Abstract
Most climate change predictions omit species interactions and interspecific variation in dispersal. Here, we develop a model of multiple competing species along a warming climatic gradient that includes temperature-dependent competition, differences in niche breadth and interspecific differences in dispersal ability. Competition and dispersal differences decreased diversity and produced so-called ‘no-analogue’ communities, defined as a novel combination of species that does not currently co-occur. Climate change altered community richness the most when species had narrow niches, when mean community-wide dispersal rates were low and when species differed in dispersal abilities. With high interspecific dispersal variance, the best dispersers tracked climate change, out-competed slower dispersers and caused their extinction. Overall, competition slowed the advance of colonists into newly suitable habitats, creating lags in climate tracking. We predict that climate change will most threaten communities of species that have narrow niches (e.g. tropics), vary in dispersal (most communities) and compete strongly. Current forecasts probably underestimate climate change impacts on biodiversity by neglecting competition and dispersal differences.
Keywords: climate change, competition, dispersal, community ecology, movement ecology, thermal performance breadth
1. Introduction
General circulation models predict that global land temperatures will increase 1.8–4°C by the year 2100 [1]. Good dispersers might track their optimal temperatures as climate change shifts thermal habitats across the landscape. As predicted, species have already begun moving in response to rapid warming [2], and these climate-induced range expansions appear to have accelerated in recent years [3]. However, not all species move fast enough to expand their range into regions made suitable by climate change, and resident species might resist range expansions [4]. We currently know little about how dispersal differences and community ecology interact to shape future biotic responses to climate change. Here, we develop a general model to explore how competition and interspecific variation in dispersal abilities might alter species diversity patterns, extinction risks and community assembly in response to climate change.
Most predictive models of species' responses to climate change only include abiotic variables to describe species distributions and thus ignore species interactions. Yet species interactions clearly influence most species' realized niches and distributions [5]. Abundant evidence suggests that rapidly changing climates will alter the strength and character of many species interactions [4,6] owing to changes in relative performance [7] and differences in dispersal capacities [8]. The few models that have incorporated species interactions suggest that these interactions strongly alter species' responses to climate change [9,10] depending on the sensitivity of individual species to environmental change and the specific pattern of species interactions within a community [11]. Given emerging empirical evidence that species interactions strongly affect responses to climate change [5], a logical first step is to explore if competition and climate change alter theoretical predictions about biodiversity losses.
Interspecific differences in dispersal also probably shape community responses to climate change. Most models consider a constant dispersal rate shared by all species [12], but range expansions in response to climate change vary dramatically among species owing to differences in both species' dispersal abilities and sensitivities to climate change [3,8]. Gilman et al. [4] predicted that dispersal differences will alter community-wide responses to climate change by bringing together allopatric species or by separating sympatric species and creating no-analogue communities. A no-analogue community is a novel combination of species that does not currently co-occur and thus have no modern analogue [13]. No-analogue communities have formed in response to past climate change [13]. Thus, future communities are likely to be a product of a complex set of responses to changes in both biotic and abiotic drivers with further constraints imposed by the different capacities of organisms to reach suitable habitats.
We develop a multi-species model to investigate how competition and interspecific differences in dispersal affect community responses to climate change. We simulate a continuous thermal gradient which we populate with competing species that differ in their thermal optima (electronic supplementary material, figure S1A). Local temperature determines each individual's fitness and the strength of competition among species with different thermal optima (electronic supplementary material, figure S1B). We evaluate how different community-wide thermal performance breadths (fundamental niches, approximated empirically as thermal performance curves [14]), different thermal competition breadths (realized niches), and different dispersal means and interspecific variances affect alpha, beta and gamma diversities [15] and the formation of no-analogue communities. We take a general approach that incorporates community ecology explicitly in contrast to previous work that applies correlative relationships between existing species' ranges and climate factors to predict future responses to climate change [12,16]. Unlike climate envelope approaches which assume that correlations between current species' distributions and abiotic variables reflect the fundamental niche [17], species' realized ranges in our model emerge mechanistically because species-specific thermal performance determines intrinsic rates of growth and competitive abilities. Therefore, we model fundamental and realized niches directly and apply a trait-based approach to understanding community responses to environmental change [18] along an environmental gradient.
2. Material and methods
(a). Model overview
We consider a 20°C temperature gradient that coincides with a 3000 m mountain or 15° latitudinal cline (electronic supplementary material, figure S1A). To minimize edge effects, we assume reflecting boundaries, whereby dynamics mirror each other at each end of the landscape. We populated the temperature gradient with 40 species that differ randomly in the temperature at which they experience highest performance (thermal optima). We varied community-wide thermal performance breadth which defines the range of temperatures across which species have positive population growth without competition (electronic supplementary material, figure S1B). Wherever possible, we used empirical data to inform our choice of parameter values (see electronic supplementary material, table S1). We also varied temperature-based competition breadth for all species simultaneously. We evaluated simulations with either the same or varying dispersal abilities among species. We first allowed communities to reach equilibrium with a constant climate. We then increased temperatures by 4°C over 100 years (0.04°C per year) to reflect predicted global temperature changes [1]. We ended simulations after community abundance distributions equilibrated.
