Abstract
This study focuses on the sense of brightness in the foraging Japanese yellow swallowtail butterfly, Papilio xuthus. We presented two red discs of different intensity on a grey background to butterflies, and trained them to select one of the discs. They were successfully trained to select either a high intensity or a low intensity disc. The trained butterflies were tested on their ability to perceive brightness in two different protocols: (i) two orange discs of different intensity presented on the same intensity grey background and (ii) two orange discs of the same intensity separately presented on a grey background that was either higher or lower in intensity than the training background. The butterflies trained to high intensity red selected the orange disc of high intensity in protocol 1, and the disc on the background of low intensity grey in protocol 2. We obtained similar results in another set of experiments with purple discs instead of orange discs. The choices of the butterflies trained to low intensity red were opposite to those just described. Taken together, we conclude that Papilio has the ability to learn brightness and darkness of targets independent of colour, and that they have the so-called simultaneous brightness contrast.
Keywords: vision, insect, compound eye, neuroethology
1. Introduction
For humans, brightness of a light is defined by its perceived location on a scale ranging from black to white, and is essential in the judgement of the physical appearance of an object. It can be compared to chromaticity that differentiates colours of equal brightness. Our perception of brightness depends on the spatial context: when we see identical objects at the same time, one surrounded by a light background and another by a dark background, we recognize the former object as being darker than the latter. This phenomenon is called simultaneous brightness contrast, but the underlying neuronal mechanism is not well understood, probably because of its complexity [1]. Although brightness is a term defined for human vision, we here extend the definition of brightness of a light to non-human animals as ‘its perceived location on the grey scale for their visual systems’.
Accumulating evidence suggests that insects use achromatic vision based on a single type of spectral receptor to detect objects, motion, pattern and depth. However, the question of whether and how insects sense ‘brightness’ has rarely been asked directly [2]. Kelber [3] applied a dual choice protocol using spectral lights in a diurnal hawkmoth, Macroglossum stellatarum, to test the effect of light intensity on their flower-visiting behaviour. She demonstrated that hawkmoths were able to learn and discriminate the relative intensity of the stimuli, and that they use either achromatic or chromatic cues depending on the training conditions [3]. Bumble-bees (Bombus terrestris) also learn and discriminate relative intensities of spectral lights [4]. In honeybees (Apis mellifera), detectability of coloured targets increased with the intensity contrast between the target and background [5]. Despite the efforts of these previous studies, the existence of ‘brightness’ sense in insects has not been directly proved.
Here, we investigate the possible presence of brightness vision in the Japanese yellow swallowtail butterfly, Papilio xuthus whose visual system is well characterized. The eyes are furnished with at least six classes of spectral receptor [6], four of which appear to be responsible for their tetrachromatic vision used when searching for flowers [7]. We previously demonstrated colour constancy [8] and simultaneous colour contrast in this species [9]. Although brightness vision has not been explored in detail, we recently demonstrated that flower-visiting Papilio butterflies discriminate differently polarized lights as different intensities of light [10], whereas egg-laying butterflies discriminate them as different colours [11]. We also found that the landing of foraging Papilio required a distinct intensity contrast between the target and background [12], indicating that intensity contrast somehow affects the butterfly's vision.
After demonstrating that Papilio could learn and discriminate two stimuli of different intensities, we tested the trained butterflies to demonstrate whether they have simultaneous brightness contrast, as is present in humans. We presented two identical targets, each on a background of a different intensity of grey. Butterflies trained to the high intensity stimulus selected the target on the grey background of lower intensity, which to the human eye is perceived as lighter, while those trained to the low intensity stimulus selected the target on the background of higher intensity. We carried out the same set of tests with two different colours and concluded that foraging Papilio has the sense of brightness as well as simultaneous brightness contrast.
2. Material and methods
(a). Animals
We used laboratory-raised spring-form females of the Japanese yellow swallowtail butterfly (P. xuthus). Eggs, laid by females captured in the field, were allowed to hatch in the laboratory. The hatched larvae were reared on fresh citrus leaves at 25–27°C under a light regime of 10 L : 14 D. The pupae were chill-treated at 4°C for at least three months and then allowed to emerge in a styrofoam box at 30 ± 2°C. The day of emergence was defined as post-emergence day 1 (PED 1).
