Abstract
Contest theory predicts the evolution of a stable mixture of different strategies for fighting. Here, we investigate the possibility that stable between-individual differences in startle-response durations influence fighting ability or ‘resource-holding potential’ (RHP) in the beadlet sea anemone, Actinia equina. Both winners and losers showed significant repeatability of pre-fight startle-response durations but mean pre-fight startle-response durations were greater for eventual losers than for eventual winners, indicating that RHP varies with boldness. In particular, individuals with short startle responses inflicted more attacks on their opponent. Both repeatability and mean-level responses were changed by the experience of fighting, and these changes varied with outcome. In losers, repeatability was disrupted to a greater extent and the mean startle-response durations were subject to a greater increase than in winners. Thus, following a fight, this behavioural correlate of RHP behaves in a way similar to post-fight changes in physiological status, which can also vary between winners and losers. Understanding the links between aggression and boldness therefore has the potential to enhance our understanding of both the evolution of animal personality and the ‘winner and loser effects’ of post-fight changes in RHP.
Keywords: contest, boldness, resource-holding potential, sea anemone, personality, fighting
1. Introduction
Contest behaviour occurs when two or more individuals directly interact during competition over the ownership of a resource and victory in a fight is typically determined by persistence, so that the encounter ends when one individual (the loser) decides to withdraw from the contest and relinquish the resource [1]. An individual's ability to persist in a fight (i.e. its fighting ability) is referred to as its ‘resource-holding potential’ (RHP). The loser might make the decision to withdraw based only on its own ‘absolute’ RHP or, if information about the opponent is available, on its RHP relative to that of the opponent [2]. For fights settled by both types of decision, a range of both morphological and physiological traits have been shown to differ between the winners and losers of fights, indicating that these traits reflect RHP [3]. Thus, the evolution of traits such as large body size or mass (see Arnott & Elwood [2] for a review), large weapons relative to the size of the body (e.g. shore crabs [4] and sea anemones [5]), well-developed musculature [6] and high performance capacities [7,8] can be explained by the need to win fights over access to critical resources. While morphological traits are not expected to change as a result of recent experience of a fight, an individual's physiological state can change dramatically as a result of recent fight experience and these changes can differ markedly between winners and losers (see Briffa & Sneddon [9] for a review). Such physiological consequences of fighting might lead to post-fight changes in behaviour and could explain ‘winner and loser’ effects, whereby RHP is altered as a result of experiencing a particular fight outcome [10–12].
Recently, much interest has focused on ‘animal personality’, the concomitant presence of (i) between-individual differences in behaviour (i.e. ‘inter-individual variation’ [13]) and (ii) relative consistency in these differences (i.e. ‘intra-individual stability’ [13,14]) over time [15] and/or ecological ‘situations’ [16]. Animal personality, defined thus, has been demonstrated in a wide range of phyla including chordates [17], arthropods [18], molluscs [19], cnidarians [20]; and both fighting [1] and personality [17] are widespread across animal taxa. Animal personality may also involve stable correlations between behaviours that are expressed under different functional ‘contexts’ and the phrase ‘behavioural syndromes’ has been used to describe such correlated suites of behaviour [16].
In the context of aggression, the Hawk–Dove game [21] shows how, under certain conditions, natural selection can result in evolutionarily stable alternative strategies of violent ‘hawkish’ behaviour and the ‘dove-like’ use of agonistic displays. The central premise of evolutionary game theory is negative frequency-dependent selection and this idea has recently been invoked more generally [22] (and in the specific context of sexual selection [13]) to offer a potential explanation for the presence of consistent inter-individual differences in behaviour. Indeed, a recent study on the swordtail Xiphophorus helleri demonstrates the presence of consistent between-individual differences in aggressiveness [23]. The presence of ‘behavioural syndromes’ has meanwhile been explained in terms of mechanistic links between behaviours that are displayed in different contexts [16]. For example, the correlation between boldness and aggressiveness in male sticklebacks (Gasterosteus aculeatus) might occur because steroid hormones produced in the testes influence responses to both predators and to rival conspecifics [24]. Regardless of the ultimate or proximate causal factors, both of these aspects of animal personality might be subject to change over time, either as a result of development [14] or as a result of experiencing specific events.
