Abstract
The ectomycorrhizal (ECM) symbiosis is the most widespread biotrophic nutritional mode in mushroom-forming fungi. ECM fungi include, though are not limited to, about 5000 described species of Agaricales from numerous, independently evolved lineages. Two central hypotheses suggest different explanations for the origin of ECM fungal diversity: (i) dual origins, initially with the Pinaceae in the Jurassic and later with angiosperms during the Late Cretaceous, and (ii) a simultaneous and convergent radiation of ECM lineages in response to cooling climate during the Palaeogene and advancing temperate ECM plant communities. Neither of these hypotheses is supported here. While we demonstrate support for asynchronous origins of ECM Agaricales, the timing of such events appears to have occurred more recently than suggested by the first hypothesis, first during the Cretaceous and later during the Palaeogene. We are also unable to reject models of rate constancy, which suggests that the diversity of ECM Agaricales is not a consequence of convergent rapid radiations following evolutionary transitions from saprotrophic to ECM habits. ECM lineages of Agaricales differ not only in age, but also in rates of diversification and rate of substitution at nuclear ribosomal RNA loci. These results question the biological uniformity of the ECM guild.
Keywords: ancestral state reconstruction, Bayesian analysis, dated phylogenies, incomplete taxon sampling, plant–fungal mutualism
1. Introduction
Mutualistic associations between organisms impact ecosystems in major ways and perform a central role in the evolution of many diverse groups [1]. The mycorrhizal symbiosis is one such mutualism whereby plants receive mineralized nutrients, increased absorption of water and protection from root parasites in exchange for providing photosynthate to their mycorrhizal fungal partners [2]. Indeed, the symbiosis was important for the colonization of land by plants [2–5]. The ancestral form of mycorrhizas in plants, the arbuscular mycorrhiza (AM), is evident from 460 million year (Myr) old fossils [3,6] and is thought to have evolved once among fungi in the phylum Glomeromycota. In contrast, another frequent type of mycorrhizal symbiosis, the ectomycorrhiza (ECM), evolved more recently and independently on numerous occasions in separate lineages of fungi [6,7]. It has been estimated that around 7750 described fungal species are ECM [8], making this artificial group considerably more diverse than the AM Glomeromycota, with around 170 described species or an estimated 1000 species [9,10]. The oldest ECM fossil, however, is only about 55 Myr old [11], while the Pinaceae, a plant family almost exclusively forming ECM, is much older (about 200 Myr) [12–14]. This implies that the ECM symbiosis should also be at least 200 Myr old. Unfortunately, the evolutionary history of ECM fungi within a geological timeline is poorly known and fraught with challenges such as a weak fossil record [15–17].
The diversity of any group of organisms depends on its age and rate of diversification through time. Niche theory predicts that each species must occupy a separate niche and, assuming a finite niche space, species diversification is expected to slow down through time as various niches are filled. Indeed, decreasing diversification rates have been observed for many groups of animals [18–20]. In mushroom-forming fungi, the ECM habit is derived from saprotrophic ancestors [7,21,22]. If a transition to ECM habit represents a switch to a new niche space followed by a rapid adaptive radiation filling these niches, then analyses of phylogenetic patterns should detect slowdowns in diversification rate after initial bursts of diversification. Transitions from saprotrophy to biotrophy in what are today many large and species-rich ECM clades could then be interpreted as adaptive and support the evolution of the ECM habit as a key innovation or a feature promoting diversification in response to ecological opportunities for fungi. However, other factors, such as geological and climatic changes, may have a larger impact on diversification rates than niche limitations [23]. The Cenozoic encompassed large climatic and vegetation changes with a general cooling trend since the Eocene thermal maximum (approx. 50 Myr ago) [24]. The cooling climate of the Cenozoic has been accompanied by an expansion of temperate ECM plant communities [25]. These changes have resulted in increasing areas of ECM habitats and new environments for ECM fungi to occupy.
Recent genomic comparisons between two deeply unrelated ECM fungi—Laccaria bicolor (Basidiomycota) and Tuber melanosporum (Ascomycota)—have highlighted major differences in genes used to establish and maintain the ECM symbiosis. These differences may suggest that the ECM condition represents a syndrome of variable traits and that ECM fungi share fewer functional similarities in their molecular ‘toolboxes’ than anticipated [26]. Because such key differences exist, estimates of diversification rates and nucleotide substitution rates of ECM fungi could differ between lineages despite sharing a similar nutritional mode and lifestyle.
The Agaricales is the largest order of Agaricomycetes, with some 13 200 described species [9], and includes at least 10 ECM lineages that account for more than half of the estimated fungal ECM species diversity [8,21]. Each of these ECM lineages has been shown to have sister clades that are non-ECM or sister to other ECM lineages nested in otherwise non-ECM clades [21,27,28]. This large inclusive group containing multiple unrelated ECM lineages is therefore suitable for testing hypotheses on ECM evolution. Here, we wish to evaluate two hypotheses previously proposed in the literature concerning the origin and diversification of ECM lineages: the first suggests that some fungal ECM lineages began to diversify during the Jurassic, together with the Pinaceae, followed by diversification of additional lineages during the Cretaceous, along with ECM angiosperms [29]. We refer to this as the ‘dual origin’ hypothesis. In contrast, Bruns et al. [30] suggested that ECM lineages began to diversify simultaneously and more recently, during and after the Late Eocene/Oligocene, when major ECM plant communities expanded during cooling climates. We refer to this second hypothesis as the ‘convergent radiation’ hypothesis.
Here we produce ultrametric trees for 10 lineages of ECM Agaricales, all within a geological time frame, to test the dual origin and convergent radiation hypotheses. Model-based methods are used to determine whether diversification rates differ between lineages and through time. The gamma statistic [31] is used to test for slowdowns in diversification with respect to simulations. The new programs SubT and DivBayes [32] are used to take into account incomplete taxon sampling when estimating rates of diversification in a Bayesian context. In addition, we use ancestral state reconstruction methods to infer ancestral plant associations (Pinaceae or angiosperm) for each ECM fungal lineage. The dual origin hypothesis predicts that older ECM clades should be ancestrally associated with Pinaceae and that younger ECM clades should be ancestrally associated with angiosperms, whereas the convergent radiation hypothesis should be neutral in this regard.