(b). Visual stimuli and illumination
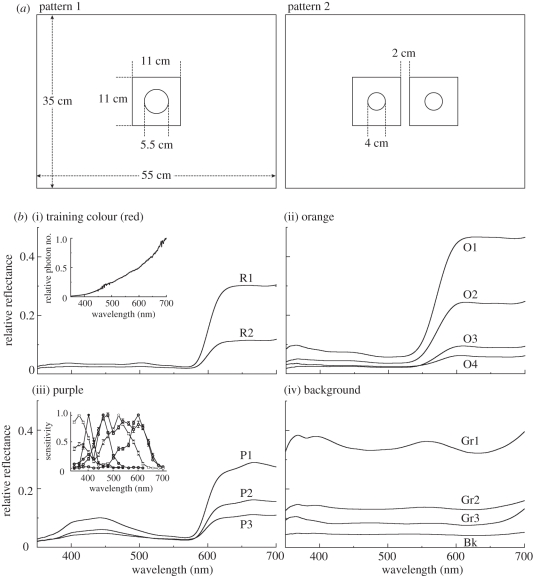
One (pattern 1) or two (pattern 2) stimuli, each consisting of a coloured disc (diameter 5.5 or 4.0 cm) surrounded by a grey square background (11 × 11 cm2), were put on a larger sheet of black paper (Kent paper, Kokuyo Japan, 35 × 55 cm2; figure 1a). The combination of the disc colour and background grey of each stimulus varied among experiments, as described below. The patterns were covered with anti-reflection glass when they were presented to butterflies in order to protect the patterns from any contamination by spilled drops of sugar solution or the animals' excreta.
Figure 1.
(a) Pattern 1 and pattern 2. (b) Reflectance spectrum of coloured (R, red; O, orange; P, purple; Gr, grey; Bk, black) papers. Numbers after the symbols indicate intensity: smaller numbers correspond to higher intensities. The insets of (i) and (ii) in (b), respectively, show the irradiation spectrum measured relative to an magnesium oxide-coated surface and the spectral sensitivities of six receptor classes.
Behavioural experiments were carried out in a cage (60 × 80 × 45 cm3) illuminated with 12 halogen lamps (300 W, Toshiba, Japan) hanging from the ceiling. The illuminance at the cage floor was about 5000 lx, which was sufficient for Papilio to discriminate coloured papers. The irradiance spectrum of the illumination is shown as the reflection of an magnesium oxide (MgO)-coated surface (inset of figure 1b(i)).
Figure 1b shows reflectance spectra of all colour, grey and black paper used in the experiments measured in the wavelength range of 300–700 nm with a spectrometer (HR2000, Ocean Optics, Inc., USA) relative to the MgO-coated surface. Colours of the discs were red (R), orange (O) and purple (P; Training colour 100, Nihonshikisai, Japan). Red was used for the training, whereas orange and purple were used in the tests (see §2c). This is because red-trained butterflies clearly distinguish orange and purple as colours different from red, the training colour, but still visit them when the red disc is absent, which enabled us to test the effect of intensity of discs and backgrounds. For each colour, we prepared two (R1, R2), three (P1, P2, P3) or four (O1, O2, O3, O4) different intensities by covering the discs with neutral density filters (Wratten gelatin filter, Kodak, USA). For the grey background, we used three different densities (Gr1, Gr2 and Gr3; NT-Raxa paper, Japan).
We calculated the relative intensity of paper i relative to the reflection intensity of the grey background of the Gr2 paper (Pi)
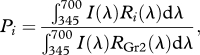 |
where I(λ) is the irradiance spectrum and Ri(λ) is the reflectance spectrum of paper i (figure 1b). The Pi values shown in table 1 where Gr2 was taken as 1.00.
Table 1.
Relative intensity (Pi) of colour papers used in this study.
| colour | R1 | R2 | O1 | O2 | O3 | O4 | P1 | P2 | P3 | Gr1 | Gr2 | Gr3 | Bk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pi | 1.72 | 0.71 | 3.22 | 1.22 | 0.68 | 0.48 | 1.71 | 0.99 | 0.73 | 2.57 | 1.00 | 0.61 | 0.33 |
Based on Pi values, we further calculated the intensity contrast (C) of each disc with respect to the background (bg):
when the intensity of the disc is lower (higher) than the background, C is negative (positive). The C values are shown in table 2.
Table 2.
Intensity contrast (C) between the disc and the background. Italic numbers indicate the stimuli selected by the trained butterflies.
| control test |
test 1/orange |
test 2/orange |
test 3/orange |
test 1/purple |
test 2/ purple |
test 3/ purple |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| experiment 1 (R1-trained) | ||||||||||||||
| disc | R1 | R2 | R1 | O2 | O2 | O4 | O2 | O2 | R1 | P1 | P1 | P3 | P2 | P2 |
| back | Gr2 | Gr2 | Gr2 | Gr2 | Gr2 | Gr2 | Gr1 | Gr3 | Gr2 | Gr2 | Gr2 | Gr2 | Gr1 | Gr3 |
| C | 0.24 | −0.15 | 0.24 | 0.09 | 0.09 | −0.32 | −0.32 | 0.30 | 0.24 | 0.23 | 0.23 | −0.14 | −0.41 | 0.21 |
| experiment 2 (R2-trained) | ||||||||||||||
| disc | R1 | R2 | R2 | O4 | O1 | O3 | O2 | O2 | R2 | P3 | P1 | P3 | P2 | P2 |
| back | Gr2 | Gr2 | Gr2 | Gr2 | Gr2 | Gr2 | Gr1 | Gr3 | Gr2 | Gr2 | Gr2 | Gr2 | Gr1 | Gr3 |
| C | 0.24 | −0.15 | −0.15 | −0.32 | 0.09 | −0.17 | −0.32 | 0.30 | 0.24 | −0.14 | 0.23 | −0.14 | −0.41 | 0.21 |
Because both Pi and C are based on physical intensity of reflection of the papers, those values may distinctly differ from the Papilio-subjective brightness, depending on which spectral channels Papilio uses for detecting brightness.