Inter-individual differences and intra-individual stability have been recently demonstrated in startle-response durations in the beadlet sea anemone, Actinia equina, under field conditions [20], a trait which is often described as ‘boldness’ [18,25,26]. Furthermore, aggressive behaviour in sea anemones has been well documented [27–34]. Actinia equina have recently been shown to settle fights using a decision rule based on ‘self-assessment’ [2], whereby an individual's persistence in a contest is based on its own RHP but is not influenced by information about the RHP of the opponent [5]. Contests in A. equina occur over ownership of space on intertidal substrates, and involve the use of organelles called nematocysts [29,35,36]. When contact is made against the epithelium of another anemone, these inject a toxin, capable of causing localized tissue necrosis [37,38]. In A. actinia, these are present in especially dense concentrations in specialized fighting tentacles called ‘acrorhagi’. After inflating its acrorhagi, an anemone will attempt to execute a manoeuvre called ‘overtopping’ in order to bring its acrorhagi into contact with the ectoderm of its rival [35]. A successful strike causes sections of the attacker's acrorhagial ectoderm, termed ‘acrorhagial peels’ [36], to be left behind on the receiver of the attack. Not every encounter, however, involves the use of the acrorhagi and a proportion of fights are settled without injuries at the tentacular contact stage [5]. In fights that do not involve acrorhagial peels, overall body size appears to be the primary morphological driver of RHP [5]. If peels do occur, then RHP appears to be determined by average nematocyst length if only one individual stings its opponent; but if both individuals use the acrorhagi, then it is the ability to strike the opponent (assessed by counting the number of peels deposited) that appears to determine the chance of victory [5]. Thus, morphological traits appear to influence RHP in A. equina, but behavioural differences between opponents can also influence the outcome. Because engaging in these contests can entail receiving damage inflicted by the opponent's nematocysts, we might also expect an individual's experiences during a fight to influence their subsequent behaviour.
While RHP (the ability to win fights) might be dependent on aggressiveness (the propensity to perform agonistic behaviour [39]), this is not necessarily the case, and the two characters are not identical. Previous studies have demonstrated the presence of behavioural syndromes involving correlations between aggressiveness and boldness (e.g. G. aculeatus [24], but see [40,41] for recent examples where these traits are not correlated), and relationships between a composite personality measure and social dominance [42]. As yet, however, no studies have directly investigated the possibility that boldness is an RHP trait. Because A. equina use self-assessment to settle fights [5], they are an ideal study species in which to investigate the role of a potential correlate of absolute RHP. As in the case of other correlates of RHP, this possibility can be investigated by determining whether the trait influences the outcome of an encounter [43]. As described in a recent study [3], the same question can be phrased in terms of asking whether measures of the trait in question differ between winners and losers. Because behavioural syndromes linking boldness to aggressiveness have been identified in a range of study species, we might expect measures of boldness to differ between winners and losers in a way similar to morphological and physiological correlates of RHP. Therefore, the aims of this study were to determine (i) the extent to which startle responses in A. equina are repeatable under standardized conditions, (ii) whether these might be subject to change in mean levels as a result of winning or losing a fight, and (iii) whether initial startle responses (i.e. before entering the fight) vary with aggressiveness (the number of peels inflicted on the opponent) during the fight and differ between eventual winners and losers of fights.
2. Methods
(a). Collecting animals and obtaining startle-response durations
Actinia equina (n = 98) were collected intertidally from Mount Batten (Plymouth, UK; grid reference: SX 48500 53117) between June and July 2010 and transported to the laboratory within 2 h of collection. Only anemones of the red/brown colour morph from the mid/upper shore were used in this study because they have been shown to be more aggressive than green/orange morphs and individuals found on the lower shore [44]. In the laboratory, each anemone was placed on a flat stone and housed individually in a plastic tank containing 700 ml of aerated and filtered sea water at 15 ± 0.5°C. After each anemone had attached to its stone and extended its tentacles, we evoked the first startle response (‘pre-fight 1’) by rapidly discharging a 50 ml syringe filled with sea water into the oral disk from a distance of approximately 2 cm [20]. This caused anemones to retract their tentacles and the duration of the response was timed from the point at which the stimulus was applied to the point at which the anemone re-opened its tentacles fully. The duration was recorded using a stopwatch and then converted into seconds prior to analysis. Care was taken to avoid direct contact between the syringe and any part of the anemone. This procedure was repeated 7 days after the initial stimulus to obtain a second startle-response duration (‘pre-fight 2’). During this 7 day period, the sea water was changed every 2–3 days and the anemones were fed aquaria marine fish flakes every 2–3 days. After 24 h, anemones were used in staged fights (see below) and then after a further 24 h, a third startle response (‘post-fight’) was obtained as above.