2. Material and methods
(a). Dataset compilation
Data matrices for the phylogenetic analyses of each lineage comprised DNA sequences of the internal transcribed spacer (ITS) or the nuclear large subunit (LSU) ribosomal RNA, the most commonly used gene regions in fungal systematics, environmental assays and fungal community studies [33]. We examined lineages of Agaricales considered to be ECM by Tedersoo et al. [7], with the following exceptions: Lyophyllum was excluded since the genus is not monophyletic [34], and the trophic status for most species is uncertain; Entoloma was excluded as we were unable to pinpoint any specific clades as ECM in a preliminary analysis; and Phaeocollybia was included as its δ15N and δ13C stable isotopic signatures are consistent with a biotrophic habit [35], and pseudopodia and hyphae from sporocarps have been traced to roots where ECM structures including Hartig nets were formed [36].
The Web tool emerencia [37,38] was used to download all ITS sequences available on GenBank associated with Hydnangiaceae, Descolea (including gasteroid allies), Hebelomateae, Tricholoma, Phaeocollybia and Cortinarius, including sequences with and without complete species annotations. Using the same principles as emerencia, LSU sequences for Catathelasma, Hygrophorus, Amanita and Inocybaceae were extracted from GenBank [39] using a local software pipeline written in Perl (script available at http://www.bio.utk.edu/matheny/Site/Alignments_%26_Data_Sets.html). For Inocybaceae, we also added 119 LSU sequences generated in-house according to protocols described earlier [40,41] (GenBank accession numbers JN974916–JN975034). Operational taxonomic units (OTUs) were formed for all lineages using single-linkage clustering [42] according to Ryberg & Matheny [43], based on phylogenetic distances (expected number of substitutions per site) in a tree generated in RAxML [44] (GTR + Γ + invariable sites). Cut-off values for each lineage are given in the electronic supplementary material. One sequence was kept for each OTU while the rest were excluded from the alignment to avoid sequence redundancies. Sequences that were unlikely to belong to the respective lineage were pruned from the alignment following Nilsson et al. [45]. Alignments were constructed in Mafft [46] using guide trees generated in RAxML [44] according to the algorithm of Ryberg & Matheny [43]. In Amanita, two OTUs forming the sister group to the rest of Amanita were regarded as outgroup taxa, and were excluded from the age and diversification rate estimates, since they are not ECM [7]. The DNA data matrices used for the phylogenetic analysis contained between 7 and 797 taxa (including outgroups) and 675–1526 characters. This represents between 20 and 96 per cent of the estimated number of described species of each lineage [9] (table 1).
Table 1.
The estimated number of described species in each lineage and the number of included species/OTUs; the probability that the lineage originated during a specific geological epoch and period; the probability (p) that the plant association is independent of the phylogeny averaged over the posterior tree distribution; and the BF support for the phylogenetic over the non-phylogenetic model as two times the difference in log BF (negative values indicate support for the non-phylogenetic model) are also shown. A difference in two times log BF greater than two is considered positive support.
| ECM lineage of Agaricales | number of species/OTUs |
Cretaceous crown group origin (p) |
Palaeogene crown group origin (p) |
independence of plant association and phylogeny |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| estimateda | included | early | late | total | Palaeocene | Eocene | Oligocene | total | p-value | ΔBF | |
| Amanita | 500 | 99 | 0.08 | 0.85 | 0.93 | 0.06 | 0.01 | 0 | 0.07 | 0.28 | 2.5 |
| Catathelasma | 4 | 2 | 0.07 | 0.52 | 0.59 | 0.19 | 0.18 | 0.02 | 0.39 | —c | —d |
| Cortinariusb | 2011 | 796 | 0 | 0.35 | 0.35 | 0.32 | 0.32 | 0 | 0.65 | 0 | 55.0 |
| Descoleab | 30 | 27 | 0 | 0.04 | 0.04 | 0.11 | 0.68 | 0.15 | 0.95 | —c | −0.4 |
| Hebelomateae | 281 | 117 | 0 | 0.01 | 0.01 | 0.09 | 0.78 | 0.11 | 0.98 | 0.26 | 15.6 |
| Hydnangiaceae | 76 | 37 | 0 | 0.03 | 0.03 | 0.07 | 0.62 | 0.26 | 0.95 | 0.37 | −1.7 |
| Hygrophorus | 100 | 21 | 0.27 | 0.55 | 0.83 | 0.11 | 0.06 | 0 | 0.17 | 0.01 | 4.7 |
| Inocybaceae | 507 | 489 | 0 | 0.20 | 0.20 | 0.39 | 0.41 | 0 | 0.80 | 0 | 10.3 |
| Phaeocollybia | 50 | 23 | 0 | 0.16 | 0.16 | 0.30 | 0.52 | 0.02 | 0.84 | —c | 0.2 |
| Tricholoma | 200 | 78 | 0.01 | 0.64 | 0.65 | 0.26 | 0.09 | 0 | 0.35 | 0.12 | 1.2 |
aAccording to Kirk et al. [9].
bIncluding gasteroid allies.
cClades with no or only one taxon with deviating plant association.
dClade where phylogenetic and non-phylogenetic model is identical.
(b). Phylogenetic analyses and lineage ages
Chronograms were reconstructed using BEAST v. 1.4.8 [47]. Four separate Markov chain Monte Carlo (MCMC) runs were performed for each lineage. Each chain was run for 60 million generations and sampled every 6000th generation. Models of molecular evolution incorporated six possible transformation rates (GTR). The proportion of invariable sites and four discrete rate categories generated from a Γ distribution were estimated to accommodate rate heterogeneity. The Yule process was used as a prior on node heights, and an uncorrelated lognormal distribution was used for the relaxed molecular clock. Normally distributed age priors for splits between ingroup and outgroup taxa for each lineage were inferred from age estimates of corresponding splits in the Basidiomycota chronogram of Ryberg & Matheny [43] (TreeBASE : S11162:Tr27334). We evaluated chain stationarity graphically using Tracer v. 1.5 [48] and by comparing split frequencies between runs in AWTY [49]. In the BEAST analyses, 20 million generations were excluded as a burn-in phase. One of the four chains for Inocybaceae was excluded since it had a mean log-likelihood score more than 10 points lower than the others; the rest of the analyses of this lineage are based on three chains. Matrices and consensus trees are available in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S11395).
The probability that a lineage originated in a certain time period was determined by the proportion of trees in the posterior distribution that had the crown node within that period. The probability that all lineages originated in a certain time period, and the probability that at least one lineage originated in a certain time period, were calculated assuming independent age estimates between lineages.
