Abstract
How do genetic variation and evolutionary change in critical species affect the composition and functioning of populations, communities and ecosystems? Illuminating the links in the causal chain from genes up to ecosystems is a particularly exciting prospect now that the feedbacks between ecological and evolutionary changes are known to be bidirectional. Yet to fully explore phenomena that span multiple levels of the biological hierarchy requires model organisms and systems that feature a comprehensive triad of strong ecological interactions in nature, experimental tractability in diverse contexts and accessibility to modern genomic tools. The water flea Daphnia satisfies these criteria, and genomic approaches capitalizing on the pivotal role Daphnia plays in the functioning of pelagic freshwater food webs will enable investigations of eco-evolutionary dynamics in unprecedented detail. Because its ecology is profoundly influenced by both genetic polymorphism and phenotypic plasticity, Daphnia represents a model system with tremendous potential for developing a mechanistic understanding of the relationship between traits at the genetic, organismal and population levels, and consequences for community and ecosystem dynamics. Here, we highlight the combination of traits and ecological interactions that make Daphnia a definitive model system, focusing on the additional power and capabilities enabled by recent molecular and genomic advances.
Keywords: keystone species, trophic cascade, ecological genomics, eco-evolutionary dynamics, ecological stoichiometry, host–parasite
1. Introduction
Among the most exciting and significant current challenges in biology is to connect the genetic basis of variation in ecologically important traits with its effects on population, community and ecosystem properties. It is well known that community composition has profound effects on ecosystem functioning, yet phenotypic variation within a single species also has the potential to scale up to ecosystem impacts [1–4]. When phenotypic variation has dramatic effects on the strengths and qualities of ecological interactions, the properties and functioning of an ecosystem can depend on within-species variation in critical traits. This can lead to a cascade of effects from evolutionary change within a single species, through alterations of ecological interactions and community composition, to ecosystem change. This chain of causation goes both ways; of course ecosystems, via ecological interactions, exert selective pressure on organisms that results in evolution. This bidirectional feedback structure now known as ‘eco-evolutionary dynamics’, in which evolutionary and ecological processes each affect the other [5], has been called the ‘newest synthesis’ in ecology and evolution [6].
To illuminate the causal chain from individual phenotypes to community and ecosystem functioning, we can gain much by harnessing the power of the genomic revolution. We see two key advantages here. First is the obvious reality that our understanding of traits and phenotypic change is improved when their molecular mechanisms are known. With molecular information, we can improve our understanding of traits that have the potential for community- and ecosystem-level effects, and begin to illuminate elements of genome structure and regulation that are involved in causing these effects. Such elements are then easily testable in other organisms. A second advantage is that molecular traits, once identified, are typically easier to assess and follow over time. Once the molecular basis of an ecologically relevant trait has been identified, molecular signatures can be rapidly assessed in additional populations or closely related species to identify systems where further exploration of the trait and its ecological interactions is most promising. Molecular information can also be tracked over time in experimental trials at a scale not possible at the level of trait values of individuals. Although it by no means diminishes the importance of basic ecological experiments, genomic knowledge will serve both the expansive goal of improving mechanistic understanding and the practical challenge of implementing observational and manipulative studies on a spatial, temporal and numerical scale not previously attainable.
To achieve this grand ambition of connecting genes, traits, populations, communities and ecosystems in ways that improve our understanding of ecology and evolution, we need to apply modern genomic tools to model organisms known to have substantial and diverse ecological roles in natural environments. Not all species are equal in their ecological impacts (‘keystone’ species and ‘ecological engineers’ are two prominent examples), and not all species are equally tractable as model organisms for genomic research. Today, species with well-defined ecological roles are increasingly becoming a focus of the genomic revolution. One such species, the freshwater zooplankter Daphnia (Arthropoda: Crustacea), offers exceptional potential to generate in-depth genomic insight into the environmental responses of a strong ecological interactor. Daphnia is known to be of pivotal ecological importance in lakes and ponds on all continents, where it is a highly efficient grazer on phytoplankton and a preferred prey item for planktivorous fish and other predators ([7]; figure 1). Long a focal genus in ecology, evolution and ecotoxicology, Daphnia has matured into a versatile genomic model with the publication of the first Daphnia genome sequence [8] and a broad series of companion papers documenting its genome biology.1
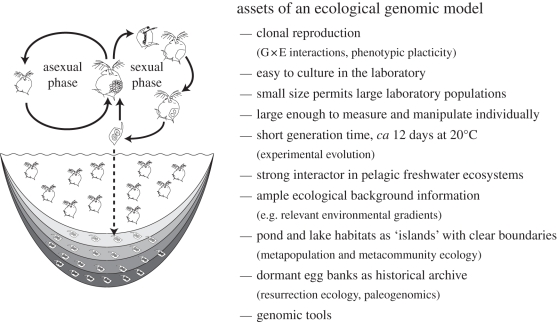
Figure 1.
Assets of Daphnia as a model organism. Most Daphnia species reproduce by cyclical parthenogenesis, which involves clonal reproduction during favourable periods, and sexual reproduction with the production of dormant stages in response to deteriorating environmental conditions [7]. Clonal reproduction is a key asset in the analysis of phenotypic plasticity and genotype-by-environment interactions. In addition, dormant eggs accumulate in layered egg banks that provide a unique opportunity to reconstruct the history of natural populations. We thank Joachim Mergeay for inspiring the design of this illustration.
The combination of modern genomic tools with well-documented, trait-based community and ecosystem impacts makes Daphnia ideal for integrative investigation of the traits and mechanisms that underlie responses to environmental change and their community- and ecosystem-level effects. In this review, we highlight broad ecological questions that the Daphnia system is positioned to address, and emphasize the additional power and potential that genomics brings to this enterprise. Because Daphnia has repeatedly been shown to be capable of rapid evolutionary change, it is an ideal system with which to explore the role of evolution in driving ecological dynamics in nature. With Daphnia, we should be able to identify phenotypes likely to affect ecological dynamics via rapid evolutionary change and the genomic characters underlying these phenotypes.
2. The ecological role of Daphnia
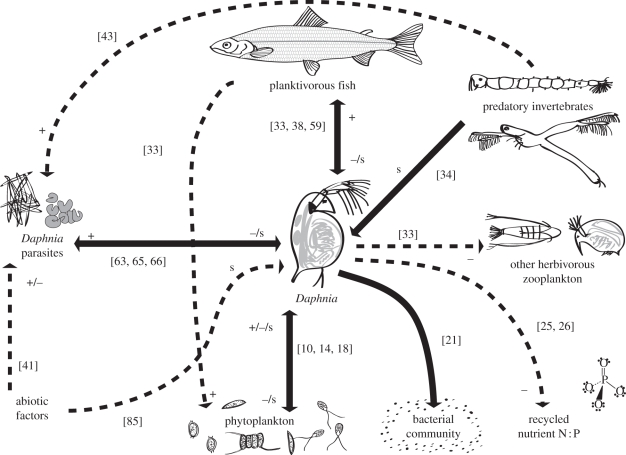
Daphnia species occur in lakes and ponds on every continent [9], where they consistently play a significant ecological role in food-web dynamics. Daphnia is a pelagic filter-feeding zooplankter with the potential for high population growth rate. Daphnia's food-web interactions, both as a primary consumer of phytoplankton and as a key food source for secondary consumers, define it as a strong ecological interactor. Its combined effects on phytoplankton grazing and nutrient cycling, along with its role as a preferred prey species for secondary consumers, cause Daphnia to occupy a uniquely significant position in the pelagic ecosystem of many lakes (figure 2). In each of these ecological processes, Daphnia populations possess a range of morphological, physiological and behavioural adaptations that influence the strength of interactions and are known to be genetically variable.