(b). Staging fights and obtaining morphological measures
Two anemones, size matched by visual estimation, were selected for each encounter. To check the effectiveness of this method of size matching, we calculated the Pearson's correlation between the masses of the opponents, using the mass measurements obtained after the staged fights (see below for details of the method for obtaining mass measurements). The close correlation (r47 = 0.96, z = 13.3, p < 0.0001) indicates that visual estimation was an effective means of matching opponents for size. Size-matched contests were staged in an attempt to standardize morphological variables that have previously been shown to influence fight outcomes [5]. Both individuals were placed into a new tank as described above, and the tank was placed behind the one-way mirror of an observation chamber. After 1 h, the two anemones were then moved to the centre of the tank such that they were in tentacle contact with each other. This was defined as the starting point of the fight and the fights were considered concluded when one of the contestants either moved away from its opponent by one pedal disk diameter (estimated visually) or retracted all of its tentacles for 10 min [5]. The contest duration was then back-calculated as the time from initial contact to the time at which the loser either first retracted its tentacles or first started moving away from the opponent. At the end of the encounter, the number of acrorhagial peels inflicted on each opponent was counted and fights were classified according to whether or not any peels were inflicted. Samples from acrorhagi that were not used in the contest (i.e. from the opposite side of the anemone to the side that was in contact with the opponent) of each individual were collected using forceps, spread on a glass microscope slide and stained using 1 per cent methylene blue solution [44]. Only undischarged nematocysts were measured because it has been shown that changes in length can occur after discharge [45]. The nematocyst lengths were measured using a binocular, confocal microscope (Leica MZ12) equipped with a Lumenera Infinity 1 camera connected to a computer. The nematocyst lengths were measured using point-to-point measurements (Lumenera Infinity Analyze v. 5.0.3). The nematocyst length of an individual was defined as the mean of 10 randomly selected nematocysts. Following the collection of the acrorhagi, the anemones were placed on an aluminium foil tray, placed in an oven (approx. 65°C) and allowed to dry for 2 days. The dried anemones were weighed using a Fisherbrand PF-203 scale. Dry mass was used instead of wet mass because water volumes stored within the coelenteron may vary considerably between individuals [5].
(c). Statistical methods
To determine the repeatability (i.e. the intra-class correlation coefficient, ICC) of startle-response durations across the three occasions (pre-fight 1, pre-fight 2 and post-fight), we calculated ANOVA-based repeatability (RA) [46], and its standard errors [47]. We determined RA for all individuals combined, for the eventual winners of fights only and for the eventual losers of fights only. To determine whether the experience of being in a fight caused a change in startle-response duration, whether any change in startle-response durations varied between the eventual winners and losers of the fights, and whether any changes were dependent on the aggressive behaviours that occurred during the fight, we used a two-within one-between repeated-measures ANOVA. This is an appropriate analysis for comparing measures taken from winners and losers from within the same fights [3]. The repeated measures were ‘occasion’ (pre-fight 1, pre-fight 2 and post-fight) and outcome (winner, loser), and the between-group factor was ‘type of fight’ (peels, no peels). To determine whether any post-fight changes in mean startle-response duration were responsible for differences in RA between winners and losers, we calculated a series of Pearson's correlation coefficients (r), which provide an appropriate measure of repeatability for data obtained across two occasions [47]. We calculated r for the correlation between pre-fight 1 and pre-fight 2, and for the correlation between pre-fight 2 and post-fight startle-response durations. These analyses were performed for all individuals combined, for the eventual winners of fights only and for the eventual losers of fights only. To determine which traits potentially linked to RHP differed between winners and losers, we performed a series of one-within, one-between repeated measures ANOVAs [3]. The repeated measure was outcome (winner and loser). Because the factors that influence fighting ability in A. equina have previously been shown to vary according to the type of fight that occurred [5], the between-group factor was ‘type of fight’ (peels and no peels). The dependent variables were mean pre-fight startle-response duration ((pre-fight 1 + pre-fight 2)/2), body mass and mean nematocyst length. A paired t-test was used to compare the number of peels received by winners and losers [5], and the Pearson's correlation coefficient was calculated in order to determine whether the number of peels that an individual inflicted on the opponent varied with the average duration of that individual's pre-fight startle responses.
3. Results
(a). The effects of experiencing a fight on startle-response duration
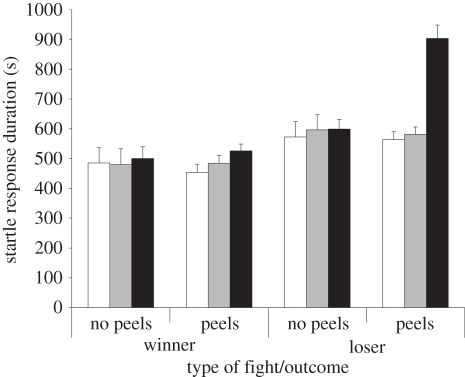
Startle-response duration did not vary between anemones that engaged in the two types of fight (F1,47 = 1.47, p = 0.23), but winners had shorter responses than did losers (F1,47 = 40.64, p < 0.0001), and response durations increased between occasions (F2,94 = 13.85, p = 0.0001). There were significant two-way interactions between outcome and type of fight (F1,47 = 4.1, p = 0.049), occasion and type of fight (F2,94 = 9.75, p = 0.0001), and outcome and occasion (F2,94 = 5.6, p = 0.005). A significant three-way interaction (F2,94 = 5.93, p = 0.004) indicated that the greatest increase in startle-response duration occurred between the pre-fight 2 and post-fight occasions in anemones that lost fights in which there were acrorhagial peels (figure 1).
Figure 1.
The three-way interaction effect between type of fight, outcome and occasion. Prior to fights, startle-response durations were greater for the eventual losers than for the eventual winners. This difference was more marked after fights because of the substantial increase in startle-response durations shown by individuals that lost fights where peels occurred (fights with peels, n = 37; fights without peels, n = 12). Error bars denote standard errors (occasion: white bars, pre-fight 1; grey bars, pre-fight 2; black bars, post-fight).