(c). Ancestral state reconstruction
The plant association (Pinaceae, angiosperm, generalist) of each OTU was derived from metadata provided in GenBank, the study in which the sequences originated or (for OTUs associated with a species name) from the taxonomic literature (e.g. [50]). Ancestral state reconstruction on the ingroup of each lineage was performed using parsimony in Mesquite [51] and Bayesian methods using BayesTraits (www.evolution.rdg.ac.uk) [52]. Each run in BayesTraits included 1.3 million generations sampled every 1000 generations with 300 000 generations discarded as the burn-in. The reversible jump Markov chain option was used to average over models [53]. Effective sample size values of the likelihood score and model parameters were determined in CODA [54]; graphically, we confirmed that log-likelihood scores had levelled off.
To evaluate if plant–host association is evolutionarily conserved, we compared Bayes factors (BFs) between runs on the observed posterior tree distribution as generated in BEAST (phylogenetic model) and on a distribution of star-shaped trees (non-phylogenetic model) [55–57]. The distribution of star-shaped trees was generated by setting the internal branch lengths to zero and tip branches equal to the distance between the root and tips of the tree for each tree in the posterior distribution. Catathelasma was not tested as it only has two available OTUs, and the phylogenetic and non-phylogenetic models are identical. For Cortinarius, the tree distributions were thinned to half the original sizes owing to computer memory constraints. The R package PHANGORN [58] was used to evaluate if ancestral host preference was randomly distributed among the tips of the trees of each lineage or evolutionarily conserved according to parsimony criteria. The observed parsimony score of each tree in the posterior distribution was compared with the parsimony score of 100 trait randomizations on that tree [59,60].
(d). Diversification and substitution rate differences between lineages
The mean substitution rate and rate distribution between branches for each lineage were estimated simultaneously with the phylogeny in BEAST v. 1.4.8. Posterior distributions were summarized in Tracer v. 1.5. Diversification rates were estimated from node heights under the Yule model using Bayesian statistics in SubT v. 1.0 [32]. Missing taxa (estimated number of taxa minus included taxa; table 1) were accounted for using ‘substitute taxa’ as described by Ryberg et al. [32]. A MCMC with 10 million generations, sampling every 10 000 generations, was implemented for every tenth tree of the posterior distribution as estimated in BEAST. The parameters for each lineage were averaged over the thinned posterior distribution of trees.
DivBayes v. 1.0 [32] was used to compare a model with equal diversification rates for all clades to models with different diversification rates using clade ages and number of species per clade. The number of species for each clade follows table 1, and a normally distributed prior estimated from the tree distribution in BEAST was used for clade stem ages. Eleven different models were tested (see electronic supplementary material). A MCMC chain with 100 million generations, sampled every 10 000 generations, was used to estimate the posterior distribution for each model. The different models were compared using BF as estimated from the harmonic means of the log-likelihood scores of the MCMC. A difference of more than two was taken as positive support for the model with highest BF [61].
We also investigated differences in rates between lineages within a clade using the B1 statistic and the Colless statistic [62], calculated using R, and by stepwise AIC in MEDUSA [63] as implemented in GEIGER [64]. MEDUSA was performed on the maximum clade credibility (MCC) tree and a difference in AIC of four was used as the cut-off for significance (the default). As we have no specific information on the position of ‘substitute taxa’ within each lineage, missing taxa were not assigned to tips of the MCC tree in this analysis. The probability of the B1 and Colless statistics observed for each tree in the posterior tree distribution was estimated from a null distribution generated by simulating 10 000 trees under full taxon sampling using the Yule model in GEIGER but where taxa had been randomly removed to match the observed number of taxa. The probability for the posterior distribution was calculated as the average probability of each tree.
(e). Analysing diversification through time
We excluded Catathelasma from analyses on rate variation through time since only two OTUs were available for this lineage. Different models of diversification (table 2) were optimized for each tree in the posterior distribution as estimated in BEAST under the ML criterion using LASER [65]. A time period equal to the OTU cut-off value (see electronic supplementary material) divided by the mean substitution rate (table 3) was excluded from the tips of the tree to account for ambiguity in OTU delimitation. The difference in AIC between the model with the highest mean AIC value for the posterior tree distribution and the Yule model (the model with lowest number of parameters) was calculated for each tree in the posterior distribution. A null distribution of differences in AIC scores was calculated for the same two models on 10 000 trees simulated as above. The gamma statistic was calculated in LASER for all trees in the posterior distribution. The null distribution was estimated from 10 000 trees with incomplete taxon sampling simulated as above [31].
Table 2.
Gamma values for trees sampled in the posterior distribution and simulated null distribution, Akaike information criteria (AIC) for five models, and the difference between the model with the lowest AIC score and the Yule model (ΔAIC). A simulated null distribution for the difference between these two models is also given. All values are means with standard deviations in parentheses.
| ECM lineage of Agaricales | gamma value |
AIC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| observed | simulated | Yule | birth–death | slowdowna | Yule 2 rate | Yule 3 rate | ΔAIC | ΔAIC simulated | |
| Amanita | 0.88 (0.69) | −3.31 (0.94) | 81.4 (23.5) | 82.1 (23.8) | 84.0 (23.5) | 81.5 (23.9) | 80.7 (23.8)b | 0.44 (2.67) | 8.79 (5.64) |
| Cortinariusc | −4.20 (0.79) | −6.24 (0.96) | −3718.6 (266.1) | −3716.6 (266.1) | −3734.2 (265.9) | −3734.5 (266.0) | −3739.0 (265.8)b | 19.53 (6.78) | 28.87 (10.0) |
| Descoleac | 0.76 (0.65) | −0.08 (1.00) | 50.4 (8.5)b | 51.4 (8.8) | 52.3 (8.5) | 51.4 (8.9) | 51.1 (8.9) | 0 | 0 |
| Hebelomateae | 1.50 (0.75) | −2.21 (0.96) | −94.6 (45.2) | −95.8 (45.6) | −92.6 (45.1) | −97.6 (45.7) | −99.0 (45.7)b | 3.85 (3.96) | 5.05 (4.44) |
| Hydnangiaceae | −1.05 (0.96) | −0.55 (1.02) | 53.7 (14.4) | 55.7 (14.5) | 52.9 (14.2) | 52.6 (14.3) | 50.7 (14.7)b | 2.48 (3.61) | 1.22 (3.03) |
| Hygrophorus | −1.11 (0.8) | −1.06 (0.97) | 80.4 (9.3) | 82.3 (9.3) | 79.2 (9.0) | 79.7 (9.0) | 78.9 (9.4)b | 1.16 (3.06) | 1.53 (3.29) |
| Inocybaceae | −1.88 (0.70) | −0.13 (1.01) | −1309.1 (135.4) | −1307 (135.4) | −1308.3 (135.6) | −1312.0 (135.2) | −1312.8 (135.4)b | 2.89 (3.33) | 2.52 (3.15) |
| Phaeocollybia | 1.28 (0.84) | −0.76 (0.96) | 57.2 (8.0) | 56.3 (9.3) | 59.1 (8.0) | 53.6 (9.8) | 52.1 (9.7)b | 4.62 (3.99) | 1.39 (3.21) |
| Tricholoma | 0.05 (0.67) | −1.54 (1.00) | 82.1 (21.5) | 83.8 (21.6) | 83.8 (21.3) | 83.0 (21.4) | 81.8 (21.5)b | 0.27 (2.41) | 3.21 (3.84) |
aDDL model in LASER.
bLowest AIC score for that lineage.
cIncluding gasteroid allies.
