Abstract
Adaptive radiations, bouts of morphological divergence coupled with taxonomic proliferation, underpin biodiversity. The most widespread model of radiations assumes a single round, or ‘early burst’, of elevated phenotypic divergence followed by a decline in rates of change or even stasis. A vertebrate-specific model proposes separate stages: initial divergence in postcranial traits related to habitat use, followed by diversification in cranial morphology linked to trophic demands. However, there is little empirical evidence for either hypothesis. Here, we show that, contrary to both models, separate large-scale radiations of actinopterygian fishes proceeded through distinct cranial and later postcranial stages of morphological diversification. Early actinopterygians and acanthomorph teleosts dispersed in cranial morphospace immediately following the end-Devonian extinction and the Cretaceous origin of the acanthomorph clade, respectively. Significant increases in postcranial morphological variation do not occur until one interval after cranial diversification commenced. Therefore, our results question the universality of the ‘general vertebrate model’. Based on the results of model-fitting exercises and application of the divergence order test, we find little evidence that the early onset of cranial diversification in these two radiations is due to elevated rates of cranial change relative to postcranial change early in their evolutionary histories. Instead, postcranial and cranial patterns are best fit by an Ornstein–Uhlenbeck model, which is characterized by constant evolutionary rates coupled with a strong central tendency. Other groups have been reported to show early saturation of cranial morphospace or tropic roles early in their histories, but it is unclear whether these patterns are attributable to dynamics similar to those inferred for our two model radiations.
Keywords: Actinopterygii, ecomorphology, cladogenesis, biodiversity, disparity, mass extinction
1. Introduction
Most models of adaptive radiation assume rapid initial divergence marked by coincident change among all traits followed by stasis [1–4]. This hypothesis was historically informed by patterns in the fossil record, yet is now tested extensively using phylogenies of extant groups and individual or paired traits (e.g. body size and ecotype) [2–5]. These studies attempt to detect a single ‘burst’ of divergence in the focal trait, with the implicit assumption that other features might show similar evolutionary patterns [2–6].
A set of alternative models—variously referred to as ‘radiation-in-stages’, the ‘habitat first rule’ and even the ‘general vertebrate model’—hold that adaptive radiations proceed as a sequence of ordered divergences along separate phenotypic lines [2,7,8]. This view has a long pedigree, implied in Osborn's identification of evolution along distinct feeding and locomotor trajectories in the type adaptive radiation: placental mammals [5]. Under the ‘general vertebrate’/stage model as originally proposed [7], divergence occurs first in habitat preference (defined by changes in body shape and size related to locomotion), then along trophic lines (defined by changes in skull and jaw morphology related to feeding) and finally through sexual selection (defined by coloration, sexual dimorphism and other traits).
Both the staged and single peak hypotheses assume an ‘early burst’ (EB) in rates of postcranial shape change, yet this is not supported by some recent phylogenetic analyses of model clades, including Galapagos finches, Caribbean anoles (but see Mahler et al. [9]) and African cichlids [3,8]. Further confounding matters, the only direct test of the stage model failed to recover evidence for distinct intervals of feeding and locomotor diversification in labrid fishes (wrasses) [10]. Detection of radiation patterns might require temporal information in conjunction with morphological and phylogenetic data, as subsequent events can overwrite earlier change [3,6,11]. Unfortunately, most model radiations have poor fossil records, and ecomorphological signal can be difficult to detect in extinct organisms [2,3].
Ray-finned fishes (Actinopterygii) are a diverse group of jawed vertebrates with a 400-Myr record of articulated fossil material, and have been the target of considerable research focused on extracting functional and ecological correlates of skeletal anatomy in recent taxa [12]. Actinopterygian evolutionary history contains multiple radiation events; particularly striking are episodes of diversification associated with major biotic crises in the Earth's history. Sallan & Coates [13] recognized a large increase in early actinopterygian richness following the end-Devonian Hangenberg extinction [14]. Friedman [15] showed that acanthomorph teleosts, a clade containing both cichlids and labrids, increased in taxonomic diversity and morphological variety following their appearance in the Late Cretaceous and after the end-Cretaceous extinction [16]. It has been argued that both events likely involved the invasion of new adaptive space and/or ecological release as a function of extinction-related competitor loss [1,13,15,17]. Here, we examine patterns of morphological diversification that we define as changes in levels of morphological variation within a clade over time, in order to test the generality of proposed models of adaptive radiation. We also subject these data to analyses intended to reveal the evolutionary dynamics responsible for producing observed phenotypic patterns.
2. Material and methods
(a). Morphospace construction and quantification of morphological diversity
In order to establish the timing of morphological divergence, we constructed separate cranial and postcranial morphospaces for early actinopterygians from the Middle Devonian through Mississippian epochs (391.8–318.1 Ma) [18] and acanthomorph teleosts from their first appearance in the Late Cretaceous through the Miocene (99.6–5.3 Ma) [18]. Cranial landmarks were assigned to capture aspects of the skull related to trophic function in modern fishes, such as overall shape, linkages between elements and jaw morphology [7,10,15,19–21]. Postcranial landmarks were placed to capture lateral body shape and fin morphologies, which are related to locomotion in living fishes and are thought to correspond to habitat use at both large and small spatial scales [7,10,15,19,22–24]. Thus, while the landmark schemes summarize morphology, they might also reflect patterns of ecological and functional variation [24].
