Abstract
Recent studies of sprinters and distance runners have suggested that variations in human foot proportions and plantarflexor muscle moment arm correspond to the level of sprint performance or running economy. Less clear, however, is whether differences in muscle moment arm are mediated by altered tendon paths or by variation in the centre of ankle joint rotation. Previous measurements of these differences have relied upon assumed joint centres and measurements of bone geometry made externally, such that they would be affected by the thickness of the overlying soft tissue. Using magnetic resonance imaging, we found that trained sprinters have shorter plantarflexor moment arms (p = 0.011) and longer forefoot bones (p = 0.019) than non-sprinters. The shorter moment arms of sprinters are attributable to differences in the location of the centre of rotation (p < 0.001) rather than to differences in the path of the Achilles tendon. A simple computer model suggests that increasing the ratio of forefoot to rearfoot length permits more plantarflexor muscle work during plantarflexion that occurs at rates expected during the acceleration phase following the sprint start.
Keywords: humans, sprinting, plantarflexor, moment arm, centre of rotation
1. Introduction
Human foot and ankle structure are known to vary substantially but the implications of this variation on locomotor function are unclear. The plantarflexor moment arms (pfMA) measured in 21 adult males by Maganaris et al. [1] were found to range between 4.7 and 6.1 cm. The lengths of the metatarsals and phalanges in adult males have been found to exhibit similar variation, with between-subject standard deviations that range from 8 to 17 per cent of their mean values [2]. While the influence of such structural variation in the human foot and ankle upon function has not received much attention, variation across species is known to correspond to differences in functional demands. Cursorial animals such as the cheetah and greyhound have long forefeet and short heels, a combination that permits rapid joint rotations [3]. By contrast, digging animals like the mole have limb structures that permit the large output forces that are needed for digging [4]. Within-species variations in joint and skeletal structure may have little connection to function if they are compensated for by muscular and nervous system adaptations, and animal studies have been performed to investigate how muscles adapt to acutely applied variations in muscle moment arm. Koh & Herzog [5] found that the muscles of rabbits adapt to retinacular release in such a way that muscle fibre excursion is maintained, but the results of Burkholder & Lieber [6] suggested that mouse muscles adapt to maintain optimal sarcomere length at a given joint angle. Human muscle has been shown to adapt rapidly to changes in training, loading and exposure to bed rest [7–9], but the relationship between muscle function and constraints owing to joint structure are generally not well understood.
Two recent studies have focused on the influence of foot and ankle structure upon human locomotor function in the form of sprinting ability, but with inconsistent results. Lee & Piazza [10] found sprinters to have shorter pfMA and longer toes than height-matched non-sprinters. Karamanidis et al. [11], however, found no significant differences in pfMA, toe lengths or midfoot lengths between a group of elite sprinters and a group of slower sprinters. The means used to quantify foot and ankle geometry in these studies were somewhat indirect. In both studies, pfMA were computed from ultrasound images of tendon excursion and measurements of joint angle rather than from the position of the Achilles tendon relative to the centre of ankle rotation. Measures of toe and midfoot lengths were made from externally identified bony landmarks with measuring tapes and without the benefit of medical imaging techniques.
pfMA has the capacity to influence locomotor performance in complex ways. A long moment arm will result in more plantarflexor moment for a given muscle force, but moment arm also determines the speed at which the plantarflexors shorten during plantarflexion [10,12]. Muscles with longer lever arms will thus shorten more rapidly during the same joint rotation and thus produce less force owing to the force–velocity property of muscle. Carrier et al. [13] examined the ratio of the lever arm of the ground reaction force to the length of the heel, which they took to represent pfMA, in human runners. They found this ‘gear ratio’ to increase in late stance, allowing the plantarflexor muscle fibres to shorten more slowly and maintain force production. It is not currently known, however, whether variation in foot proportions contributes to the ability to generate propulsive muscle forces, perhaps by influencing the gear ratio.
The purpose of the present study was to determine if there are differences in the skeletal structure of the foot and ankle between two groups of humans with different functional abilities and histories: trained sprinters and non-sprinters. In this study, we used magnetic resonance (MR) imaging to quantify bone lengths directly and to make geometric determination of pfMA. The use of MR imaging permitted identification of the centre of ankle rotation which was not possible in previous studies in which ultrasound was used to track tendon excursion, and also facilitated the measurement of bony geometry that was unimpeded by soft tissue. We hypothesized that sprinters would have longer forefoot bones and shorter pfMA, which would reduce plantarflexor shortening velocity and increase plantarflexor force during acceleration at the start of a sprint race. It is hoped that characterization of within-species differences in pfMA and toe length that correspond to human sprinting ability will lead to an improved understanding of how joint mechanics and bone geometry affect human and animal locomotor function in general.
2. Methods
We studied two groups with eight male subjects in each (table 1). The first group was composed of sprinters who were involved in regular sprint training and competition. The second group consisted of height-matched individuals who were never trained or had competed in sprinting. Inclusion criteria for the sprinter subjects was current engagement in competitive sprinting and at least 3 years of continuous sprint training. Six sprinter subjects competed in the 100 m dash, with personal best times ranging from 10.5 to 11.1 s, and two reported 200 m personal best times of 21.4 and 24.1 s. Subjects were excluded if they had a recent history of lower extremity injuries, joint pain six months prior to data collection or any obvious movement abnormalities.