(c). Procedure of behavioural experiments
We carried out two behavioural experiments to test the capacity for brightness vision in Papilio. In experiment 1, we trained butterflies to a red disc of high intensity (R1), whereas in experiment 2 the butterflies were trained to a red disc of low intensity (R2; figure 2a). Each experiment consisted of three sessions: training, control test and a set of three independent tests. Figure 2b shows the time schedule of the behavioural experiments.
Figure 2.
(a) Photograph of the arena, showing a butterfly being trained (experiment 2). Abbreviations are given in figure 1. (b) Time schedule of behavioural experiments. The sequence of three tests after day 10 was not fixed. Two sets of test 1–3 after day 10 were performed in two different colours, the sequence of which was not fixed.
In any session, we released only one butterfly into the cage, and the butterflies were taken out from the storage box only when they were used in the experiments. We started to train butterflies to take drops of sucrose solution on a disc covered with anti-reflection glass on PED 2. Prior to training, butterflies were not fed to maximize their feeding motivation. We used 3 per cent sucrose solution on PED 2 in any training situation. After PED 3, we increased the concentration of sucrose solution up to 5 per cent as a reward to keep the butterflies healthy.
(i). Experiment 1
In the training sessions, butterflies were trained to search for a reward on an R1 disc. This session was divided into two steps. First, we presented pattern 1 with an R1 disc on Gr2 and forced butterflies to feed from sucrose on the disc on PED 2 (figure 2b). We continued this for an additional 3 days, during which most butterflies became adept at visiting the disc spontaneously. The second step started on PED 6 and lasted for 3 days, during which we presented pattern 2 with an R1 disc and an R2 disc, both on Gr2 (figure 2b). Using pattern 2, we fed butterflies on the R1 disc, and let them take sucrose solution on the R1 disc for at least 10 times a day. Because butterflies approached the stimuli on the floor from various directions, the relative position of two stimuli itself changed for butterflies over time. But, to minimize the effects of any positional learning owing to static structures such as the cage frame, we switched the position of the discs after every third visit.
A control test was carried out on PED 9 to check whether the butterflies could correctly select R1 over R2 (figure 2b). First, we presented the pattern 1 with an R1 disc and fed the R1-trained butterfly with about 10 µl of 10 per cent sucrose solution on the disc to stimulate feeding motivation. Then, we presented the pattern 2 with the R1 and R2 discs on Gr2 without providing any reward, allowed the butterfly to visit the stimuli five times, and counted the number of visits to each disc. Here, we defined a ‘visit’ as the behaviour in which the butterfly landed and touched a disc with an extended proboscis. The visiting butterflies always probed around the disc edge, but never on the edge between the grey square and the black sheet, indicating that the butterflies learned to compare the discs but not the grey squares. After every third visit, we changed the position of the discs to minimize any positional effects. In the following tests, we used the butterflies that selected the R1 disc at least three times in the five trials. The criteria were applied in all tests described below.
From PED 10, we performed three independent tests (1, 2 and 3) using pattern 2. A butterfly was subjected to only one of the three tests in a single day (figure 2b). The butterfly was allowed to visit either one of the stimuli five times in a single test. The order of the tests was randomized for each individual to avoid any effect of a fixed test order. In the tests, we used the same procedure to evaluate the butterflies' choice as in the control test. Test 1 was a colour discrimination test: we presented R1 on Gr2 with either O2 or P1 on Gr2. Test 2 was an intensity discrimination test with O2 versus O4, or P1 versus P3, all on Gr2. Test 3 was a test for simultaneous brightness contrast: we presented two identical colour discs, either O2 or P2, on two different intensities of grey (Gr1 and Gr3). Note that any paper used in test 3 was never used in the training session.
After each test, we checked the motivation of butterflies by presenting pattern 2 with R1 and R2 on Gr2. If butterflies visited R1 at least once, then we concluded that the motivation was intact and used the data for further analysis. We then presented pattern 1 used for the first step of the training and fed the butterflies with sucrose solution put on the R1 disc, until they recoiled their proboscis, indicating satiation.