Early actinopterygian morphospaces were constructed from 69 published lateral reconstructions of actinopterygian taxa. Taxa were binned by geological stage with the exception of Devonian forms, which were combined in a single interval. Some reconstructions were based on taxa with missing data; however, analyses excluding these species produced identical results. Seventy homologous two-dimensional landmarks and sliding semilandmarks were digitized in ImageJ [25], with equal numbers assigned to the crania and postcrania. Cranial landmarks were assigned to capture the morphology of the jaws, opercular series, orbits and overall dimensions in lateral view, all of which are likely affected by trophic adaptations [7,19–21]. Postcranial landmarks describe the lateral body outline and shape as well as relative positions of the fins.
Acanthomorph morphospaces were constructed from 22 homologous two-dimensional landmarks and sliding semilandmarks originally digitized by Friedman [15], using the Tps package [26], for 605 taxa with articulated specimens. Similar to Friedman [15], these taxa were binned into six multi-stage intervals, of which the first four are relevant to our analyses. The four cranial landmarks capture important areas of muscle insertion (supraoccipital crest), points of movement (craniovertebral and jaw joints) and together summarize overall shape of the skull, including relative gape [20,21]. The 18 postcranial landmarks and sliding semilandmarks capture lateral body shape, the length and position of median fins, and the shape and size of the caudal fin. The difference in the number of early actinopterygian and acanthomorph taxa over similar time spans reflects either the ‘pull of the recent’ (an increase in fossil record quality and completeness in younger strata) or a genuine increase in ray-finned fish richness over time [27,28]. Anatomical correlates of significant relative warps (RWs) generated by all analyses are described in the electronic supplementary material.
Taxa were Procrustes aligned using the procedures described by Friedman [15]. RWs for both datasets were generated using tpsRelw v. 1.45 with default settings [26] (figure 1; electronic supplementary material). Warp scores for individual taxa in each dataset are available from the authors. We quantified morphological diversity, or disparity [29], as multi-variate variance (sum of univariate variances on retained ordination axes [15,30]). We estimated 95% confidence intervals for each temporal bin from 10 000 bootstrapped pseudoreplicates produced in R [15,31] (figure 2; electronic supplementary material). One- and two-tailed tests were performed using t-statistics calculated from bootstrapped estimates of standard error in order to evaluate hypotheses of directional and adirectional changes in disparity between successive bins [32]. In addition, likelihood ratios were calculated for each transition using the procedure of Finarelli and Flynn [33], with values greater than 8 considered to indicate substantial shifts in morphological diversity [15,31,33] (electronic supplementary material).
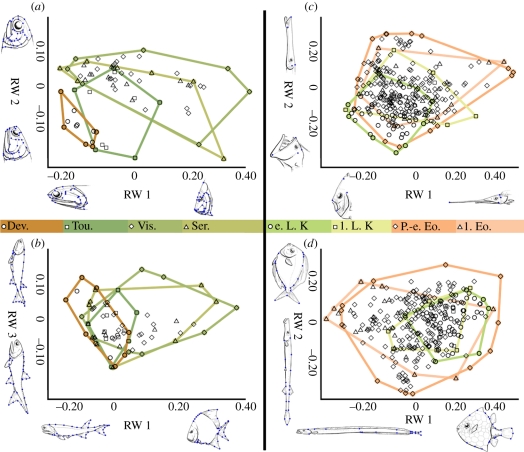
Figure 1.
Morphospaces. (a) Early actinopterygian cranial morphospace (n = 69). (b) Early actinopterygian postcranial morphospace (n = 69). Relative warp (RW) 3 is shown for clarity. RW 2 is driven by difference between the two eel-like tarrasiid genera, both of which exhibit highly divergent body forms, and all other taxa in a more constricted version of their distribution along RW3. When the Tarrasiidae are excluded, the morphospce and disparity results are identical except for exclusion of that RW (electronic supplementary material). (c) Acanthomorph cranial morphospace (n = 304). (d) Acanthomorph postcranial morphospace (n = 304). Convex hulls colour-coded by interval according to the official colour scheme of the International Commission on Stratigraphy.
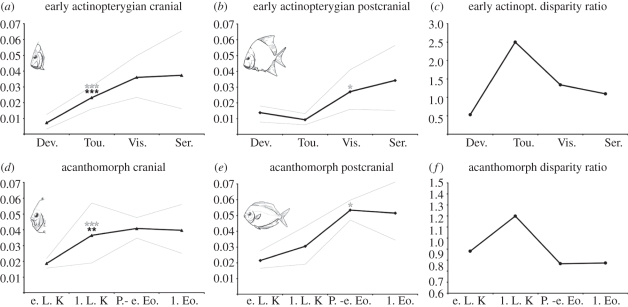
Figure 2.