(b). Population growth dynamics
We assumed that per capita birth (b) and death (d) rates follow skew-normal [19] and inverse skew-normal functions, of the difference between a species' thermal optimum (z) and the local temperature (T), respectively, with equal variances (σ2) and skew scalar (λ). We used a skew-normal distribution because most thermal performance curves are right-shifted towards warmer temperatures [20]. Per capita growth rate equals:
| 2.1 |
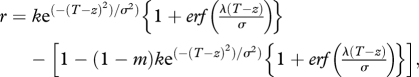 |
2.2 |
or more simply,
| 2.3 |
where k is a constant that standardizes the maximum r to one and m quantifies a background death rate that does not vary with temperature. We assumed a thermal niche breadth with standard deviation σ equal to 5°C, consistent with the mean observed among empirical estimates [21] (see the electronic supplementary material, table S1). The function in the brackets introduces a right-skewed distribution with a negative shape parameter λ. We chose the skew-normal because it can be fitted with a minimum of parameters (T, σ, λ), its results can be directly compared with the symmetric Gaussian distribution commonly used in niche-based studies when λ = 0, and it replicates the shape of prior distributions that have been shown to fit empirical data well [21]. We set λ = −2.7, corresponding to a thermal performance curve asymmetry of 3.4, which equals the mean of the observed thermal performance curve asymmetries in Deutsch et al. [21] (see the electronic supplementary material, table S1). Thermal curve asymmetry measures how far the critical thermal minimum is from the optimum relative to the distance between the optimum and the critical thermal maximum. The details of the skew-normal distribution, its implementation, and model results when assuming a symmetric normal distribution are provided in the electronic supplementary material. Qualitative predictions were equivalent between models assuming either a skewed performance curve or a symmetric performance curve after correcting for changes in thermal performance breadth between the two distributions (see the electronic supplementary material, figures S2 and S3).
If r varies with temperature, then carrying capacity (K) also must vary with temperature because of its dependence on r. Let a and c represent the linear relationships between birth and death rates and population size. Then carrying capacity equals:
| 2.4 |
Thus, lower per capita growth rates in suboptimal thermal environments result in lower carrying capacities as long as density-dependent slopes a and c remain constant.
(c). Temperature-dependent competition
We also varied the relationship between competition among species and their thermal optima, which would be the case if resource uptake depends on temperature-dependent performance [5,22,23]. We assume that competitive coefficients (α) vary as a Gaussian function of temperature:
| 2.5 |
where the thermal competitive niche breadth is determined by variance δ2 and skew scalar λ. In this formulation, a species competes more strongly with species with more similar thermal optima. In our model, alpha equals one for intraspecific competition. Lower competitive niche variance decreases the strength of competition among species with divergent thermal performance optima. By altering the breadth of thermal performance (the fundamental niche width) versus the breadth of thermal competition (the realized niche width), we can examine cases where species distributions depend more or less on fundamental versus realized niches.
The effect of all individuals in a patch on a focal individual equals the sum of the product of all the competitive coefficients of the focal species and all other species and their abundances in that patch or:
| 2.6 |
where S indicates the total number of species. Alphas are re-standardized relative to the focal species' competitive effect on itself to satisfy the definition of a competitive coefficient as per capita competition relative to intraspecific competition.
Discrete logistic population growth for the ith species depends on the fit between each species' optimal thermal environment and its local environment, its density, and the density of other competitors and their fit with the local environment:
| 2.7 |
(d). Dispersal
We modelled dispersal probabilities with a Laplace distribution (back-to-back exponential functions) to match the commonly observed leptokurtic dispersal kernel:
| 2.8 |
where x is the displacement from u focal habitat and d determines mean dispersal distance in both dimensions. We discretized the spatial gradient and multiplied each population's abundance by its probability of remaining and the sum of all immigration probabilities of that species into that patch multiplied by their abundances. We varied dispersal among species by randomly drawing from a lognormal distribution with mean D and variance ɛ2. The lognormal distribution significantly fit most of the distributions of interspecific dispersal variance we found, including plants, amphibians, birds and mammals (see the electronic supplementary material, table S1). We explored a range of dispersal means from 0 to 1 spatial units per year or distances that approximate 0–0.2°C shifts in temperature per year, which brackets the range observed in natural species (mean 0.02°C yr–1 shift in latitude and a 0.01°C yr–1 shift in altitude; see the electronic supplementary material, table S1). We explored the impact of variance in mean dispersal distance by altering its standard deviation among species from 0 (no interspecific dispersal variation, as has commonly been assumed) to 1.0.
(e). Simulations and response variables
We evaluated each parameter combination 25 times and calculated mean changes in alpha, beta and gamma (inverse-Simpson's) diversity for each model following either stable or changing climates. We also recorded the mean number of novel and lost species interactions at equilibrium after climate change. Novel species interactions refer to new range overlaps following climate change (no-analogue community assembly). Lost species interactions describe those interactions that disappeared after climate change (community disassembly).