Table 1.
Subject characteristics.
| sprinters (n = 8) | non-sprinters (n = 8) | p-value | |
|---|---|---|---|
| stature (cm) | 177.4 ± 7.2 | 177.1 ± 7.0 | 0.944 |
| body mass (kg) | 76.3 ± 7.9 | 82.0 ± 8.9 | 0.252 |
| age (years) | 21.3 ± 2.5 | 24.0 ± 2.6 | 0.051 |
| foot length (cm) | 27.5 ± 1.2 | 27.5 ± 0.9 | 0.946 |
(a). Imaging
MR images of the right foot and ankle of each subject were acquired on a 3.0 T Siemens Trio scanner (Siemens; Erlangen, Germany). Subjects were positioned supine on the scanner bed with both knees flexed to 30° and resting on a foam pad. The right ankle was placed on an MR-compatible ankle-positioning device that was constructed from plastic and fastened to the scanner gantry prior to imaging. This device supported the foot while permitting the sagittal plane ankle position to be set. Images were acquired with the ankle positioned at 15° dorsiflexion, neutral ankle position, and 15° plantarflexion using a Siemens 4-Channel Flex coil placed around the anterior aspect of the ankle and secured using sandbags and elements 1 to 3 of the Siemens Spine Matrix. Scans were made in each ankle position while subjects remained relaxed. Following these scans, subjects were asked to maximally co-contract their ankle musculature while not moving the foot within the positioning device. The Achilles tendon and a point midway between the second and third metatarsal heads were palpated and identified using adhesive MR-sensitive external skin markers (Beekley Corp., Bristol, CT, USA). The high signal intensity registered by these markers provided landmarks for slice positioning and were used to ensure that lateral motion did not occur during scanning. Imaging was performed at the magnet isocentre and verbal communication between the subject and research team was facilitated through the Siemens MR console. T2-weighted two-dimensional True FISP images were acquired with sagittal orientation aligned with the Achilles tendon and a point midway between the second and third metatarsal heads while the subject rested (field of view = 300 mm, 1.2 × 1.2 × 4 mm resolution, echo time = 2.2 ms, repetition time = 4.4 ms, flip angle = 50°).
(b). Image processing
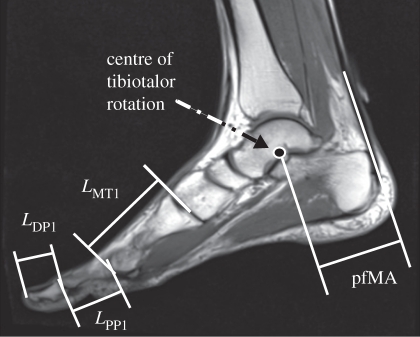
Images were post-processed with custom-written MatLab programs (The Mathworks, Natick, MA, USA). Centres of rotation between the tibia and talus were determined from pseudo-sagittal plane (bisecting the length of the Achilles tendon and the second metatarsal bone) images made in dorsiflexion and plantarflexion using a geometric method similar to that of Reuleaux [14] that has previously been described by Maganaris et al. [15]. The Achilles tendon line of action was defined as a line drawn down the midline of the tendon on the image taken at neutral ankle position. The pfMA was measured by finding the shortest distance from the tibiotalar centre of rotation (CoR) to the Achilles tendon line of action on the neutral-position scan (figure 1). Centres of tibiotalar rotation were located relative to a tibia-fixed coordinate system whose y-axis was oriented along the long axis of the tibia (with superior being positive). The origin of this coordinate system was the intersection of the y-axis and the tibial cortex and the x-axis was directed posterior and perpendicular to the y-axis (figure 2). The pfMA of a randomly selected sprinter subject was found 10 times from the same set of images and the coefficient of variation was found to be 3.5 per cent. The standard deviations of the anteroposterior and superior–inferior CoR positions were 1.45 and 2.39 mm, respectively. The lengths of the first metatarsal and the proximal and distal phalanges were measured from two-dimensional images recreated from three-dimensional MR data using OsiriX software (Pixmeo, Geneva, Switzerland). Lengths of bones were measured along the long axis of the bone between the intersections of the long axis and the cortex at the distal and proximal ends.
Figure 1.
Magnetic resonance image of a sagittal cross section of the foot and ankle in neutral position showing measurements of bone lengths and pfMA. Symbols are defined in table 2.
Figure 2.
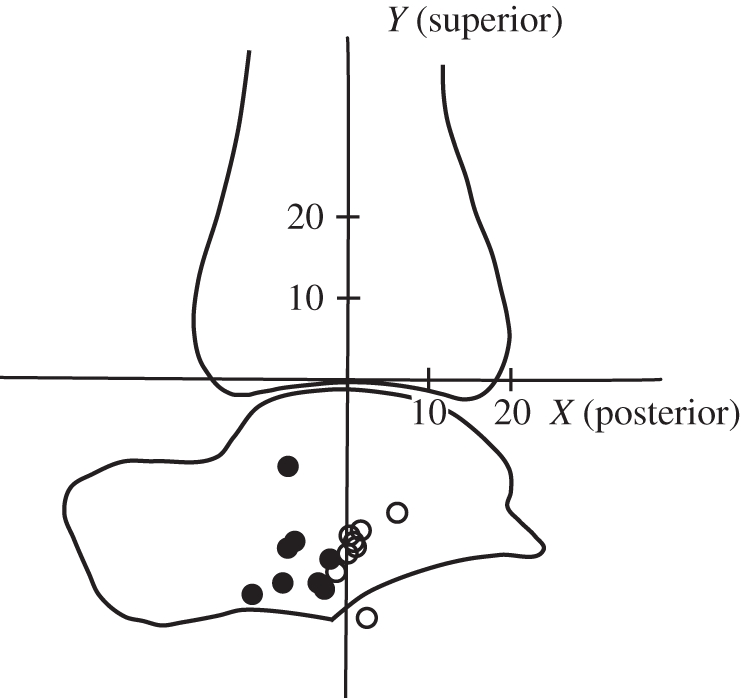
Sprinters' centres of rotation between the tibia (Ti) and talus (Ta) were located farther posterior relative to the long axis of the tibia than those of non-sprinters. Unfilled circles, sprinter; filled circles, non-sprinter. Units for both axes are in mm.