Table 3.
Mean nucleotide substitution rates and species diversification rates. Mean and 95% credibility intervals (in parentheses) for the posterior distributions are provided. Diversification rates are averaged over every tenth tree of the posterior tree distribution.
| ECM lineage of Agaricales | mean nucleotide substitution rate (substitutions per billion years) | net diversification rate according to a Yule model (speciations per lineage per million years) |
|---|---|---|
| Amanita | 1.52 (1.11–2.00)a | 0.08 (0.06–0.11) |
| Catathelasma | 0.32 (0.23–0.42)a | 0.03 (0.01–0.08) |
| Cortinariusc | 5.84 (3.96–7.92)b | 0.14 (0.10–0.20) |
| Descoleac | 2.50 (1.52–3.64)b | 0.07 (0.04–0.13) |
| Hebelomateae | 2.59 (1.62–3.70)b | 0.14 (0.09–0.22) |
| Hydnangiaceae | 1.42 (0.849–2.05)b | 0.09 (0.05–0.14) |
| Hygrophorus | 0.62 (0.38–0.91)a | 0.04 (0.03–0.08) |
| Inocybaceae | 2.64 (1.79–3.60)a | 0.09 (0.06–0.12) |
| Phaeocollybia | 4.61 (2.92–6.45)b | 0.08 (0.05–0.13) |
| Tricholoma | 3.66 (2.57–4.86)b | 0.06 (0.04–0.09) |
aBased on nuclear large subunit of ribosomal DNA (LSU).
bBased on ITS1-5.8S-ITS2.
cIncluding gasteroid allies.
3. Results and discussion
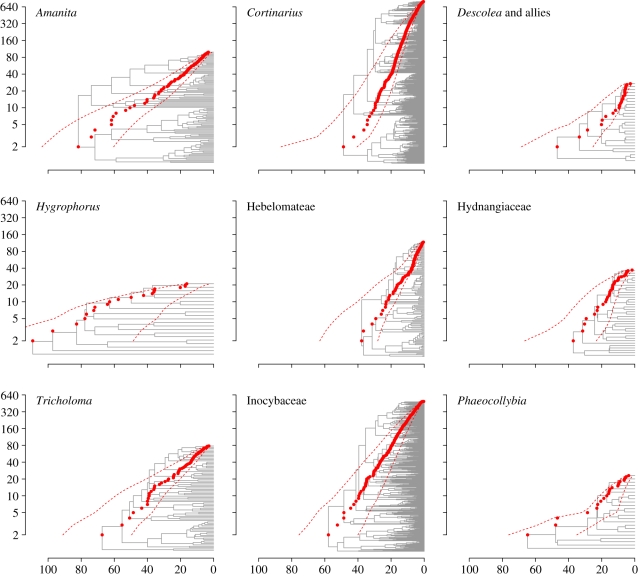
ECM lineages of Agaricales differ significantly in evolutionary rates and have crown group origins in at least two different geological epochs of the Mesozoic and Cenozoic (figure 1; trees including tip information are presented in the electronic supplementary material). In addition, we failed to detect any strong evidence of niche limitation on net rates of diversification. Our results suggest that at least two temporally separate crown group origins of ECM Agaricales occurred, one during the Late Cretaceous and one during the Eocene (p = 0.004; table 1). Owing to uncertainties in clade age estimates, however, we cannot confidently conclude that ECM Agaricales diversified during more than two epochs. Stem ages provide less support for origins during two distinct epochs but do support origins during the Cretaceous and Palaeogene. It is only for Hygrophorus that a Jurassic stem origin cannot be rejected (p = 0.19; electronic supplementary material). As the exact sister group is not known with confidence for many clades [21,27,28], the stem ages here may be overestimated for several groups, which may also account for some of the higher variation in stem origins than crown origins. While it is clear that not all ECM lineages share a concentrated origin during the Palaeogene (p < 0.01), as predicted by the ‘convergent radiation’ hypothesis [30], there is some support that all six ECM lineages of the Agaricoid clade, one of several major subgroups of Agaricales, radiated simultaneously during the Palaeogene (table 1) [21].
Figure 1.
Species accumulation curves (in red) and MCC trees (in grey) for nine ECM lineages of Agaricales (Catathelasma excluded). Red dots represent the species accumulation curve for the MCC tree and dashed red lines represent a 95% credibility interval for the age of each node depth. The x-axis gives the age in million years, and the y-axis the number of species (log-scaled). The six lineages in the Agaricoid clade are presented in the centre and right-hand side columns.
The ‘dual origin’ hypothesis predicts that older lineages should be ancestrally associated with Pinaceae and younger lineages with angiosperms. A prerequisite to investigate this prediction using ancestral state reconstruction is that plant association is dependent on evolutionary history [66]. Randomization tests and comparison of phylogenetic and non-phylogenetic models were used to investigate if fungal ECM plant association at the level of angiosperm, Pinaceae or generalist association is correlated with the phylogeny. The randomization test only supported that plant association is conserved in Cortinarius, Hygrophorus and Inocybaceae (table 1). However, the comparisons of phylogenetic and non-phylogenetic models also show positive support (difference in 2 × log BF > 2) for evolutionarily conserved plant associations in Amanita and Hebelomateae. Three of the five lineages where the plant association was not indicated as evolutionarily conserved have a very low variation in host association. All extant species of Catathelasma associate with Pinaceae; all Descolea and allies (Descomyces, Setchelliogaster, Timgrovea) associate with angiosperms; and only one of the included Phaeocollybia species is associated with angiosperms, while the rest associate with Pinaceae (indeed, most species of Phaeocollybia are Pinaceae associates [67]). With no variation in plant association, the specific position in the tree of a taxon does not matter as they all have the same plant association. If only one taxon differs, the change will be restricted to one branch. This led to the same parsimony score regardless of the phylogenetic position of the taxon. It also produced little difference in likelihood between the phylogenetic and non-phylogenetic model. Nevertheless, the low variation in plant association observed in Phaeocollybia and Descolea is highly unlikely given the distribution of plant association over all groups (p < 0.01). Unknown aspects of their biology or constraints in dispersal in these groups may restrict their general plant association. The failure to reject independence of plant association from the phylogeny for Hydnangiaceae and Tricholoma may be owing to lack of power, and these results should be re-evaluated in light of denser taxon sampling.