(b). Repeatability of startle-response duration
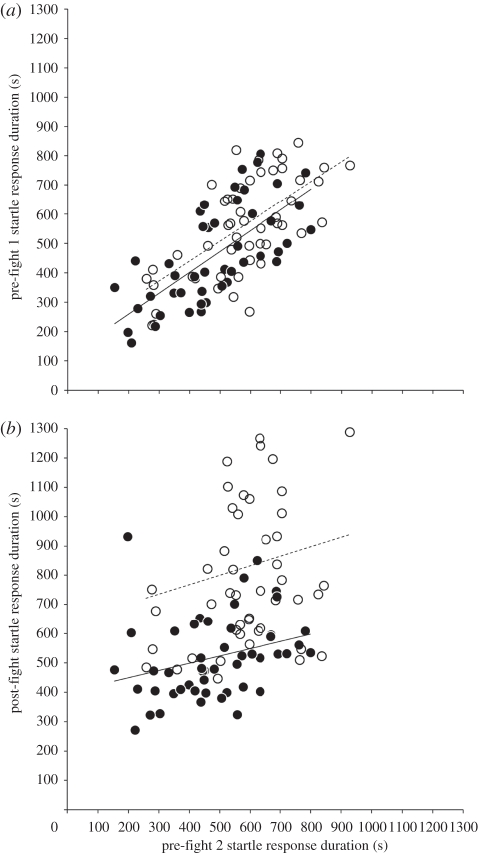
There was significant repeatability in startle-response duration for all individuals (winners and losers) combined (RA = 0.31 ± s.e. = 0.06; F97,196 = 2.35, p < 0.0001) and for winners only (RA = 0.45 ± s.e. = 0.1; F48,98 = 3.47, p < 0.0001), but not for losers (RA = 0.07 ± s.e. = 0.02; F48,98 = 1.22, p = 0.2). For all individuals (winners and losers) combined, there were significant positive correlations between pre-fight 1 and pre-fight 2 (r96 = 0.7, z = 5.12, p < 0.0001; figure 2a), and between pre-fight 2 and post-fight occasions (r96 = 0.34, z = 3.43, p = 0.0006; figure 2b). For winners only, there were significant correlations between pre-fight 1 and pre-fight 2 (r47 = 0.7, z = 5.83, p < 0.0001; figure 2a), and between pre-fight 2 and post-fight occasions (r47 = 0.29, z = 2.03, p = 0.043; figure 2b). For losers only, there was a significant correlation between pre-fight 1 and pre-fight 2 (r47 = 0.64, z = 5.12, p < 0.0001; figure 2a), but there was no significant correlation between pre-fight 2 and post-fight occasions (r47 = 0.18, z = 1.25, p = 0.21; figure 2b).
Figure 2.
Correlations between successive startle-response durations for winners (black circles) and losers (white circles). There were significant correlations for both winners and losers between (a) pre-fight 2 and pre-fight 1 occasions, but the correlation between (b) pre-fight 2 and post-fight occasions was weaker for winners and absent for losers. Trend lines (winners, solid line; losers, dashed line) are added for illustrative purposes.
(c). Differences between winners and losers
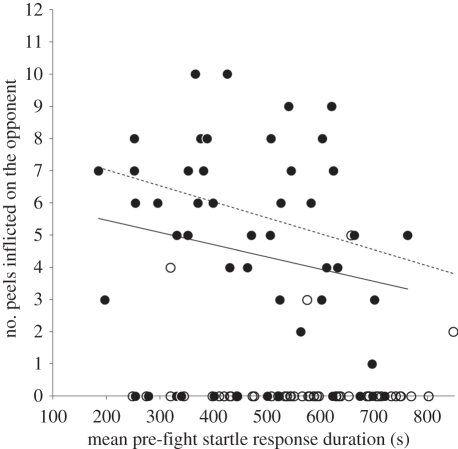
There was no difference in mean pre-fight startle-response duration between fights that did and did not involve peels (F1,47 = 0.1, p = 0.8), but pre-fight startle responses were of shorter duration in winners than in losers (F1,47 = 11.5, p = 0.0014; figure 1). There was no interaction effect between the outcome and the type of fight (F1,47 = 0.001, p = 0.98). There was no difference in mass between fights that did and did not involve peels (F1,47 = 0.1, p = 0.76), but the mass of winners was greater than that of losers (F1,47 = 13, p = 0.0008). There were no significant correlations between startle-response durations and mass (see electronic supplementary material). There was a non-significant trend for an interaction effect (F1,47 = 3.4, p = 0.07) caused by the difference in mass between winners and losers being more marked in fights that did not involve peels compared with fights that did involve peels. There was no difference in mean nematocyst length between fights that did and did not involve peels (F1,47 = 0.3, p = 0.59), nor between winners and losers (F1,47 = 2.6, p = 0.12), and there was no interaction effect between type of fight and outcome on mean nematocyst length (F1,47 = 2.3, p = 0.14). However, a paired t-test comparing mean nematocyst length in winners and losers indicated that, overall, winners had longer nematocysts than did losers (t48 = 2.7, p = 0.009). Winners inflicted more acrorhagial peels on their rival than did losers (t48 = 9.3, p = 0.0001) and, among all individuals, there was a strong negative relationship between the mean duration of the pre-fight startle-response durations and the number of peels inflicted on the opponent (r96 = −0.31, z = 3.12, p = 0.0018); when individuals that did not inflict peels on the opponent (n = 57; 45 losers plus 12 winners) were excluded from the analysis, the correlation remained significant (r39 = −0.36, z = 2.3, p = 0.022; figure 3).