(c). Statistical testing
To test our hypotheses that the forefoot bones of sprinters are longer than those of non-sprinters and that the Achilles tendons of sprinters have smaller pfMA, unpaired, one-tailed t-tests were performed. Differences in stature, body mass, age, foot length, and x- and y-coordinates of the CoR were tested for using unpaired two-tailed t-tests. A paired, two-tailed t-test was performed to compare the pfMA found at resting and contracted states. The level of significance was set at α = 0.05 for all tests.
(d). Computer simulation
We developed a simple computer model to investigate the influence of variation in foot proportions on plantarflexor work during an isolated maximal plantarflexion contraction (figure 3). A foot segment with very low mass was confined to planar rotation with a revolute that represented the ankle joint. To simulate variation in foot proportions across simulations, the revolute joint position relative to the common plantarflexor tendon insertion at the extreme posterior of the foot was changed in 5 mm increments from 45 to 70 mm (the range for pfMA measured for our subjects) while the length of the foot was kept constant at 280 mm. The distal end of the foot was connected to a wall by a revolute joint that was permitted to slide without friction along the wall while the revolute constraint force was monitored. Three Hill-type muscle-tendon actuators [16] representing the soleus and the lateral and medial heads of the gastrocnemius were included in the model and force-generating properties for each muscle (optimal fibre length, tendon slack length, maximum isometric force; electronic supplementary material, table S1) were specified according to measurements made in cadaver specimens by Arnold et al. [17]. These actuators incorporated active and passive muscle force–length behaviour, muscle force–velocity relations and tendon force–length curves as specified by Delp et al. [18]. A full description of the details of the model is presented in the electronic supplementary material.
Figure 3.
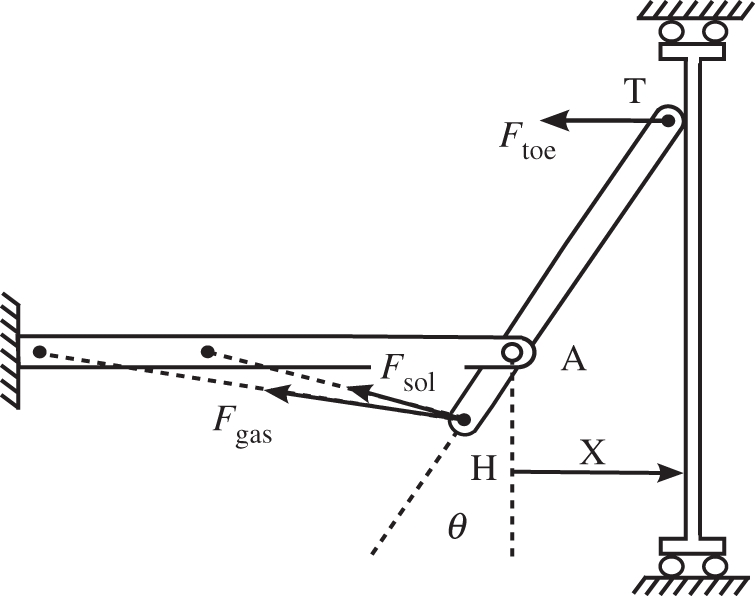
Computer model used in forward-dynamic simulations of the toe pushing against a retreating wall with maximal effort. The prescribed wall speed (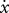 ) was varied between simulations, as was the proportion of rearfoot length (HA) to forefoot length (AT), but foot length (HT) was held constant.
) was varied between simulations, as was the proportion of rearfoot length (HA) to forefoot length (AT), but foot length (HT) was held constant.
During each simulation the plantarflexor actuators were excited maximally as the foot pushed against the wall, which receded at a constant velocity. This velocity was varied across simulations between 0.4 and 4 m s−1, producing a range of ankle joint velocities approximately representative of the velocities occurring during the push-off of walking [19], the start of sprinting [20] and maximal speed sprinting [21]. The combined work done by the three muscles between neutral ankle position and the position at which contact between the toe and the wall was broken (or 50° plantarflexion if this occurred first) was found by trapezoidal integration of the wall reaction force with respect to wall displacement.