(ii). Experiment 2
In experiment 2, we tested whether the butterflies could be trained to select a target of lower intensity, including simultaneous brightness contrast effect. Here, we used the same procedures as those of the training and tests in experiment 1. In the initial training phase, the butterflies were fed on an R2 disc on Gr2, and they were subsequently trained to discriminate R2 from R1 (figure 2a).
On PED 9, we carried out the control test by using pattern 2 with R1 and R2 discs on Gr2 to check whether butterflies were trained to discriminate the R2 target (figure 2b). We then performed tests 1–3 with pattern 2 from PED 10. In test 1 (colour discrimination), we presented R2 with either O4 or P3 on Gr2. In test 2 (intensity discrimination), we used patterns of O1 versus O3 and P1 versus P3, all on Gr2. In test 3 (contrast test), two identical discs of either O2 or P2 were presented on different intensities of grey, Gr1 and Gr3. After each test, we checked the motivation as described above.
(d). Statistical analysis
We calculated both mean and standard error of responses to each stimulus in each test (figures 3 and 4). Choices among tested individuals varied considerably. Therefore, we first counted the number of individuals that selected one stimulus four or five times in five trials (more than 80% selection). We then carried out a binomial test by calculating the probability that the observed number of individuals showed the same preference for each disc (p) by
where n is the number of all tested individuals, s is the number of individuals selected more than four times out of five visits and r is one-sixteenth, which is the probability that one particular stimulus of pattern 2 (figure 1a) is selected in four out of five trials. A p-value of 0.05 indicates that the chance of the observed selection occurring is 5 per cent if the selection was random. When p < 0.05 for the first stimulus and p > 0.05 for the second stimulus we concluded that there was a significant preference for the first stimulus over the second stimulus.
Figure 3.
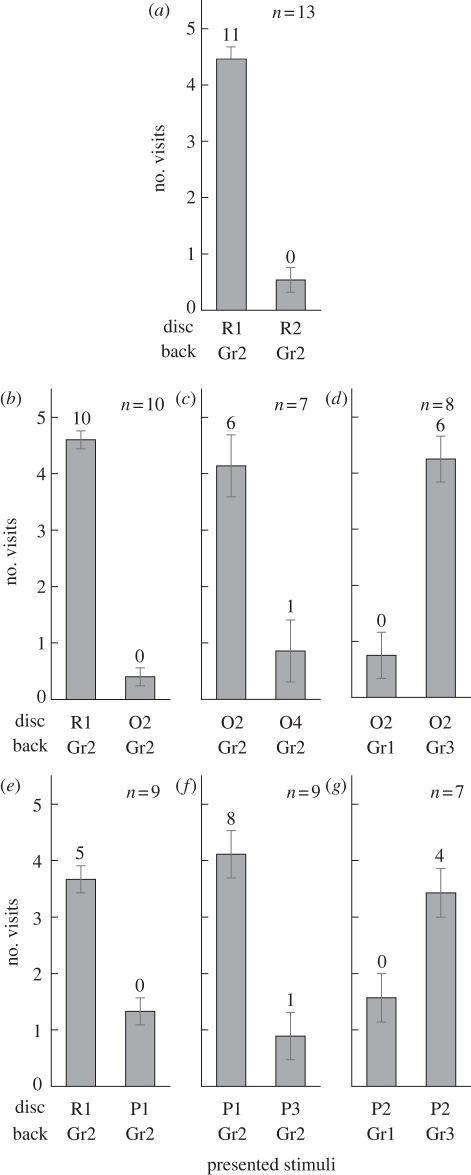
Effects of training to high intensity red (R1). (a) Control test (R1; p = 3.90 × 10−12, R2; p = 0.43, binomial test). (b) Test 1 (colour discrimination) with orange (O). R1; p = 9.09 × 10−13, O1; p = 0.52. (c) Test 2 (intensity discrimination) with O. O2; p = 3.91 × 10−7, O4; p = 0.27. (d) Test 3 (simultaneous brightness contrast) with O. Gr1; p = 0.60, Gr3; p = 1.47 × 10−6. (e) Test 1 with purple (P). R1; p = 9.28 × 10−5, P1; p = 0.56). (f) Test 2 with V. P1; p = 1.96 × 10−9, P3; p = 0.34. (g) Test 3 with P. Gr1; p = 0.64, Gr3; p = 4.4 × 10−4. Coloured paper details shown in figure 1. Numbers above each column indicates the number of individuals that visited the disc more than four times out of their five visits. Error bars show s.e.
Figure 4.