Mean disparity curves. (a) Early actinopterygian cranial disparity curve (n = 69). (b) Early actinopterygian postcranial disparity curve (n = 69). (c) Ratio between early actinopterygian cranial and postcranial disparity over time. (d) Early actinopterygian cranial disparity curve (n = 304). (e) Early actinopterygian postcranial disparity curve (n = 304). (f) Ratio between early actinopterygian cranial and postcranial disparity over time. Thin lines represent 95% confidence intervals. Check marks indicate significant likelihood ratios for change since the previous interval. White asterisks indicate one-tailed significance (testing the directional hypothesis of no increase in morphological diversity). Black asterisks indicate two-tailed significance (testing the adirectional hypothesis of no change in morphological diversity). Significance levels are denoted using asterisks as follows: 0.05 (*), 0.01 (**) and 0.001 (***) (electronic supplementary material).
(b). Phylogenetic comparative methods and model fitting
Supertrees were constructed for early actinopterygians and acanthomorphs (electronic supplementary material). Polytomies were randomly resolved for each round of model fitting (electronic supplementary material). Branch lengths were determined by randomly assigning an absolute age to each terminal taxon from a designated range specified by geological data, with a minimum length of 1 Ma for all internal and terminal branches. Each of the models of trait evolution discussed in the following section was fitted to the early actinopterygian and acanthomorph dataset with maximum likelihood using the software package geiger [34] as implemented in R [31]. Models were fitted independently for scores along each RW. This procedure was repeated for each of the topologies (early actinopterygians, n = 1000; acanthomorphs, n = 100) produced by our procedure for randomly resolving soft polytomies (electronic supplementary material).
Our analyses draw on three statistically explicit evolutionary models, first developed for the study of living radiations: Brownian motion (BM), Ornstein–Uhlenbeck (OU) and Pagel's δ [35–37]. These were fit to individual significant RWs (electronic supplementary material). BM is the most basic model, representing gradual change at a constant rate, resulting in pure diffusive drift. It assumes a constant-variance random walk in which all trait changes are equiprobable. OU adds a central tendency to BM, representing some source of constraint, adaptive peak or other factor enforcing a normal distribution during all trait evolution. Potential for further dispersion is inversely related to the distance between the current trait value and the optimum, θ, tempered by selection strength, α, for the optimum. Pagel's δ is another elaboration of basic BM that includes an additional parameter, δ, which is used to as a power value for rescaling node heights within the tree. The resultant value of δ indicates whether morphological change is concentrated early (δ < 1) or late (δ > 1) in the history of a clade, and thus whether evolutionary rates increased or decreased over time [36] (electronic supplementary material). In a situation where δ is 1, this model is equivalent to BM. Therefore, Pagel's δ is similar to the ‘EB’ model developed by Harmon et al. [3] and the ‘time model’ used by Mahler et al. [9] for ultrametric trees, in that rates change monotonically from an initial value along all branches [36] (electronic supplementary material). While other, often more complex, models of evolutionary rate change have been used elsewhere [38], Pagel's δ as described earlier should be sufficient to test whether changes in rates of cranial and postcranial evolution closely correspond to the predictions of the ‘general vertebrate model’, with BM and OU representing plausible alternatives (electronic supplementary material).
Our first analysis directly tested the stage model by applying two variations of Pagel's δ. The more complicated of these estimated separate δ values for cranial and postcranial datasets, whereas the simpler version imposed a common δ for the two partitions (electronic supplementary material). Because the latter model represents a special case of the former, their fit to data can be compared statistically using the G-test. Often referred to as the likelihood-ratio test, the test statistic is a function of the ratio of log likelihoods for the two models. Significance is assessed using a χ2 distribution with degrees of freedom equal to the difference in the number of free parameters between the more complex and simpler models. We calculated p-values for each of the resolved, stratigraphically calibrated phylogenies produced by the procedures described earlier.
Our second analysis compared the fits of the three basic models along each morphospace axis. We calculated Akaike's information criterion (AICc; a sample-size corrected version of AIC) [39] and Akaike weights for each of the competing models. First, we combined likelihoods generated from model fitting across all significant RWs within each anatomical partition to gauge the relative support for competing models within a given dataset. AICc scores were derived from these net likelihoods, allowing calculation of mean Akaike weights derived from all phylogenetic realizations (electronic supplementary material). In order to summarize overall trait evolution, we generated model-averaged parameter estimates for each axis of our early actinopterygian and acanthomorph morphospaces. Results of all model-fitting exercises are reported in the electronic supplementary material.