Figure 2.
Idealized interaction web for Daphnia in a lake ecosystem. Solid lines represent direct interactions and dashed lines represent indirect interactions. Plus (+) and minus (−) signs indicate positive or negative demographic interactions, while s represents natural selection acting on Daphnia populations. Citations refer to example studies that have documented each interaction and are discussed in the main text.
(a). Top-down control of algae and clearing of lakes
Daphnia have a greater ability to graze down phytoplankton than any other freshwater zooplankton (figure 3a, [10]), and a major ecosystem impact is the seasonal increase in water transparency driven by consumer–resource dynamics between Daphnia and phytoplankton known as the ‘clear-water phase’ [14]. The annual timing and duration of the clear-water phase has been demonstrated in several whole-lake ecosystems to depend on not just the abundance of Daphnia, but also which Daphnia species is present [15].
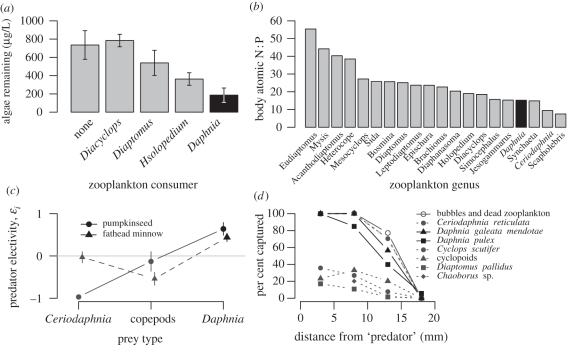
Figure 3.
Species belonging to the genus Daphnia are exceptional among zooplankton because of their (a) high grazing rates on phytoplankton, (b) low N : P ratios that make them a high-quality (P-rich) food source, (c) tendency to be the preferred prey of fishes, which results largely from(d) their inability to evade capture as demonstrated by the capture efficiency of a suction tube in laboratory trials. Graphs redrawn from (a) [10], (b) [11], (c) [12], (d) [13].
Much of the among-species variation in grazing impact by Daphnia is related to body size. Large-bodied species graze at higher rates than small-bodied taxa, which they typically outcompete [16]. Even within a Daphnia species, body-size variation can lead to differential impacts on plankton communities [17]. This means there is ample phylogenetic and genetic variation in the capacity of Daphnia populations to control phytoplankton abundance and productivity. This, in combination with the capacity of Daphnia to adapt genetically to changes in food quality [18,19], demonstrates the potential ecosystem impacts of genetic variation for traits related to Daphnia grazing. For example, planktivory by phenotypically divergent alewife populations has driven life-history divergence among Daphnia ambigua populations [20] in a group of New England lakes already known to exhibit cascading trophic effects from the alewives to the zooplankton community and subsequent phytoplankton productivity [2].
(b). Impacts on microbial communities
Because Daphnia is a generalist grazer, its feeding affects the biomass and structure of microbial communities by consuming protozoans and large bacteria in addition to phytoplankton [21]. Indeed, the composition of Daphnia diets varies seasonally and is sometimes dominated by bacteria, especially methanotrophs [22]. The degree to which daphnids feed on bacteria depends on the mesh-size of the filter combs on their thoracic appendages. Mesh size is species-specific, independent of body size, shows extensive heritable phenotypic plasticity, and can be genetically variable within species [23]. Daphnia may also have a non-trophic ecological connection with bacteria by enabling the vertical dispersal of bacteria that ‘hitchhike’ on their bodies as they move through thermally stratified water during diurnal vertical migration cycles [24].
(c). Impacts on nutrient loading and stoichiometry
Because different zooplankton taxa have distinct elemental (carbon : nitrogen : phosphorus, C : N : P) requirements and release C, N and P to the environment in distinct ratios, grazer community composition alone can determine whether pelagic primary production is N- or P-limited [25]. Daphnia stands out as having among the highest P contents and lowest body N : P of all zooplankton taxa (figure 3b; [11]). The high growth rates of Daphnia and the accompanying high P requirements combine with the low N : P ratio of its excretion to result in strong ecological interactions compared with other crustacean zooplankton. For example, when Daphnia becomes abundant early in the season, its feeding can suppress cyanobacterial blooms even when the ratio of available N : P is quite low, thus suppressing N-fixation and keeping soluble N much lower than in the absence of Daphnia grazing [26].
Understanding the linkages between high growth rates, high P demand and high P content in Daphnia is a promising avenue for connecting genetic variation in this taxon to ecosystem impacts. P requirements for Daphnia vary genetically among populations, with greater P demand in animals from habitats with shorter growing seasons, and hence higher growth rates [27]. In the laboratory, selection for rapid growth results in a heritable response [28], and both the structure of ribosomal rDNA [29] and specific alleles of the phosphoglucose isomerase (Pgi) locus [30] have been associated with variation in growth rates and P demand among Daphnia clones.
(d). Daphnia as prey
Daphnia plays a central role in lake pelagic ecosystems not only down the food web as a grazer, but also up the food web as an important prey for predatory invertebrates and planktivorous fish [31]. Its high P content makes it a particularly nutritious food resource (figure 3b; [11]), and its relatively large body size and limited escape ability make it a preferred prey item (figure 3c,d; [12,13]). Whole-lake experimental manipulations of planktivory identified Daphnia pulex as a ‘keystone species’ [32], while manipulations of nutrient supply in lakes with contrasting food webs have highlighted consistent effects of Daphnia on pelagic primary production across a wide range of nutrient levels [33].
Despite its high vulnerability to predation, there is considerable phylogenetic diversity among Daphnia species and genetic variation within species in antipredator traits [34–36]. Many traits show extensive phenotypic plasticity, and genetic variation for this plasticity [37,38]. This variation directly affects vulnerability to predation and therefore body size and population density, both of which affect grazing pressure on phytoplankton, with the result that the indirect impact on ecosystems of variation in antipredator traits is likely substantial.