3. Results
(a). Structural differences between groups
Sprinters were found to have shorter pfMA and longer forefoot bones than non-sprinters (table 2). Sprinters had pfMA measured with muscles at rest that were 7 mm (12%) shorter than those of non-sprinters (p = 0.011). The combined length of the first phalanges was 3.5 mm (6.2%) greater in sprinters (p = 0.010). Sprinters' first metatarsals were 2.9 mm (4.3%) longer (p = 0.050) and the combined length of the phalanges and metatarsal in sprinters was also significantly longer (p = 0.019). Sprinters' CoR were located farther posterior relative to the tibia than the CoR of non-sprinters (figure 2 and table 2; p < 0.001).
Table 2.
Measurements of foot and ankle structure. (LDP1, length of first distal phalanx; LPP1, length of first proximal phalanx; LMT1, length of first metatarsal; LR1, LDP1 + LPP1+MT1, length of first ray; XCoR, anteroposterior position of the centre of tibiotalar rotation relative to long axis of the tibia; YCoR, superior–inferior position of the centre of tibiotalar rotation. *p ≤ 0.05.)
| sprinters (n = 8) | non-sprinters (n = 8) | p-value | |
|---|---|---|---|
| LDP1 (mm) | 26.8 ± 1.2 | 25.2 ± 2.3 | 0.052 |
| LPP1 (mm) | 32.9 ± 1.5 | 31.0 ± 1.7 | 0.017* |
| LDP1 + LPP1 (mm) | 59.7 ± 2.0 | 56.2 ± 3.2 | 0.010* |
| LMT1 (mm) | 70.1 ± 2.0 | 67.2 ± 4.2 | 0.050* |
| LR1 (mm) | 129.8 ± 3.7 | 123.4 ± 7.0 | 0.019* |
| pfMAresting (mm) | 51.5 ± 3.9 | 58.5 ± 6.6 | 0.011* |
| pfMAcontracted (mm) | 52.9 ± 4.8 | 58.7 ± 5.4 | 0.021* |
| LR1 : pfMAresting [1] | 2.5 ± 0.2 | 2.1 ± 0.2 | 0.001* |
| XCoR (mm) | 1.2 ± 2.1 | −6.0 ± 3.1 | <0.001* |
| YCoR (mm) | −21.0 ± 3.9 | −21.8 ± 5.1 | 0.730 |
(b). Contraction state during imaging
Differences in pfMA between sprinters and non-sprinters were found both from images made with the muscles at rest and from images made with contracted muscles (p = 0.011 and p = 0.021, respectively). However, significant differences in pfMA were not found between resting and contracted states for either sprinters, non-sprinters or all subjects taken together (p = 0.133, p = 0.887 and p = 0.294, respectively). Similarly, no significant differences in the anteroposterior or superior–inferior positions of the CoR were found between the resting and contracted states for either group or for all subjects (all p > 0.104).
(c). Computer simulation results
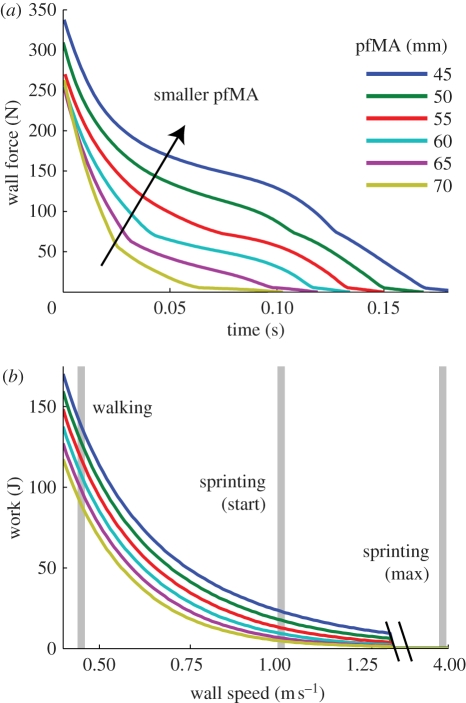
The computer model revealed substantial differences in simulated plantarflexor work as the distance from the ankle to the heel was varied while foot length was held constant. Smaller rearfoot lengths (accompanied by longer forefoot lengths) increased the force applied to the wall by the toe and prolonged toe contact (figure 4a). When the wall retreated at speeds that were consistent with the first steps of a sprint race, the foot configuration with the shortest rearfoot resulted in 3.9 times more muscle work than did the configuration with the longest rearfoot (figure 4b). At slower plantarflexion velocities consistent with walking, a shorter rearfoot also resulted in greater muscle work, but at faster velocities representative of maximal sprinting, the muscles were capable of doing only negligible amounts of work for any prescribed foot geometry.
Figure 4.
(a) Simulated toe-wall contact forces. A greater ratio of forefoot length to rearfoot length (R/r) permitted greater force at the toe and extended the time of toe contact. (b) Ankle plantarflexor work for different rates of plantarflexion and different rearfoot lengths. At ankle rotation velocities similar to those reported for the sprint start, the shortest rearfoot length simulated permits the production of 3.5 times more work than the longest rearfoot length. At maximal sprinting plantarflexion speeds, the shortening velocities of the muscle fibres are so great that the muscle cannot do work on the wall.
