Abstract
Whether lateralization of communicative signalling in non-human primates might constitute prerequisites of hemispheric specialization for language is unclear. In the present study, we examined (i) hand preference for a communicative gesture (clapping in 94 captive chimpanzees from two research facilities) and (ii) the in vivo magnetic resonance imaging brain scans of 40 of these individuals. The preferred hand for clapping was defined as the one in the upper position when the two hands came together. Using computer manual tracing of regions of interest, we measured the neuroanatomical asymmetries for the homologues of key language areas, including the inferior frontal gyrus (IFG) and planum temporale (PT). When considering the entire sample, there was a predominance of right-handedness for clapping and the distribution of right- and left-handed individuals did not differ between the two facilities. The direction of hand preference (right- versus left-handed subjects) for clapping explained a significant portion of variability in asymmetries of the PT and IFG. The results are consistent with the view that gestural communication in the common ancestor may have been a precursor of language and its cerebral substrates in modern humans.
Keywords: handedness, gestural communication, hemispheric specialization, origins of language, primates
1. Introduction
Hemispheric specialization of the brain and right-handedness has historically been considered a specific trait of human evolution [1–3]. Most language functions involve a greater activation of the left hemisphere, including Wernicke's and Broca's areas, two key regions of the language cortical network involved in the comprehension and production of signals within the temporal and the frontal cortex, respectively [4–9]. Leftward size asymmetries have been reported, particularly among right-handed individuals for the planum temporale (PT) [10–12], but such a leftward bias is not entirely consistent across studies for the inferior frontal gyrus (IFG) [13,14], which includes Brodmann's cytoarchitectonic areas 44 and 45 [15]. The PT and IFG overlap with Wernicke's and Broca's areas; therefore, these anatomical asymmetries may correspond to the functional dominance of the left hemisphere for language.
Left lateralization for language has been historically linked to right-handedness for manipulative actions [16,17], but 70 per cent of left-handed humans also show a similar left lateralization [18–20], suggesting that the direction of handedness for manipulation is not a perfect predictor of hemispheric lateralization for language. An alternative model claims that handedness for gestural communication may constitute a better predictor [21,22] of hemispheric specialization for language. Right-hand dominance in humans (almost 90% are right-handed [23]) is not only associated with manipulation, but also with communicative gestures, including signing in deaf people [24,25], manual movements when people are talking [26] and pointing gestures by infants [27]. Interestingly, signing, pointing or symbolic actions—abilities that might play an active role in the development of children's communicative skills [28]—elicit a stronger degree of right-handedness than non-communicative manual actions in young children, potentially indicating a greater involvement of the left hemisphere for communicative signalling [27,29–32]. Furthermore, evidence of links between speech and gesture production in humans is consistent with the notion of a single integrated communication system within the left cerebral hemisphere for both vocal and gestural communication [22,33–37]. Finally, functional brain imaging studies have shown a ‘speech-like’ activation of Broca's area in the left hemisphere for sign production in deaf individuals [38,39]. These collective findings raise important questions regarding the role of gestures in the origin of language in humans [22,40,41].
Because chimpanzees (Pan troglodytes) are our closest phylogenetic relatives, this species may be a relevant model for investigating the potential precursors of left hemisphere specialization for language in humans. It is well known that chimpanzees produce intentional manual gestures to communicate with social partners in various social contexts, such as play, threat, greeting, invitation for grooming, shared excitation, reassurance-seeking after stress and food begging [42–46]. It has been further shown that great apes have a much more flexible [44,46] and much less context-dependent gestural, compared with vocal, system of communication [47], and are able to adjust their gestural behaviours to the responses and the attentional state of the recipient [48–52]. These features of the gestural system have been considered as evidence of continuities with key properties of human language, such as flexibility, intentionality and referential properties, which some believe support the gestural origins theory of language evolution [53–56].
Within this theoretical framework, there are some questions about whether the gestural system in non-human primates involves a left hemisphere specialization, as it does in human language. To our knowledge, the studies on non-human primates that investigated hand preferences for gestural communication have all reported a significant preponderance of right-handedness for different categories of gestures, including intraspecific (e.g. hand slap) and human-directed gestures (e.g. food-begging extended arm) in both captive chimpanzees [57–59] and olive baboons (Papio anubis) [60,61]. Similarly, although less well documented, evidence of asymmetries in gestures has also been reported in bonobos (Pan paniscus) [62] and gorillas (Gorilla gorilla) [63]. Interestingly, according to different assessment methods (i.e. region-of-interest manual tracing of brain imaging scans [64–67], voxel-based morphometry [68] and post-mortem morphological analyses of the PT tissues [69]), investigations of neuroanatomical asymmetries in great apes—and chimpanzees specifically—have reported leftward biases for both the IFG and PT (considered as the homologue regions that overlap Broca's and Wernicke's areas in humans [70]). Leftward bias has been confirmed at microcellular level by cytoarchitectonic studies for Brodmann's area 22 (which makes up part of the PT) [71], but not for Brodmann's areas 44 and 45 (i.e. Broca's area) [72].