Our inferred clade ages of ECM Agaricales are younger than the Pinaceae and in most cases younger or comparable in age with ECM angiosperms [14,68], results inconsistent with the ‘dual origin’ hypothesis [29]. In the parsimony ancestral state reconstruction, Hygrophorus, which is relatively old (table 1), is inferred to be associated with Pinaceae, the younger Cortinarius and Inocybaceae are inferred to be originally associated with angiosperms (electronic supplementary material), lending some support to the notion that older ECM lineages originated with Pinaceae. The Bayesian analyses neither support nor reject that older lineages are associated with Pinaceae, as we could not infer high probabilities for any particular plant association at the root (highest median PP is 0.65 for generalist association at the root for Hebelomateae). The reason that the original association cannot be confidently inferred in the model-based method is probably owing to the relatively high transformation rates between states. The inferred median transformation rates, for lineages where the plant association was inferred to be evolutionarily conserved, varied between 0.00 and 0.21 changes Myr−1, with the median highest rate between specific states varying between 0.01 and 0.21 changes Myr−1. The large difference in transformation rates between groups and the fact that plant association is completely conserved in some groups suggest that there may be differences in how plant association is constrained in different fungal ECM lineages.
Diversification rates vary significantly between ECM fungal lineages, which indicate that lineage-specific factors are important to determine rates of diversification. When accounting for missing taxa using SubT, Cortinarius and Hebelomateae exhibit the fastest net diversification rate (speciation rate—extinction rate), four times higher than the clade with the slowest rate, Catathelasma (p < 0.05; table 3). When comparing models that permit different diversification rates for different sets of lineages using the number of species and stem age for each lineage in DivBayes, a model implementing three different rate categories (1: Cortinarius, Hebelomateae and Inocybaceae; 2: Amanita, Descolea and allies, Hydnangiaceae, Phaeocollybia and Tricholoma; 3: Catathelasma and Hygrophorus) is best supported among tested alternatives. This rate-variable model receives strong support as a better fit than models with equal diversification rates for all lineages (difference in two times BFs (2 × Δ log BF) > 6) and positive support as a better fit than any other tested model (2 × Δ log BF > 2; electronic supplementary material).
Unfortunately, it is not clear which morphological, anatomical or molecular traits, if any, have promoted changes in diversification rates of ECM Agaricales. The six ECM lineages that are nested in the Agaricoid clade [21] are all among the seven lineages with the highest mean diversification rate estimate, the seventh being Amanita. All these seven lineages occur in clades characterized by a multi-nucleate basidiospore condition, whereas the other three clades (Hygrophorus, Catathelasma and Tricholoma) have predominantly uninucleate spores [67,69,70]. Heterokaryotic spores have been suggested to facilitate establishment of individuals after long-distance dispersal through self-fertilization [69,71], possibly facilitating the establishment of separate populations. However, most species of the Agaricoid clade and Amanita are probably homokaryotic [69]. It should be noted that the diversification rate of Tricholoma is not significantly lower than for Amanita and four of the lineages in the Agaricoid clade. It remains to be explored whether the entire Agaricoid clade is characterized by a high diversification rate, or if it is the combination with the ECM habit that results in high diversification rates. This is a topic worthy of further research.
We find that substitution rates vary considerably both among and within clades. The mean substitution rate at the ITS locus for six ECM lineages ranged between 1.4 and 5.8 substitutions per site per billion years, with non-overlapping 95% credibility intervals between extremes. These rates fall within the same range as previously estimated for this region in fungi [72,73]. Nucleotide substitution rates of LSU are estimated between 0.32 and 2.6 substitutions per site per billion years, with non-overlapping 95% credibility intervals between the two with highest and the two with lowest rates (table 3). The LSU rate of Takamatsu & Matsuda [72] lies between the lowest and highest rates in this study. Substitution rate variation between branches was similar for the 10 lineages, with a mean standard deviation for the lognormal distribution of the relaxed molecular clock ranging between 0.46 and 0.68; the 95% credibility intervals overlapped among all lineages. Generally, substitution rates may correlate with several life-history traits. In animals and plants, shorter generation times and herbaceous habit often yield faster substitution rates [74]. Life-history traits for ECM fungi are difficult to study as most of them cannot be actively cultivated.
We found no evidence for slowdowns in diversification rate in any of the ECM lineages, indicating that the diversification of ECM fungi has not been niche-limited. In contrast, rates of diversification seem to have been relatively constant through time. The mean gamma values from the posterior tree distributions were higher than the mean gamma values for simulated trees in six of nine lineages (Catathelasma excluded; see §2). The gamma values for the posterior tree distributions were not significantly lower than the simulated null distribution in any lineage taking into account incomplete taxon sampling (table 2). The slowdown model (DDL or logistic variant of the density-dependent speciation rate model) was not indicated as most suitable for any lineage. Rather, a model implementing separate diversification rates during three different periods (3-rate model) had the lowest average AIC [75] scores for eight of the nine lineages. The first rate was not supported as the highest for the majority of the trees in the posterior distribution for any of the eight lineages, and no general trend for shifts in diversification rates could be inferred. Furthermore, no lineage had higher support for the 3-rate model than what can be expected under random variation of a constant-rate model (table 2). As a consequence, we find no significant support for variation in diversification rates of ECM Agaricales through time.
Measures of tree symmetry did not show significant variation in diversification rates within any of the nine lineages (Catathelasma excluded; see §2). However, there was a tendency towards asymmetric trees in the Inocybaceae using the B1 statistic (p = 0.068) [62]. MEDUSA [63] indicated two rate changes in Cortinarius, and one rate change each in Hebelomateae and Phaeocollybia, with subclades having higher rates of diversification (see electronic supplementary material). The inferred increases in diversification rate of separate clades within Cortinarius, Hebelomateae and Phaeocollybia could bias against a slowdown in diversification rates in these taxa [76].
The lack of evidence for evolutionary slowdowns suggests that transitions to the ECM habit did not promote rapid radiations into new niche spaces. This implies that fungal diversification is not niche-limited and may depend on allopatry or parapatry for speciation and coexistence [77,78]. However, the radiation can still be adaptive, but far from filling all niches, and it is possible that a continuous diversification is facilitated by an expanding niche space enabled by cooling climate and expansion of ECM temperate forests after the Eocene thermal maximum. Density-dependent patterns may also be compromised by structured extinctions.