4. Discussion
The results of the present study show that there are differences in the skeletal structure of the foot and ankle between two groups of healthy human subjects with different levels of locomotor performance and which also vary in the functional demands they place on their limbs. Sprinters were found to have significantly longer forefoot bones and shorter pfMA than those of non-sprinters (table 2), confirming our hypotheses. For the first time, to our knowledge, these differences have been documented using measurements made from MR images, and differences in pfMA have been accounted for by a posterior shift of the CoR in sprinters rather than differences in the Achilles tendon path (figure 2). The results of simulations performed using a simple computer model show that the differences in foot proportions we found experimentally have the capacity to increase plantarflexor work substantially during the acceleration phase of sprinting (figure 4b). Simulations performed with shorter rearfoot lengths were found to increase muscle force by reducing muscular shortening velocities, and to increase the time of contact with the ground (figure 4a).
Our measurements of pfMA were similar to those of previous investigators who have used similar means to quantify Achilles tendon moment arms in healthy subjects. Previous studies report mean values of pfMA measured at 0° ankle flexion ranging from 48 to 60 mm and with standard deviations of 3–4.3 mm [15,22,23]. Unlike previous authors, however, we did not find differences between pfMA measured with muscles at rest and those measured with the plantarflexors contracted. Maganaris and colleagues [22] reported moment arms that were approximately 10–15 mm greater when subjects maximally contracted. The differences between the present results and those of Maganaris et al. [15] may be owing to differences in the ankle-strapping technique between the two studies. When we applied a similar technique during pilot tests, we found that it reduced the pfMA measured in the passive condition. It is also possible that these differences were owing to our subjects' performing co-contractions of the ankle plantarflexors and dorsiflexors rather than plantarflexing against a support.
Our results are similar to those reported by Lee & Piazza [10]. In that study, external measurements from the tip of the first toe to the first metatarsal head were made to approximate toe length, and an ultrasound probe was used to track Achilles tendon excursion during plantarflexion. In the current study, MR imaging was used to make direct measurements of the lengths of the first phalanges and first metatarsal. MR-based measurement of pfMA also permitted location of the ankle CoR that permitted insight into the mechanism underlying differences in moment arm. We found smaller differences in pfMA (12% compared with 25%) than those previously reported by Lee & Piazza [10], and these disparities may be derived from incorrect assumptions related to the tendon excursion method made in the earlier study. Computation of muscle moment arms from tendon excursion is based on the Principle of Virtual Work, which requires that the energy stored in the tendon does not change during the joint rotation [24]. However, it is not possible to maintain constant tendon force in vivo, thus leading to measurement errors. Also, the mechanical properties of tendons in sprinters differ from non-sprinters [25], complicating the assessment of pfMA differences using tendon excursion. In addition, the subjects studied by Lee & Piazza [10] maximally contracted their plantarflexors during measurement of tendon excursion, which may have resulted in additional tendon shortening with plantarflexion among the sprinters that would have been interpreted as a smaller moment arm.
Very few studies have investigated differences in foot and ankle structure and how it relates to functional abilities in humans. Several authors have used indirect measurements to compare foot structure and running economy and sprinting performance, however, none of those investigators who measured pfMA located the ankle CoR [10,11,26,27]. The results of the present study reveal differences between sprinters and non-sprinters in the anteroposterior location of the tibiotalar CoR relative to the tibia. This finding raises important questions about whether between-subject variation in muscle moment arm is generally owing to differences in CoR location and, if so, whether the CoR location depends on bone shapes or if it is modulated by specific patterns of muscle activity. We examined our sagittal MR images to determine if there were differences in talar dome curvature that might be expected to determine joint kinematics [28], but we did not find any. Further investigation is needed to identify the influence of surface geometry and ligaments on joint kinematics and muscle moment arms in functionally different groups.
The finding that pfMA are shorter and forefoot bones are longer in sprinters suggests that foot proportions may influence the capacity for acceleration. The computer simulation shows that a longer forefoot and shorter pfMA permits the plantarflexors to do more work at certain velocities (figure 4b). At slower velocities, this additional work output is not likely to be of use, because each foot proportion simulated results in muscle work being done that was far greater than the approximately 26 J necessary for walking [19]. At higher speeds consistent with the start of a sprint race, however, the model shows that sprinter-like foot proportions convey a substantial advantage. At speeds consistent with maximal sprinting, our model suggests that plantarflexion occurs at a rate too high for any positive muscular work to be done. The plantarflexors of bipeds such as wild turkeys and wallabies isometrically contract during high speed running and hopping, modulating stiffness and aiding in calcaneal tendon energy storage and return [29,30]. Similarly, horses are able to run at high speeds with very short digital flexor muscles that facilitate energy storage and return by extremely long tendons [31]. While we did not measure the ground reaction force moment arms and varying pfMA necessary to compute actual gear ratios, it is reasonable to expect that longer forefoot bones coupled with shorter pfMA would result in a larger gear ratio. Larger gear ratios have been found to occur during the late stance phases of accelerating human runners [13] and similar differences in foot proportions have been noted in elite animal sprinters [3,4].
There are many factors known to contribute to elite sprint performance, and the present results do not demonstrate that foot proportions or plantarflexor tendon leverage is a primary determinant of sprinting ability. Muscular strength and the proportion of fast-twitch fibres to slow-twitch fibres are correlated with maximal running velocity [32]. The muscle fibres of sprinters, especially sprinters' fast twitch fibres, have been reported to have faster fibre conduction velocities than those of distance runners [33]. The knee extensors and plantarflexors of sprinters are thicker, have smaller pennation angles and longer muscle fascicles than those of distance runners and non-sprinters [10,34,35]. Similar differences in muscle architecture have been documented between highly skilled sprinters and less-skilled sprinters [36], although Karamanidis et al. [11] failed to identify such differences in a similar study. It is unknown how any benefits conveyed by foot and ankle structure might be compared with those that follow from these characteristics and others.
