Postcopulatory fertilization bias as a form of cryptic sexual selection. Evolution 62, 1137–1148 10.1111/j.1558-5646.2008.00356.x (doi:10.1111/j.1558-5646.2008.00356.x) [DOI] [PubMed] [Google Scholar]
- 27.Mckean K. A., Nunney L. 2005. Bateman's principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution 59, 1510–1517 10.1554/04-657 (doi:10.1554/04-657) [DOI] [PubMed] [Google Scholar]
- 28.Rolff J. 2002. Bateman's principle and immunity. Proc. R. Soc. Lond. B 269, 867–872 10.1098/rspb.2002.1959 (doi:10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolff J., Armitage S. A. O., Coltman D. W. 2005. Genetic constraints and sexual dimorphism in immune defense. Evolution 59, 1844–1850 10.1111/j.0014-3820.2005.tb01831.x (doi:10.1111/j.0014-3820.2005.tb01831.x) [DOI] [PubMed] [Google Scholar]
- 30.Svensson E. I., McAdam A. G., Sinervo B. 2009. Intralocus sexual conflict over immune defense, gender load, and sex specific signaling in a natural lizard population. Evolution 63, 3124–3135 10.1111/j.1558-5646.2009.00782.x (doi:10.1111/j.1558-5646.2009.00782.x) [DOI] [PubMed] [Google Scholar]
- 31.Maklakov A. A., Simpson S. J., Zajitschek F., Hall M. D., Dessmann J., Clissold F., Raubenheimer D., Bonduriansky R., Brooks R. C. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066 10.1016/j.cub.2008.06.059 (doi:10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- 32.Roulin A., Almasi B., Jenni L. 2010. Temporal variation in glucocorticoid levels during the resting phase is associated in opposite way with maternal and paternal melanic coloration. J. Evol. Biol. 23, 2046–2053 10.1111/j.1420-9101.2010.02086.x (doi:10.1111/j.1420-9101.2010.02086.x) [DOI] [PubMed] [Google Scholar]
- 33.Renfree M. B., Wilson J. D., Short R. V., Shaw G., George F. W. 1992. Steroid-hormones content of the gonads of the Tammar wallaby during sexual differentiation. Biol. Reprod. 47, 644–647 10.1095/biolreprod47.4.644 (doi:10.1095/biolreprod47.4.644) [DOI] [PubMed] [Google Scholar]
- 34.Hau M. 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144 10.1002/bies.20524 (doi:10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- 35.Roff D. A. 1997. Evolutionary quantitative genetics. New York, NY: International Thomson Publishing [Google Scholar]
- 36.Ketterson E. D., Nolan V., Sandell M. 2005. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 166, S85–S98 10.1086/444602 (doi:10.1086/444602) [DOI] [PubMed] [Google Scholar]
- 37.Møller A. P., Garamszegi L. Z., Gil D., Hurtrez-Bousses S., Eens M. 2005. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav. Ecol. Sociobiol. 58, 534–544 10.1007/s00265-005-0962-2 (doi:10.1007/s00265-005-0962-2) [DOI] [Google Scholar]
- 38.Mank J. E. 2007. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. Am. Nat. 169, 142–149 10.1086/510103 (doi:10.1086/510103) [DOI] [PubMed] [Google Scholar]
- 39.Mills S. C., Grapputo A., Koskela E., Mappes T. 2007. Quantitative measure of sexual selection with respect to the operational sex ratio: a comparison of selection indices. Proc. R. Soc. B 274, 143–150 10.1098/rspb.2006.3639 (doi:10.1098/rspb.2006.3639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koskela E., Mappes T., Ylönen H. 1997. Territorial behaviour and reproductive success of bank vole Clethrionomys glareolus females. J. Anim. Ecol. 66, 341–349 10.2307/5980 (doi:10.2307/5980) [DOI] [Google Scholar]
- 41.Mills S. C., Alatalo R. V., Koskela E., Mappes J., Mappes T., Oksanen T. A. 2007. Signal reliability compromised by genotype by environment interaction and potential mechanisms for its preservation. Evolution 61, 1748–1757 10.1111/j.1558-5646.2007.00145.x (doi:10.1111/j.1558-5646.2007.00145.x) [DOI] [PubMed] [Google Scholar]