Figure 3.
The significant negative correlation among both winners (black circles) and losers (white circles) between mean pre-fight startle-response duration and the number of peels inflicted on the opponent. Some individuals across the range of observed startle-response durations did not inflict peels on their opponent, and when these are excluded from the analysis the significant correlation remains. The solid trend line illustrates the correlation for all data and the dashed line illustrates the correlation for only those individuals that inflicted peels.
4. Discussion
In a recent study, we demonstrated high repeatability in the startle responses of A. equina under field conditions [20]. This result was indicative of the presence of animal personality in sea anemones but it was also possible that some of the repeatability detected in the field was influenced by extraneous biologically relevant factors, such as differences in micro-habitats and recent experiences that were not controlled for, rather than the ‘internal factors’ that are normally considered to be responsible for animal personality [22]. The present study demonstrates significant repeatability under laboratory conditions, where environmental variables were controlled. Thus, animal personalities are indeed present in A. equina. Moreover, consistent variation in startle-response duration is linked to the ability to inflict peels on the opponent and (as it differed between winners and losers [3]) to RHP.
In another recent study [5], we demonstrated that two morphological variables correlate with fighting success in A. equina. In fights with no acrorhagial stinging, body size is the key driver of RHP, whereas average nematocyst length is the key determinant in fights where only one individual stings. If both individuals inflict peels, however, then it is the opponent that receives the greatest number of peels that makes the decision to withdraw from the contest. Thus, the ability to attack the opponent with the acrorhagi influences the chance of winning in these contests. This behavioural measure of performance during the fight is analogous to the trait of ‘aggressiveness’ that has been described in diverse animal taxa including several fish species (see Conrad et al. [48] for a review); tortoises, Eurotestudo boettgeri [41]; house crickets, Acheta domesticus [49]; and social sea anemones, Anthopleura elegantissima [30]. Here, we show that a similar measure in A. equina is inversely proportional to the mean duration of startle responses measured prior to the fight. Although the agonistic behaviour of the opponent might be expected to modify a focal individual's ability to inflict peels, this key determinant of agonistic success is linked to consistent between-individual differences in startle-response duration. This is similar to the result recently reported for X. helleri [23] that consistent between-individual differences in aggressiveness do not preclude the possibility of plasticity in aggressiveness dependent on the RHP of the opponent.
Winners were bolder than losers in terms of showing shorter startle-response durations. In the case of large body size, muscles or weapons, or high energy reserves, the contribution of these traits to fighting prowess seems obvious. Further studies are required to determine exactly how high boldness might contribute to RHP, but one possibility is that individuals that are quick to recover from a perturbation might have greater opportunities for using their acrorhagi to attack their opponent during a contest, as evidenced by the significant inverse association between startle-response duration and peels inflicted.
Variation in personality was not limited to differences in mean-level responses. When repeatability was calculated across all three occasions, although significant, it was lower than the range reported when anemones were startled over three occasions in the field (0.31 compared with 0.62–0.9 in situ [20]). However, the correlation-based repeatability between the two pre-fight startle-response durations only was 0.64 for eventual losers of fights and 0.7 for the eventual winners, such that in the absence of fighting, repeatability estimates in the laboratory were within the range of those obtained under field conditions. When consecutive measures of startle-response duration were interrupted by a fight, this high repeatability was reduced for winners (0.29) and there was no significant repeatability in the case of losers. Thus, the presence of fighting, especially for losers, appears to disrupt the rank-order of individual startle-response durations. In addition to this post-fight change in repeatability, there was an increase in mean of level startle-response duration, particularly in individuals that lost a fight involving acrorhagial stinging. It was these individuals that received the greatest number of stings during the fight.
While these fight experiences influence subsequent boldness, we have yet to investigate the effects of fighting experience on subsequent fighting behaviour and RHP. However, the post-fight change in startle-response duration indicates that at least one correlate of RHP changes as a result of being in a fight. Moreover, for individuals that lose the fight, startle responses are of longer duration compared with the duration before the fight. As short startle responses are associated with high aggressiveness and with winning, it is reasonable to hypothesize that this change might lead to a reduction in aggressiveness and in RHP during subsequent fights. It is not yet clear why receiving injuries leads to an increase in the duration of post-fight startle responses, but one possible explanation is that increased caution would be beneficial in the presence of hostile competitors, as a strategy for avoiding further injuries. In the current study, we did not assess the longevity of this change in mean startle-response duration or post-contest repeatability in startle-response duration (rather, we assessed repeatability prior to the contest and repeatability of responses on either side of the contest). It is clear, though, that in addition to animal personality, A. actinia show behavioural plasticity in response to engaging in fights, particularly if these fights are highly escalated and involve damage. Studies on a range of taxa [18,23,50] have shown that personality and behavioural plasticity are not mutually exclusive. Thus, post-fight changes in behaviour might contribute to winner and loser effects in other animals where personality is present, and the possibility of causal links between post-fight changes in animal personality and winner and loser effects warrants further investigation.