Our final analysis tested for staged shifts in evolutionary divergence by implementing the divergence order test (DOT) [11] using the R package ape [40]. This method uses phylogenetically independent contrasts generated from trait values of terminal taxa and ancestral states reconstructed for internal nodes using maximum likelihood [41] and a BM model of trait evolution. Contrasts are then used to weight the ages of the internal nodes in order to produce a mean age of trait divergence (W). A bootstrapping procedure that incorporates uncertainty surrounding ancestral reconstructions places confidence limits on estimates of W [11]. This simple statistic permits direct comparison of mean ages of divergence between traits. In cases of staged divergence, the absolute value of the difference between estimates of W for two traits, D, will be greater than 0.
In our implementation of the DOT, ancestral states and their standard errors (s.d.) were reconstructed for 100 random resolutions of the supertrees. Bootstrapped mean ages (200 per resolved tree) were generated using ancestral states drawn from a node-specific normal distribution based on reconstructed ancestral states and their associated error. The number of D greater than 0 for all bootstrap repetitions (20 000 per dataset) divided by the total number of values was used to generate a one-tailed p-value. This revealed whether there were any significant differences between the mean divergence times for cranial and postcranial RWs [11] (electronic supplementary material).
3. Results
(a). Morphospace axes and anatomical correlates
For early actinopterygians, the first five RWs were retained as they explained more than 5 per cent of overall variance in either the cranial or postcranial data (electronic supplementary material). The first four RWs were examined for the acanthomorph cranial and postcranial datasets, based on the same selection criterion. For both radiations, cranial RWs captured aspects of relative gape length, gape and suspensorium angle, and skull aspect ratio. The denser sampling of cranial landmarks possible for the sample of early actinopterygians resulted in ordinations that also directly capture variation in jaw depth, maxilla shape, pre-orbital proportions and orbit size. All these aspects of cranial morphology are related to trophic demands [20,21]. Gape, mandible dimensions and the angle of jaw suspension are associated with food preference and force production during biting or suction feeding [20,21]. Orbit size and position have been related to nocturnal or diurnal hunting in modern reef fishes [19]. In contrast, the postcranial RWs for both radiations were related to changes in functional traits associated with locomotion and habitat choice, including body depth, axial elongation, caudal fin shape and aspect, and median fin size and position [22–24].
(b). Patterns of morphological diversification
We found that cranial disparity increased significantly at the start of both the early actinopterygian (Devonian–Tournaisian: two-tailed t-test p < 0.001, likelihood ratio, LR = 159.18; figure 2 and electronic supplementary material) and acanthomorph diversification events (early-late Late Cretaceous: p = 0.0019, LR = 8.78; figure 2 and electronic supplementary material). In contrast to the cranial results, the most striking increases in postcranial disparity take place one geological interval after the onset of cranial diversification (Tournaisian–Visean: p = 0.090, LR = 9.92 × 107; late-Late Cretaceous: p = 0.062, LR = 9.92 × 105; figure 2 and electronic supplementary material).
Cranial and postcranial morphospaces confirmed that actinopterygian morphological diversification occurred in the aftermath of the Hangenberg extinction and immediately following the origination of acanthomorph teleosts (figure 1) [13,15]. Disparity increases were in line with previously reported taxonomic increases and the origins of new subclades in both groups [13–16]. Because the landmarks are related to functional and ecological morphology, these diversification events can be classified as adaptive radiations (sensu Schluter [1]). Patterns in the data, particularly staged increases in cranial and then postcranial disparity, can therefore be interpreted in those terms.
(c). Evolutionary dynamics underlying patterns of diversification: models of trait evolution
We wanted to determine whether our ‘head first’ disparity patterns were attributable to a common mechanism. It is often assumed that rapid accumulation of morphological diversity within a radiation represents evidence of coincident elevation in rates of phenotypic change [1–3,8,29,42]. However, many processes besides evolutionary rate shifts can produce similar disparity trajectories. These include the presence of internal (architectural or developmental) or external (ecological) constraints [3,29,42], which can be modelled by an OU process.
In order to dissect the dynamics behind observed disparity patterns, we tested for temporal asymmetries in rates of cranial and postcranial change. Results of fitting coincident and staged Pagel's δ to the two datasets indicated that rates of phenotypic change were greater in the observed radiation intervals: after the Hangenberg extinction for early actinopterygians (δ > 1; the latter half of the sampled history of the clade), and early in clade history for acanthomorphs (δ < 1; the first half of the sampled history of the clade; see electronic supplementary material). We found significant support for staged rate increases for early actinopterygian RWs 1 and 3 and acanthomorph RWs 2–4 (electronic supplementary material). However, cranial δ was greater than postcranial δ for all of these axes. This indicates concentration of postcranial change earlier in phylogenetic history relative to cranial change, meaning that observed patterns of morphological diversification were decoupled from shifts in evolutionary rate. While early actinopterygian RWs 2 and 4 and acanthomorph RW 1 do indicate a ‘head first’ pattern of relative rate changes, the difference in δ values is not significant for these axes (electronic supplementary material).