Acknowledgements
This manuscript benefitted from comments on earlier versions by Elizabeth Sheedy and two anonymous reviewers. The work was supported by National Science Foundation award DEB-0949517 to P.B.M. and N. L. Bougher, and the Hesler Endowment Fund. We thank the University of Tennessee, College of Arts and Sciences, Office of Research, and the Department of Ecology and Evolutionary Biology for providing additional means of support. Geri Ragghianti provided assistance with use of the Newton cluster at the University of Tennessee.
References
- 1.Margulis L., Fester R. 1991. Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. Cambridge, MA: MIT Press; [PubMed] [Google Scholar]
- 2.Smith S. E., Read D. 2008. Mycorrhizal symbiosis, 3rd edn. London, UK: Academic Press (Elsevier) [Google Scholar]
- 3.Redecker D. 2000. Glomalean fungi from the Ordovician. Science 289, 1920–1921 10.1126/science.289.5486.1920 (doi:10.1126/science.289.5486.1920) [DOI] [PubMed] [Google Scholar]
- 4.Wang B., Yeun L. H., Xue J.-Y., Liu Y., Ané J.-M., Qiu L. 2010. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol. 186, 514–525 10.1111/j.1469-8137.2009.03137.x (doi:10.1111/j.1469-8137.2009.03137.x) [DOI] [PubMed] [Google Scholar]
- 5.Pirozynski K., Malloch D. 1975. The origin of land plants: a matter of mycotrophism. Biosystems 6, 153–164 10.1016/0303-2647(75)90023-4 (doi:10.1016/0303-2647(75)90023-4) [DOI] [PubMed] [Google Scholar]
- 6.Wang B., Qiu L. 2006. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16, 299–363 10.1007/s00572-005-0033-6 (doi:10.1007/s00572-005-0033-6) [DOI] [PubMed] [Google Scholar]
- 7.Tedersoo L., May T. W., Smith M. E. 2010. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20, 217–263 10.1007/s00572-009-0274-x (doi:10.1007/s00572-009-0274-x) [DOI] [PubMed] [Google Scholar]
- 8.Rinaldi A., Comandini O., Kuyper T. 2008. Ectomycorrhizal fungal diversity: separating the wheat from the chaff. Fungal Divers. 33, 1–45 [Google Scholar]
- 9.Kirk P., Cannon P., Minter D., Stalpers J. 2008. Dictionary of fungi, 10th edn. Wallingford, UK: CAB International [Google Scholar]
- 10.Kivlin S. N., Hawkes C. V., Treseder K. K. 2011. Global diversity and distribution of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 43, 2294–2303 10.1016/j.soilbio.2011.07.012 (doi:10.1016/j.soilbio.2011.07.012) [DOI] [Google Scholar]
- 11.LePage B. A., Currah R. S., Stockey R. A., Rothwell G. W. 1997. Fossil ectomycorrhizae from the Middle Eocene. Am. J. Bot. 84, 410–412 10.2307/2446014 (doi:10.2307/2446014) [DOI] [PubMed] [Google Scholar]
- 12.Wang X. Q., Tank D. C., Sang T. 2000. Phylogeny and divergence times in Pinaceae: evidence from three genomes. Mol. Biol. Evol. 17, 773–781 [DOI] [PubMed] [Google Scholar]
- 13.LePage B. A. 1999. The evolution, biogeography and palaeoecology of the Pinaceae based on fossil and extant representatives. ISHS Acta Hortocult. 615, 29–52 [Google Scholar]
- 14.Clarke J. T., Warnock R. C. M., Donoghue P. C. J. 2011. Establishing a time-scale for plant evolution. New Phytol. 192, 266–301 10.1111/j.1469-8137.2011.03794.x (doi:10.1111/j.1469-8137.2011.03794.x) [DOI] [PubMed] [Google Scholar]
- 15.Cairney J. W. 2000. Evolution of mycorrhiza systems. Die Naturwissenschaft. 87, 467–475 10.1007/s001140050762 (doi:10.1007/s001140050762) [DOI] [PubMed] [Google Scholar]
- 16.Matheny P. B., et al. 2009. Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J. Biogeogr. 36, 577–592 10.1111/j.1365-2699.2008.02055.x (doi:10.1111/j.1365-2699.2008.02055.x) [DOI] [Google Scholar]
- 17.Berbee M. L., Taylor J. W. 2010. Dating the molecular clock in fungi—how close are we? Fungal Biol. Rev. 24, 1–16 10.1016/j.fbr.2010.03.001 (doi:10.1016/j.fbr.2010.03.001) [DOI] [Google Scholar]
- 18.Rüber L., Zardoya R. 2005. Rapid cladogenesis in marine fishes revisited. Evolution 59, 1119–1127 [PubMed] [Google Scholar]
- 19.McPeek M. A. 2008. The ecological dynamics of clade diversification and community assembly. Am. Nat. 172, E270–E284 10.1086/593137 (doi:10.1086/593137) [DOI] [PubMed] [Google Scholar]
- 20.Phillimore A. B., Price T. D. 2008. Density-dependent cladogenesis in birds. PLoS Biol. 6, e71. 10.1371/journal.pbio.0060071 (doi:10.1371/journal.pbio.0060071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matheny P. B., et al. 2006. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98, 982–995 10.3852/mycologia.98.6.982 (doi:10.3852/mycologia.98.6.982) [DOI] [PubMed] [Google Scholar]
- 22.Hibbett D. S., Matheny P. B. 2009. The relative ages of ectomycorrhizal mushrooms and their plant hosts estimated using Bayesian relaxed molecular clock analyses. BMC Biol. 7, 13. 10.1186/1741-7007-7-13 (doi:10.1186/1741-7007-7-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steeman M. E., et al. 2009. Radiation of extant cetaceans driven by restructuring of the oceans. Syst. Biol. 58, 573–585 10.1093/sysbio/syp060 (doi:10.1093/sysbio/syp060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachos J., Pagani M., Sloan L., Thomas E., Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 10.1126/science.1059412 (doi:10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 25.Tallis J. H. 1991. Plant community history: long-term changes in plant distribution and diversity, 1st edn. London, UK: Chapman and Hall [Google Scholar]
- 26.Plett J. M., Martin F. 2011. Blurred boundaries: lifestyle lessons from ectomycorrhizal fungal genomes. Trends Genet. 27, 14–22 10.1016/j.tig.2010.10.005 (doi:10.1016/j.tig.2010.10.005) [DOI] [PubMed] [Google Scholar]