It is unclear whether differences in foot and ankle skeletal structure are adaptations to sprint training or hereditary, but there is evidence that human skeletal strength and form are altered by certain forms of athletic training. Prolonged participation in activities such as running and gymnastics promotes increases in lower leg bone thickness and greater radii cortical areas, respectively [37,38]. Athletes involved in sports in which one hand is primarily used, such as bowling and tennis, have thicker and denser humeri and radii on the dominant side [39,40]. Torsion deformation of the humerus has been documented in handball and baseball players [41,42]. These adaptations may function as a safety mechanism that corresponds to increased range of motion of the throwing shoulder [42]. Biewener & Bertram [43] documented reductions in tibia length during development when the limbs of chicks were denervated. However, the authors found no differences in bone length between sedentary and exercised animals. Carpenter & Carter [44] used a computer model to simulate straightening of a congenitally bowed tibia in response to repeated loading. It is unknown, however, if human bone lengths or muscle moment arms adapt in similar ways in response to sport training.
Certain limitations affected our study. We were able to perform MR scans of only eight subjects in each group. The sprinters we tested were for the most part club track athletes who may not be classified as ‘elite’ but who were well trained (average training: 6.5 ± 2.8 years). We measured pfMA only for neutral ankle position and assumed that rotations of the foot relative to the tibia were represented by tibiotalar rotation. Another limitation is that, like those of previous authors [15] who measured Achilles tendon moment arms, our analysis was two-dimensional. The axis of tibiotalar rotation, however, is known to vary in its location and orientation throughout plantarflexion [45], and rotations out of the sagittal plane could not be accounted for using our planar imaging techniques. The simplified ankle joint and muscle-tendon geometry in our computer model caused pfMA to decrease with plantarflexion; a more complex model might have incorporated a mechanism by which pfMA would remain constant or increase slightly with plantarflexion—behaviour that has been reported in vivo [15].
The results of the present study are significant because they are the first indication that variation in the location of the CoR are responsible for differences in joint leverage between functionally different but otherwise healthy groups of humans. Our simple computer simulations show that foot proportions consistent with the pfMA and forefoot bone lengths we measured provide considerable force generating advantages to those with ‘sprinter-like’ feet. Further research is needed to identify the structural mechanisms that account for differences in CoR and to determine whether differences in CoR and forefoot bone lengths are determined by genetics or are an adaptation to training.
Acknowledgements
The experimental protocol was approved by the Institutional Review Board of The Pennsylvania State University and participants gave informed consent prior to data collection.
We would like to thank the coaches of the Penn State Track Club, who assisted with the recruiting of participants, and the staff of the Penn State Social, Life, and Engineering Sciences Imaging Centre (SLEIC).
References
- 1.Maganaris C. N., Baltzopoulos V., Tsaopoulos D. 2006. Muscle fibre length-to-moment arm ratios in the human lower limb determined in vivo. J. Biomech. 39, 1663–1668 10.1016/j.jbiomech.2005.04.025 (doi:10.1016/j.jbiomech.2005.04.025) [DOI] [PubMed] [Google Scholar]
- 2.Dogan A., Uslu M., Aydinlioglu A., Harman M., Akpinar F. 2007. Morphometric study of the human metatarsals and phalanges. Clin. Anat. 20, 209–214 10.1002/ca.20348 (doi:10.1002/ca.20348) [DOI] [PubMed] [Google Scholar]
- 3.Hudson P. E., Corr S. A., Payne-Davis R. C., Clancy S. N., Lane E., Wilson A. M. 2011. Functional anatomy of the cheetah (Acinonyx jubatus) hindlimb. J. Anat. 218, 363–374 10.1111/j.1469-7580.2010.01310.x (doi:10.1111/j.1469-7580.2010.01310.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildebrand M. 1994. Analysis of vertebrate structure, 4th edn. New York, NY: J. Wiley [Google Scholar]
- 5.Koh T. J., Herzog W. 1998. Increasing the moment arm of the tibialis anterior induces structural and functional adaptation: implications for tendon transfer. J. Biomech. 31, 593–599 10.1016/S0021-9290(98)00052-9 (doi:10.1016/S0021-9290(98)00052-9) [DOI] [PubMed] [Google Scholar]
- 6.Burkholder T. J., Lieber R. L. 1998. Sarcomere number adaptation after retinaculum transection in adult mice. J. Exp. Biol. 201, 309–316 [PubMed] [Google Scholar]