Here, we have demonstrated that a measure of boldness that shows high within-individual consistency prior to fights changes as a result of the experience of being in a fight. Changes in both mean-level responses and in the stability of differences between individuals are particularly marked in the losers of fights. The pattern of variation in boldness between individuals that experience different fight outcomes shows marked similarities to variation in the physiological correlates of RHP, where a range of factors have been shown to differ between winners and losers [9,11]. Indeed, startle responses and other correlates of RHP (e.g. investment in weapons, mass and energetic status) might be regulated by common physiological mechanisms. The potential for such links has been documented in previous studies [24], and here we show for the first time that boldness can be an RHP trait; not only did startle responses vary between outcomes, but these consistent between-individual differences in boldness varied with fighting prowess (the ability to inflict peels on the opponent) in A. equina. In a recent review, it was argued that sexual selection could contribute to the evolution of animal personality [13]. The fact that boldness can influence RHP indicates that selection for high RHP could also play a role in the evolution of intraspecific variation in behaviour.
Acknowledgements
We are grateful to two anonymous reviewers for their constructive comments on the manuscript.
References
- 1.Briffa M., Sneddon L. U. 2010. Contest behavior. In Evolutionary behavioral ecology (eds Westneat D., Fox C.), pp. 246–265 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Arnott G., Elwood R. W. 2009. Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004 10.1016/j.anbehav.2009.02.010 (doi:10.1016/j.anbehav.2009.02.010) [DOI] [Google Scholar]
- 3.Briffa M., Elwood R. W. 2010. Repeated measures analysis of contests and other dyadic interactions: problems of semantics, not statistical validity. Anim. Behav. 80, 583–588 10.1016/j.anbehav.2010.06.009 (doi:10.1016/j.anbehav.2010.06.009) [DOI] [Google Scholar]
- 4.Sneddon L. U., Huntingford F. A., Taylor A. C. 1997. Weapon size versus body size as a predictor of winning in fights between shore crabs, Carcinus maenas (L.). Behav. Ecol. Sociobiol. 41, 237–242 10.1007/s002650050384 (doi:10.1007/s002650050384) [DOI] [Google Scholar]
- 5.Rudin F. S., Briffa M. 2011. The logical polyp: assessments and decisions during contests in the beadlet anemone Actinia equina. Behav. Ecol. 22, 1278–1285 10.1093/beheco/arr125 (doi:10.1093/beheco/arr125) [DOI] [Google Scholar]
- 6.Mowles S. L., Cotton P. A., Briffa M. 2011. Flexing the abdominals: do bigger muscles make better fighters? Biol. Lett. 7, 358–360 10.1098/rsbl.2010.1079 (doi:10.1098/rsbl.2010.1079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mowles S. L., Cotton P. A., Briffa M. 2009. Aerobic capacity influences giving-up decisions in fighting hermit crabs: does stamina constrain contests? Anim. Behav. 78, 735–740 10.1016/j.anbehav.2009.07.003 (doi:10.1016/j.anbehav.2009.07.003) [DOI] [Google Scholar]
- 8.Mowles S. L., Cotton P. A., Briffa M. 2010. Whole-organism performance capacity predicts resource-holding potential in the hermit crab Pagurus bernhardus. Anim. Behav. 80, 277–282 10.1016/j.anbehav.2010.05.004 (doi:10.1016/j.anbehav.2010.05.004) [DOI] [Google Scholar]
- 9.Briffa M., Sneddon L. U. 2007. Physiological constraints on contest behaviour. Funct. Ecol. 21, 627–637 10.1111/j.1365-2435.2006.01188.x (doi:10.1111/j.1365-2435.2006.01188.x) [DOI] [Google Scholar]
- 10.Hsu Y., Wolf L. 1999. The winner and loser effect: integrating multiple experiences. Anim. Behav. 57, 903–910 10.1006/anbe.1998.1049 (doi:10.1006/anbe.1998.1049) [DOI] [PubMed] [Google Scholar]
- 11.Hsu Y., Earley R. L., Wolf L. L. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. Camb. Philos. Soc. 81, 33–74 10.1017/S146479310500686X (doi:10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 12.Hsu Y. 2001. The winner and loser effect: what fighting behaviours are influenced? Anim. Behav. 61, 777–786 10.1006/anbe.2000.1650 (doi:10.1006/anbe.2000.1650) [DOI] [Google Scholar]