(e). Daphnia as host
Daphnia have numerous bacterial, fungal and microsporidian parasites (reviewed by Ebert [39]) and the occurrence, severity and duration of parasite epidemics in Daphnia are influenced by both abiotic and biotic factors. The wide variation among lakes in the incidence of Daphnia parasitism can often be explained by environmental characteristics, such as population age and pond size [40] or lake basin shape [41]. The presence of additional Daphnia species that are less susceptible to parasitism creates a dilution effect that inhibits epidemic outbreaks in Daphnia dentifera [42]. Predators are also important: consumption of Daphnia by the dipteran larvae Chaoborus helps spread Metschnikowia by releasing fungal spores [43], while vertical migration by Daphnia to avoid fish predation brings putative hosts closer to spore-rich sediments [44]. And although direct costs of antipredator defences have been difficult to identify, recent evidence suggests an intriguing trade-off between the expression of morphological antipredator traits and vulnerability to parasitism [45].
Host–parasite dynamics involving a critical species like Daphnia can be expected to have ecosystem consequences. Duffy [46] found that phytoplankton density increased as epidemics progressed in enclosures containing D. dentifera and Metschnikowia. Another effect of Daphnia pathogen infection at the ecosystem level has been shown for elemental stoichiometry and nutrient transfer across trophic levels. Parasite infection has been shown to affect the C, N and P content of Daphnia in two different systems. In the case of chytridomycete infection in Daphnia pulicaria, infected animals contained less N and P and were lower in essential fatty acids than uninfected animals, in addition to being smaller [47]. Daphnia magna infected by the endobacterium Pasteuria differ in body C : P and C : N ratios from uninfected hosts [48], together with a feedback in which the C : P ratio of D. magna food resources influences the prevalence and virulence of Pasteuria [49].
3. The importance of cyclic parthenogenesis
Daphnia is a cyclic parthenogen and one of the few genomic model organisms capable of both sexual and asexual reproduction (figure 1), although dual reproductive modes are common across the tree of life (including many fungi, ‘protists’, plants and invertebrates). Sexual reproduction is linked to diapause in Daphnia because the product of sex is always a protective casing containing two dormant eggs that are independent products of meiosis and sexual recombination [7]. Diapausing eggs do not hatch immediately but either float to the shore, where they may be spatially dispersed by water birds, terrestrial mammals and humans [50], or sink to the sediments where they can facilitate temporal population persistence. Those deposited on the lake bottom can survive in sediments for decades, where they play an important role in permitting dispersal across generations or may even permit a species to re-establish after being absent from the water column for many years [51,52]. In addition, diapausing eggs recovered from chronologically layered lake sediments have enabled the development of the field of ‘resurrection ecology’ [53,54] in Daphnia and other freshwater zooplankton. Eggs are induced to hatch in the laboratory, making it possible to reconstruct evolutionary change using living animals from historical time sequences. The reservoir of living genetic and phenotypic history available in dormant eggs, combined with their temporal layering in lake sediments, is a truly remarkable resource with few parallels in other study systems.
While the phenomenon of cyclic parthenogenesis is interesting in its own right, clonal reproduction is also a powerful experimental tool. The ability to raise large cohorts of genetically identical animals provides opportunities to answer ecological, evolutionary and molecular questions that are otherwise unapproachable. For example, clonal reproduction is valuable for exploring the molecular basis of phenotypic plasticity, for which Daphnia has long been a leading study system [55,56]. Clones are also useful for exploring changes in gene expression in response environmental conditions. Here, the ability to gather replicate measures from genetically identical individuals exposed to uniform experimental treatments serves as a direct control for developmental noise and a baseline against which to compare responses to differing environments. This capability is used productively in studies we discuss below [8,57].
4. Capacity for rapid evolutionary response
The capacity of Daphnia populations to undergo rapid evolutionary change in natural systems has received considerable attention, especially in the last decade. A number of examinations of rapid evolution in Daphnia capitalize on the unique development of ‘resurrection ecology’, and most of these studies have focused on systems with a documented history of anthropogenic environmental change.
(a). Dietary tolerance of cyanobacteria
Hairston et al. [18] used clones of Daphnia galeata hatched from the sediments of Lake Constance to reconstruct the evolution of tolerance to dietary cyanobacteria. Lineages hatched from diapausing eggs deposited on the lake bottom in the 1960s, before cyanobacterial blooms had become frequent, were much more sensitive to cyanobacteria in their diets than were clones hatched from sediments deposited at the end of the 1970s, after their immediate ancestors had experienced 15 years of eutrophication-caused blooms. In essence, the D. galeata population evolved increased tolerance to cyanobacteria and decreased growth rate plasticity [18].
(b). Antipredator traits
There is ample among-population genetic variation for antipredator traits in Daphnia, typically fitting a pattern of local adaptation [58]. A comparison of Daphnia melanica from lakes in the Sierra Nevada of CA, USA, with and without a history of fish introductions showed that introduced predators drove Daphnia towards smaller body sizes and earlier reproduction [59]. These fixed genetic differences among populations were further augmented by contributions of adaptive plasticity in response to chemical cues from salmonid fish [60].
Cousyn et al. [38] found rapid behavioural evolution of D. magna in response to changes in predator regime by hatching diapausing eggs from the sediments of a former fish culture pond. During changes in the intensity of fish stocking, the Daphnia population evolved striking alterations of the plasticity of phototactic behaviour in response to fish chemical cues. Lineages resurrected from periods of more intensive fish stocking displayed greater overall plasticity in phototaxis than those from periods of lower fish density [38].
(c). Host–parasite co-evolution
The susceptibility of Daphnia to pathogens can evolve rapidly in response to infectious agents. The susceptibility of D. magna to the bacterial pathogen Pasteuria ramosa, for instance, is largely genetic, with minimal influence of plasticity, and can evolve rapidly [61]. Parasite-mediated selection can alter the outcome of clonal competition in experimental metapopulations of D. magna, with different parasite species causing different Daphnia clones to dominate [62]. Parasite-mediated selection can also act in a disruptive manner, resulting in a bimodal distribution of susceptibility, as observed for D. dentifera exposed to the fungal parasite Metschnikowia [63]. Most importantly, the genetic variation that exists for parasite susceptibility is ecologically relevant because it affects epidemic characteristics in wild populations [64]. In one of the best examples of an eco-evolutionary feedback loop in the wild, Duffy et al. [65] showed that the termination of Metschnikowia epidemics in D. dentifera is a direct result of host evolution rather than environmental conditions.
Decaestecker et al. [66] used ‘resurrection ecology’ to show that the interaction between D. magna and Pasteuria co-evolves in a pattern consistent with the ‘Red Queen’ hypothesis, i.e. that hosts must continually evolve to evade co-evolving parasites. Host lineages obtained by hatching diapausing eggs from sediment layers and then exposed to parasite populations from preceding, contemporary or future time periods experienced greatest infectivity when exposed to contemporary parasites [66].
5. Linking genes to ecologically relevant trait variation
The publication of the D. pulex genome [8] brings Daphnia research into a new era in which we face the challenging task of connecting genome structure, function and expression with variation in ecologically important traits. Recent studies have met various successes. For example, we know that genetically based dietary tolerance of toxic Microcystis aeruginosa cyanobacteria allows D. galeata to persist in the face of eutrophication [18]. A candidate-gene study of D. magna identified three genes (of seven candidates) that were each upregulated 8–10-fold in individuals exposed to toxin-producing strains of Microcystis relative to those exposed to non-toxic strains [67]. These genes code for enzymes involved in glycolysis, protein catabolism and the tricarboxylic acid cycle. Notably, all three belong to expanded gene families that result from ancestral gene duplications [67].