- 42.Oksanen T. A., Alatalo R. V., Horne T. J., Koskela E., Mappes J., Mappes T. 1999. Maternal effort and male quality in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 266, 1495–1499 10.1098/rspb.1999.0806 (doi:10.1098/rspb.1999.0806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills S. C., Grapputo A., Jokinen I., Koskela E., Mappes T., Oksanen T. A., Poikonen T. 2009. Testosterone-mediated effects on fitness-related phenotypic traits and fitness. Am. Nat. 173, 475–487 10.1086/597222 (doi:10.1086/597222) [DOI] [PubMed] [Google Scholar]
- 44.Mills S. C., Grapputo A., Jokinen I., Koskela E., Mappes T., Puttonen T. 2010. Fitness trade-offs mediated by immunosuppression costs in a small mammal. Evolution 64, 166–179 10.1111/j.1558-5646.2009.00820.x (doi:10.1111/j.1558-5646.2009.00820.x) [DOI] [PubMed] [Google Scholar]
- 45.Schroderus E., Jokinen I., Koivula M., Koskela E., Mappes T., Mills S. C., Oksanen T. A., Poikonen T. 2010. Intra- and intersexual trade-offs between testosterone and immune system: implications for sexual antagonistic selection. Am. Nat. 176, E90–E97 10.1086/656264 (doi:10.1086/656264) [DOI] [PubMed] [Google Scholar]
- 46.Zysling D. A., Greives T. J., Breuner C. W., Casto J. M., Dernas G. E., Ketterson E. D. 2006. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Horm. Behav. 50, 200–207 10.1016/j.yhbeh.2006.03.004 (doi:10.1016/j.yhbeh.2006.03.004) [DOI] [PubMed] [Google Scholar]
- 47.Mokkonen M., Koskela E., Mappes T., Mills S. C. In press Sexual antagonism for testosterone maintains multiple mating behaviour. J. Anim. Ecol. (doi:10.1111/j.1365-2656.2011.01903.x) [DOI] [PubMed] [Google Scholar]
- 48.Day T., Bonduriansky R. 2004. Intralocus sexual conflict can drive the evolution of genomic imprinting. Genetics 167, 1537–1546 10.1534/genetics.103.026211 (doi:10.1534/genetics.103.026211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oksanen T. A., Jokinen I., Koskela E., Mappes T., Vilpas H. 2003. Manipulation of offspring number and size: benefits of large body size at birth depend upon the rearing environment. J. Anim. Ecol. 72, 321–330 10.1046/j.1365-2656.2003.00703.x (doi:10.1046/j.1365-2656.2003.00703.x) [DOI] [Google Scholar]
- 50.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. The Auk 104, 116–121 [Google Scholar]
- 51.Field A. 2005. Discovering statistics using SPSS. Oxford, UK: The Alden Press [Google Scholar]
- 52.Gockel J., Harr B., Schlotterer C., Arnold W., Gerlach G., Tautz D. 1997. Isolation and characterization of microsatellite loci from Apodemus flavicollis (Rodentia, Muridae) and Clethrionomys glareolus (Rodentia, Cricetidae). Mol. Ecol. 6, 597–599 10.1046/j.1365-294X.1997.00222.x (doi:10.1046/j.1365-294X.1997.00222.x) [DOI] [PubMed] [Google Scholar]
- 53.Rikalainen K., Grapputo A., Knott E., Koskela E., Mappes T. 2008. A large panel of novel microsatellite markers for the bank vole (Myodes glareolus). Mol. Ecol. Res. 8, 1164–1168 10.1111/j.1755-0998.2008.02228.x (doi:10.1111/j.1755-0998.2008.02228.x) [DOI] [PubMed] [Google Scholar]
- 54.Koivula M., Koskela E., Mappes T., Oksanen T. A. 2003. Cost of reproduction in the wild: manipulation of reproductive effort in the bank vole. Ecology 84, 398–405 10.1890/0012-9658(2003)084[0398:CORITW]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0398:CORITW]2.0.CO;2) [DOI] [Google Scholar]
- 55.Mappes T., Koskela E. 2004. Genetic basis of the trade-off between offspring number and quality in the bank vole. Evolution 58, 645–650 [PubMed] [Google Scholar]
- 56.Mappes T., Koivula M., Koskela E., Oksanen T. A., Savolainen T., Sinervo B. 2008. Frequency and density-dependent selection on life-history strategies: a field experiment. PLoS ONE 3, e1687. 10.1371/journal.pone.0001687 (doi:10.1371/journal.pone.0001687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byers J. A., Waits L. P. 2006. Good genes sexual selection in nature. Proc. Natl Acad. Sci. USA 103, 16 343–16 345 10.1073/pnas.0608184103 (doi:10.1073/pnas.0608184103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinervo B., DeNardo D. F. 1996. Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution 50, 1299–1313 10.2307/2410670 (doi:10.2307/2410670) [DOI] [PubMed] [Google Scholar]