Since Pagel's δ does not provide strong or even consistent evidence for monotonic rate changes corresponding to a naive reading of our disparity patterns, we compared Pagel's δ with competing models that assume constant rates of evolutionary change: BM and OU. We found that Pagel's δ, and thus change in evolutionary rate, was a strong fit for only a single axis (early actinopterygian cranial RW 1); all other significant RWs were best fit by the OU model. When likelihoods are pooled across all significant axes within each anatomical partition for each clade, the Akaike weight for the OU model is approximately 1. Strong preference for the OU model suggests that monotonic shifts in rates of phenotypic change are not responsible for observed disparity patterns. Instead, there appear to be strong central tendencies involved in diversification along both cranial and postcranial axes, possibly resulting from adaptive peaks or other constraints.
(d). Evolutionary dynamics underlying patterns of diversification: divergence order test
DOT mean node ages (divergence times) for early actinopterygians and acanthomorphs generally fell within a 2 million year range some time after the midpoint of the two intervals, and postdating the end-Devonian and end-Cretaceous extinctions, respectively (electronic supplementary material). No clear pattern of divergence emerges from these analyses, and the vast majority of pairwise age differences (D) were not significant (69 of 73 pairwise comparisons, representing all possible RW combinations within clades excluding self-comparisons; electronic supplementary material). In interpreting these results, it should be noted that the DOT relies on a simple BM model for ancestral state reconstruction [11], which was not the best fit for any tested RW (electronic supplementary information).
In summary, the DOT results are broadly consistent with the results of our model-fitting exercises: the ‘head first’ pattern is not easily attributable to corresponding changes in relative rates of cranial and postcranial evolution. This agrees with a recent large-scale model-based analysis of multiple living radiations that showed little evidence for increased rates of phenotypic change early in the history of clades [3,42]. Other studies of single groups have supported ‘burst’-like dynamics above simple constant-rate BM [9], but many of these did not consider models involving constant rates of change coupled with constraint (such as OU). In cases where morphological diversification is decoupled from rate change, detection of divergence patterns might require a different approach than using rate-change models, such as direct observation of morphological change using fossil, laboratory or other data with a temporal aspect [3].
4. Discussion
Our temporal disparity data show evidence for the existence of cranial and postcranial stages in two separate vertebrate radiations. Interestingly, the ‘head first’ sequence observed here is the opposite of that proposed by the original ‘general vertebrate’/‘radiation-in-stages’ model, which had not been tested using data that directly bear on historical patterns of morphological diversity [2–9]. The first stage of both the early actinopterygian and acanthomorph radiations was defined by increased cranial disparity. Most change involved those functional and morphological elements associated with feeding, such as jaws and oral muscle attachment [20–22].
The second stage of both actinopterygian radiations was characterized by increased postcranial disparity. For actinopterygians, changes in body shape are expected in cases of habitat partitioning and/or greater specialization [7]. Disparity of heads and bodies eventually fell back into line after the initial offset, perhaps as adaptive space became saturated (figure 2). Alternatively, the initial path of diversification might limit the possibilities for subsequent diversification of other traits. Such a situation could explain the correlation of cranial and postcranial traits in some living, mature actinopterygian radiations [10].
Given the lack of evidence for evolutionary rate shifts, the existence of a ‘head first’ sequence both in the Palaeozoic and Mesozoic–Cenozoic actinopterygian radiations might reflect responses to similar patterns of environmental and faunal perturbation as well as opportunity. In the aftermath of the end-Devonian extinction, global ecosystems were fundamentally altered and many vertebrate functional roles are likely to have been empty [12,17,43]. Likewise, it is expected that acanthomorphs did not occupy all potential adaptive space at their origin in the Late Cretaceous, and the group seems to have colonized functional roles vacated by victims of the end-Cretaceous extinction [15,44]. Common circumstances might lead to common patterns, and it is possible that our results are contingent rather than general.
However they occurred, the patterns observed here contradict the ‘EB’ and ‘general vertebrate’ ‘habitat first’ models, and question whether a ‘head first’ diversification pattern might have occurred in additional radiations. For example, Richman & Price [45] proposed that cranial trophic innovations (prey size/bill size) appeared before habitat-related body morphologies in the evolutionary history of Phylloscopus warblers, seemingly contradicting their ‘habitat first’ model of speciation [8]. Indeed, Price [46] later amended his interpretation, suggesting that either diet or habitat might have driven diversification, and could not select between these alternatives owing to a lack of temporal data. More relevant to our examples, the endemic cichlid radiations of the three main East African rift lakes diversified along similar trajectories in which head and jaw disparity accumulates at a higher rate than would be expected from postcranial traits [6,47,48].
As with our radiations, the ‘head first’ pattern of cichlid diversification need not reflect differential evolutionary rates. In fact, recent model-fitting analyses of several clades of living cichlids could not find support for an association between initial morphological diversification and change in evolutionary rates [3]. Indeed, there was a clear tendency for fish radiations, involving data from cichlids and other actinopterygian clades, to be best fit by OU models. The shared trajectory is therefore consistent with greater constraints on cranial than postcranial morphology (or, more specifically, the arrangement of landmarks selected by researchers). The primary axes of variation within the cichlid cranial data are very similar to those for our fossil clades, with taxon position driven by functional characteristics such as gape size. This might indicate that actinopterygians share architectural constraints and/or a limited number of potential ecologies, and that these constrain morphological evolution in a predictable fashion [47–49].