With respect to trophic interactions between Daphnia and its predators, a number of morphological, behavioural and life-history responses have been documented (reviewed by Lass & Spaak [34]). One study identified seven known genes (from 31 candidates) and three novel genes with the greatest upregulation when Daphnia is exposed to predatory insect cues during development [68]. The known genes fit into three categories: morphogenetic factors, juvenile hormone pathway genes and insulin signalling pathway genes. Other studies in the context of naturally occurring trait variation involve the interaction between ultraviolet radiation tolerance and vulnerability to predation in D. melanica in western North America. Populations of this species inhabiting small ponds of the Olympic Mountains do not possess UV-protective melanin pigmentation, perhaps because UV radiation is less intense than at higher elevations [69]. In larger, high altitude lakes of the Sierra Nevada, D. melanica display intense melanin pigmentation, but this trait has evolved rapidly in lakes with introduced fish predators [70]. The intensity of pigmentation is plastic in response to UV radiation in the ancestral fishless populations, while those with introduced predators have uniformly low melanin pigmentation regardless of UV treatment. The molecular basis of this loss in plasticity correlates with changes in the expression of two genes in the melanin pathway [70].
Because Daphnia are easily grown clonally, the candidate-gene approach is relatively easy to apply, yet it is biased towards known genes. An alternative is to use high-throughput functional genomics (e.g. microarrays or RNA-seq) to measure genome-wide expression patterns. An early study investigated differences in gene expression between sexes to identify genes involved in parthenogenesis and sex determination [71]. In this and other microarray studies [8], the finding that unknown genes are particularly likely to exhibit differential expression emphasizes the bias inherent in candidate-gene studies. Additional microarray studies have focused on exposure to environmental contaminants, both in D. pulex [72] and D. magna [73], reflecting the long history of both taxa as indicator species in ecotoxicology. Vandegehuchte et al. [73] screened for DNA methylation in zinc-exposed D. magna, opening the possibility that epigenetic control may be involved in adaptation to environmental change in Daphnia.
Proteomics is a burgeoning field for Daphnia, as advances in mass spectrometry enable analyses based upon the published D. pulex genome sequence [74]. Researchers have begun to investigate changes in protein expression in response to environmental perturbations, such as identifying cold-induced and cold-repressed proteins [75]. The proteomic response to hypoxia includes the expected increase in haemoglobin expression but also increases in a number of carbohydrate-degrading enzymes [76]. Proteomics is just beginning to be applied to trait variation among Daphnia species and genotypes [77], and this should become widespread in the future. Protein expression can also be explored in targeted candidate studies or used as a diagnostic marker for physiological conditions such as nutritional status [78].
A transcriptome study with direct relevance to the critical ecological role of Daphnia concerns the organism's functional genomic response to alterations in the stoichiometric balance of C and P in its algal food source [57]. As noted above, Daphnia have especially high P demands so the response of a particular Daphnia species to P limitation could have broad implications for aquatic ecosystems. A key finding of Jeyasingh et al. [57] is that P limitation influences gene expression in both direct and indirect ways: while some of the differentially expressed genes are directly involved in P metabolism, many unrelated metabolic pathways also show changes in gene expression. The authors suggest that this is likely a response to biochemical changes in algae that result from P-limited growth, which implies that the specific compensatory mechanisms of individual producer taxa to nutrient limitation could have considerable bearing on the upward transfer of energy in food webs.
6. Perspectives on genomics of an ecological model system
The genus Daphnia is remarkable for both its central ecological role in freshwater pelagic ecosystems and the features of its reproductive biology that enable exceptional experimental flexibility and control. Considering the extensive body of research on Daphnia, we now provide our perspective on the most promising opportunities for investigation provided by new molecular and genomic tools.
(a). Paleogenomics
While ‘resurrection ecology’ is a powerful approach to reconstruct evolutionary dynamics in natural populations during the past, the method is limited to the time period for which dormant eggs remain viable and can be hatched (approx. 30–80 years). Direct examination of DNA broadens this timeline because even when the eggs are no longer viable, the DNA they contain can still be analysed. Once we identify the genes that underlie ecologically relevant trait variation, we could use the molecular signatures of trait changes to infer the sequence of historical phenotypic evolution contained in a layered dormant egg bank. This approach would extend the time axis for historical reconstructions in Daphnia to hundreds or even thousands of years [52].
(b). Hunting for ecologically relevant genes
An important step in our search for mechanisms of adaptation will be finding genes linked to specific traits and identifying their function. These studies will not only be critical to understand the ecology of Daphnia, but will also inform studies of other model and non-model species. Once a function of a gene is identified or its link with a specific environmental variable is established, homologues of this gene in other species can be analysed for their relevance to similar ecological gradients. We see two broad methodological approaches to identify ecologically relevant genes, each of which has advantages and disadvantages:
(i). Bottom-up, hypothesis-based investigations of quantitative trait loci, expression quantitative trait loci and candidate genes
In this approach, traits are chosen that are known in advance to be ecologically relevant. Mapping quantitative trait loci (QTLs) involves crossing lineages that differ in traits of interest, while the candidate gene approach involves screening the expression of putative candidate loci chosen because of their function in other organisms. A combination of these approaches, expression quantitative trait loci (eQTLs), treats gene regulation as the phenotype of interest. The candidate-gene expression method is exemplified by the study of D. melanica differentially adapted to fish-inhabited versus fishless lakes of the Sierra Nevadas [70]. Notably, this study took the traditional candidate-gene approach one step further, by demonstrating gene expression differences in response to environmental cues in multiple natural populations with different ecological histories.
(ii). Top-down, hypothesis-neutral explorations using genome scans and genome-wide expression analysis
An alternative to the targeted approaches that focus on particular traits and candidate loci is to apply high-throughput genomic analyses, such as genome scans, gene expression arrays (transcriptomics) or proteomics to identify genes and gene pathways that show signatures of selection or environmentally determined expression. These methods have the advantage of having less bias because candidate traits and genes are not selected in advance. Genome scans apply a large array of markers to populations along strong environmental gradients and identify signatures of evolution at the genome level through screening for outlier behaviour of markers [79]. Although this method identifies genomic regions rather than genes, an advantage is that the signatures of selection come directly from natural populations. This approach has recently been applied to Belgian D. magna populations that occur across known selection gradients, identifying several genomic regions that exhibit signatures of selection owing to both natural and anthropogenic environmental stressors [80]. At the gene transcription level, microarray and RNA-seq technologies have enabled assessment of global gene expression for questions of direct relevance to ecological and ecosystem processes. The recent study of changes in D. pulex gene expression in response to differing C : P ratios in their phytoplankton food source is a compelling example because many of the genes involved had no direct connection to P metabolism [57], a finding that would likely have been overlooked in a candidate-gene study.