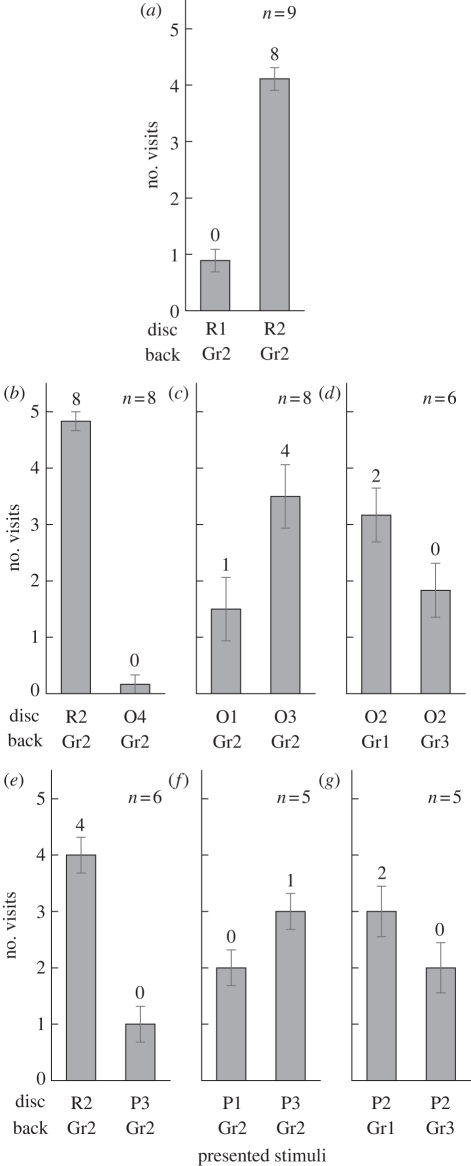
Effects of training to low intensity red (R2). (a) Control test (R1; p = 0.56, R2; p = 1.96 × 10−9, binomial test). (b) Test 1 (colour discrimination) with orange (O). R2; p = 2.33 × 10−10, O4; p = 0.60. (c) Test 2 (intensity discrimination) with O. O1; p = 0.32, O3; p = 8.25 × 10−4. (d) Test 3 (simultaneous brightness contrast) with O. Gr1; p = 0.045, Gr3; p = 0.68. (e) Test 1 with purple (P). R2; p = 2.01 × 10−4, P3; p = 0.68. (f) Test 2 with P. P1; p = 0.68, P3; p = 0.27. (g) Test 3 with P. Gr1; p = 0.032, Gr3; p = 0.72. For colour papers, see figure 1. Numbers above each column indicates the number of individuals that visited the disc more than four times of their five visits. Error bars show s.e.
3. Results
(a). Experiment 1: learning high intensity red
We used 55 individuals for experiment 1. The number of individuals that visited either the high intensity red (R1) or the low intensity red (R2) in the control test at least five times was 13 (figure 3a), and they were accepted as successfully trained individuals. The mean number of visits to R1 per individual was 4.46, and 11 out of 13 visited R1 four or five times (binomial test, p < 0.01). There were no individuals that selected R2 four or five times in their five visits.
Most individuals that completed the control test became adept at visiting the R1 disc in the first 3 days of the training session. We tried to use all the 13 individuals for the three tests with orange (O) and purple (P) colours, six tests in total (figure 3b–g), but seven individuals dropped out owing to damage to the wings, antennae and other body parts, and therefore, only six individuals completed all the tests.
Ten of the 13 individuals were tested on O discs (figure 3b). When presented R1 and O2 side by side in test 1, all butterflies successfully selected R1 (p < 0.01) based on their colour vision. When two O discs of different intensities (O2 and O4) were presented in test 2, there was a significant preference for O2 over O4 (p < 0.01). Only one individual visited O4 four times in its five visits (figure 3c). In test 3, two O2 discs were presented, one on a grey background of higher intensity (Gr1) and another on a grey background of lower intensity (Gr3). The butterflies selected the O2 disc on Gr3 significantly more often than the O2 disc on Gr1 (p < 0.01). No butterfly selected the O2 disc on Gr1 four or more times (figure 3d).
Nine of the R1-trained individuals were tested on P discs (figure 3e). Results with P discs were very similar to those with O discs. The butterflies selected R1 significantly more often (p < 0.01) when it was presented with P1 side by side (figure 3e). Most of them selected P1 (p < 0.01) over P3 in test 2 (figure 3f). In test 3, even though the presented discs themselves were identical in intensity, P2 on Gr3 was more frequently selected than P2 on Gr1. Four of the seven individuals tested selected P2 on Gr3 four or five times (figure 3g, p < 0.01).
(b). Experiment 2: learning low intensity red
Of the 51 individuals used in experiment 2, nine were successfully trained, visiting R2 (p < 0.01) over R1 in the control test (figures 2a and 4a). Five out of the nine completed all the following six tests, whereas another four contributed to some of the tests. Most of the tested individuals became adept at selecting and landing on the R2 disc in the first 3 days of the training session. However, in test 2 and 3, butterflies often flew very close to the cage floor for a long time looking for a landing place, and therefore, took longer to complete five visits.