To explore lags in how species track changing climates, we calculated the difference in the abundance-weighted mean and species-specific thermal optima for the 20 warmest adapted species immediately after climate change. We only include the warmest adapted species to provide the most direct comparison between models with and without competition. Otherwise, we would have included species near the landscape temperature minimum that cannot track their optimal climate because it has disappeared. The warmest adapted 50 per cent of species have thermal performance distributions that do not overlap significantly with lost thermal environments.
3. Results
(a). Stable climates: community-wide niche width variation
When climates do not change, differences in both thermal performance and competitive breadth alter community diversity patterns. The highest (inverse-Simpson's) gamma and alpha diversities occur when thermal performance and competitive breadths are broadest, such that individuals have high fitness over a broad range of temperatures (see the electronic supplementary material, figure S4). No species were lost across this range of parameter values before climate change. Therefore, changes in inverse-Simpson's gamma diversity reflect altered evenness rather than richness. Broader thermal performance and competition breadths also allow species to inhabit overlapping niches, thereby increasing alpha diversity. Gamma diversity also peaks at low thermal performance breadths (σ = 0.5) regardless of competitive niche breadth because species with small ranges seldom interact with each other. Beta diversity becomes highest at narrow fundamental and realized niche breadths because few species coexist in the same habitat.
(b). Stable climates: different dispersal means and variances
Gamma diversity decreases with higher community-wide dispersal because dispersal broadens abundance distributions, which causes more competition. This competition exacerbates disparities in abundances where thermal optima are clustered by random chance (see the electronic supplementary material, figure S5). Alpha diversity changes only slightly across the parameter range. Beta diversity decreases when species disperse well and vary less in their dispersal means because higher dispersal results in wider species distributions and thus less species turnover.
(c). Climate change: community-wide niche width variation
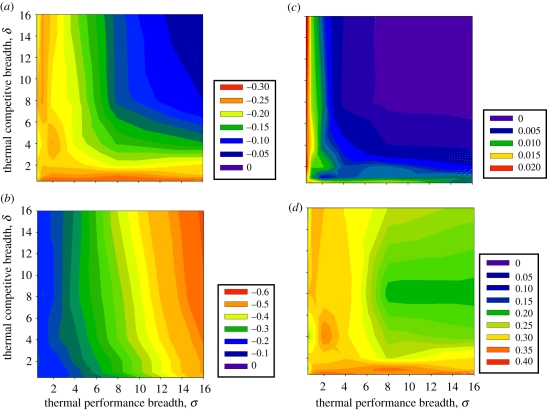
The most species become extinct during climate change when species have narrow niche widths (σ < 2) regardless of whether they originated from narrow thermal performance or competitive breadths (figure 1a). Most species become extinct when their temperature optimum disappears from the coldest region of the climate gradient and other warm-adapted species out-compete them for warmer habitats. Broader thermal performance curves prevent some of these extinctions by allowing more species to persist in warmer regions of the gradient. Additional extinctions occur when species with initially low abundances do not survive the transitory period of low-fitness during climate change.
Figure 1.
Changes in diversity and species interactions after climate change in relation to performance and competition niche breadths. Diversity responses are indicated as percentages of original metrics at equilibrium after climates change, including (a) per cent extinctions, (b) per cent change in inverse-Simpson's gamma diversity and per cent, (c) novel, and (d) lost species interactions. Note that scales differ across subpanels for clearer presentation.
Although broader thermal performance moderates the effects of climate change on species richness, it exacerbates the effects on gamma diversity by decreasing evenness (figure 1b) and increasing beta diversity (see the electronic supplementary material, figure S6). This result occurs because warming strongly increases the abundances of the warmest adapted species following ecological release in novel hot regions, which maintains a subset of the original species in hot environments (e.g. tropical lowlands). The maintenance of these warm-adapted species potentially counters some, but not all, of the predicted ‘lowland biotic attrition’ following climate change in tropical areas [24]. This result requires that thermal performance breadths are broad enough to provide hot-adapted species with the plasticity needed to allow them to persist in these novel hot environments [25].
Assuming sufficient and equal migration among species (D = 0.5; ɛ = 0), usually only one novel species interaction (1–2% of initial species interactions) forms following climate change across the thermal and competitive niche breadths analysed (figure 1c). A novel species interaction forms when climate change reduces the abundance of a species that inhabits a range which separates two other species' ranges before climate change. By eliminating this ‘competitive wedge’, the two outer species can compete with each other. Most novel species interactions occur with narrow niches (σ < 1), where the most species become extinct (cf. figure 1a versus c). Extinctions positively correlate with novel species interactions (r2 = 0.74), supporting the idea that extinctions of wedge species produce novel competitive interactions in the model.