The focus of this study was on assessing whether variation in asymmetries in the IFG and PT are associated with hand preferences for manual gestures in captive chimpanzees. There is one previous study in captive chimpanzees that reported neuroanatomical correlates between asymmetries in the IFG but not the PT with hand preferences for human-directed food-begging gestures (i.e. extended arm towards humans) [66]. Here, we focused on clapping gestures because they have several unique features in terms of social cognition and handedness that make them particularly interesting for this research topic. First, clapping is an auditory rather than visual gesture that has been frequently described as a communicative signal that is uniquely produced by great apes (gorillas, chimpanzees, bonobos and orangutans) but not by more distantly related monkeys [50,52,73,74]. Moreover, in captive chimpanzees, because the frequency of clapping has been shown to increase when a human recipient is inattentive in the presence of out-of-reach food, this gesture has the specific function of capturing the attention of an otherwise inattentive individual [52,75,76]. Second, most of the clapping behaviours in chimpanzees are asymmetrically performed between the two hands, namely with one hand in the upper position that slaps the other hand in the lower position when produced in the horizontal plane, resulting in a sound [77]. Finally, clapping has been shown to elicit exclusive hand preferences in a majority of captive chimpanzees [77], suggesting that hand use is not sensitive to postural or situational factors and therefore has the potential to better reflect underlying inherent specialization of the left or right hemisphere. In this study, we investigated hand preferences for clapping behaviours in two cohorts of captive chimpanzees and then subsequently compared the patterns of neuroanatomical asymmetry in the PT and IFG in those subjects that were found to consistently clap with the right or left hand. If clapping as a communicative behaviour is lateralized and associated with asymmetries in the PT and IFG, then we hypothesized that (i) significantly more individuals would be right- than left-handed for clapping and (ii) significant differences in asymmetries in the PT and IFG would be found between left- and right-handed subjects.
2. Methods
(a). Subjects
Hand preferences for clapping were assessed in two cohorts of chimpanzees comprising 241 individuals (139 females and 102 males) ranging in age from 2 to 47 years of age (mean = 19.03, s.e. = 1.13). Among this sample, 94 subjects (68 females, 26 males) were observed to clap on at least one occasion. The first cohort of clapping individuals comprised 43 subjects (35 females, 8 males) out of the 111 chimpanzees that have been observed at the Yerkes National Primate Research Center (YNPRC) in Atlanta, GA. The second cohort comprised 51 subjects (33 females, 18 males) out of the 130 chimpanzees that have been observed at The University of Texas MD Anderson Cancer Center (UTMDACC) in Bastrop, TX. In vivo magnetic resonance imaging (MRI) scans were previously obtained from a sample of 40 chimpanzees (32 females, 8 males) for whom hand-use data for clapping were recorded.
(b). Procedure
(i). Hand use measures for clapping
The preferred hand for clapping was defined as the one in the upper position when the two hands came together (figure 1). From April 2007 to March 2011, data were collected between 10.00 and 18.00 h in an observational day during two 2-h observation sessions, one in the morning and one in the afternoon. Social groups were randomly observed during an observation session, especially during feeding times and experimental sessions when clapping frequencies increase in order to get the attention of humans distributing food. An all-occurrences sampling procedure was used and a hand-use response of right or left was recorded opportunistically whenever clapping was observed. If a sequence of claps occurred with the same hand without an interruption of at least 2 s, then the entire sequence was considered a single instance of clapping.
Figure 1.
An adult female chimpanzee claps with the right hand in the upper position in order to get the attention of a human observer.
(ii). Magnetic resonance image collection
For collecting in vivo MRI scans, 11 anaesthetized chimpanzees were scanned using a 1.5 T MRI machine and 29 were scanned with a 3 T machine. The full details of the procedure of image collection are provided in the electronic supplementary material (point 1; see also [66]).
(iii). Quantification of the regions of interest
The brain regions of interest included the IFG and PT, and were restricted to 40 subjects for whom clapping data and MRI scans were available. Using a mouse-driven computer-guided cursor with the software Analyze v. 7.0, the surface area of a given cerebral region of interest was quantified by tracing the area manually in each of the slices of the brain scan where the region was present and then the cumulative measures of all slices were added to yield a single value. Such a quantification in both right and left hemispheres allowed for the assessment of the neuroanatomical asymmetry of this region in each subject.
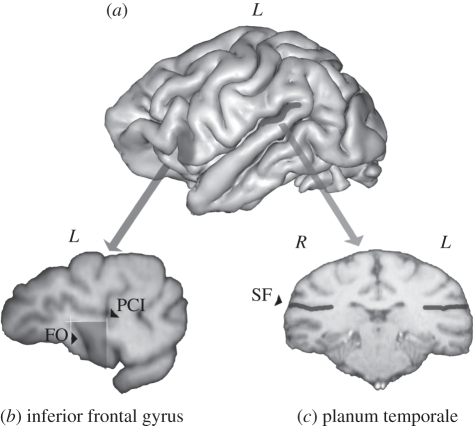
Inferior frontal gyrus. For quantifying the IFG, we used the methodology described in previous studies [64,66]. The MRI scans were aligned in the parasagittal plane and cut into 1 mm slices. The IFG was defined as the portion of cortical surface bounded anteriorly by the fronto-orbital sulcus (FO), posteriorly by the precentral–inferior sulcus (PCI) and superiorly by the inferior frontal sulcus (figure 2b). For each slice, the anterior border of the IFG was traced in following the FO sulcus. The superior border of the IFG was traced by a straight line from the extremity of the FO sulcus until it reached the PCI sulcus. Then, as the posterior border of the IFG, a straight line was dropped vertically to the bottom edge of the brain. Consecutive parasagittal slices were traced from the lateral surface of the IFG to the most medial slice immediately preceding the insula. This region could be measured in both hemispheres in 35 subjects. Ambiguity in the sulci landmarks precluded measurement of the IFG in five subjects.
Figure 2.
(a) Three-dimensional reconstruction of a chimpanzee brain indicating the inferior frontal gyrus (IFG) and the planum temporale (PT). (b) 1 mm slice in the parasagittal plane with the IFG traced on the slice (FO, fronto-orbital sulcus; PCI, precentral–inferior sulcus). (c) 1 mm slice in the coronal plane with the sylvian fissure (SF) traced on the slice.