The R2-trained individuals were tested with O discs (figure 4b–d) as well as the P discs (figure 4e–g). The butterflies successfully discriminated R2 from O4 or from P3 in the colour discrimination test 1 (p < 0.01). In the intensity discrimination test 2 with O discs, O3 (p < 0.01) was selected over O1 (figure 4c). Half of the tested individuals visited O3 four or more times in their five visits. In test 2 with P discs of two different intensities, P1 and P3 (figure 4f), only one individual visited P3 four or more times (p = 0.27), whereas none visited P1 more than three times (p = 0.68). The selections of both P1 and P3 were statistically insignificant, indicating that the selection of either P1 or P3 was random. In test 3 with two identical O discs on different intensities of a grey background (Gr1 and Gr3), the O disc on Gr1 was selected significantly more often (p < 0.05) than that on Gr3 (figure 4d). The same was true in test 3 with two P2 discs: the P2 disc on Gr1 was selected significantly more often (p < 0.05) than that on Gr3 (figure 4g). However, less than half of the tested individuals visited the disc on Gr1 more than three times.
4. Discussion
(a). Effect of training
For studying possible brightness vision, achromatic grey stimuli would have been preferred because the effect of colour vision can be more easily minimized. However, we had to use coloured discs because it is virtually impossible to train Papilio to a grey stimulus [13]. Therefore, we carefully designed the tests to minimize any possible effect of colour-opponent mechanisms.
We used a red disc for training, so the butterflies learned the red colour [13]. We first checked this effect in test 1, where we presented a red disc alongside either an orange or a purple disc, which the butterflies had never encountered before. The butterflies clearly discriminated red from those new colours (figures 3b,e and 4b,e) demonstrating their capacity for colour discrimination. We used these non-selected new colours to test the effect of intensity under conditions where the effect of colour vision was minimal.
Next, we presented two orange or two purple discs of different intensities on an identical grey background (Gr2). Therefore, the only cue the butterflies could use was the difference in intensity of the discs. The R1-trained butterflies selected the target of higher intensity significantly more often (figure 3c,f), and the R2-trained butterflies appeared to select the target of lower intensity (figure 4c,f), indicating that they had successfully learned to discriminate intensity differences regardless of spectral content.
(b). Innate preference and learning of intensity difference
The selection results were clearer in the R1-trained butterflies (figure 3) than the R2-trained butterflies (figure 4). This is because of the innate preference for higher intensity stimuli: when a trained Papilio is faced with two targets of the training colour of differing intensities, the butterfly almost always selects the target with the higher intensity [10]. Therefore, the results of the R1-trained butterflies contain the effects of both learned and innate preference (figure 3c,f), whereas those of the R2-trained butterflies were obscured by the innate preference for higher intensity targets (figure 4c,f). The innate preference appeared to be quite robust and it was difficult to change as long as the training was performed with single-target patterns. We could change the preference to a lower intensity target only by training butterflies to a rewarding lower intensity target presented simultaneously with an unrewarding higher intensity target (figure 4a). This indicates that the butterflies learn intensity difference in relative terms, and learning absolute intensity is very difficult or may even be impossible.
As also shown in hawkmoths [3] and honeybees [14,15], training to a specific intensity is much more difficult than to a specific colour. This seems to be a common feature among diurnal flower-visiting insects, where colour vision is the primary sense for locating flowers. This might be due to the fact that light levels during the day are extremely variable, particularly with a cloud cover and in shade. Therefore, for diurnal insects, intensity could be less reliable than spectral content during the day. The butterflies also have innate colour preference [13], but the colour preference can be adjusted quite easily depending on circumstances [1]. The adjustability of colour preference is most likely an adaptation for dealing with variation in the colours of nectar-rewarding flowers in natural environments. Presumably, fresh flowers tend to express brighter colour and produce more nectar compared with old flowers [16]. The robustness of innate preference towards higher intensity targets is probably an adaptation to locate more rewarding flowers of a certain colour.
(c). Sense of brightness and simultaneous brightness contrast
The prerequisites of demonstrating simultaneous contrast are that the butterflies (i) can compare two-coloured discs, each presented on a grey square background, (ii) have the sense of brightness, and (iii) have the ability to learn relative brightness. As described above, these points have been all confirmed in our experiments.
In test 3, we presented two identical discs of O2 or P2, each on a square of a higher (Gr1) or a lower intensity grey (Gr3); the only difference in the pattern was the intensity of the grey background. Since none of these papers, including the grey background, were used in the training sessions, the tested butterflies were unable to use memories formed during training relating to the absolute reflection spectra of these papers. The R1-trained butterflies selected a disc presented on Gr3 (figure 3d,g), whereas the R2-trained butterflies selected a disc on Gr1 (figure 4d,g). The most plausible explanation for the results is that the disc on Gr3 appeared ‘brighter’ and the disc on Gr1 appeared ‘darker’ for butterflies, and thus they selected a disc based on their sense of brightness and memory of rewarding brightness. In addition, their brightness vision is spatial-context dependent. In other words, it involves simultaneous brightness contrast.