Climate change generally results in the loss of species interactions rather than the creation of new ones (figure 1c versus d), and this effect increases with narrower fundamental or realized niche breadths. Extinction is the major source of lost species interactions. On average, extinction causes 71 per cent of the initial species interactions to disappear. The rest of the interactions disappear when an intermediary species increases in abundance and creates a competitive wedge between interacting species. This wedge species usually increases in abundance because of the extinction of other competitors or a slight advantage over other species during the transitory dynamics that occur during climate change. Most lost species interactions not attributed to extinctions occur with broad fitness or competitive thermal niche widths (see the electronic supplementary material, figure S7).
(d). Climate change: variation in dispersal means and variances
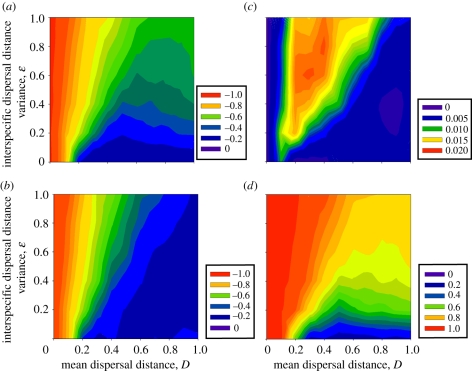
With no dispersal variation, 98 per cent of species become extinct when they do not disperse (left side, figure 2a). A majority of species, 74 per cent, also become extinct at low dispersal levels when they cannot fully track their optimal thermal environment (D = 0.1). Fewer species become extinct (13–24%) when species disperse well enough to track suitable climates (greater than 0.2 spatial units per year). At high mean dispersal distances and no dispersal variance, gamma diversity differs little, few novel species interactions form and most lost species interactions result from extinctions because all species track climate similarly (figures 2b–d; electronic supplementary material, figure S8). Note that we measured the change in diversity relative to pre-disturbance levels such that the reduced changes in diversity with higher dispersal are not merely the result of differences in range sizes before climate change.
Figure 2.
Changes in diversity and species interactions with interspecific competition after climate change in relation to changes in mean dispersal among communities and interspecific dispersal variation. Diversity responses are indicated as percentages of original metrics at equilibrium after climates change, including (a) per cent extinctions, (b) per cent change in inverse-Simpson's gamma diversity and per cent, (c) novel, and (d) lost species interactions. Both performance and competitive niche breadth standard deviations were set to 5.0°C.
Interspecific dispersal differences strongly affect model outcomes. Greater dispersal differences among species decrease community diversity and initiate dramatic increases in no-analogue community formation (figure 2c,d). More than half of the species disappear with moderate to high interspecific dispersal variation. This effect weakens at higher mean dispersal levels once a greater number of species can track climate changes. With lognormally distributed interspecific dispersal variation, the mean dispersal distance does not change, but many species disperse less than the mean and a few disperse more than the mean. For instance, when the lognormal dispersal variance equals one, 85 per cent of species disperse less than the mean. Any variance in dispersal thus can generate species losses directly because some species disperse less than required to track climate change. However, dispersal variance also directly causes extinction as shown by the increased extinction risk even when most species disperse well (D ≫ 0.2; upper right, figure 2a). Here, the few best dispersing species track climate change, out-compete the poor dispersers and cause extinctions through competition. As a result of these extinctions and uneven distributions at lower mean dispersal rates, gamma diversity also decreases with increasing dispersal variance (figure 2b).
Species form more competitive interactions with novel partners when species differ in dispersal rate, especially at moderate mean dispersal distance and high dispersal variance (figure 2c). Many species extinctions occur in this parameter space which allows species to colonize open habitats and interact with novel competitors normally constrained to a distant part of the climate gradient. Greater dispersal variation allows for more interactions between poor and good dispersers. This effect dissipates at higher mean dispersal because more species disperse better than needed to track climate change, and most species shift ranges at the same rate: even if they can disperse faster, species will not shift ranges faster than climate change dictates.
(e). Climate change: direct effects of competition
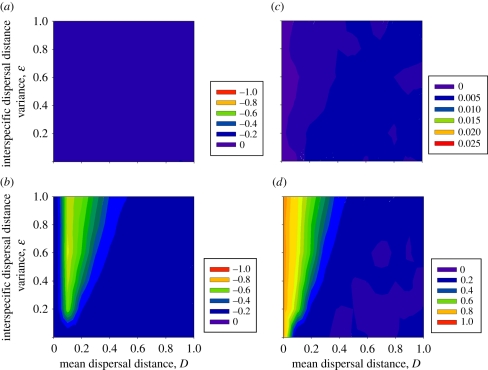
Competition strongly determines biodiversity changes following climate change across a broad dispersal parameter range (cf. figures 2 and 3). Without competition, only a few species become extinct, and extinction does not depend strongly on dispersal mean or variance (figure 3a). In addition, many cold-adapted species can persist at the landscape temperature minimum even after their optimal habitat disappears. These polar or mountaintop species can persist despite climate change because their absolute rates of increase remain positive in the absence of competition from warmer adapted species. With competition, these species become extinct once better adapted competitors arrive. Competition greatly increases extinction risks by lowering fitness and population abundances, which increases extinction risks during climate-induced transitory fitness declines. Competition also allows species that track climate change better than others to out-compete and ultimately eradicate species in cooler regions.