- 13.Schuett W., Tregenza T., Dall S. R. X. 2010. Sexual selection and animal personality. Biol. Rev. Camb. Philos. Soc. 85, 217–246 10.1111/j.1469-185X.2009.00101.x (doi:10.1111/j.1469-185X.2009.00101.x) [DOI] [PubMed] [Google Scholar]
- 14.Stamps J., Groothuis T. G. G. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. Camb. Philos. Soc. 85, 301–325 10.1111/j.1469-185X.2009.00103.x (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 15.Stamps J. A. 2007. Growth–mortality trade-offs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 16.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 17.Gosling S. D., John O. P. 1999. Personality dimensions in nonhuman animals: a cross-species review. Curr. Dir. Psychol. Sci. 8, 69–75 10.1111/1467-8721.00017 (doi:10.1111/1467-8721.00017) [DOI] [Google Scholar]
- 18.Briffa M., Rundle S. D., Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 10.1098/rspb.2008.0025 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinn D. L., Moltschaniwskyj N. A. 2005. Personality traits in dumpling squid (Euprymna tasmanica): context-specific traits and their correlation with biological characteristics. J. Comp. Psychol. 119, 99–110 10.1037/0735-7036.119.1.99 (doi:10.1037/0735-7036.119.1.99) [DOI] [PubMed] [Google Scholar]
- 20.Briffa M., Greenaway J. 2011. High in situ repeatability of behaviour indicates animal personality in the beadlet anemone Actinia equina (Cnidaria). PLoS ONE 6, e21963. 10.1371/journal.pone.0021963 (doi:10.1371/journal.pone.0021963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J. M., Parker G. A. 1976. The logic of asymmetric contests. Anim. Behav. 24, 159–175 10.1016/S0003-3472(76)80110-8 (doi:10.1016/S0003-3472(76)80110-8) [DOI] [Google Scholar]
- 22.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 23.Wilson A. J., de Boer M., Arnott G., Grimmer A. 2011. Integrating personality research and animal contest theory: aggressiveness in the green swordtail Xiphophorus helleri. PLoS ONE 6, e28024. 10.1371/journal.pone.0028024 (doi:10.1371/journal.pone.0028024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntingford F. A. 1976. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 24, 245–260 10.1016/S0003-3472(76)80034-6 (doi:10.1016/S0003-3472(76)80034-6) [DOI] [Google Scholar]
- 25.Budaev S. V. 1997. ‘Personality’ in the guppy (Poecilia reticulata): a correlational study of exploratory behavior and social tendency. J. Comp. Psychol. 111, 399–411 10.1037/0735-7036.111.4.399 (doi:10.1037/0735-7036.111.4.399) [DOI] [Google Scholar]
- 26.Brown C., Jones F., Braithwaite V. 2005. In situ examination of boldness–shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim. Behav. 70, 1003–1009 10.1016/j.anbehav.2004.12.022 (doi:10.1016/j.anbehav.2004.12.022) [DOI] [Google Scholar]
- 27.Turner V. L. G., Lynch S. M., Paterson L., León-Cortés J., Thorpe J. 2003. Aggression as a function of genetic relatedness in the sea anemone Actinia equina (Anthozoa: Actiniaria). Mar. Ecol. Prog. Ser. 247, 85–92 10.3354/meps247085 (doi:10.3354/meps247085) [DOI] [Google Scholar]
- 28.Ayre D. J., Grosberg R. K. 1995. Aggression, habituation, and clonal coexistence in the sea anemone Anthopleura elegantissima. Am. Nat. 146, 427–453 10.1086/285808 (doi:10.1086/285808) [DOI] [Google Scholar]
- 29.Brace R. C., Pavey J., Quicke D. L. J. 1979. Intraspecific aggression in the colour morphs of the anemone Actinia equina: the ‘convention’ governing dominance ranking. Anim. Behav. 27, 553–561 10.1016/0003-3472(79)90190-8 (doi:10.1016/0003-3472(79)90190-8) [DOI] [Google Scholar]
- 30.Ayre D., Grosberg R. 2005. Behind anemone lines: factors affecting division of labour in the social cnidarian Anthopleura elegantissima. Anim. Behav. 70, 97–110 10.1016/j.anbehav.2004.08.022 (doi:10.1016/j.anbehav.2004.08.022) [DOI] [Google Scholar]
- 31.Ayre D. J. 1982. Inter-genotype aggression in the solitary sea anemone Actinia tenebrosa. Mar. Biol. 68, 199–205 10.1007/BF00397607 (doi:10.1007/BF00397607) [DOI] [Google Scholar]
- 32.Purcell J. E., Kitting C. L. 1982. Intraspecific aggression and population distributions of the sea anemone Metridium senile. Biol. Bull. 162, 345–359 10.2307/1540988 (doi:10.2307/1540988) [DOI] [Google Scholar]
