Abstract
Although parasites and their hosts often coexist in a set of environmentally differentiated populations connected by gene flow, few empirical studies have considered a role of environmental variation in shaping correlations between traits of hosts and parasites. Here, we studied for the first time the association between the frequency of adaptive parasitic common cuckoo Cuculus canorus phenotypes in terms of egg matching and level of defences exhibited by its reed warbler Acrocephalus scirpaceus hosts across seven geographically distant populations in Europe. We also explored the influence of spring climatic conditions experienced by cuckoos and hosts on cuckoo–host egg matching. We found that between-population differences in host defences against cuckoos (i.e. rejection rate) covaried with between-population differences in degree of matching. Between-population differences in host egg phenotype were associated with between-population differences in parasitism rate and spring climatic conditions, but not with host level of defences. Between-population differences in cuckoo egg phenotype covaried with between-population differences in host defences and spring climatic conditions. However, differences in host defences still explained differences in mimicry once differences in climatic conditions were controlled, suggesting that selection exerted by host defences must be strong relative to selection imposed by climatic factors on egg phenotypes.
Keywords: Acrocephalus scirpaceus, coevolution, Cuculus canorus, environmental effect, climatic conditions, egg mimicry
1. Introduction
Coevolution often occurs over wide spatial and temporal scales. Understanding the spatio-temporal dynamic of the coevolutionary process is a central issue in current evolutionary ecology [1]. Theoretical and model-based approaches have proved useful in identifying a variety of factors that may influence such a dynamic [1–4], and pointed out difficulties in making inferences on the dynamic of the process through the examination of only one or a reduced subset of localities where the interaction takes place [5].
Empirical studies have often resorted to the study of coevolution over wide geographical scales of the interaction as an effective way of making inferences on the spatio-temporal dynamics of coevolution [6–10]. Because adaptive traits of interacting species often vary among populations, these studies aimed to substantiate whether complementary traits were closely correlated across the distributional range of the interaction as evidence of coevolution [7,9,11,12]. Trait covariation, however, should not unequivocally be interpreted as being due to reciprocal selection as it may emerge in unilaterally evolved systems as well [13]. Furthermore, the existence of trait covariation across the distributional range of the interaction does not imply perfect trait matching between interacting organisms at the population level. Examples of trait mismatch, where the most extreme trait in one species does not produce an ecological effect in the most extreme trait of the interacting species, include studies of trait correlation between pollinators and plant floral morphologies (reviewed by Anderson et al. [10]), between parasites and the size of the plant defensive apparatus [8], or between prey and predator traits [9]. Trait mismatch has commonly been considered the consequence of the existence of coevolutionary coldspots, where the interacting species may not experience reciprocal selection or due to gene flow into hotspots that hampers local adaptation [1,2]. However, recent findings have revealed that the direction of local trait mismatch (or armament imbalances) between plants and insects was related to the level of pollinator specialization and to plant mating system, suggesting the existence of predictable patterns of trait mismatch across the range of interaction [10].
Environmental conditions have been identified as a key factor determining the outcome of ecological interactions between species, thereby affecting the direction and strength of local coevolutionary selection [14]. Local environmental effects may, for instance, affect the growth dynamics (i.e. productivity) of one of the two species involved in the interaction, thus favouring local trait mismatch [8]. In addition, recent theoretical models have suggested that correlation between traits of interacting species may emerge if the climatic conditions favoured similar traits in both the interacting species [13]. Although the possible role of climatic conditions on the dynamic of coevolutionary interactions has been largely considered in theoretical studies [13,15], and its potential effects inferred using short generation times in systems under thermally controlled conditions [16,17], few empirical studies have considered a possible role of climatic conditions in shaping local levels of trait matching in parasite–host interactions over large geographical scales.
An ideal system for assessing possible environmental effects on trait mismatch involves the spatially structured interaction between the obligate avian brood parasite common cuckoo Cuculus canorus (hereafter cuckoo) and some of their favourite hosts. Cuckoo females lay their eggs in the nests of host species, and leave parental care of their offspring to unrelated foster parents [18]. Once the cuckoo egg hatches, the young cuckoo readily displaces all nest content by ejecting host eggs and/or nest-mates [19]. Consequently, the cuckoo chick grows up alone, having eliminated all host reproduction [20]. Cuckoo parasitism, therefore, imposes strong selection that has led to effective anti-cuckoo defensive mechanisms in their hosts, mainly discrimination and removal of cuckoo eggs [21–23]. This has selected for further elaborated counter-defences in the cuckoo to overcome host defences, such as more accurate host egg phenotype matching by the cuckoo [24].
A large number of studies have shown that selection pressure imposed by European brood parasites (i.e. common and great spotted cuckoos Clamator glandarius) on their hosts varies among populations, leading to differences in the frequency of adaptive host phenotypes/genotypes [25–27]. For example, the level of magpie host defensive phenotypes (i.e. rejection rate) in populations exposed to parasitism depends on selective pressure exerted by the parasitic great spotted cuckoo (i.e. parasitism rate) over large [28] and small [29] geographical scales. A similar pattern was reported for the common cuckoo and its favourite reed warbler Acrocephalus scirpaceus host in 14 populations distributed across Europe [30]. Interestingly, these patterns were independent of geographical and genetic distance among the sampled host populations, implying that local parasite pressure is important in shaping host phenotype [28,30]. So far, however, a test of the association between the frequency of adaptive cuckoo phenotypes in terms of egg matching and host defences is still lacking, which precludes any firm conclusion about the spatial organization of coevolutionary interactions in avian brood parasite–host systems.