Figure 3.
Changes in diversity and species interactions after climate change as in figure 2, but without interspecific competition. Note that scales differ across subpanels within a figure, but remain the same for each subpanel to aid comparisons between figures 2 and 3. (a) Per cent extinctions, (b) per cent change in inverse-Simpson's gamma diversity and per cent, (c) novel, and (d) lost species interactions.
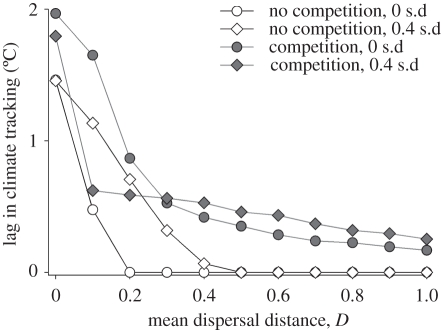
Competition slows range expansions in response to climate change. In most instances, species tracked climate more slowly with interspecific competition than without (figure 4). For instance, when D = 0.1 and ɛ = 0, species lag their optimal niche by 1.7°C with competition when compared with 0.5°C without competition immediately after climate change (figure 4). With interspecific dispersal variance, the same pattern holds except at low mean dispersal distances (D = 0.1, 0.2). With low mean dispersal distances, species track climate change faster with competition because all the poor dispersers become extinct, leaving only the best dispersers that can track climate change. Competition slows climate tracking by decreasing population abundances and thus decreasing the absolute number of migrants colonizing newly optimal habitat. In addition, maladapted species do not vacate habitats as rapidly as they become conducive to colonization by warmer adapted species.
Figure 4.
The lag in climate tracking induced by competition. The lag in climate tracking indicates how many degrees Celsius the average species' abundance-weighted mean position on the thermal gradient differs from its optimal thermal position. We depict this pattern for the 20 extant species with the warmest optima for reasons explained in the main text. We depict patterns with interspecific competition (black) and without competition (white) and for the case with interspecific variance in dispersal (diamonds: 0.4 s.d.) and without (circles: 0 s.d.) across a gradient of mean dispersal.
4. Discussion
Several models of biotic responses to changing climates predict species redistributions and extinctions [12,26]. These predictions generally have ignored species interactions. Yet, species interactions often determine species ranges [5], and individualistic responses to climate could produce no-analogue communities [4,13]. Recent empirical studies suggest that competition can facilitate climate change-induced extinctions [27,28]. Here, we explore maybe the most common way in which competition could affect community responses to climate change: species differ in their dispersal ability, which alters their rate of response to climate change and changes their competitive interactions. Most predictions of community responses to climate change have assumed that either all species track climate or no species do [12]. This assumption ignores the ubiquitous variation in dispersal among species. These variable dispersal rates can influence species interactions by shifting species ranges apart and disassembling communities [29] or by pushing species together and assembling no-analogue communities [4].
We show that interspecific competition and dispersal variation, both alone and together, dramatically alter community responses to climate change by elevating extinction risks, altering diversity patterns and creating no-analogue communities. Competition affects community responses to climate change through three inter-related mechanisms: (i) decreasing population abundances, which increase extinction risk and slow climate tracking; (ii) preventing species from colonizing newly available environments; and (iii) causing the extinction of species that would otherwise persist owing to their broad thermal performance profiles.
In the first mechanism, competition reduces the abundances of all species and increases differences in population abundances among species. Generally, reduced abundances increase extinction risks during the transitory low-fitness period induced by climate change. Reduced abundances also decrease the absolute number of migrants that can colonize environments made habitable through recent climate change, given our assumption that migration is proportional to abundance. In addition, competition creates asymmetries in species' abundances by decreasing abundances in those species that randomly have more similar thermal optima. Climate change deepens these asymmetries, which can lead to extinctions.
In the second mechanism, competition lowers the fitness of migrants into newly suitable habitats. Competition increases the lag in climate tracking by up to 1.2°C over models without competition (figure 4). This effect cannot be attributed to lower species abundances; models without competition and with 90 per cent lower carrying capacities did not result in lags as strong as those observed with competition. Competition at range boundaries can explain this competition-fuelled slowing of range expansion as elucidated by work on species replacements during invasions [10]. Maladapted individuals that remain in habitats do not immediately become extirpated. New colonists face strong competition initially, thus slowing growth rates in new habitats. Such a mechanism is potentially widespread as empirical evidence indicates that warmer adapted species generally colonize warming areas faster than resident species disappear [30]. Newly emerging climate space will not probably be free of competitors, and species migrating into that space will need to compete with residents. In this ‘boxcar effect’, each species is blocked from colonizing the next cooler habitat by the next resident competitor, slowing the climate responses of all species. Just like boxcars on a train, many species can only climb climate gradients as fast as species further up the line.