- 33.Grosberg R. K., Ayre D. J. 1997. Is there a relationship between multilocus homozygosity and dominance rank in sea anemones? A reply to Zeh and Zeh. Am. Nat. 149, 790–793 10.1086/286022 (doi:10.1086/286022) [DOI] [Google Scholar]
- 34.Zeh J. A., Zeh D. W. 1997. Homozygosity, self-recognition, and aggressive ability in the sea anemone, Anthopleura elegantissima. Am. Nat. 149, 785–793 10.1086/286021 (doi:10.1086/286021) [DOI] [Google Scholar]
- 35.Williams R. B., LeMagnen J., MacLeod P. 1978. Some recent observations on the acrorhagi of sea anemones. J. Mar. Biol. Assoc. UK 58, 787–788 10.1017/S0025315400041448 (doi:10.1017/S0025315400041448) [DOI] [Google Scholar]
- 36.Bigger C. H. 1982. The cellular basis of the aggressive acrorhagial response of sea anemones. J. Morphol. 173, 259–278 10.1002/jmor.1051730303 (doi:10.1002/jmor.1051730303) [DOI] [PubMed] [Google Scholar]
- 37.Nüchter T., Benoit M., Engel U., Ozbek S., Holstein T. W. 2006. Nanosecond-scale kinetics of nematocyst discharge. Curr. Biol. 16, R316–R318 10.1016/j.cub.2006.03.089 (doi:10.1016/j.cub.2006.03.089) [DOI] [PubMed] [Google Scholar]
- 38.Bartosz G., Finkelshtein A., Przygodzki T., Bsor T., Nesher N., Sher D., Zlotkin E. 2008. A pharmacological solution for a conspecific conflict: ROS-mediated territorial aggression in sea anemones. Toxicon 51, 1038–1050 10.1016/j.toxicon.2008.01.017 (doi:10.1016/j.toxicon.2008.01.017) [DOI] [PubMed] [Google Scholar]
- 39.Earley R. L., Hsu Y., Wolf L. L. 2000. The use of standard aggression testing methods to predict combat behaviour and contest outcome in Rivulus marmoratus dyads (Teleostei: Cyprinodontidae). Ethology 106, 743–761 10.1046/j.1439-0310.2000.00586.x (doi:10.1046/j.1439-0310.2000.00586.x) [DOI] [Google Scholar]
- 40.Le Vin A. L., Mable B. K., Taborsky M., Heg D., Arnold K. E. 2011. Individual variation in helping in a cooperative breeder: relatedness versus behavioural type. Anim. Behav. 82, 467–477 10.1016/j.anbehav.2011.05.021 (doi:10.1016/j.anbehav.2011.05.021) [DOI] [Google Scholar]
- 41.Mafli A., Wakamatsu K., Roulin A. 2011. Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann's tortoises. Anim. Behav. 81, 859–863 10.1016/j.anbehav.2011.01.025 (doi:10.1016/j.anbehav.2011.01.025) [DOI] [Google Scholar]
- 42.David M., Auclair Y., Cézilly F. 2011. Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Anim. Behav. 81, 219–224 10.1016/j.anbehav.2010.10.008 (doi:10.1016/j.anbehav.2010.10.008) [DOI] [Google Scholar]
- 43.Hardy I. C. W., Field S. A. 1998. Logistic analysis of animal contests. Anim. Behav. 56, 787–792 10.1006/anbe.1998.0833 (doi:10.1006/anbe.1998.0833) [DOI] [PubMed] [Google Scholar]
- 44.Manuel R. L. 1988. British Anthozoa. London, UK: Academic Press [Google Scholar]
- 45.Godknecht A., Tardent P. 1988. Discharge and mode of action of the tentacular nematocysts of Anemonia sulcata (Anthozoa: Cnidaria). Mar. Biol. 100, 83–92 10.1007/BF00392958 (doi:10.1007/BF00392958) [DOI] [Google Scholar]
- 46.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. The Auk 2, 116–121 [Google Scholar]
- 47.Nakagawa S., Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 85, 935–956 10.1111/j.1469-185X.2010.00141.x (doi:10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 48.Conrad J. L., Weinersmith K. L., Brodin T., Saltz J. B., Sih A. 2011. Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J. Fish Biol. 78, 395–435 10.1111/j.1095-8649.2010.02874.x (doi:10.1111/j.1095-8649.2010.02874.x) [DOI] [PubMed] [Google Scholar]
- 49.Wilson A. D. M., Whattam E. M., Bennett R., Visanuvimol L., Lauzon C., Bertram S. M. 2009. Behavioral correlations across activity, mating, exploration, aggression, and antipredator contexts in the European house cricket, Acheta domesticus. Behav. Ecol. Sociobiol. 64, 703–715 10.1007/s00265-009-0888-1 (doi:10.1007/s00265-009-0888-1) [DOI] [Google Scholar]
- 50.Rieucau G., Morand-Ferron J., Giraldeau L. A. 2010. Group size effect in nutmeg mannikin: between-individuals behavioral differences but same plasticity. Behav. Ecol. 21, 684–689 10.1093/beheco/arq039 (doi:10.1093/beheco/arq039) [DOI] [Google Scholar]