- 27.Binder M., Larsson K.-H., Matheny P. B., Hibbett D. S. 2009. Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102, 865–880 10.3852/09-288 (doi:10.3852/09-288) [DOI] [PubMed] [Google Scholar]
- 28.Garnica S., Weiss M., Walther G., Oberwinkler F. 2007. Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycol. Res. 111, 1019–1029 10.1016/j.mycres.2007.03.019 (doi:10.1016/j.mycres.2007.03.019) [DOI] [PubMed] [Google Scholar]
- 29.Halling R. E. 2001. Ectomycorrhizae: co-evolution, significance, and biogeography. Ann. Miss. Bot. Garden 88, 5–13 10.2307/2666128 (doi:10.2307/2666128) [DOI] [Google Scholar]
- 30.Bruns T. D., et al. 1998. A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Mol. Ecol. 7, 257–272 10.1046/j.1365-294X.1998.00337.x (doi:10.1046/j.1365-294X.1998.00337.x) [DOI] [Google Scholar]
- 31.Pybus O. G., Harvey P. H. 2000. Testing macro-evolutionary models using incomplete molecular phylogenies. Proc. R. Soc. Lond. B 267, 2267–2272 10.1098/rspb.2000.1278 (doi:10.1098/rspb.2000.1278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryberg M., Nilsson R. H., Matheny P. B. 2011. DivBayes and SubT: exploring species diversification using Bayesian statistics. Bioinformatics 27, 2439–2440 10.1093/bioinformatics/btr405 (doi:10.1093/bioinformatics/btr405) [DOI] [PubMed] [Google Scholar]
- 33.Horton T. R., Bruns T. D. 2001. The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol. Ecol. 10, 1855–1871 10.1046/j.0962-1083.2001.01333.x (doi:10.1046/j.0962-1083.2001.01333.x) [DOI] [PubMed] [Google Scholar]
- 34.Hofstetter V., Clémençon H., Vilgalys R., Moncalvo M. 2002. Phylogenetic analyses of the Lyophylleae (Agaricales, Basidiomycota) based on nuclear and mitochondrial rDNA sequences. Mycol. Res. 106, 1043–1059 10.1017/S095375620200641X (doi:10.1017/S095375620200641X) [DOI] [Google Scholar]
- 35.Trudell S. A., Rygiewicz P. T., Edmonds R. L. 2004. Patterns of nitrogen and carbon stable isotope ratios in macrofungi, plants and soils in two old-growth conifer forests. New Phytol. 164, 317–335 10.1111/j.1469-8137.2004.01162.x (doi:10.1111/j.1469-8137.2004.01162.x) [DOI] [PubMed] [Google Scholar]
- 36.Norvell L. L. 1998. Observations on development, morphology and biology in Phaeocollybia. Mycol. Res. 102, 615–630 10.1017/S0953756297005431 (doi:10.1017/S0953756297005431) [DOI] [Google Scholar]
- 37.Ryberg M., Kristiansson E., Sjökvist E., Nilsson R. H. 2009. An outlook on the fungal internal transcribed spacer sequences in GenBank and the introduction of a web-based tool for the exploration of fungal diversity. New Phytol. 181, 471–477 10.1111/j.1469-8137.2008.02667.x (doi:10.1111/j.1469-8137.2008.02667.x) [DOI] [PubMed] [Google Scholar]
- 38.Nilsson R. H., Kristiansson E., Ryberg M., Larsson H. 2005. Approaching the taxonomic affiliation of unidentified sequences in public databases: an example from the mycorrhizal fungi. BMC Bioinform. 6, 178. 10.1186/1471-2105-6-178 (doi:10.1186/1471-2105-6-178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson D. A., Karsch-Mizrachi I., Lipman D. J., Ostell J., Sayers E. W. 2010. GenBank. Nucl. Acids Res. 39, 32–37 10.1093/nar/gkq1079 (doi:10.1093/nar/gkq1079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Judge B. S., Ammirati J. F., Lincoff G. H., Trestrail J. H., Matheny P. B. 2010. Ingestion of a newly described North American mushroom species from Michigan resulting in chronic renal failure: Cortinarius orellanosus. Clin. Toxicol. 48, 545–549 10.3109/15563650.2010.495346 (doi:10.3109/15563650.2010.495346) [DOI] [PubMed] [Google Scholar]
- 41.Matheny P. B. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 35, 1–20 10.1016/j.ympev.2004.11.014 (doi:10.1016/j.ympev.2004.11.014) [DOI] [PubMed] [Google Scholar]
- 42.Jain A. K., Murty M. N., Flynn P. J. 1999. Data clustering: a review. ACM Comput. Surv. 31, 264–323 10.1145/331499.331504 (doi:10.1145/331499.331504) [DOI] [Google Scholar]
- 43.Ryberg M., Matheny P. B. 2011. Dealing with incomplete taxon sampling and diversification of a large clade of mushroom-forming fungi. Evolution 65, 1862–1878 10.1111/j.1558-5646.2011.01251.x (doi:10.1111/j.1558-5646.2011.01251.x) [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 10.1093/bioinformatics/btl446 (doi:10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 45.Nilsson R. H., Ryberg M., Sjökvist E. 2011. Rethinking taxon sampling in the light of environmental sequencing. Cladistics 27, 197–203 10.1111/j.1096-0031.2010.00336.x (doi:10.1111/j.1096-0031.2010.00336.x) [DOI] [PubMed] [Google Scholar]
- 46.Katoh K., Asimenos G., Toh H. 2009. Bioinformatics for DNA sequence analysis. Methods Mol. Biol. 537, 39–64 10.1007/978-1-59745-251-9 (doi:10.1007/978-1-59745-251-9) [DOI] [PubMed] [Google Scholar]
- 47.Drummond A. J., Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214. 10.1186/1471-2148-7-214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rambaut A., Drummond A. J. 2007. Tracer v. 1.4. See http://beast.bio.ed.ac.uk/Tracer/ref
- 49.Nylander J. A. A., Wilgenbusch J. C., Warren D. L., Swofford D. L. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24, 581–583 10.1093/bioinformatics/btm388 (doi:10.1093/bioinformatics/btm388) [DOI] [PubMed] [Google Scholar]
- 50.Knudsen H., Vesterholt J. 2008. Funga Nordica: agaricoid, boletoid, and cyphelloid genera, 1st edn. Copenhagen, Denmark: Nordsvamp [Google Scholar]
- 51.Maddison W. P., Maddison D. R. 2010. Mesquite: a modular system for evolutionary analysis. Version 2.75. See http://mesquiteproject.org [Google Scholar]