In the third mechanism, species that lose optimal habitat (e.g. at the mountaintop) become extinct with competition. Extinction occurs even before species' rates of increase fall below replacement because warmer adapted competitors gain an increasing advantage and eventually out-compete these species and drive them off the mountaintop or pole (although not immediately as per mechanism no. (ii)).
(a). Model predictions about changes in biological diversity
Given the aforementioned mechanisms, under what circumstances can we expect that competition and dispersal variation will alter community responses to climate change? Climate change will cause the largest change in communities comprised species with (i) narrow niches; (ii) low mean dispersal rates; and (iii) large interspecific differences in dispersal.
(i). Niche breadth and climate change
More species become extinct and no-analogue communities tend to form when thermal performance and competitive niche breadths are narrow. Smaller niche breadths lead to smaller range sizes, which subsequently increases extinction risks when habitat disappears. Such results could also apply to community-wide differences in niche breadth. If species ranges tend to be smaller on tropical versus temperate mountains [29,31] and species thermal performance curves are narrower (e.g. amphibian thermal performance breadth decreases 5°C from 50° to 10° latitude [32]), then we would expect more extinctions in the tropics [33] under equal amounts of temperature change.
(ii). Low dispersal rates
Many extinctions occur from climate change when species disperse at sufficient, but not excessive, dispersal rates. Under this scenario, most species cannot keep up with climate change because of competition from species in warmer habitats. This scenario generates the greatest asymmetries in species' abundances. Smaller population sizes and more variance in population sizes among species combine to create a strong extinction risk.
(iii). Interspecific variance in dispersal and climate change
The variance in dispersal among species played as great a role as mean dispersal. Here again, the available empirical evidence suggests that dispersal ability often varies widely among species in the same communities [4]. This result depends strongly on competition. Variation in dispersal capacities creates strong differences among species in their abilities to track climate change and thus maintain fitness in a changing environment. The best dispersers over-run the worst dispersers and cause their extinction. Therefore, we can expect that communities comprising species with widely different dispersal abilities will be most at risk of extinctions, changes in diversity and the formation of no-analogue communities (figure 2). Moreover, this process will keep the best dispersers in a community at the expense of more sedentary species.
The lognormal distribution assumed here could over-emphasize contributions from variance given the strong asymmetrical concentration of dispersal means at lower mean dispersal rates and rarity of long-distance types. Currently, our understanding of community-level variation in dispersal capacity is poor because most movement studies focus on single species, and community-level examinations of range expansion confound dispersal capacity with the impacts of species interactions and habitat suitability. Even with these limitations, variation in dispersal capacity is typically large, and often strongly skewed.
(b). Empirical patterns
We built a model that is not specific to any single community in order to build general insights into the importance of potential mechanisms. Yet, abundant evidence indicates that temperature strongly affects both the fundamental and the realized niche, so the major assumptions of this model are likely to be general [22]. Parametrizing a more specific model would require information on the thermal performance curves and temperature-dependence of competitive effects between each species along a climate gradient. Not surprisingly, this information is seldom available. Simply extracting ‘niche’ data from a species' current range is insufficient because part of the realized range is likely to be confounded with species interactions [34].
We also need information on interspecific differences in dispersal ability among species that compete along the same climatic gradient. To our knowledge, this data has never been collected. We instead evaluated the variance in dispersal among species within the same taxonomic group. The standard deviation of this dispersal data averaged 1.9, much greater than even the parameter range evaluated in this paper (see the electronic supplementary material, table S1). Moreover, this value probably underestimates the variance among competitors in a real community which are likely to be more phylogenetically distant and thus differ more in dispersal ability. Effects of interspecific variance on community patterns could be even greater than predicted within our parameter range. Recent work suggests that species are tracking latitudinal shifts in climate change, but with a great deal of variance among species [3]. We predict that the large variance in climate change responses and lags in certain communities (e.g. elevational) [3,8] might be explained, at least in part, by interspecific competition and dispersal differences.
(c). Future considerations
Mechanistic models [35] and those parametrized for specific systems [11] will be valuable additions in future research. We also need to explore other species interactions, such as predator–prey, mutualism and host–parasite [10], as well as mixtures of interaction types (i.e. community modules, [4]). We did not consider that naive competitors might interact more strongly than long-established competitors. However, species that have never interacted before lack a coevolutionary history with each other and thus might interact more strongly (if character displacement has not occurred) and exacerbate effects. We also did not assess ‘hotter is better’ scenarios [20], whereby species with higher thermal optima also have higher absolute fitness. Under such a scenario, the warm-adapted species would be favoured to an even greater degree, and this asymmetry could cause stronger effects on community dynamics. We assumed continuous climatic changes—increased climate variance or extreme climatic events could cause stronger effects. We also assumed a univariate change in temperature. Yet, climate change is likely to involve changes in multiple climate parameters, which might alter each species response depending on individual sensitivities to each of these many changing factors. Discerning which factors might be important to incorporate in a multi-factor climate gradient will depend on the individual system to which predictions are applied. Lastly, we omitted evolutionary responses, which are likely to dampen community impacts for organisms with short-generation times and high genetic variance [36]. However, adaptation is not a climate change cure-all. Interspecific competition can prevent adaptation to climate change by species that otherwise have the ability to evolve into a new niche in order to escape a disappearing niche [37,38].