Planum temporale. For quantifying the PT [65,67], the MRI scans were aligned in the coronal plane and virtually cut into 1 mm slices. The anterior border of the PT was defined by the most frontal slice showing Heschl's gyrus. The posterior border was defined as the most caudal slice showing the sylvian fissure. The flat surface of the PT, corresponding to the depth of the sylvian fissure, was measured on each slice and then summed across all slices for each hemisphere (figure 2c). The PT surface area (in square millimetres) was computed as the sum of the cumulative PT surface measures across all slices [12]. This region could be measured in both hemispheres in 38 subjects. The PT could not be traced in two apes owing to an unclear delineation of Heschl's gyrus.
(c). Data analysis
The hand preferences for clapping were determined according to two methods. First, on the basis of total left and right responses of hand use for clapping, an individual handedness index (HI) was calculated by using the following formula: HI = (R − L)/(R + L), with R and L representing total right- and left-hand responses, respectively. The HI values varied on a continuum from −1.0 to 1.0, with the sign indicating the direction of hand preference. Positive values indicated right-hand preferences and negative values indicated left-hand preferences. The absolute value of the HI score (ABS-HI) reflected the strength of individual hand preferences. Second, a more conservative method consisted of calculating an individual z-score based on total left- and right-hand clapping responses in order to classify the subjects as left-handed (z ≤ −1.96), right-handed (z ≥ 1.96) or ambiguously handed (−1.96 < z < 1.96). According to the z-score technique, subjects that produced fewer than four responses were classified as ambiguously handed, regardless of the direction of hand-use responses. For the IFG and PT, an asymmetry quotient (AQ) was obtained by using the following formula: AQ = (R − L)/[(R + L) × 0.5], where R and L represent the size of the right and left regions, respectively. AQ values also varied on a continuum, with positive values indicating a right hemisphere bias and negative values indicating a left hemisphere bias.
3. Results
(a). Hand preferences for clapping
Basic descriptive data on the frequency of hand use in clapping are provided in the electronic supplementary material (point 2). Among the total sample of 94 chimpanzees who were observed to clap, 42 were classified as right-handed, 28 as left-handed and 24 were classified as ambiguously handed (including 20 subjects that produced fewer than four responses). Among the 74 subjects that produced a minimum of four responses, 63 subjects showed exclusive hand preference (i.e. ABS-HI = 1.00), whereas only 11 chimpanzees did not (i.e. ABS-HI < 1.00). Based on a chi-square goodness-of-fit test, there was a trend towards a predominance in the number of right- compared with left-handed subjects: χ2 (1, n = 70) = 2.80, p = 0.09. The mean HI score of the 94 subjects (mean = 0.26, s.e. = 0.09) revealed a significant right-hand bias according to a one-sample t-test, t93 = 2.71, p < 0.01. All results are summarized in table 1. Further detailed analyses presented in the electronic supplementary material (points 3–5) demonstrated that there was no effect of time, population, sex or number of responses (using a funnel plot; see [78]) on the patterns of hand preferences.
Table 1.
Distribution of hand preferences and degree of group-level manual bias. no. L, number of left-handed subjects; no. R, number of right-handed subjects; no. A, number of ambiguously handed subjects; n < 4, number of subjects that produced fewer than four clapping responses and were subsequently categorized as ambiguously handed according to the z-score; N, sample of subjects; M. HI, mean handedness index score of N individuals that corresponds to degree of population-level handedness—the sign indicates the direction of the manual bias (negative value: left-hand bias, positive value: right-hand bias); s.e., standard error of the mean. Bold indicates significance at p < 0.01.
| samples | no. L | no. R | no. A | n < 4 | N | M. HI | s.e. |
|---|---|---|---|---|---|---|---|
| all subjects | 28 | 42 | 4 | 20 | 94 | 0.26 | 0.09 |
| population YNPRC | 14 | 22 | 3 | 4 | 43 | 0.19 | 0.14 |
| population UTMDACC | 14 | 20 | 1 | 16 | 51 | 0.31 | 0.13 |
| males | 5 | 10 | 2 | 9 | 26 | 0.45 | 0.17 |
| females | 23 | 32 | 2 | 11 | 68 | 0.18 | 0.11 |
(b). Neuroanatomical asymmetries
The 38 subjects for whom the asymmetry of the PT was measured showed a significant leftward bias for the PT (AQ =−0.13, s.e. = 0.02), according to a one-sample t-test (t37 =−5.14, p < 0.001). The 35 subjects that were measured on the IFG did not show a significant population-level neuroanatomical bias (AQ = 0.09, s.e. = 0.06), t35 = 1.53, p > 0.10. In the next analyses, we investigated whether neuroanatomical asymmetries for the PT and IFG were associated with the direction of handedness for clapping.
(c). Neuroanatomical correlates of hand preferences for clapping
Among the 40 individuals for whom brain MRI scans were analysed, according to the z-score, 10 were classified as ambiguously handed, 7 of whom produced fewer than four responses. For more statistical power, we decided to include these ambiguous subjects in the neuroanatomical analyses and determined the direction of their hand preferences based on the sign of their individual HI score. Among these 10 subjects, 6 had a positive HI value and were included in the right-handed group, and 4 had a negative value and were included in the left-handed group. Thus, for these analyses, there were 24 right-handed and 16 left-handed subjects.
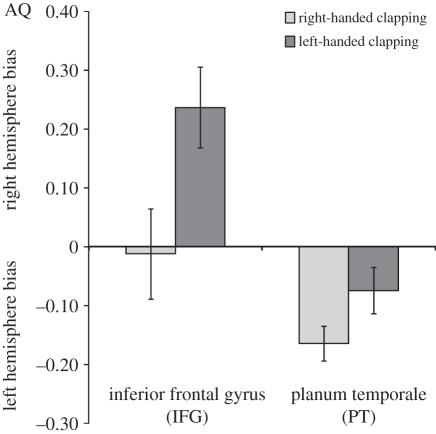
We conducted a mixed-model analysis of variance with AQs for the IFG and the PT serving as the repeated measure while sex and handedness were between-group factors. We found a significant main effect for handedness in clapping on the AQ scores (F1,31 = 7.74, p < 0.01; figure 3). Right-handed chimpanzees had greater leftward asymmetries compared with left-handed individuals. As an alternative analytic approach, we also correlated the individual HI scores for clapping with the AQ scores for the PT and IFG. The HI scores for clapping were significantly negatively correlated with the AQ scores for both the PT (r38 =−0.39, p < 0.05) and IFG (r35 = −0.35, p < 0.05).