It is of course reasonable that a strongly visual animal such as a butterfly has the property of simultaneous brightness contrast. We have to note, however, that the patterns we used for the training and control test had two stimuli, one with positive and another with negative contrast C (table 2). The results of the tests could be explained by postulating that the butterflies somehow learned the sign of the value of C, and selected a stimulus based on it. So far we have not been able to unambiguously exclude this possibility.
(d). Perspectives
Papilio compound eyes are complex with at least six classes of spectral receptors, five of which have peak sensitivities in the ultraviolet (UV), violet (V), blue (B), green (G) and red (R) wavelength region, respectively, whereas the remaining one has a broad-band (BB) sensitivity with a half-bandwidth of 210 nm in the range 420–630 nm (inset of figure 1b(iii)). The spectral receptors are embedded in the ommatidia in three fixed combinations, making the eye a collection of three types of spectrally heterogeneous ommatidia. The three types of ommatidia are distributed randomly, at least locally [6]. When searching for flowers, Papilio butterflies use tetrachromatic vision based on the UV, B, G and R receptors [7]; the excluded V and BB receptors exist in a particular type of ommatidium (type II; see Arikawa et al. [17]).
If brightness vision of Papilio relies on a dedicated receptor class to detect the general luminance of the environment, then the BB receptor [18] seems to be the immediate candidate. We recently found that intensity contrast between the target and background is crucial for landing in foraging Papilio. Although the spectral mechanism responsible for this is not yet known, the behavioural results could be explained by postulating the involvement of either the BB receptor system or the set of four receptors for colour vision [12].
On the other hand, studies in other insect species have indicated that achromatic vision, not necessarily brightness vision, relies basically on a single type of spectral receptor in the retina. The optomotor response in flies is based on the photoreceptors R1–6, whose spectral sensitivity is identical throughout the eye [19]. In honeybees and bumble-bees, G receptors appear to mediate the detection of small targets, motion and pattern, and the measurement of distance by optic flow [20–23]. In several species of butterfly, the action spectrum of the optomotor response resembles the G receptor sensitivity [24]. G receptors are the most common class of photoreceptor, and they exist in all ommatidia in all insect species so far studied, providing the basis for the best spatial resolution. If G receptors have a similar role in Papilio, then the most plausible candidate for the sense of brightness would be a population of G receptors in the distal tier of the retina filling the hexagonal lattice of ommatidia completely [25,26]. In any case, identification of input photoreceptor channel(s) for brightness vision requires further behavioural analyses.
Acknowledgements
The authors thank Dr Hideki Innan for discussing the statistical analysis and Drs Thomas W. Cronin, Doekele G. Stavenga and Aidan Vey for critical reading of the manuscript. This work was supported by the Japan Society for the Promotion of Science (JSPS) grants-in-aid for Scientific Research no. 21770078 to M.K. and no. 21247009 to K.A., and a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) grant (Elucidation of biological mechanisms of photoresponse and development of advanced technologies using light) no. INSECT-1101 to K.A.
References
- 1.Adelson E. H. 2000. Lightness perception and lightness illusions. In The new cognitive neurosciences (ed. Gazzaniga M.), pp. 339–351 Cambridge, MA: MIT Press [Google Scholar]
- 2.Kelber A., Osorio D. 2010. From spectral information to animal colour vision: experiments and concepts. Proc. R. Soc. B 277, 1617–1625 10.1098/rspb.2009.2118 (doi:10.1098/rspb.2009.2118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelber A. 2005. Alternative use of chromatic and achromatic cues in a hawkmoth. Proc. R. Soc. B 272, 2143–2147 10.1098/rspb.2005.3207 (doi:10.1098/rspb.2005.3207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke R., Lotto R. B. 2009. Visual processing of the bee innately encodes higher-order image statistics when the information is consistent with natural ecology. Vision Res. 49, 1455–1464 10.1016/j.visres.2009.02.021 (doi:10.1016/j.visres.2009.02.021) [DOI] [PubMed] [Google Scholar]
- 5.Hempel de Ibarra N., Vorobyev M., Brandt R., Giurfa M. 2000. Detection of bright and dim colours by honeybees. J. Exp. Biol. 203, 3289–3298 [DOI] [PubMed] [Google Scholar]