5. Conclusions
We show that interspecific competition and dispersal differences could greatly elevate extinction risk, alter community diversity and create no-analogue communities following climate change. Such effects have been discussed previously as concepts [4,5], but never evaluated in a general theoretical framework. Importantly, competition substantially slows species' abilities to track changing climates, even when they otherwise disperse well enough owing to the ‘boxcar effect’. However, strong dispersers can overcome this effect and supplant poor dispersers. Variation in dispersal among species—ubiquitous in nature—resulted in the most dramatic biodiversity losses. The species that face the greatest extinction risks might not be limited to those that disperse less than climate change absolutely requires, but also apply to those that disperse poorly relative to their warm-adapted competitors. Based on the highly skewed distributions of dispersal ability within taxonomic groups, the most realistic parameter space for most natural communities probably lies at the top of figure 2, where dispersal variation is greatest, no-analogue community formation was most evident and species losses were substantial. Because species interact and differ in dispersal ability, we might be vastly underestimating climate change impacts on biodiversity. This means that current predictions underlying biodiversity threats used by governments and conservation organizations could be conservative. We challenge ecologists to incorporate species interactions and dispersal differences into future predictions of biodiversity under climate change, and we suggest that conservation biologists should consider concentrating protection efforts on those species that disperse poorly and interact strongly.
Acknowledgements
We thank S. Alonzo, D. Skelly, P. Zarnetske and two anonymous reviewers for comments that improved the manuscript. The original idea was cooked up in the Species Range Dynamics working group supported by NCEAS and NESCent. Comments by members of this group greatly contributed to this project.
References
- 1.IPCC 2007. Climate change 2007: the physical science basis. Working group I contribution to the fourth assessment report of the IPCC. Cambridge University Press, Cambridge, UK [Google Scholar]
- 2.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 3.Chen I.-C., Hill J. K., Ohlemuller R., Roy D. B., Thomas C. D. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 10.1126/science.1206432 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 4.Gilman S. E., Urban M. C., Tewksbury J., Gilchrist G. W., Holt R. D. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331 10.1016/j.tree.2010.03.002 (doi:10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 5.Davis A. J., Jenkinson L. S., Lawton J. H., Shorrocks B., Wood S. 1998. Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 10.1038/35842 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- 6.Van der Putten W. H., Macel M., Visser M. E. 2010. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Phil. Trans. R. Soc. B 365, 2025–2034 10.1098/rstb.2010.0037 (doi:10.1098/rstb.2010.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huey R. B., Deutsch C. A., Tewksbury J. J., Vitt L. J., Hertz P. E., Perez H. J. A., Garland T. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948 10.1098/rspb.2008.1957 (doi:10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angert A. L., Crozier L. G., Rissler L. J., Gilman S. E., Tewksbury J. J., Chunco A. J. 2011. Do species' traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689 10.1111/j.1461-0248.2011.01620.x (doi:10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 9.Kissling W. D., Field R., Korntheuer H., Heyder U., Bohning-Gaese K. 2010. Woody plants and the prediction of climate-change impacts on bird diversity. Phil. Trans. R. Soc. B 365, 2035–2045 10.1098/rstb.2010.0008 (doi:10.1098/rstb.2010.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorcroft P. R., Pacala S. W., Lewis M. A. 2006. Potential role of natural enemies during tree range expansions following climate change. J. Theor. Biol. 241, 601–616 10.1016/j.jtbi.2005.12.019 (doi:10.1016/j.jtbi.2005.12.019) [DOI] [PubMed] [Google Scholar]
- 11.Ives A. R. 1995. Predicting the response of populations to environmental change. Ecology 76, 926–941 10.2307/1939357 (doi:10.2307/1939357) [DOI] [Google Scholar]
- 12.Thomas C. D., et al. 2004. Extinction risk from climate change. Nature 427, 145–148 10.1038/nature02121 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 13.Williams J. W., Jackson S. T. 2007. Novel climates, no-analog communities, and ecological surprises. Front Ecol. Environ. 5, 475. 10.1890/070037 (doi:10.1890/070037) [DOI] [Google Scholar]
- 14.Huey R. B., Stevenson R. D. 1979. Integrating thermal physiology and ecology of ectotherms: discussion of approaches. Am. Zool. 19, 357–366 [Google Scholar]