Figure 3.
Degree and direction of neuroanatomical asymmetries (AQ scores) for the inferior frontal gyrus (IFG) and the planum temporale (PT) in left-handed (n = 13 for the IFG; n = 16 for the PT) and right-handed subjects (n = 21 for the IFG; n = 22 for the PT) for clapping. AQ values varied on a continuum with positive values indicating right hemisphere biases and negative values indicating a left hemisphere bias.
4. Discussion
Several findings were revealed in this study. First, this is the first demonstration of predominance of right-handedness for clapping, a communicative gesture produced by great apes. As previously reported in another study [77], we found that clapping elicits nearly exclusive hand preference at the individual level that is consistent across time. Interestingly, as can be seen in the electronic supplementary material, figure S2 (point 6), the degree of population-level right-handedness for clapping is consistent with that reported for other categories of gestures in captive chimpanzees, such as human-directed food begging [58] and species-specific signals, including threat, extended arm and hand slap [59]. The degree of right-handedness for gestural communication is much more pronounced than for non-communicative motor actions in chimpanzees, such as coordinated bimanual actions and simple reaching [58,59]. Similar differences in the degree of right-handedness between communicative and non-communicative actions have also been reported in captive baboons [60,61], bonobos [62] and humans [27,29–32]. Collectively, these findings support the hypothesis that a specific left-lateralized gestural system might be involved in the production of communicative signalling [60] in the common ancestor.
Second, we confirmed previous reports of leftward neuroanatomical asymmetries in chimpanzees for the PT [65,67,69], but not for the IFG [64,66]. Interestingly, the latter findings are consistent with the human literature, for which reports of leftward neuroanatomical asymmetries are also inconsistent for the IFG [13,14], but not for the PT [10–12]. However, the absence of population-level asymmetries reported in the present study for the IFG might be related to a sample size limitation because only the chimpanzees that produced clapping behaviours were included in the analyses. However, the overall sample of the available MRI scans of the Yerkes chimpanzees (n = 82) showed leftward neuroanatomical asymmetries for the IFG [79].
Third, we found that neuroanatomical asymmetries for both the IFG and PT were associated with individual differences in hand preferences for clapping. These neuroanatomical correlates of an auditory communicative gesture are congruent with previous studies in chimpanzees showing that hand preference for human-directed food-begging gestures predicts variation of the neuroanatomical asymmetry in the IFG [66] and of the grey matter asymmetries in the PT [67]. In contrast, it has been previously reported that handedness for non-communicative motor actions (bimanual coordinated task and unimanual reaching) does not significantly correlate with the IFG and PT homologues [66,80]. Thus, these findings support the notion that the gestural communication system in chimpanzees might involve a common cerebral substrate with human language and could have been the precursor of the cerebral substrate for language in the common ancestor of humans and chimpanzees at least 5–7 Myr ago.
Within an evolutionary framework, the associations found between handedness for clapping and asymmetries in the IFG and PT are particularly interesting because of the cognitive and motor processes underlying this behaviour in chimpanzees. Notably, the use of clapping as an attention-getting behaviour has only been reported in great apes and not in more distantly related monkeys. Moreover, clapping behaviours in great apes are auditory signals that have been shown to be used as attention-getting sounds especially when the recipient is not attentive [50,52]. In other words, the use of clapping is a manifestation of the ability of the great apes to adjust the modality of the signal to the attentional state of the recipient. Therefore, in contrast to monkeys, clapping might involve a specific feature of social cognition in great apes that is ultimately related to the prerequisites of the language brain specialization. Such high-level social cognitive behaviour might therefore be a significant landmark in the evolution of communication and of the brain in our common ancestors that led to language and its specialization in the left hemisphere.
One issue raised in this study is whether clapping would similarly stimulate cortical activation in the left IFG and PT instead of simply being linked to variation in neuroanatomy. Although no functional neuroimaging data are available for communicative clapping gestures in chimpanzees at the present time, two functional brain imaging studies using positron emission tomography (PET) suggest that such a hypothesis would be supported. First, a study on grasping in chimpanzees has demonstrated that the neuroanatomical landmark of the motor hand area (KNOB) overlaps with the functional maps generated by PET analysis [81], indicating that neuroanatomical asymmetry of the brain might predict functional asymmetry in chimpanzees. Second, in another study in three captive chimpanzees, the IFG has been shown to be activated in the left hemisphere during a communicative context that included either pointing gestures, atypical attention-getting sounds or the simultaneous expression of both [82]. These signals were produced towards a human observer in the presence of out-of-reach food. Indeed, captive chimpanzees have been shown to be able to learn and intentionally use atypical sounds in order to get the attention of a human observer [83]. These findings may indicate that the production of intentional communicative signalling—regardless of the communicative strategies and modalities (vocal or gestural)—involves a functional lateralized activation of homologues of language areas that is congruent with the neuroanatomical asymmetries in the IFG [66]. Because the production of clapping is part of the intentional communicative system that is variably used by the chimpanzees according to the attentional state of the recipient [52,75,76], we might expect that the neuroanatomical correlates of clapping would overlap with functional activation of these regions.