- 52.Pagel M., Meade A., Barker D. 2004. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 53, 673–684 10.1080/10635150490522232 (doi:10.1080/10635150490522232) [DOI] [PubMed] [Google Scholar]
- 53.Pagel M., Meade A. 2006. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am. Nat. 167, 808–825 10.1086/503444 (doi:10.1086/503444) [DOI] [PubMed] [Google Scholar]
- 54.Plummer M., Best N., Cowls K., Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7–11 [Google Scholar]
- 55.Mooers A. Ø., Vamosi S. M., Schluter D. 1999. Using phylogenies to test macroevolutionary hypotheses of trait evolution in cranes (Gruinae). Am. Nat. 154, 249–259 10.1086/303226 (doi:10.1086/303226) [DOI] [PubMed] [Google Scholar]
- 56.Vanderpoorten A., Goffinet B. 2006. Mapping uncertainty and phylogenetic uncertainty in ancestral character state reconstruction: an example in the moss genus Brachytheciastrum. Syst. Biol. 55, 957–971 10.1080/10635150601088995 (doi:10.1080/10635150601088995) [DOI] [PubMed] [Google Scholar]
- 57.Ryberg M., Larsson E., Jacobsson S. 2010. An evolutionary perspective on morphological and ecological characters in the mushroom family Inocybaceae (Agaricomycotina, Fungi). Mol. Phylogenet. Evol. 55, 431–442 10.1016/j.ympev.2010.02.011 (doi:10.1016/j.ympev.2010.02.011) [DOI] [PubMed] [Google Scholar]
- 58.Schliep K. P. 2010. phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 10.1093/bioinformatics/btq706 (doi:10.1093/bioinformatics/btq706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddison W., Slatkin M. 1991. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45, 1184–1197 10.2307/2409726 (doi:10.2307/2409726) [DOI] [PubMed] [Google Scholar]
- 60.Faith D., Cranston P. 1991. Could a cladogram this short have arisen by chance alone? On permutation tests for cladistic structure. Cladistics 7, 1–28 10.1111/j.1096-0031.1991.tb00020.x (doi:10.1111/j.1096-0031.1991.tb00020.x) [DOI] [Google Scholar]
- 61.Kass R. E., Raftery A. E. 1995. Bayes factors. J. Am. Stat. Assoc. 90, 773–795 10.2307/2291091 (doi:10.2307/2291091) [DOI] [Google Scholar]
- 62.Kirkpatrick M., Slatkin M. 1993. Searching for evolutionary patterns in the shape of a phylogenetic tree. Evolution 47, 1171–1181 10.2307/2409983 (doi:10.2307/2409983) [DOI] [PubMed] [Google Scholar]
- 63.Alfaro M. E., Santini F., Brock C., Alamillo H., Dornburg A., Rabosky D. L., Carnevale G., Harmon L. J. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106, 13 410–13 414 10.1073/pnas.0811087106 (doi:10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harmon L. J., Weir J. T., Brock C. D., Glor R. E., Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131 10.1093/bioinformatics/btm538 (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 65.Rabosky D. L. 2006. LASER: a maximum likelihood toolkit for detecting temporal shifts in diversification rates from molecular phylogenies. Evol. Bioinform. Online 2, 257–260 [PMC free article] [PubMed] [Google Scholar]
- 66.Losos J. B. 2011. Seeing the forest for the trees: the limitations of phylogenies in comparative biology. Am. Nat. 177, 709–727 10.1086/660020 (doi:10.1086/660020) [DOI] [PubMed] [Google Scholar]
- 67.Singer 1986. The Agaricales in modern taxonomy, 4th ed. Königstein, Germany: Koeltz Scientific Books [Google Scholar]
- 68.Bell C. D., Soltis D. E., Soltis P. S. 2010. The age and diversification of the angiosperms re-revisited. Am. J. Bot. 97, 1296–1303 10.3732/ajb.0900346 (doi:10.3732/ajb.0900346) [DOI] [PubMed] [Google Scholar]
- 69.Horton T. R. 2006. The number of nuclei in basidiospores of 63 species of ectomycorrhizal homobasidiomycetes. Mycologia 98, 233–238 10.3852/mycologia.98.2.233 (doi:10.3852/mycologia.98.2.233) [DOI] [PubMed] [Google Scholar]
- 70.Kühner R. 1977. Variation of nuclear behaviour in the homobasidiomycetes. Trans. Br. Mycol. Soc. 68, 1–16 10.1016/S0007-1536(77)80145-9 (doi:10.1016/S0007-1536(77)80145-9) [DOI] [Google Scholar]
- 71.Vellinga E. C., Wolfe B. E., Pringle A. 2009. Global patterns of ectomycorrhizal introductions. New Phytol. 181, 960–973 10.1111/j.1469-8137.2008.02728.x (doi:10.1111/j.1469-8137.2008.02728.x) [DOI] [PubMed] [Google Scholar]
- 72.Takamatsu S., Matsuda S. 2004. Estimation of molecular clocks for ITS and 28S rDNA in Erysiphales. Mycoscience 45, 340–344 10.1007/s10267-004-0187-7 (doi:10.1007/s10267-004-0187-7) [DOI] [Google Scholar]
- 73.Kasuga T., White T. J., Taylor J. W. 2001. Letter to the editor: estimation of nucleotide substitution rates in eurotiomycete fungi. Mol. Biol. Evol. 19, 2318–2324 [DOI] [PubMed] [Google Scholar]
- 74.Bromham L. 2009. Why do species vary in their rate of molecular evolution? Biol. Letters 5, 401–404 10.1098/rsbl.2009.0136 (doi:10.1098/rsbl.2009.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723 10.1109/TAC.1974.1100705 (doi:10.1109/TAC.1974.1100705) [DOI] [Google Scholar]
- 76.Rabosky D. L. 2009. Extinction rates should not be estimated from molecular phylogenies. Evolution 4, 1816–1824 10.1111/j.1558-5646.2009.00926.x (doi:10.1111/j.1558-5646.2009.00926.x) [DOI] [PubMed] [Google Scholar]
- 77.Gittenberger E. 1991. What about non-adaptive radiation? Biol. J. Linn. Soc. 43, 263–272 10.1111/j.1095-8312.1991.tb00598.x (doi:10.1111/j.1095-8312.1991.tb00598.x) [DOI] [Google Scholar]
- 78.Rundell R. J., Price T. D. 2009. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol. Evol. 24, 394–399 10.1016/j.tree.2009.02.007 (doi:10.1016/j.tree.2009.02.007) [DOI] [PubMed] [Google Scholar]