- 15.Rosenzweig M. L. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press [Google Scholar]
- 16.La Sorte F. A., Jetz W. 2010. Projected range contractions of montane biodiversity under global warming. Proc. R. Soc. B 277, 3401–3410 10.1098/rspb.2010.0612 (doi:10.1098/rspb.2010.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soberon J. 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 10.1111/j.1461-0248.2007.01107.x (doi:10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- 18.Norberg J., Swaney D. P., Dushoff J., Lin J., Casagrandi R., Levin S. A. 2001. Phenotypic diversity and ecosystem functioning in changing environments: a theoretical framework. Proc. Natl Acad. Sci. USA 98, 11 376–11 381 10.1073/pnas.171315998 (doi:10.1073/pnas.171315998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzalini A. 2005. The skew-normal distribution and related multivariate families (with discussion). Scand. J. Stat. 32, 159–200 10.1111/j.1467-9469.2005.00426.x (doi:10.1111/j.1467-9469.2005.00426.x) [DOI] [Google Scholar]
- 20.Huey R. B., Kingsolver J. G. 1989. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 4, 131–135 10.1016/0169-5347(89)90211-5 (doi:10.1016/0169-5347(89)90211-5) [DOI] [PubMed] [Google Scholar]
- 21.Deutsch C. A. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 10.1073/pnas.0709472105 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park T. 1954. Experimental studies of interspecies competition. 2. Temperature, humidity and competition in two species of Tribolium. Physiol. Zool. 27, 177–238 [Google Scholar]
- 23.Gill D. E. 1972. Intrinsic rates of increase, saturation densities, and competitive ability. I. An experiment with Paramecium. Am. Nat. 106, 461–471 10.1086/282787 (doi:10.1086/282787) [DOI] [Google Scholar]
- 24.Colwell R. K., Brehm G., Cardelus C. L., Gilman A. C., Longino J. T. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 10.1126/science.1162547 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- 25.Feeley K. J., Silman M. R. 2010. Biotic attrition from tropical forests correcting for truncated temperature niches. Global Change Biol. 16, 1830–1836 10.1111/j.1365-2486.2009.02085.x (doi:10.1111/j.1365-2486.2009.02085.x) [DOI] [Google Scholar]
- 26.Malcolm J. R., Liu C., Neilson R. P., Hansen L., Hannah L. 2006. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 20, 538–548 10.1111/j.1523-1739.2006.00364.x (doi:10.1111/j.1523-1739.2006.00364.x) [DOI] [PubMed] [Google Scholar]
- 27.Sinervo B., et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 10.1126/science.1184695 (doi:10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 28.Jankowski J. E., Robinson S. K., Levey D. J. 2010. Squeezed at the top: interspecific aggression may constrain elevational ranges in tropical birds. Ecology 91, 1877–1884 10.1890/09-2063.1 (doi:10.1890/09-2063.1) [DOI] [PubMed] [Google Scholar]
- 29.Sheldon K. S., Yang S., Tewksbury J. J. 2011. Climate change and community disassembly: impacts of warming on tropical and temperate montane communities. Ecol. Lett. 14, 1191–1200 10.1111/j.1461-0248.2011.01689.x (doi:10.1111/j.1461-0248.2011.01689.x) [DOI] [PubMed] [Google Scholar]
- 30.Kelly A. E., Goulden M. L. 2008. Rapid shifts in plant distribution with recent climate change. Proc. Natl Acad. Sci. USA 105, 11 823–11 826 10.1073/pnas.0802891105 (doi:10.1073/pnas.0802891105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janzen D. H. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 10.1086/282487 (doi:10.1086/282487) [DOI] [Google Scholar]
- 32.Ghalambor C. K., Huey R. B., Martin P. R., Tewksbury J. J., Wang G. 2005. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17 10.1093/icb/icj003 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 33.Tewksbury J., Huey R. B., Deutsch C. A. 2008. Putting the heat on tropical animals. Science 320, 1296–1297 10.1126/science.1159328 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 34.Araujo M. B., Guisan A. 2006. Five (or so) challenges for species distribution modeling. J. Biogeogr. 33, 1677–1688 10.1111/j.1365-2699.2006.01584.x (doi:10.1111/j.1365-2699.2006.01584.x) [DOI] [Google Scholar]
- 35.Vasseur D. A., McCann K. S. 2005. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am. Nat. 166, 184–198 10.1086/431285 (doi:10.1086/431285) [DOI] [PubMed] [Google Scholar]
- 36.Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 10.1111/j.1365-294X.2007.03413.x (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 37.Johansson J. 2008. Evolutionary responses to environmental changes: how does competition affect adaptation? Evolution 62, 421–435 10.1111/j.1558-5646.2007.00301.x (doi:10.1111/j.1558-5646.2007.00301.x) [DOI] [PubMed] [Google Scholar]
- 38.Urban M. C., De Meester L., Vellend M., Stoks R., Vanoverbeke J. In press. A crucial step toward realism: responses to climate change from an evolving metacommunity perspective. Evol. Appl. 10.1111/j.1752-4571.2011.00208.x (doi:10.1111/j.1752-4571.2011.00208.x) [DOI] [PMC free article] [PubMed] [Google Scholar]