In summary, our findings support the hypothesis that language might have evolved from an initial gestural or simple multi-modal communication that was present in the common ancestor of humans and chimpanzees 5–7 Myr ago [41]. Recently, it has been reported that Brodmann's areas 44 and 45 in the human brain, the constituent parts of Broca's area, are nearly seven times larger than in chimpanzees [72]. Taken together, these findings suggest that with increasing selection for cognitive representation and motor control of language and speech in early humans, there was selective expansion in Broca's area in the human brain, particularly within the left hemisphere, and this may account for the robust expression of language lateralization in modern humans [54].
Acknowledgements
This research was supported by Fondation Fyssen, NIH grants no. NS-42867, NS-73134, HD-56232 and HD-60563 and Cooperative Agreement RR-15090. We thank Yerkes National Primate Research Center, The University of Texas MD Anderson Cancer Center and their respective veterinary staffs for assistance in MRI. Further assistance was provided from Jamie Russell and Jennifer Schaeffer. American Psychological Association guidelines for the treatment of animals were followed during all aspects of this study. The photograph in figure 1 was taken by Molly J. Gardner.
References
- 1.Crow T. 2004. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): a reply to Rogers' review of the speciation of modern Homo sapiens. Laterality 9, 233–242 10.1080/13576500342000374 (doi:10.1080/13576500342000374) [DOI] [Google Scholar]
- 2.Ettlinger G. F. 1988. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex 24, 389–398 [DOI] [PubMed] [Google Scholar]
- 3.Warren J. M. 1980. Handedness and laterality in humans and other animals. Physiol. Psychol. 8, 351–359 [Google Scholar]
- 4.Cooper D. L. 2006. Broca's arrow: evolution, prediction and language in the brain. Anat. Rec. 289, 9–24 10.1002/ar.b.20088 (doi:10.1002/ar.b.20088) [DOI] [PubMed] [Google Scholar]
- 5.Poeppel D., Hickok G. 2004. Towards a new functional anatomy of language. Cognition 92, 1–12 10.1016/j.cognition.2003.11.001 (doi:10.1016/j.cognition.2003.11.001) [DOI] [PubMed] [Google Scholar]
- 6.Lieberman P. 2007. The evolution of human speech: its anatomical and neural bases. Curr. Anthropol. 48, 39–66 10.1086/509092 (doi:10.1086/509092) [DOI] [Google Scholar]
- 7.Broca P. 1865. Sur le siège de la faculté du langage articulé. Bull. Soc. Anthropol. Paris 6, 377–393 10.3406/bmsap.1865.9495 (doi:10.3406/bmsap.1865.9495) [DOI] [Google Scholar]
- 8.Wernicke K. 1874. Der aphasische symptomencomplex on Aphasias. Eine psychologische studie auf anatomischer basis. Breslau, Germany: Cohn & Weigert [Google Scholar]
- 9.Fadiga L., Craighero L. 2007. Cues on the origin of language. From electrophysiological data on mirror neurons and motor representations. In On being moved: from mirror neurons to empathy (ed. Bråten S.), pp. 101–110 Amsterdam, The Netherlands: John Benjamins [Google Scholar]
- 10.Geschwind N., Levitsky W. 1968. Human brain: left–right asymmetries in temporal speech region. Science 161, 186–187 10.1126/science.161.3837.186 (doi:10.1126/science.161.3837.186) [DOI] [PubMed] [Google Scholar]
- 11.Beaton A. A. 1997. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: a review of the evidence. Brain Lang. 60, 255–322 10.1006/brln.1997.1825 (doi:10.1006/brln.1997.1825) [DOI] [PubMed] [Google Scholar]
- 12.Shapleske J., Rossell S. L., Woodruff P. W., David A. S. 1999. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Rev. 29, 26–49 10.1016/S0165-0173(98)00047-2 (doi:10.1016/S0165-0173(98)00047-2) [DOI] [PubMed] [Google Scholar]
- 13.Foundas A. L., Eure K. F., Luevano L. F., Weinberger D. R. 1998. MRI asymmetries of Broca's area: the pars triangularis and pars opercularis. Brain Lang. 64, 282–296 10.1006/brln.1998.1974 (doi:10.1006/brln.1998.1974) [DOI] [PubMed] [Google Scholar]
- 14.Keller S. S., Crow T., Foundas A., Amunts K., Roberts N. 2009. Broca's area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 109, 29–48 10.1016/j.bandl.2008.11.005 (doi:10.1016/j.bandl.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 15.Amunts K., Schleicher A., Buergel U., Mohlberg H., Uylings H. B. M., Zilles K. 1999. Broca's region re-visited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 412, 319–341 (doi:10.1002/(SICI)1096-9861(19990920)412:2<319::AID-CNE10>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 16.Galaburda A. M., Sanides F., Geschwind N. 1978. Human brain. Cytoarchitectonic left–right asymmetries in the temporal speech region. Arch. Neurol. 35, 812–817 10.1001/archneur.1978.00500360036007 (doi:10.1001/archneur.1978.00500360036007) [DOI] [PubMed] [Google Scholar]
- 17.Naidich T. P., Hof P. R., Gannon P. J., Yousry T. A., Yousry I. 2001. Anatomical substrates of language: emphasizing speech. Neuroimaging Clin. N. Am. 11, 305–341 [PubMed] [Google Scholar]