(c). Linking genomics to ecosystem characteristics
We have sought to describe how Daphnia is a key model system to connect genomic information with ecosystem consequences. Obvious candidates for study are genes that influence grazing rates, body size and toxin resistance and, in turn, are linked to top-down control of phytoplankton and associated ecosystem characteristics. Genes related to diapause initiation and termination, seasonality and climate change, predator avoidance and parasite resistance could indirectly affect water clarity, detrital deposition, benthic decomposition and nutrient remineralization, as well as the species composition and abundance of algal communities. The connection between biogeochemical cycles and the genes affecting Daphnia growth rate and nutrient requirements is another promising avenue to assess ecosystem impacts of specific genes.
Understanding the causal relationships between genes and traits on one level, and communities and ecosystems on another, will require quantitative methods that explicitly consider and define these connections. This has previously been addressed via demography [81] or by partitioning changes at higher levels of organization into the separate contributions of evolution, non-heritable phenotypic change and environmental change [82], and we encourage further development of these and other methods.
(d). Genomics and eco-evolutionary dynamics
Ecological genomics can contribute strongly to our understanding of the interplay between ecological and evolutionary dynamics, at the level of populations [82,83] as well as at the level of communities (evolving metacommunity concept; [84]) and ecosystems [2–4]. Finding the genomic signature of rapid evolutionary adaptation may provide insight into the frequency of parallel evolution and why some traits can evolve faster than others. Moreover, the use of molecular tools will enable large-scale studies, thus enabling us to fully incorporate spatial signals that represent different levels of gene exchange. This is important, as rapid evolutionary tracking of the environment has the potential to change the relative importance of local and regional processes in responding to environmental change. For instance, a mesocosm experiment showed that genetic adaptation of local D. magna to higher temperatures may reduce the immigration success of genotypes from warmer regions [85]. Identifying the genomic signature of adaptation to major environmental challenges at a regional scale to evaluate the relative importance of local versus regional processes, and to couple these to community and ecosystem consequences, will likely be a productive line of inquiry.
7. Conclusion
The critical ecological role played by Daphnia in the functioning of lake ecosystems around the world provides a stimulating real-world backdrop against which we can design, perform and evaluate studies of Daphnia genome biology. While a sequenced genome is a necessary prerequisite that will enable such investigations, the ecological role of Daphnia together with its key assets for experimental work will be central in exploiting the genome to advance understanding in ecological genomics. In this endeavour, it will be important to keep in mind that the linkages between genomics and ecology are bidirectional: understanding genome evolution and its relationship with phenotype depends on knowing the ecological context of the organism and the nature of selection. Not only will our understanding of Daphnia ecology be informed by new discoveries in the genome; the deep knowledge of Daphnia ecology and evolution that has been developed over the past century should be used to guide genome investigations. The publication of the D. pulex genome is but a start on the task of developing a truly integrative understanding of biological interactions from genes to ecosystems.
Acknowledgements
We thank Eawag for hosting the 2009 PhD Summer School in Kastanienbaum, Switzerland, where the idea for this paper originated. We also thank two anonymous referees for providing helpful comments on the manuscript. B.E.M. was supported by a Graduate Research Fellowship from the US National Science Foundation (NSF). L.D.M. was supported by K. U. Leuven Research Fund grants GOA/2008/006 and PF/2010/007. M.E.P. was supported by grants from the US NIH and NSF. N.G.H. was supported by grants from the James S. McDonnell Foundation and the US NSF.
Endnote
Daphnia: the companion papers for the genome sequence. BioMed Central Thematic Series. http://www.biomedcentral.com/series/Daphnia
References
- 1.Fussmann G. F., Loreau M., Abrams P. A. 2007. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 21, 465–477 10.1111/j.1365-2435.2007.01275.x (doi:10.1111/j.1365-2435.2007.01275.x) [DOI] [Google Scholar]
- 2.Post D. M., Palkovacs E. P., Schielke E. G., Dodson S. I. 2008. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology 89, 2019–2032 10.1890/07-1216.1 (doi:10.1890/07-1216.1) [DOI] [PubMed] [Google Scholar]
- 3.Palkovacs E. P., Post D. M. 2009. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology 90, 300–305 10.1890/08-1673.1 (doi:10.1890/08-1673.1) [DOI] [PubMed] [Google Scholar]
- 4.Bassar R. D., et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621 10.1073/pnas.0908023107 (doi:10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post D. M., Palkovacs E. P. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640 10.1098/rstb.2009.0012 (doi:10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoener T. W. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429 10.1126/science.1193954 (doi:10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 7.Lampert W. 2011. Daphnia: development of a model organism in ecology and evolution. In Excellence in Ecology. Book 21 (ed. Kinne O.). Oldendorf/Luhe: International Ecology Institute [Google Scholar]
- 8.Colbourne J. K., et al. 2011. The ecoresponsive genome of Daphnia pulex. Science 331, 555–561 10.1126/science.1197761 (doi:10.1126/science.1197761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamowicz S. J., Petrusek A., Colbourne J. K., Hebert P. D. N., Witt J. D. S. 2009. The scale of divergence: a phylogenetic appraisal of intercontinental allopatric speciation in a passively dispersed freshwater zooplankton genus. Mol. Phylogenet. Evol. 50, 423–436 10.1016/j.ympev.2008.11.026 (doi:10.1016/j.ympev.2008.11.026) [DOI] [PubMed] [Google Scholar]