- 7.Dawson B., Fitzsimons M., Green S., Goodman C., Carey M., Cole K. 1998. Changes in performance, muscle metabolites, enzymes and fibre types after short sprint training. Eur. J. Appl. Physiol. Occup. Physiol. 78, 163–169 10.1007/s004210050402 (doi:10.1007/s004210050402) [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc A., Gogia P., Schneider V., Krebs J., Schonfeld E., Evans H. 1988. Calf muscle area and strength changes after five weeks of horizontal bed rest. Am. J. Sports Med. 16, 624–629 10.1177/036354658801600612 (doi:10.1177/036354658801600612) [DOI] [PubMed] [Google Scholar]
- 9.Williams P. E., Goldspink G. 1973. The effect of immobilization on the longitudinal growth of striated muscle fibres. J. Anat. 116, 45–55 [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S. S. M., Piazza S. J. 2009. Built for speed: musculoskeletal structure and sprinting ability. J. Exp. Biol. 212, 3700–3707 10.1242/jeb.031096 (doi:10.1242/jeb.031096) [DOI] [PubMed] [Google Scholar]
- 11.Karamanidis K., Albracht K., Braunstein B., Moreno Catala M., Goldmann J.-P., Brüggemann P. 2011. Lower leg musculoskeletal geometry and sprint performance. Gait Posture 34, 138–141 10.1016/j.gaitpost.2011.03.009 (doi:10.1016/j.gaitpost.2011.03.009) [DOI] [PubMed] [Google Scholar]
- 12.Nagano A., Komura T. 2003. Longer moment arm results in smaller joint moment development, power and work outputs in fast motions. J. Biomech. 36, 1675–1681 [DOI] [PubMed] [Google Scholar]
- 13.Carrier D., Heglund N., Earls K. 1994. Variable gearing during locomotion in the human musculoskeletal system. Science 265, 651–653 10.1126/science.8036513 (doi:10.1126/science.8036513) [DOI] [PubMed] [Google Scholar]
- 14.Reuleaux F. 1876. The kinematics of machinery: outlines of a theory of machines. New York, NY: Macmillan [Google Scholar]
- 15.Maganaris C. N., Baltzopoulos V., Sargeant A. J. 1998. Changes in Achilles tendon moment arm from rest to maximum isometric plantarflexion: in vivo observations in man. J. Physiol. 510, 977–985 10.1111/j.1469-7793.1998.977bj.x (doi:10.1111/j.1469-7793.1998.977bj.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schutte L. M. 1992. Using musculoskeletal models to explore strategies for improving performance in electrical stimulation-induced leg cycle ergometry. PhD thesis, Stanford University, CA, USA. [Google Scholar]
- 17.Arnold E. M., Ward S. R., Lieber R. L., Delp S. L. 2010. A model of the lower limb for analysis of human movement. Ann. Biomed. Eng. 38, 269–279 10.1007/s10439-009-9852-5 (doi:10.1007/s10439-009-9852-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delp S. L., Loan J. P., Hoy M. G., Zajac F. E., Topp E. L., Rosen J. M. 1990. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans. Biomed. Eng. 37, 757–767 10.1109/10.102791 (doi:10.1109/10.102791) [DOI] [PubMed] [Google Scholar]
- 19.Winter D. A. 1983. Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin. Orthop. Relat. Res. 175, 147–154 [PubMed] [Google Scholar]
- 20.Slawinski J., Bonnefoy A., Ontanon G., Leveque J. M., Miller C., Riquet A., Chèze L., Dumas R. 2010. Segment-interaction in sprint start: analysis of 3D angular velocity and kinetic energy in elite sprinters. J. Biomech. 43, 1494–1502 10.1016/j.jbiomech.2010.01.044 (doi:10.1016/j.jbiomech.2010.01.044) [DOI] [PubMed] [Google Scholar]
- 21.Bezodis I. N., Kerwin D. G., Salo A. I. T. 2008. Lower-limb mechanics during the support phase of maximum-velocity sprint running. Med. Sci. Sports Exerc. 40, 707–715 10.1249/MSS.0b013e318162d162 (doi:10.1249/MSS.0b013e318162d162) [DOI] [PubMed] [Google Scholar]
- 22.Maganaris C. N., Baltzopoulos V., Sargeant A. J. 2000. In vivo measurement-based estimations of the human Achilles tendon moment arm. Eur. J. Appl. Physiol. 83, 363–369 10.1007/s004210000247 (doi:10.1007/s004210000247) [DOI] [PubMed] [Google Scholar]
- 23.Fath F., Blazevich A. J., Waugh C. M., Miller S. C., Korff T. 2010. Direct comparison of in vivo Achilles tendon moment arms obtained from ultrasound and MR scans. J. Appl. Physiol. 109, 1644–1652 10.1152/japplphysiol.00656.2010 (doi:10.1152/japplphysiol.00656.2010) [DOI] [PubMed] [Google Scholar]
- 24.An K. N., Takahashi K., Harrigan T. P., Chao E. Y. 1984. Determination of muscle orientations and moment arms. J. Biomech. Eng. 106, 280–282 10.1115/1.3138494 (doi:10.1115/1.3138494) [DOI] [PubMed] [Google Scholar]
- 25.Arampatzis A., Karamanidis K., Morey-Klapsing G., De Monte G., Stafilidis S. 2007. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J. Biomech. 40, 1946–1952 10.1016/j.jbiomech.2006.09.005 (doi:10.1016/j.jbiomech.2006.09.005) [DOI] [PubMed] [Google Scholar]