As to whether ‘head first’ is also ‘feeding first’, it is notable that in a previous study of oak subclades Ackerly et al. [11] found evidence that traits associated with resource use (the α trait: leaf area [11], growth rate, tiering [50]) might have diversified before those linked to habitat use (the β trait: climatic tolerance [11], substrate choice). The α trait has been synonymized with feeding morphologies and the β trait with habitat/body form in the rockfish Sebastes [51], although the latter was also linked to eye size, a trophic characteristic here and elsewhere [19,47]. Because of the uncertainties associated with reconstructing ancestral states from living taxa, Ackerly et al. [11] proposed a diversification model of ‘α early, β throughout’, in which resource-related traits evolved slowly and became fixed early, whereas habitat traits changed rapidly, deleting traces of earlier evolution [2,8]. One advantage of using fossil and other temporal data is that trait values for early branches can be directly observed, helping to ameliorate this potential source of error.
The generality of a ‘head/feeding first’ model, and the real extent of the relationship between evolutionary rates and morphological divergence will be revealed through further examination of adaptive radiations with temporal data. Any group with distinct structures linked to feeding and habitat use is a candidate for such analyses. Drawing an example from invertebrates, there is some evidence from the Carboniferous radiation of insects that dietary diversification began before the appearance of associated body morphologies, but available data are not definitive [52,53]. Fortunately, it should be straightforward to divide published morphometric and character datasets into various trait complexes and explore the patterns that emerge.
It is important to note that while this ‘head first’ pattern might be present in other diversification events, the real world is more complicated than any model. For example, traits could be associated with more than one functional unit (with form limited by trade-off) and divergent morphologies can have similar implications (many-to-one mapping) [54]. Any one pattern of divergence, whether ‘feeding first’ or ‘EB’, is unlikely to define all radiations. The proliferation of increasingly sophisticated phylogenetic comparative approaches places us in an ideal position to test the predictions of existing models of adaptive radiation, and it is only by further documentation of patterns in additional clades, both extinct and extant, that the conditions under which certain models apply can be better constrained.
Acknowledgements
The authors thank M. Coates, M. Webster, D. Bapst, J. Mitchell, M. Alfaro, L. Harmon and two anonymous reviewers for their helpful comments. L.C.S. is supported by the National Science Foundation (DEB-0917922 and DDIG DEB-1011002), the Palaeontological Association, the Paleontological Society, the American Society of Ichthyologists and Herpetologists, the Evolving Earth Foundation and the Committee on Evolutionary Biology (University of Chicago). M.F. acknowledges the support of the Natural Environment Research Council (NE/I005536/1), the Fell Fund of the University of Oxford, the National Science Foundation (DGE-0228235), the Environmental Protection Agency (FP916730), the Paleontological Society, the Evolving Earth Foundation and the Lerner-Grey Fund of the American Museum of Natural History.
References
- 1.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University [Google Scholar]
- 2.Gavrilets S., Losos J. 2009. Adaptive radiation: contrasting theory with data. Science 323, 732–737 10.1126/science.1157966 (doi:10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- 3.Harmon L. J., et al. 2010. Early bursts of body size and shape evolution are rare in comparative data. Evolution 64, 2385–2396 [DOI] [PubMed] [Google Scholar]
- 4.Simpson G. G. 1953. The major features of evolution. New York, NY: Columbia University Press [Google Scholar]
- 5.Osborn H. F. 1902. The law of adaptive radiation. Am. Nat. 36, 353–363 10.1086/278137 (doi:10.1086/278137) [DOI] [Google Scholar]
- 6.Uyeda J. C., Hansen T. F., Arnold S. J., Pienarr J. 2011. The million year wait for macroevolutionary bursts. Proc. Natl Acad. Sci. USA 108, 15 908–15 913 10.1073/pnas.1014503108 (doi:10.1073/pnas.1014503108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streelman J. T., Danley P. D. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 18, 126–131 10.1016/S0169-5347(02)00036-8 (doi:10.1016/S0169-5347(02)00036-8) [DOI] [Google Scholar]