- 10.Brett M. T., Wiackowski K., Lubnow F. S., Muellersogler A., Elser J. J., Goldman C. R. 1994. Species-dependent effects of zooplankton on planktonic ecosystem processes in Castle Lake, California. Ecology 75, 2243–2254 10.2307/1940880 (doi:10.2307/1940880) [DOI] [Google Scholar]
- 11.Elser J. J., et al. 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 10.1038/35046058 (doi:10.1038/35046058) [DOI] [PubMed] [Google Scholar]
- 12.Hambright K. D., Hall R. O. 1992. Differential zooplankton feeding behaviors, selectivities, and community impacts of two planktivorous fishes. Environ. Biol. Fish. 35, 401–411 10.1007/BF00004992 (doi:10.1007/BF00004992) [DOI] [Google Scholar]
- 13.Drenner R. W., Strickler J. R., O'Brien W. J. 1978. Capture probability: role of zooplankter escape in selective feeding of planktivorous fish. J. Fish. Res. Board Can. 35, 1370–1373 10.1139/f78-215 (doi:10.1139/f78-215) [DOI] [Google Scholar]
- 14.Lampert W., Schober U. 1978. Regular pattern of spring algal bloom and extremely clear water in Lake Constance as a result of climatic conditions and planktonic interactions. Arch. Hydrobiol. 82, 364–386 [Google Scholar]
- 15.Hairston N. G., Kearns C. M., Perry Demma L., Effler S. W. 2005. Species-specific Daphnia phenotypes: a history of industrial pollution and pelagic ecosystem response. Ecology 86, 1669–1678 10.1890/03-0784 (doi:10.1890/03-0784) [DOI] [Google Scholar]
- 16.Kreutzer C., Lampert W. 1999. Exploitative competition in differently sized Daphnia species: a mechanistic explanation. Ecology 80, 2348–2357 [Google Scholar]
- 17.Compte J., Brucet S., Gascon S., Bolix D., Sala J., Lopez-Flores R., Quintana X. D. 2009. Impact of different developmental stages of Daphnia magna (Straus) on the plankton community under different trophic conditions. Hydrobiologia 635, 45–56 10.1007/s10750-009-9860-3 (doi:10.1007/s10750-009-9860-3) [DOI] [Google Scholar]
- 18.Hairston N. G., Holtmeier C. L., Lampert W., Weider L. J., Post D. M., Fischer J. M., Cáceres C. E., Fox J. A., Gaedke U. 2001. Natural selection for grazer resistance to toxic cyanobacteria: evolution of phenotypic plasticity? Evolution 55, 2203–2214 [DOI] [PubMed] [Google Scholar]
- 19.Sarnelle O., Gustafsson S., Hansson L. A. 2010. Effects of cyanobacteria on fitness components of the herbivore Daphnia. J. Plankton Res. 32, 471–477 10.1093/plankt/fbp151 (doi:10.1093/plankt/fbp151) [DOI] [Google Scholar]
- 20.Walsh M. R., Post D. M. 2011. Interpopulation variation in a fish predator drives evolutionary divergence in prey in lakes. Proc. R. Soc. B 278, 2628–2637 10.1098/rspb.2010.2634 (doi:10.1098/rspb.2010.2634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langenheder S., Jürgens K. 2001. Regulation of bacterial biomass and community structure by metazoan and protozoan predation. Limnol. Oceangr. 46, 121–134 10.4319/lo.2001.46.1.0121 (doi:10.4319/lo.2001.46.1.0121) [DOI] [Google Scholar]
- 22.Taipale S., Kankaala P., Tiirola M., Jones R. I. 2008. Whole-lake dissolved inorganic C-13 additions reveal seasonal shifts in zooplankton diet. Ecology 89, 463–474 10.1890/07-0702.1 (doi:10.1890/07-0702.1) [DOI] [PubMed] [Google Scholar]
- 23.Bednarska A., Dawidowicz P. 2007. Change in filter-screen morphology and depth selection: uncoupled responses of Daphnia to the presence of filamentous cyanobacteria. Limnol. Oceanogr. 52, 2358–2363 10.4319/lo.2007.52.6.2358 (doi:10.4319/lo.2007.52.6.2358) [DOI] [Google Scholar]
- 24.Grossart H.-P., Dziallas C., Leunert F., Tang K. W. 2010. Bacteria dispersal by hitchhiking on zooplankton. Proc. Natl Acad. Sci. USA 107, 11 959–11 964 10.1073/pnas.1000668107 (doi:10.1073/pnas.1000668107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterner R. W., Elser J. J. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press [Google Scholar]
- 26.Hambright K. D., Hairston N. G., Schaffner W. R., Howarth R. W. 2007. Grazer control of nitrogen fixation: synergisms in the feeding ecology of two freshwater crustaceans. Fund. Appl. Limnol. 170, 89–101 10.1127/1863-9135/2007/0170-0089 (doi:10.1127/1863-9135/2007/0170-0089) [DOI] [Google Scholar]
- 27.Elser J. J., O'Brien W. J., Dobberfuhl D. R., Dowling T. E. 2000. The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J. Evol. Biol. 13, 845–853 10.1046/j.1420-9101.2000.00215.x (doi:10.1046/j.1420-9101.2000.00215.x) [DOI] [Google Scholar]
- 28.Gorokhova E., Dowling T. E., Weider L. J., Crease T. J., Elser J. J. 2002. Functional and ecological significance of rDNA intergenic spacer variation in a clonal organism under divergent selection for production rate. Proc. R. Soc. Lond. B 269, 2373–2379 10.1098/rspb.2002.2145 (doi:10.1098/rspb.2002.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weider L. J., Glenn K. L., Kyle M., Elser J. J. 2004. Associations among ribosomal (r) DNA intergenic spacer length, growth rate, and C : N : P stoichiometry in the genus Daphnia. Limnol. Oceanogr. 49, 1417–1423 10.4319/lo.2004.49.4_part_2.1417 (doi:10.4319/lo.2004.49.4_part_2.1417) [DOI] [Google Scholar]
- 30.Jeyasingh P. D., Weider L. J., Sterner R. W. 2009. Genetically-based trade-offs in response to stoichiometric food quality influence competition in a keystone aquatic herbivore. Ecol. Lett. 12, 1229–1237 10.1111/j.1461-0248.2009.01368.x (doi:10.1111/j.1461-0248.2009.01368.x) [DOI] [PubMed] [Google Scholar]
- 31.Carpenter S. R., Kitchell J. F. 1993. The trophic cascade in lakes. Cambridge, UK: /New York, NY: Cambridge University Press [Google Scholar]
- 32.Ives A. R., Carpenter S. R., Dennis B. 1999. Community interaction webs and zooplankton responses to planktivory manipulations. Ecology 80, 1405–1421 10.1890/0012-9658(1997)080[1405:CIWAZR]2.0.CO;2 (doi:10.1890/0012-9658(1997)080[1405:CIWAZR]2.0.CO;2) [DOI] [Google Scholar]
- 33.Carpenter S. R., et al. 2001. Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol. Monogr. 71, 163–186 10.1890/0012-9615(2001)071[0163:TCNALP]2.0.CO;2 (doi:10.1890/0012-9615(2001)071[0163:TCNALP]2.0.CO;2) [DOI] [Google Scholar]
- 34.Lass S., Spaak P. 2003. Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491, 221–239 10.1023/A:1024487804497 (doi:10.1023/A:1024487804497) [DOI] [Google Scholar]
- 35.Petrusek A., Tollrian R., Schwenk K., Haas A., Laforsch C. 2009. A ‘crown of thorns’ is an inducible defense that protects Daphnia against an ancient predator. Proc. Natl Acad. Sci. USA 106, 2248–2252 10.1073/pnas.0808075106 (doi:10.1073/pnas.0808075106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spanier K., Leese F., Mayer C., Colbourne J., Gilbert D., Pfrender M., Tollrian R. 2010. Predator-induced defences in Daphnia pulex: selection and evaluation of internal reference genes for gene expression studies with real-time PCR. BMC Mol. Biol. 11, 50. 10.1186/1471-2199-11-50 (doi:10.1186/1471-2199-11-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boersma M., Spaak P., De Meester L. 1998. Predator-mediated plasticity in morphology, life history, and behavior of Daphnia: the uncoupling of responses. Am. Nat. 152, 237–248 10.1086/286164 (doi:10.1086/286164) [DOI] [PubMed] [Google Scholar]