- 59.Clemens L. G., Gladue B. A., Coniglio L. P. 1978. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm. Behav. 10, 40–53 10.1016/0018-506X(78)90023-5 (doi:10.1016/0018-506X(78)90023-5) [DOI] [PubMed] [Google Scholar]
- 60.Kruuk L. E. B., Slate J., Wilson A. J. 2008. New answers for old questions: the evolutionary quantitative genetics of wild animal populations. Annu. Rev. Ecol. Evol. Syst. 39, 525–548 10.1146/annurev.ecolsys.39.110707.173542 (doi:10.1146/annurev.ecolsys.39.110707.173542) [DOI] [Google Scholar]
- 61.Reik W., Dean W., Walter J. 2001. Epigenetic reprogramming in mammalian development. Science 293, 1089–1093 10.1126/science.1063443 (doi:10.1126/science.1063443) [DOI] [PubMed] [Google Scholar]
- 62.Reik W., Walter J. 2001. Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2, 21–32 10.1038/35047554 (doi:10.1038/35047554) [DOI] [PubMed] [Google Scholar]
- 63.Haig D. 1997. Parental antagonism, relatedness asymmetries, and genomic imprinting. Proc. R. Soc. Lond. B 264, 1657–1662 10.1098/rspb.1997.0230 (doi:10.1098/rspb.1997.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mainguy J., Cote S. D., Festa-Bianchet M., Coltman D. W. 2009. Father-offspring phenotypic correlations suggest intralocus sexual conflict for a fitness-linked trait in a wild sexually dimorphic mammal. Proc. R. Soc. B 276, 4067–4075 10.1098/rspb.2009.1231 (doi:10.1098/rspb.2009.1231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radwan J., et al. 2006. Metabolic costs of sexual advertisement in the bank vole, Clethrionomys glareolus. Evol. Ecol. Res. 8, 859–869 [Google Scholar]
- 66.Ericson E., Pylvanainen I., Fernandez-Ricaud L., Nerman O., Warringer J. B. A. 2006. Genetic pleiotropy in Saccharomyces cerevisiae quantified by high-resolution phenotypic profiling. Mol. Genet. Genomics 275, 605–614 10.1007/s00438-006-0112-1 (doi:10.1007/s00438-006-0112-1) [DOI] [PubMed] [Google Scholar]
- 67.Hodgkin J. 1998. Seven types of pleiotropy. Int. J. Dev. Biol. 42, 501–505 [PubMed] [Google Scholar]
- 68.Waxman D., Peck J. R. 1998. Pleiotropy and the preservation of perfection. Science 279, 1210–1213 10.1126/science.279.5354.1210 (doi:10.1126/science.279.5354.1210) [DOI] [PubMed] [Google Scholar]
- 69.Mank J. E., Hultin-Rosenberg L., Zwahlen M., Ellegren H. 2008. Pleiotropic constraint hampers the resolution of sexual antagonism in vertebrate gene expression. Am. Nat. 171, 35–43 10.1086/523954 (doi:10.1086/523954) [DOI] [PubMed] [Google Scholar]
- 70.Klemme I., Ylonen H., Eccard J. A. 2008. Long-term fitness benefits of polyandry in a small mammal, the bank vole Clethrionomys glareolus. Proc. R. Soc. B 275, 1095–1100 10.1098/rspb.2008.0038 (doi:10.1098/rspb.2008.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pryke S. R., Griffith S. C. 2009. Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch. Science 323, 1605–1607 10.1126/science.1168928 (doi:10.1126/science.1168928) [DOI] [PubMed] [Google Scholar]
- 72.Kallio E. R., Begon M., Henttonen H., Koskela E., Mappes T., Vaheri A., Vapalahti O. 2009. Cyclic hantavirus epidemics in humans predicted by rodent host dynamics. Epidemics 1, 101–107 10.1016/j.epidem.2009.03.002 (doi:10.1016/j.epidem.2009.03.002) [DOI] [PubMed] [Google Scholar]
- 73.Huitu O., Jokinen I., Korpimaki E., Koskela E., Mappes T. 2007. Phase dependence in winter physiological condition of cyclic voles. Oikos 116, 565–577 10.1111/j.0030-1299.2007.15488.x (doi:10.1111/j.0030-1299.2007.15488.x) [DOI] [Google Scholar]
- 74.Mokkonen M., Kokko H., Koskela E., Lehtonen J., Mappes T., Martiskainen H., Mills S. C. 2011. Negative frequency-dependent selection of sexually antagonistic alleles in Myodes glareolus. Science 334, 972–974 10.1126/science.1208708 (doi:10.1126/science.1208708) [DOI] [PubMed] [Google Scholar]