- 18.Knecht S., Dräger B., Deppe M., Bobe L., Lohmann H., Flöel A., Ringelstein E. B., Henningsen H. 2000. Handedness and hemispheric language dominance in healthy humans. Brain 123, 2512–2518 10.1093/brain/123.12.2512 (doi:10.1093/brain/123.12.2512) [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen T., Milner B. 1977. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann. NY Acad. Sci. 299, 355–369 10.1111/j.1749-6632.1977.tb41921.x (doi:10.1111/j.1749-6632.1977.tb41921.x) [DOI] [PubMed] [Google Scholar]
- 20.McKeever W. F., Seitz K. S., Krutsch A. J., van Eys P. P. 1995. On language laterality in normal dextrals and sinistrals: results from the bilateral object naming latency task. Neuropsychologia 33, 1627–1635 10.1016/0028-3932(95)00042-9 (doi:10.1016/0028-3932(95)00042-9) [DOI] [PubMed] [Google Scholar]
- 21.Bellugi U. 1991. The link between hand and brain: implications from a visual language. In Advances in cognition, education and deafness (ed. Martin D.), pp. 11–35 Washington, DC: Gallaudet University Press [Google Scholar]
- 22.Kimura D. 1993. Neuromotor mechanisms in human communication. Oxford, UK: Oxford University Press [Google Scholar]
- 23.Annett M. 1985. Left, right, hand and brain: the right shift theory. Hillsdale, NJ: Erlbaum [Google Scholar]
- 24.Vaid J., Bellugi U., Poizner H. 1989. Hand dominance for signing: clues to brain lateralization. Neuropsychologia 27, 949–960 10.1016/0028-3932(89)90070-5 (doi:10.1016/0028-3932(89)90070-5) [DOI] [PubMed] [Google Scholar]
- 25.Grossi G., Semenza C., Corazza S., Volterra V. 1996. Hemispheric specialization for sign language. Neuropsychologia 34, 737–740 10.1016/0028-3932(96)00008-5 (doi:10.1016/0028-3932(96)00008-5) [DOI] [PubMed] [Google Scholar]
- 26.Kimura D. 1973. Manual activity during speaking: I. Right-handers. Neuropsychologia 11, 45–50 10.1016/0028-3932(73)90063-8 (doi:10.1016/0028-3932(73)90063-8) [DOI] [PubMed] [Google Scholar]
- 27.Blake J., O'Rourke P., Borzellino G. 1994. Form and function in the development of pointing and reaching gestures. Infant Behav. Dev. 17, 195–203 10.1016/0163-6383(94)90055-8 (doi:10.1016/0163-6383(94)90055-8) [DOI] [Google Scholar]
- 28.Iverson J. M., Goldin-Meadow S. 2005. Gesture paves the way for language development. Psychol. Sci. 16, 367–371 10.1111/j.0956-7976.2005.01542.x (doi:10.1111/j.0956-7976.2005.01542.x) [DOI] [PubMed] [Google Scholar]
- 29.Bates E., O'Connel B., Vaid J., Sledge P., Oakes L. 1986. Language and hand preference in early development. Dev. Neuropsychol. 2, 1–15 10.1080/87565648609540323 (doi:10.1080/87565648609540323) [DOI] [Google Scholar]
- 30.Bonvillian J. D., Richards H. C., Dooley T. T. 1997. Early sign language acquisition and the development of hand preference in young children. Brain Lang. 58, 1–22 10.1006/brln.1997.1754 (doi:10.1006/brln.1997.1754) [DOI] [PubMed] [Google Scholar]
- 31.Vauclair J., Imbault J. 2009. Relationships between manual preferences for object manipulation and pointing gestures in infants and toddlers. Dev. Sci. 12, 1060–1069 10.1111/j.1467-7687.2009.00850.x (doi:10.1111/j.1467-7687.2009.00850.x) [DOI] [PubMed] [Google Scholar]
- 32.Cochet H., Vauclair J. 2010. Pointing gestures produced by toddlers from 15 to 30 months: different functions, hand shapes and laterality patterns. Infant Behav. Dev. 33, 432–442 10.1016/j.infbeh.2010.04.009 (doi:10.1016/j.infbeh.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 33.McNeill D. 1992. Hand and mind. Chicago, IL: University of Chicago Press [Google Scholar]
- 34.Bernardis P., Gentilucci M. 2006. Speech and gesture share the same communication system. Neuropsychologia 44, 178–190 10.1016/j.neuropsychologia.2005.05.007 (doi:10.1016/j.neuropsychologia.2005.05.007) [DOI] [PubMed] [Google Scholar]
- 35.Willems R., Özyürek A., Hagoort P. 2007. When language meets action: the neural integration of gesture and speech. Cereb. Cortex 17, 2322–2333 10.1093/cercor/bhl141 (doi:10.1093/cercor/bhl141) [DOI] [PubMed] [Google Scholar]
- 36.Gentilucci M., Dalla Volta R. 2008. Spoken language and arm gestures are controlled by the same motor control system. Q. J. Exp. Psychol. 61, 944–957 10.1080/17470210701625683 (doi:10.1080/17470210701625683) [DOI] [PubMed] [Google Scholar]
- 37.Bernardis P., Bello A., Pettenati P., Stefanini S., Gentilucci M. 2008. Manual action affect vocalizations of infants. Exp. Brain Res. 184, 599–603 10.1007/s00221-007-1256-x (doi:10.1007/s00221-007-1256-x) [DOI] [PubMed] [Google Scholar]
- 38.Corina D. P., San Jose-Robertson L., Guillemin A., High J., Braun A. R. 2003. Language lateralization in a bimanual language. J. Cogn. Neurosci. 15, 718–730 10.1162/jocn.2003.15.5.718 (doi:10.1162/jocn.2003.15.5.718) [DOI] [PubMed] [Google Scholar]
- 39.Emmorey K., Mehta S., Grabowski T. J. 2007. The neural correlates of sign versus word production. Neuroimage 36, 202–208 10.1016/j.neuroimage.2007.02.040 (doi:10.1016/j.neuroimage.2007.02.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendon A. 1991. Some considerations for a theory of language origins. Man 26, 199–221 10.2307/2803829 (doi:10.2307/2803829) [DOI] [Google Scholar]
- 41.Meguerditchian A., Cochet H., Vauclair J. 2011. From gesture to language: ontogenetic and phylogenetic perspectives on gestural communication and its cerebral lateralization. In Primate communication and human language: vocalisation, gestures, imitation and deixis in humans and non-humans (eds Vilain A., Schwartz J. L., Abry C., Vauclair J.), pp. 89–118 Amsterdam, The Netherlands: John Benjamins [Google Scholar]