- 38.Cousyn C., De Meester L., Colbourne J. K., Brendonck L., Verschuren D., Volckaert F. 2001. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA 98, 6256–6260 10.1073/pnas.111606798 (doi:10.1073/pnas.111606798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebert D. 2005. Ecology, epidemiology, and evolution of parasitism in Daphnia. [Internet]. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information. Available from: http://www.ncbi.nlm.nih.gov/books/NBK2036/ [Google Scholar]
- 40.Ebert D., Hottinger J. W., Pajunen V. I. 2001. Temporal and spatial dynamics of parasite richness in a Daphnia metapopulation. Ecology 82, 3417–3434 10.1890/0012-9658(2001)082[3417:TASDOP]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[3417:TASDOP]2.0.CO;2) [DOI] [Google Scholar]
- 41.Cáceres C. E., Hall S. R., Duffy M. A., Tessier A. J., Helmle C., MacIntyre S. 2006. Physical structure of lakes constrains epidemics in Daphnia populations. Ecology 87, 1438–1444 10.1890/0012-9658(2006)87[1438:PSOLCE]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1438:PSOLCE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 42.Hall S. R., Becker C. R., Simonis J. L., Duffy M. A., Tessier A. J., Cáceres C. E. 2009. Friendly competition: evidence for a dilution effect among competitors in a planktonic host–parasite system. Ecology 90, 791–801 10.1890/08-0838.1 (doi:10.1890/08-0838.1) [DOI] [PubMed] [Google Scholar]
- 43.Cáceres C. E., Knight C. J., Hall S. R. 2009. Predator-spreaders: predation can enhance parasite success in a planktonic host–parasite system. Ecology 90, 2850–2858 10.1890/08-2154.1 (doi:10.1890/08-2154.1) [DOI] [PubMed] [Google Scholar]
- 44.Decaestecker E., De Meester L., Ebert D. 2002. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc. Natl Acad. Sci. USA 99, 5481–5485 10.1073/pnas.082543099 (doi:10.1073/pnas.082543099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin M., Laforsch C., Lohr J. N., Wolinska J. 2011. Predator-induced defense makes Daphnia more vulnerable to parasites. Evolution 65, 1482–1488 10.1111/j.1558-5646.2011.01240.x (doi:10.1111/j.1558-5646.2011.01240.x) [DOI] [PubMed] [Google Scholar]
- 46.Duffy M. A. 2007. Selective predation, parasitism, and trophic cascades in a bluegill–Daphnia–parasite system. Oecologia 153, 453–460 10.1007/s00442-007-0742-y (doi:10.1007/s00442-007-0742-y) [DOI] [PubMed] [Google Scholar]
- 47.Forshay K. J., Johnson P. T. J., Stock M., Peñalva C., Dodson S. I. 2008. Festering food: chytridomycete pathogen reduces quality of Daphnia host as a food resource. Ecology 89, 2692–2699 10.1890/07-1984.1 (doi:10.1890/07-1984.1) [DOI] [PubMed] [Google Scholar]
- 48.Frost P. C., Ebert D., Smith V. H. 2008. Bacterial infection changes the elemental composition of Daphnia magna. J. Anim. Ecol. 77, 1265–1272 10.1111/j.1365-2656.2008.01438.x (doi:10.1111/j.1365-2656.2008.01438.x) [DOI] [PubMed] [Google Scholar]
- 49.Frost P. C., Ebert D., Smith V. H. 2008. Responses of a bacterial pathogen to phosphorus limitation of its aquatic invertebrate host. Ecology 89, 313–318 10.1890/07-0389.1 (doi:10.1890/07-0389.1) [DOI] [PubMed] [Google Scholar]
- 50.Havel J. E., Shurin J. B. 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnol. Oceanogr. 49, 1229–1238 10.4319/lo.2004.49.4_part_2.1229 (doi:10.4319/lo.2004.49.4_part_2.1229) [DOI] [Google Scholar]
- 51.Hairston N. G., Kearns C. M. 2002. Temporal dispersal: ecological and evolutionary aspects of zooplankton egg banks and the role of sediment mixing. Integr. Comp. Biol. 42, 481–491 10.1093/icb/42.3.481 (doi:10.1093/icb/42.3.481) [DOI] [PubMed] [Google Scholar]
- 52.Mergeay J., Vanoverbeke J., Verschuren D., De Meester L. 2007. Extinction, recolonization, and dispersal through time in a planktonic crustacean. Ecology 88, 3032–3043 10.1890/06-1538.1 (doi:10.1890/06-1538.1) [DOI] [PubMed] [Google Scholar]
- 53.Hairston N. G., Van Brunt R. A., Kearns C. M., Engstrom D. R. 1995. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76, 1706–1711 10.2307/1940704 (doi:10.2307/1940704) [DOI] [Google Scholar]
- 54.Kerfoot W. C., Robbins J. A., Weider L. J. 1999. A new approach to historical reconstruction: combining descriptive and experimental paleolimnology. Limnol. Oceanogr. 44, 1232–1247 10.4319/lo.1999.44.5.1232 (doi:10.4319/lo.1999.44.5.1232) [DOI] [Google Scholar]
- 55.Simon J.-C., Pfrender M. E., Tollrian R., Tagu D., Colbourne J. K. 2011. Genomics of environmentally induced phenotypes in two extremely plastic arthropods. J. Hered. 102, 512–525 10.1093/jhered/esr020 (doi:10.1093/jhered/esr020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tollrian R., Leese F. 2010. Ecological genomics: steps towards unraveling the genetic basis of inducible defenses in Daphnia. BMC Biol. 8, 51. 10.1186/1741-7007-8-51 (doi:10.1186/1741-7007-8-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeyasingh P. D., Ragavendran A., Paland S., Lopez J. A., Sterner R. W., Colbourne J. K. 2011. How do consumers deal with stoichiometric constraints? Lessons from functional genomics using Daphnia pulex. Mol. Ecol. 20, 2341–2352 10.1111/j.1365-294X.2011.05102.x (doi:10.1111/j.1365-294X.2011.05102.x) [DOI] [PubMed] [Google Scholar]
- 58.De Meester L. 1996. Evolutionary potential and local genetic differentiation in a phenotypically plastic trait of a cyclical parthenogen, Daphnia magna. Evolution 50, 1293–1298 10.2307/2410669 (doi:10.2307/2410669) [DOI] [PubMed] [Google Scholar]
- 59.Fisk D. L., Latta L. C., Knapp R. A., Pfrender M. E. 2007. Rapid evolution in response to introduced predators I: rates and patterns of morphological and life-history trait divergence. BMC Evol. Biol. 7, 22. 10.1186/1471-2148-7-22 (doi:10.1186/1471-2148-7-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latta L. C., Bakelar J. W., Knapp R. A., Pfrender M. E. 2007. Rapid evolution in response to introduced predators II: the contribution of adaptive plasticity. BMC Evol. Biol. 7, 21. 10.1186/1471-2148-7-21 (doi:10.1186/1471-2148-7-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Little T. J., Watt K., Ebert D. 2006. Parasite–host specificity: experimental studies on the basis of parasite adaptation. Evolution 60, 31–38 [PubMed] [Google Scholar]