- 26.Scholz M. N., Bobbert M. F., Van Soest A. J., Clark J. R., Van Heerden J. 2008. Running biomechanics: shorter heels, better economy. J. Exp. Biol. 211, 3266–3271 10.1242/jeb.018812 (doi:10.1242/jeb.018812) [DOI] [PubMed] [Google Scholar]
- 27.Raichlen D. A., Armstrong H., Lieberman D. E. 2011. Calcaneus length determines running economy: implications for endurance running performance in modern humans and Neandertals. J. Hum. Evol. 60, 299–308 10.1016/j.jhevol.2010.11.002 (doi:10.1016/j.jhevol.2010.11.002) [DOI] [PubMed] [Google Scholar]
- 28.Leardini A., O'Connor J. J., Catani F., Giannini S. 1999. A geometric model of the human ankle joint. J. Biomech. 32, 585–591 10.1016/S0021-9290(99)00022-6 (doi:10.1016/S0021-9290(99)00022-6) [DOI] [PubMed] [Google Scholar]
- 29.Biewener A. A., Konieczynski D. D., Baudinette R. V. 1998. In vivo muscle force–length behavior during steady-speed hopping in tammar wallabies. J. Exp. Biol. 201, 1681–1694 [DOI] [PubMed] [Google Scholar]
- 30.Roberts T. J., Marsh R. L., Weyand P. G., Taylor C. R. 1997. Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113–1115 10.1126/science.275.5303.1113 (doi:10.1126/science.275.5303.1113) [DOI] [PubMed] [Google Scholar]
- 31.Wilson A. M., McGuigan M. P., Su A., Van Den Bogert A. J. 2001. Horses damp the spring in their step. Nature 414, 895–899 10.1038/414895a (doi:10.1038/414895a) [DOI] [PubMed] [Google Scholar]
- 32.Mero A., Luhtanen P., Viitasalo J., Komi P. 1981. Relationships between the maximal running velocity, muscle fiber characteristics, force production and force relaxation of sprinters. Scand. J. Sports Sci. 3, 16–22 [Google Scholar]
- 33.Sadoyama T., Masuda T., Miyata H., Katsuta S. 1988. Fibre conduction velocity and fibre composition in human vastus lateralis. Eur. J. Appl. Physiol. Occup. Physiol. 57, 767–771 10.1007/BF01076001 (doi:10.1007/BF01076001) [DOI] [PubMed] [Google Scholar]
- 34.Abe T., Kumagai K., Brechue W. F. 2000. Fascicle length of leg muscles is greater in sprinters than distance runners. Med. Sci. Sports. Exerc. 32, 1125–1129 10.1097/00005768-200006000-00014 (doi:10.1097/00005768-200006000-00014) [DOI] [PubMed] [Google Scholar]
- 35.Abe T., Fukashiro S., Harada Y., Kawamoto K. 2001. Relationship between sprint performance and muscle fascicle length in female sprinters. J. Physiol. Anthropol. Appl. Human. Sci. 20, 141–147 10.2114/jpa.20.141 (doi:10.2114/jpa.20.141) [DOI] [PubMed] [Google Scholar]
- 36.Kumagai K., Abe T., Brechue W. F., Ryushi T., Takano S., Mizuno M. 2000. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J. Appl. Physiol. 88, 811–816 [DOI] [PubMed] [Google Scholar]
- 37.MacDougall J. D., Webber C. E., Martin J., Ormerod S., Chesley A., Younglai E. V., Gordon C. L., Blimkie C. J. 1992. Relationship among running mileage, bone density, and serum testosterone in male runners. J. Appl. Physiol. 73, 1165–1170 [DOI] [PubMed] [Google Scholar]
- 38.Ward K. A., Roberts S. A., Adams J. E., Mughal M. Z. 2005. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone 36, 1012–1018 10.1016/j.bone.2005.03.001 (doi:10.1016/j.bone.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 39.Calbet J. A., Moysi J. S., Dorado C., Rodríguez L. P. 1998. Bone mineral content and density in professional tennis players. Calcif. Tissue Int. 62, 491–496 10.1007/s002239900467 (doi:10.1007/s002239900467) [DOI] [PubMed] [Google Scholar]
- 40.Young K. C., Sherk V. D., Bemben D. A. 2011. Inter-limb musculoskeletal differences in competitive ten-pin bowlers: a preliminary analysis. J. Musculoskelet. Neuronal Interact. 11, 21–26 [PubMed] [Google Scholar]
- 41.Warden S. J., Bogenschutz E. D., Smith H. D., Gutierrez A. R. 2009. Throwing induces substantial torsional adaptation within the midshaft humerus of male baseball players. Bone 45, 931–941 10.1016/j.bone.2009.07.075 (doi:10.1016/j.bone.2009.07.075) [DOI] [PubMed] [Google Scholar]
- 42.Pieper H. G. 1998. Humeral torsion in the throwing arm of handball players. Am. J. Sports Med. 26, 247–253 [DOI] [PubMed] [Google Scholar]
- 43.Biewener A. A., Bertram J. E. 1994. Structural response of growing bone to exercise and disuse. J. Appl. Physiol. 76, 946–955 [DOI] [PubMed] [Google Scholar]
- 44.Carpenter R. D., Carter D. R. 2010. Computational simulation of spontaneous bone straightening in growing children. Biomech. Model Mechanobiol. 9, 317–328 10.1007/s10237-009-0178-x (doi:10.1007/s10237-009-0178-x) [DOI] [PubMed] [Google Scholar]
- 45.de Asla R. J., Wan L., Rubash H. E., Li G. 2006. Six DOF in vivo kinematics of the ankle joint complex: application of a combined dual-orthogonal fluoroscopic and magnetic resonance imaging technique. J. Orthop. Res. 24, 1019–1027 10.1002/jor.20142 (doi:10.1002/jor.20142) [DOI] [PubMed] [Google Scholar]