- 42.Goodall J. 1986. The chimpanzees of Gombe: patterns of behaviour. Cambridge, MA: Harvard University Press [Google Scholar]
- 43.Leavens D. A. 2004. Manual deixis in apes and humans. Interact. Stud. 5, 387–408 10.1075/is.5.3.05lea (doi:10.1075/is.5.3.05lea) [DOI] [Google Scholar]
- 44.Pika S., Liebal K., Call J., Tomasello M. 2005. The gestural communication of apes. Gesture 5, 41–56 10.1075/gest.5.1.05pik (doi:10.1075/gest.5.1.05pik) [DOI] [Google Scholar]
- 45.Pika S., Mitani J. C. 2006. Referential gestural communication in wild chimpanzees (Pan troglodytes). Curr. Biol. 16, 191–192 10.1016/j.cub.2006.02.037 (doi:10.1016/j.cub.2006.02.037) [DOI] [PubMed] [Google Scholar]
- 46.Call J., Tomasello M. 2007. The gestural communication of apes and monkeys. New York, NY: LEA [Google Scholar]
- 47.Pollick A. S., de Waal F. B. M. 2007. Ape gestures and language evolution. Proc. Natl Acad. Sci. USA 104, 8184–8189 10.1073/pnas.0702624104 (doi:10.1073/pnas.0702624104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebal K., Pika S., Call J., Tomasello M. 2004. To move or not to move: how apes alter the attentional state of others. Interact. Stud. 5, 199–219 10.1075/is.5.2.03lie (doi:10.1075/is.5.2.03lie) [DOI] [Google Scholar]
- 49.Leavens D. A., Russell J. L., Hopkins W. D. 2005. Intentionality as measured in the persistence and elaboration of communication by chimpanzees (Pan troglodytes). Child Dev. 76, 291–306 10.1111/j.1467-8624.2005.00845.x (doi:10.1111/j.1467-8624.2005.00845.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poss S. R., Kuhar C., Stoinski T. S., Hopkins W. D. 2006. Differential use of attentional and visual communicative signaling by orangutans (Pongo pygmaeus) and gorillas (Gorilla gorilla) in response to the attentional status of a human. Am. J. Primatol. 68, 978–992 10.1002/ajp.20304 (doi:10.1002/ajp.20304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cartmill E. A., Byrne R. W. 2007. Orangutans modify their gestural signalling according to their audience's comprehension. Curr. Biol. 17, 1345–1348 10.1016/j.cub.2007.06.069 (doi:10.1016/j.cub.2007.06.069) [DOI] [PubMed] [Google Scholar]
- 52.Leavens D. A., Russell J. L., Hopkins W. D. 2010. Multimodal communication by captive chimpanzees (Pan troglodytes). Anim. Cogn. 13, 33–40 10.1007/s10071-009-0242-z (doi:10.1007/s10071-009-0242-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hewes G. W. 1973. Primate communication and the gestural origin of language. Curr. Anthropol. 14, 5–24 10.1086/201401 (doi:10.1086/201401) [DOI] [Google Scholar]
- 54.Corballis M. C. 2002. From hand to mouth: the origins of language. Princeton, NJ: Princeton University Press [Google Scholar]
- 55.Meguerditchian A., Vauclair J. 2008. Vocal and gestural communication in nonhuman primates and the question of the origin of language. In Learning from animals? Examining the nature of human uniqueness (eds Roska-Hardy L. S., Neumann-Held E. M.), pp. 61–85 London, UK: Psychology Press [Google Scholar]
- 56.Arbib M. A., Liebal K., Pika S. 2008. Primate vocalization, gesture, and the evolution of human language. Curr. Anthropol. 49, 1053–1063 10.1086/593015 (doi:10.1086/593015) [DOI] [PubMed] [Google Scholar]
- 57.Hopkins W. D., Cantero M. 2003. From hand to mouth in the evolution of language: the influence of vocal behaviour on lateralized hand use in manual gestures by chimpanzees. Dev. Sci. 6, 55–61 10.1111/1467-7687.00254 (doi:10.1111/1467-7687.00254) [DOI] [Google Scholar]
- 58.Hopkins W. D., Russell J. L., Freeman H., Buehler N., Reynolds E., Schapiro S. J. 2005. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes). Psychol. Sci. 6, 487–493 10.1111/j.0956-7976.2005.01561.x (doi:10.1111/j.0956-7976.2005.01561.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meguerditchian A., Vauclair J., Hopkins W. D. 2010. Captive chimpanzees use their right hand to communicate with each other: implications for the origin of the cerebral substrate for language. Cortex 46, 40–48 10.1016/j.cortex.2009.02.013 (doi:10.1016/j.cortex.2009.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meguerditchian A., Vauclair J. 2006. Baboons communicate with their right hand. Behav. Brain Res. 171, 170–174 10.1016/j.bbr.2006.03.018 (doi:10.1016/j.bbr.2006.03.018) [DOI] [PubMed] [Google Scholar]
- 61.Meguerditchian A., Vauclair J. 2009. Contrast of hand preferences between communicative gestures and non-communicative actions in baboons: implications for the origins of hemispheric specialization for language. Brain Lang. 108, 167–174 10.1016/j.bandl.2008.10.004 (doi:10.1016/j.bandl.2008.10.004) [DOI] [PubMed] [Google Scholar]
- 62.Hopkins W. D., Vauclair J. In press. Evolution of behavioral and brain asymmetries in primates. In Handbook of language evolution (eds Tallerman M., Gibson K.). Oxford, UK: Oxford University Press [Google Scholar]
- 63.Shafer D. D. 1993. Patterns of hand preference in gorillas and children. In Primate laterality: current behavioral evidence of primate asymmetries (eds Ward J. P., Hopkins W. D.), pp. 267–283 New York, NY: Springer [Google Scholar]