- 62.Haag C. R., Ebert D. 2004. Parasite-mediated selection in experimental metapopulations of Daphnia magna. Proc. R. Soc. Lond. B 271, 2149–2155 10.1098/rspb.2004.2841 (doi:10.1098/rspb.2004.2841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duffy M. A., Brassil C. E., Hall S. R., Tessier A. J., Cáceres C. E., Conner J. K. 2008. Parasite-mediated disruptive selection in a natural Daphnia population. BMC Evol. Biol. 8, 80. 10.1186/1471-2148-8-80 (doi:10.1186/1471-2148-8-80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altermatt F., Ebert D. 2008. Genetic diversity of Daphnia magna populations enhances resistance to parasites. Ecol. Lett. 11, 918–928 10.1111/j.1461-0248.2008.01203.x (doi:10.1111/j.1461-0248.2008.01203.x) [DOI] [PubMed] [Google Scholar]
- 65.Duffy M. A., Hall S. R., Cáceres C. E., Ives A. R. 2009. Rapid evolution, seasonality, and the termination of parasite epidemics. Ecology 90, 1441–1448 10.1890/08-1130.1 (doi:10.1890/08-1130.1) [DOI] [PubMed] [Google Scholar]
- 66.Decaestecker E., Gaba S., Raeymaekers J. A. M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L. 2007. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature 450, 870–873 10.1038/nature06291 (doi:10.1038/nature06291) [DOI] [PubMed] [Google Scholar]
- 67.Schwarzenberger A., Courts C., von Elert E. 2009. Target gene approaches: gene expression in Daphnia magna exposed to predator-borne kairomones or to microcystin-producing and microcystin-free Microcystis aeruginosa. BMC Genomics 10, 527. 10.1186/1471-2164-10-527 (doi:10.1186/1471-2164-10-527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyakawa H., et al. 2010. Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev. Biol. 10, 45. 10.1186/1471-213X-10-45 (doi:10.1186/1471-213X-10-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miner B. E., Kerr B. 2011. Adaptation to local ultraviolet radiation conditions among neighbouring Daphnia populations. Proc. R. Soc. B 278, 1306–1313 10.1098/rspb.2010.1663 (doi:10.1098/rspb.2010.1663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scoville A. G., Pfrender M. E. 2010. Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl Acad. Sci. USA 107, 4260–4263 10.1073/pnas.0912748107 (doi:10.1073/pnas.0912748107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eads B. D., Colbourne J. K., Bohuski E., Andrews J. 2007. Profiling sex-biased gene expression during parthenogenetic reproduction in Daphnia pulex. BMC Genomics 8, 464. 10.1186/1471-2164-8-464 (doi:10.1186/1471-2164-8-464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw J. R., Colbourne J. K., Davey J. C., Glaholt S. P., Hampton T. H., Chen C. Y., Folt C. L., Hamilton J. W. 2007. Gene response profiles for Daphnia pulex exposed to the environmental stressor cadmium reveals novel crustacean metallothioneins. BMC Genomics 8, 477. 10.1186/1471-2164-8-477 (doi:10.1186/1471-2164-8-477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandegehuchte M. B., Vandenbrouck T., Coninck D. D., De Coen W. M., Janssen C. R. 2010. Can metal stress induce transferable changes in gene transcription in Daphnia magna? Aquat. Toxicol. 97, 188–195 10.1016/j.aquatox.2009.07.013 (doi:10.1016/j.aquatox.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 74.Fröhlich T., Arnold G. J., Fritsch R., Mayr T., Laforsch C. 2009. LC-MS/MS-based proteome profiling in Daphnia pulex and Daphnia longicephala: the Daphnia pulex genome database as a key for high throughput proteomics in Daphnia. BMC Genomics 10, 171. 10.1186/1471-2164-10-171 (doi:10.1186/1471-2164-10-171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwerin S., Zeis B., Lamkemeyer T., Paul R., Koch M., Madlung J., Fladerer C., Pirow R. 2009. Acclimatory responses of the Daphnia pulex proteome to environmental changes. II. Chronic exposure to different temperatures (10 and 20 degrees C) mainly affects protein metabolism. BMC Physiol. 9, 8. 10.1186/1472-6793-9-8 (doi:10.1186/1472-6793-9-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeis B., et al. 2009. Acclimatory responses of the Daphnia pulex proteome to environmental changes. I. Chronic exposure to hypoxia affects the oxygen transport system and carbohydrate metabolism. BMC Physiology 9, 7. 10.1186/1472-6793-9-7 (doi:10.1186/1472-6793-9-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerke P., Börding C., Zeis B., Paul R. J. 2011. Adaptive haemoglobin gene control in Daphnia pulex at different oxygen and temperature conditions. Comp. Biohem. Physiol. A 159, 56–65 10.1016/j.cbpa.2011.01.017 (doi:10.1016/j.cbpa.2011.01.017) [DOI] [PubMed] [Google Scholar]
- 78.Elser J. J., Peace A. L., Kyle M., Wojewodzic M., McCrackin M. L., Andersen T., Hessen D. O. 2010. Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol. Lett. 13, 1256–1261 10.1111/j.1461-0248.2010.01519.x (doi:10.1111/j.1461-0248.2010.01519.x) [DOI] [PubMed] [Google Scholar]
- 79.Vasemagi A., Primmer C. R. 2005. Challenges for identifying functionally important genetic variation: the promise of combining complementary research strategies. Mol. Ecol. 14, 3623–3642 10.1111/j.1365-294X.2005.02690.x (doi:10.1111/j.1365-294X.2005.02690.x) [DOI] [PubMed] [Google Scholar]
- 80.Orsini L., Spanier K., De Meester L. In press Genomic signature of natural and anthropogenic stress in wild populations of the waterflea Daphnia magna: validation in space, time and experimental evolution. Mol. Ecol. (doi:10.5061/dryad.384rr593) [DOI] [PubMed] [Google Scholar]
- 81.Coulson T., Benton T. G., Lundberg P., Dall S. R. X., Kendall B. E. 2006. Putting evolutionary biology back in the ecological theatre: a demographic framework mapping genes to communities. Evol. Ecol. Res. 8, 1155–1171 [Google Scholar]
- 82.Ellner S. P., Geber M. A., Hairston N. G. 2011. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603–614 10.1111/j.1461-0248.2011.01616.x (doi:10.1111/j.1461-0248.2011.01616.x) [DOI] [PubMed] [Google Scholar]
- 83.Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 10.1111/j.1461-0248.2005.00812.x (doi:10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 84.Urban M. C., et al. 2008. The evolutionary ecology of metacommunities. Trends Ecol. Evol. 23, 311–317 10.1016/j.tree.2008,02.007 (doi:10.1016/j.tree.2008,02.007) [DOI] [PubMed] [Google Scholar]
- 85.Van Doorslaer W., et al. 2009. Local adaptation to higher temperatures reduces immigration success of genotypes from a warmer region in the water flea Daphnia. Glob. Change Biol. 15, 3046–3055 10.1111/j.1365-2486.2009.01980.x (doi:10.1111/j.1365-2486.2009.01980.x) [DOI] [Google Scholar]