- 6.Arikawa K. 2003. Spectral organization of the eye of a butterfly, Papilio. J. Comp. Physiol. A 189, 791–800 10.1007/s00359-003-0454-7 (doi:10.1007/s00359-003-0454-7) [DOI] [PubMed] [Google Scholar]
- 7.Koshitaka H., Kinoshita M., Vorobyev M., Arikawa K. 2008. Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc. R. Soc. B 275, 947–954 10.1098/rspb.2007.1614 (doi:10.1098/rspb.2007.1614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita M., Arikawa K. 2000. Colour constancy of the swallowtail butterfly, Papilio xuthus. J. Exp. Biol. 203, 3521–3530 [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M., Takahashi Y., Arikawa K. 2008. Simultaneous color contrast in the foraging swallowtail butterfly, Papilio xuthus. J. Exp. Biol. 211, 3504–3511 10.1242/jeb.017848 (doi:10.1242/jeb.017848) [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita M., Yamazato K., Arikawa K. 2011. Polarization-based brightness discrimination in the foraging butterfly, Papilio xuthus. Phil. Trans. R. Soc. B 366, 688–696 10.1098/rstb.2010.0200 (doi:10.1098/rstb.2010.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelber A. 1999. Why ‘false’ colours are seen by butterflies. Nature 402, 251. 10.1038/46204 (doi:10.1038/46204) [DOI] [PubMed] [Google Scholar]
- 12.Koshitaka H., Arikawa K., Kinoshita M. 2011. Intensity contrast as a crucial cue for butterfly landing. J. Comp. Physiol. A 197, 1105–1112 10.1007/s00359-011-0671-4 (doi:10.1007/s00359-011-0671-4) [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita M., Shimada N., Arikawa K. 1999. Colour vision of the foraging swallowtail butterfly Papilio xuthus. J. Exp. Biol. 202, 95–102 [DOI] [PubMed] [Google Scholar]
- 14.Kaiser W. 1975. The relationship between visual movement detection and colour vision in insects. In The compound eye and vision in insects (ed. Horridge G. A.), pp. 359–377 Oxford, UK: Clarendon Press [Google Scholar]
- 15.Srinivasan M. V. 2011. Honeybees as a model for the study of visually guided flight, navigation, and biologically inspired robotics. Physiol. Rev. 91, 413–460 10.1152/physrev.00005.2010 (doi:10.1152/physrev.00005.2010) [DOI] [PubMed] [Google Scholar]
- 16.Weiss M. R. 1991. Floral colour changes as cues for pollinators. Nature 354, 227–229 10.1038/354227a0 (doi:10.1038/354227a0) [DOI] [Google Scholar]
- 17.Arikawa K., Mizuno S., Scholten D. G. W., Kinoshita M., Seki T., Kitamoto J., Stavenga D. G. 1999. An ultraviolet absorbing pigment causes a narrow-band violet receptor and a single-peaked green receptor in the eye of the butterfly Papilio. Vision Res. 39, 1–8 10.1016/S0042-6989(98)00070-4 (doi:10.1016/S0042-6989(98)00070-4) [DOI] [PubMed] [Google Scholar]
- 18.Arikawa K., Mizuno S., Kinoshita M., Stavenga D. G. 2003. Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of a butterfly, Papilio xuthus. J. Neurosci. 23, 4527–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi S., Wolf R., Desplan C., Heisenberg M. 2008. Motion vision is independent of color in Drosophila. Proc. Natl Acad. Sci. USA 105, 4910–4915 10.1073/pnas.0711484105 (doi:10.1073/pnas.0711484105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giurfa M., Vorobyev M. 1998. The angular range of achromatic target detection by honey bees. J. Comp. Physiol. A 183, 101–110 10.1007/s003590050238 (doi:10.1007/s003590050238) [DOI] [Google Scholar]
- 21.Giurfa M., Vorobyev M., Brandt R., Posner B., Menzel R. 1997. Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Physiol. A 180, 235–243 10.1007/s003590050044 (doi:10.1007/s003590050044) [DOI] [Google Scholar]
- 22.Giurfa M., Vorobyev M., Kevan P., Menzel R. 1996. Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A 178, 699–709 10.1007/BF00227381 (doi:10.1007/BF00227381) [DOI] [Google Scholar]
- 23.Lehrer M., Srinivasan M. V., Zhang S. W. 1990. Visual edge detection in the honeybee and its chromatic properties. Proc. R. Soc. Lond. B 238, 321–330 10.1098/rspb.1990.0002 (doi:10.1098/rspb.1990.0002) [DOI] [Google Scholar]
- 24.Horridge G. A., Marcelja L., Jahnke R. 1984. Color vision in butterflies 1. Single colour experiments. J. Comp. Physiol. A 155, 529–542 10.1007/BF00611917 (doi:10.1007/BF00611917) [DOI] [Google Scholar]
- 25.Takemura S., Kinoshita M., Arikawa K. 2005. Photoreceptor projection reveals heterogeneity of lamina cartridges in the visual system of the Japanese yellow swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 483, 341–350 10.1002/cne.20446 (doi:10.1002/cne.20446) [DOI] [PubMed] [Google Scholar]
- 26.Takemura S. Y., Arikawa K. 2006. Ommatidial type-specific interphotoreceptor connections in the lamina of the swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 494, 663–672 10.1002/cne.20830 (doi:10.1002/cne.20830) [DOI] [PubMed] [Google Scholar]