- 64.Cantalupo C., Hopkins W. D. 2001. Asymmetrical Broca's area in great apes. Nature 414, 505. 10.1038/35107134 (doi:10.1038/35107134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cantalupo C., Pilcher D., Hopkins W. D. 2003. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia 41, 1975–1981 10.1016/S0028-3932(02)00288-9 (doi:10.1016/S0028-3932(02)00288-9) [DOI] [PubMed] [Google Scholar]
- 66.Taglialatela J. P., Cantalupo C., Hopkins W. D. 2006. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport 17, 923–927 10.1097/01.wnr.0000221835.26093.5e (doi:10.1097/01.wnr.0000221835.26093.5e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hopkins W. D., Nir T. 2010. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): the effect of handedness and comparison within findings in humans. Behav. Brain Res. 208, 436–443 10.1016/j.bbr.2009.12.012 (doi:10.1016/j.bbr.2009.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopkins W. D., Taglialatela J. P., Meguerditchian A., Nir T., Schenker N. M., Sherwood C. C. 2008. Gray matter asymmetries in chimpanzees as revealed by voxel-based morphology. Neuroimage 42, 491–497 10.1016/j.neuroimage.2008.05.014 (doi:10.1016/j.neuroimage.2008.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gannon P. J., Holloway R. L., Broadfield D. C., Braun A. R. 1998. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's language area homolog. Science 279, 220–222 10.1126/science.279.5348.220 (doi:10.1126/science.279.5348.220) [DOI] [PubMed] [Google Scholar]
- 70.Bailey P., von Bonin G., McCulloch W. S. 1950. The isocortex of the chimpanzee. Champaign, IL: University of Illinois Press [Google Scholar]
- 71.Spocter M. A., Hopkins W. D., Garrison A. R., Bauernfeind A. L., Stimpson C. D., Hof P. R., Sherwood C. C. 2010. Wernicke's area homologue in chimpanzees (Pan troglodytes) and its relation to the appearance of modern human language. Proc. R. Soc. B 277, 2165–2174 10.1098/rspb.2010.0011 (doi:10.1098/rspb.2010.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schenker N. M., Hopkins W. D., Spocter M. A., Garrison A. R., Stimpson C. D., Erwin J. M., Hof P. R., Sherwood C. C. 2010. Broca's area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry, and comparison to humans. Cereb. Cortex 20, 730–742 10.1093/cercor/bhp138 (doi:10.1093/cercor/bhp138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopkins W. D., De Waal F. B. M. 1995. Behavioral laterality in captive bonobos (Pan paniscus). Int. J. Primatol. 2, 261–276 10.1007/BF02735481 (doi:10.1007/BF02735481) [DOI] [Google Scholar]
- 74.Kalan A. K., Rainey H. J. 2009. Hand-clapping as a communicative gesture by wild female swamp gorillas. Primates 50, 273–275 10.1007/s10329-009-0130-9 (doi:10.1007/s10329-009-0130-9) [DOI] [PubMed] [Google Scholar]
- 75.Hostetter A. B., Cantero M., Hopkins W. D. 2001. Differential use of vocal and gestural communication in response to the attentional status of a human. J. Comp. Psychol. 115, 337–343 10.1037//0735-7036.115.4.337 (doi:10.1037//0735-7036.115.4.337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leavens D. A., Hostetter A. B., Wesley M. J., Hopkins W. D. 2004. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Anim. Behav. 67, 467–476 10.1016/j.anbehav.2003.04.007 (doi:10.1016/j.anbehav.2003.04.007) [DOI] [Google Scholar]
- 77.Fletcher A. W. 2006. Clapping in chimpanzees: evidence of exclusive hand preference in a spontaneous, bimanual gesture. Am. J. Primatol. 68, 1081–1088 10.1002/ajp.20308 (doi:10.1002/ajp.20308) [DOI] [PubMed] [Google Scholar]
- 78.Palmer A. R. 2002. Chimpanzee right-handedness reconsidered: evaluating the evidence with funnel plots. Am. J. Phys. Anthropol. 118, 191–199 10.1002/ajpa.10063 (doi:10.1002/ajpa.10063) [DOI] [PubMed] [Google Scholar]
- 79.Hopkins W. D., Cantalupo C. 2008. Theoretical speculations on the evolution of hemispheric specialization. Curr. Dir. Psychol. Sci. 17, 233–237 10.1111/j.1467-8721.2008.00581.x (doi:10.1111/j.1467-8721.2008.00581.x) [DOI] [Google Scholar]
- 80.Hopkins W. D., Cantalupo C. 2004. Handedness in chimpanzees is associated with asymmetries in the primary motor cortex but not with homologous language areas. Behav. Neurosci. 118, 1176–1183 10.1037/0735-7044.118.6.1176 (doi:10.1037/0735-7044.118.6.1176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins W. D., Taglialatela J. P., Russell J. L., Nir T. M., Schaeffer J. A. 2010. Cortical representation of lateralized grasping in chimpanzees (Pan troglodytes): a combined MRI and PET study. PLoS ONE 510, e13383. 10.1371/journal.pone.0013383 (doi:10.1371/journal.pone.0013383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taglialatela J. P., Russell J. L., Schaeffer J. A., Hopkins W. D. 2008. Communicative signaling activates ‘Broca's’ homologue in chimpanzees. Curr. Biol. 18, 343–348 10.1016/j.cub.2008.01.049 (doi:10.1016/j.cub.2008.01.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopkins W. D., Taglialatela J. P., Leavens D. A. 2007. Chimpanzees differentially produce novels vocalizations to capture the attention of a human. Anim. Behav. 73, 281–286 10.1016/j.anbehav.2006.08.004 (doi:10.1016/j.anbehav.2006.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]