- 8.Glor R. E. 2010. Phylogenetic insights on adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 41, 251–270 10.1146/annurev.ecolsys.39.110707.173447 (doi:10.1146/annurev.ecolsys.39.110707.173447) [DOI] [Google Scholar]
- 9.Mahler D. L., Revell L. J., Glor R. E., Losos J. B. 2010. Ecological opportunity and the rate of morphological evolution in the diversification of greater Antillen anoles. Evolution 64, 2731–2745 10.1111/j.1558-5646.2010.01026.x (doi:10.1111/j.1558-5646.2010.01026.x) [DOI] [PubMed] [Google Scholar]
- 10.Collar D. C., Wainwright P. C., Alfaro M. E. 2008. Integrated diversification of locomotion and feeding in labrid fishes. Biol. Lett. 4, 84–86 10.1098/rsbl.2007.0509 (doi:10.1098/rsbl.2007.0509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackerly D. D., Schwilk D. W., Webb C. O. 2006. Niche evolution and adaptive radiation: testing the order of trait divergence. Ecology 87, S50–S61 10.1890/0012-9658(2006)87[50:NEAART]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[50:NEAART]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 12.Nelson J. S. 2006. Fishes of the world. New York, NY: Wiley [Google Scholar]
- 13.Sallan L. C., Coates M. I. 2010. End-Devonian extinction and a bottleneck in the evolution of modern jawed vertebrates. Proc. Natl Acad. Sci. USA 107, 10 131–10 135 10.1073/pnas.0914000107 (doi:10.1073/pnas.0914000107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardiner B. 1993. Osteichthyes: basal Actinopterygians. In Fossil record II (ed. Benton M. J.), pp. 611–619 London, UK: Chapman & Hall [Google Scholar]
- 15.Friedman M. 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc. R. Soc. B 277, 1675–1683 10.1098/rspb.2009.2177 (doi:10.1098/rspb.2009.2177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson C. 1993. An overview of the early fossil record of acanthomorphs. Bull. Mar. Sci. 52, 29–59 [Google Scholar]
- 17.Erwin D. H. 1998. The end and the beginning: recoveries from mass extinctions. Trends Ecol. Evol. 13, 344–349 10.1016/S0169-5347(98)01436-0 (doi:10.1016/S0169-5347(98)01436-0) [DOI] [PubMed] [Google Scholar]
- 18.Gradstein F. M., Ogg J. G., Smith A. G. 2004. A geologic time scale. Cambridge, UK: Cambridge University Press [Google Scholar]
- 19.Goatley C. H. R., Bellwood D. R., Bellwood O. 2010. Fishes on coral reefs: changing roles over the past 240 million years. Paleobiology 36, 415–427 10.1666/09035.1 (doi:10.1666/09035.1) [DOI] [Google Scholar]
- 20.Westneat M. W. 2004. The evolution of levers and linkages in the feeding mechanisms of fishes. Integr. Comp. Biol. 44, 378–389 10.1093/icb/44.5.378 (doi:10.1093/icb/44.5.378) [DOI] [PubMed] [Google Scholar]
- 21.Wainwright P. C., Bellwood D. R. 2002. Ecomorphology of feeding in coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P. F.), pp. 33–55 San Diego, CA: Academic Press [Google Scholar]
- 22.Webb P. W. 1982. Locomotor patterns in the evolution of actinopterygian fishes. Am. Zool. 22, 378–389 [Google Scholar]
- 23.Lauder G. V., Nauen J. C., Drucker E. G. 2002. Experimental hydrodynamics and evolution: function of median fins in ray-finned fishes. Integr. Comp. Biol. 42, 1009–1017 10.1093/icb/42.5.1009 (doi:10.1093/icb/42.5.1009) [DOI] [PubMed] [Google Scholar]
- 24.Lauder G. V., Drucker E. G., Nauen J. C., Wilga C. D. 2003. Experimental hydrodynamics and locomotion. In Vertebrate biomechanics and evolution (eds Bels V. L., Gasc J.-P., Casinos A.), pp. 117–135 London, UK: BIOS [Google Scholar]
- 25.Rasband W. S. 2011. ImageJ. Bethesda, MD: U.S. National Institutes of Health; See http://imagej.nih.gov/ij/. [Google Scholar]
- 26.Rohlf F. J. 2007. Tps package. Stony Brook, NY: SUNY Stony Brook [Google Scholar]
- 27.Raup D. M. 1979. Bias in the fossil record of species and genera. Bull. Carnegie Mus. Nat. Hist. 13, 85–91 [Google Scholar]
- 28.Alroy J., et al. 2008. Phanerozoic trends in the global diversity of marine invertebrates. Science 321, 97–100 10.1126/science.1156963 (doi:10.1126/science.1156963) [DOI] [PubMed] [Google Scholar]
- 29.Foote M. 1996. Models of morphological diversification. In Evolutionary paleobiology (eds Jablonski D., Erwin D., Lipps J.), pp. 62–86 Chicago, IL: University of Chicago Press [Google Scholar]
- 30.Ciampaglio C. N., Kemp M., McShea D. W. 2001. Detecting changes in morphospace occupation in the fossil record: characterization and analysis of measures of disparity. Paleobiology 27, 695–715 (doi:10.1666/0094-8373(2001)027<0695:DCIMOP>2.0.CO;2) [DOI] [Google Scholar]
- 31.R Development Core Team. 2008. R: a language and environment for statistical computing. See http://www.R-project.org.
- 32.Zelditch M. L., Swiderski D. L., Sheets H. D., Fink W. L. 2004. Geometric morphometrics for biologists: a primer. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 33.Finarelli J. A., Flynn J. J. 2007. The evolution of encephalization in caniform carnivorans. Evolution 61, 1758–1777 10.1111/j.1558-5646.2007.00131.x (doi:10.1111/j.1558-5646.2007.00131.x) [DOI] [PubMed] [Google Scholar]
- 34.Harmon L. J., Weir J. T., Brock C. D., Glor R. E., Challenger W. 2008. GIEGER: investigating evolutionary radiations. Bioinformatics 24, 129–131 10.1093/bioinformatics/btm538 (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 35.Uhlenbeck G. E., Ornstein L. S. 1930. On the theory of Brownian motion. Phys. Rev. 36, 823–841 10.1103/PhysRev.36.823 (doi:10.1103/PhysRev.36.823) [DOI] [Google Scholar]
- 36.Pagel M. 1994. Detecting correlated evolution in phylogenies: a general model for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 10.1098/rspb.1994.0006 (doi:10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 37.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zool. Scripta 26, 331–348 10.1111/j.1463-6409.1997.tb00423.x (doi:10.1111/j.1463-6409.1997.tb00423.x) [DOI] [Google Scholar]
- 38.Venditti C., Meade A., Pagel M. 2011. Multiple routes to mammalian diversity. Nature 479, 393–396 10.1038/nature10516 (doi:10.1038/nature10516) [DOI] [PubMed] [Google Scholar]
- 39.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Automat. Control 19, 716–723 10.1109/TAC.1974.1100705 (doi:10.1109/TAC.1974.1100705) [DOI] [Google Scholar]
- 40.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R package. Bioinformatics 23, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 41.Schluter D., Price T., Mooers A. O., Ludwig D. Likelihood of ancestor states in adaptive radiations. Evolution 51, 1699–1711 10.2307/2410994 (doi:10.2307/2410994) [DOI] [PubMed] [Google Scholar]
- 42.Yoder J. B., et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 581–596 10.1111/j.1420-9101.2010.02029.x (doi:10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 43.Sallan L. C., Kammer T. W., Ausich W. I., Cook L. A. 2011. Persistent predator–prey dynamics revealed by mass extinction. Proc. Natl Acad. Sci. USA 108, 8335–8338 10.1073/pnas.1100631108 (doi:10.1073/pnas.1100631108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman M. 2009. Ecological selectivity among marine teleost fishes during the end-Cretaceous extinction. Proc. Natl Acad. Sci. USA 106, 5218–5223 10.1073/pnas.0808468106 (doi:10.1073/pnas.0808468106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richman A. D., Price T. 1992. Evolution of ecological differences in Old World leaf warblers. Nature 355, 817–821 10.1038/355817a0 (doi:10.1038/355817a0) [DOI] [PubMed] [Google Scholar]
- 46.Price T. 2008. Speciation in birds. Greenwood Village, CO: Roberts and Company [Google Scholar]
- 47.Young K. A., Snoeks J., Seehausen O. 2009. Morphological diversity and the roles of contingency, changes and determinism in African cichlid radiations. PLoS ONE 4, e4740. 10.1371/journal.pone.0004740 (doi:10.1371/journal.pone.0004740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper W. J., Parsons K., McIntyre A., Kern B., McGee-Moore A., Albertson R. C., Humphries S. 2010. Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift lakes. PLoS ONE 5, e9551. 10.1371/journal.pone.0009551 (doi:10.1371/journal.pone.0009551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson P. S. L., Friedman M., Brazeau M. D., Rayfield E. J. 2011. The initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature 476, 206–209 10.1038/nature10207 (doi:10.1038/nature10207) [DOI] [PubMed] [Google Scholar]
- 50.Ausich W. I., Bottjer D. J. 1982. Tiering in suspension-feeding communities on soft substrata throughout the Phanerozoic. Science 216, 173–174 10.1126/science.216.4542.173 (doi:10.1126/science.216.4542.173) [DOI] [PubMed] [Google Scholar]
- 51.Ingram T. 2011. Speciation along a depth gradient in a marine adaptive radiation. Proc. R. Soc. B 278, 613–618 10.1098/rspb.2010.1127 (doi:10.1098/rspb.2010.1127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward P., Labandiera C., Laurin M., Berner R. A. 2006. Confirmation of Romer's gap as a low oxygen interval constraining the timing of initial arthropod and vertebrate terrestrialization. Proc. Natl Acad. Sci. USA 103, 16 818–16 822 10.1073/pnas.0607824103 (doi:10.1073/pnas.0607824103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labandiera C. 2007. The origin of herbivory on land: the initial pattern of live tissue consumption in arthropods. Insect Sci. 14, 259–275 [Google Scholar]
- 54.Wainwright P. C., Alfaro M. E., Bolnick D. I., Hulsey D. C. 2005. Many-to-one mapping of form to function: a general principle of organismal design? Integr. Comp. Biol. 45, 256–262 10.1093/icb/45.2.256 (doi:10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]