The reed warbler, one of the most commonly used cuckoo hosts in the Palaearctic [31], breeds in reed-beds surrounding lakes, ponds and ditches across Europe [32]. Populations are connected by gene flow and migration [33,34], and show a pattern of genetic isolation by distance [35]. Previous studies have found that both cuckoo parasitism rate and rejection of cuckoo eggs by reed warblers vary spatially [24,36,37]. Also, rapid temporal increase in cuckoo egg matching in a recently parasitized reed warbler population has been observed [38], suggesting that there is scope for evolutionary response of the parasite across a large geographical scale. Here we first aim to test the hypothesis that cuckoo eggs match the phenotype of their hosts better in populations of reed warblers that show higher levels of egg rejection.
Our second specific aim was to test the role of general climatic variation on cuckoo–reed warbler egg phenotype matching. Recent work has emphasized an important role of environmental gradients in shaping correlations between traits of interacting species over large geographical scales [13,14]. A number of studies have found that, though eggshell pigmentation has a genetic basis in birds [39,40], environmental factors can influence bird egg coloration by their direct effects on the physiological status of laying females, but also indirectly via their effects on food availability [41–44]. In the framework of the coevolutionary interactions between cuckoos and their hosts, a long-term study in which cuckoo egg matching was monitored over 24 consecutive breeding seasons found that cuckoo mimicry on reed warbler eggs was better in springs with higher rainfall [45]. Interestingly, the effect of climatic conditions on egg phenotype was different for cuckoos and their reed warbler hosts [45].
It is therefore important to address the possibility that variation in cuckoo–host egg phenotype matching across populations could be partly due to abiotic environmental effects, some of which may favour similar egg traits in cuckoos and their hosts. On a large geographical scale, differences in climatic conditions that laying females of the cuckoo and their reed warbler host experience in the different populations are major factors affecting caterpillar [46], and insect, spider and small snail abundance [47,48], these being the preferred prey of cuckoos and reed warblers, respectively [32]. Hence, if egg coloration were in part environmentally determined, mimicry should be more similar among populations that experience similar environmental conditions irrespective of gene flow and the selective regime exerted by hosts on cuckoo egg phenotypes. In the same vein, populations experiencing similar climatic conditions should be more similar in the appearance of both cuckoo and reed warbler eggs.
2. Material and methods
(a). Data collection
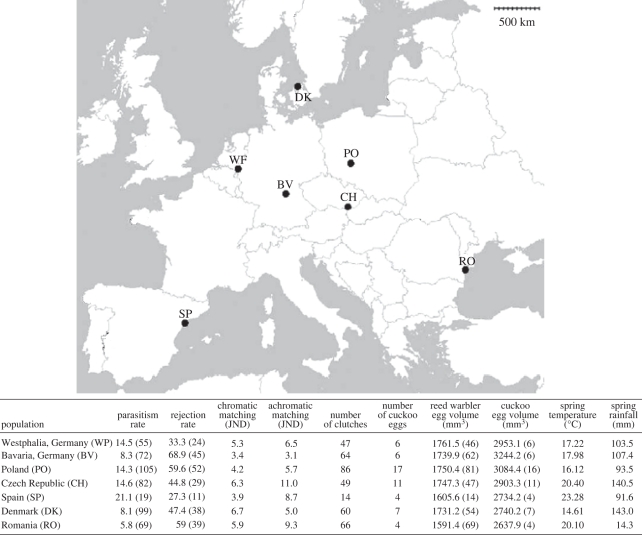
We studied cuckoo–host egg matching in 2003 and 2004 in all reed warbler populations found to be parasitized by cuckoos (n = 7) in a recent meta-populational study on reed warbler–cuckoo interactions (see [37] for further details; figure 1). In each population, we systematically searched for reed warbler nests during three weeks of the breeding season of the host. Previous experience from working with the reed warbler–cuckoo system confirmed that the three-week frame would suffice to encompass a representative part of host and cuckoo behaviours (i.e. more than 80% of the first clutches) across the entire breeding season (B. G. Stokke 2011, unpublished data). Nests were followed after they were found (usually at the nest-building or early laying stage) until fledging, which allowed us to determine the rate of parasitism in every population. We used current rate of cuckoo parasitism for each population as a measure of parasite-mediated selection on hosts. Based on photographs taken of cuckoo eggs that were later visually checked against reference clutches, we verified that all the cuckoo eggs we found were identified as belonging to the reed warbler gens. Finally, we used rejection rate of a standard non-mimetic egg in every population reported by Stokke et al. [30] as a standardized correlate of the selective pressure exerted by reed warbler hosts on cuckoo egg phenotypes [28]. We disregarded using rejection of natural cuckoo eggs because this is affected by differences in level of mimicry in each population. Furthermore, previous spatial analyses have reported a strong correlation between differences in rejection of non-mimetic and mimetic eggs across different host populations [49], which would support the use of non-mimetic models to estimate population differences in levels of host selection on cuckoo eggs. The standard non-mimetic eggs were real Chinese quail Coturnix chinensis egg painted pale blue resembling the redstart Phoenicurus phoenicurus cuckoo egg type, and which had approximately the same size as real cuckoo eggs [30].
Figure 1.
Geographical location of the seven sampled reed warbler populations parasitized by the European cuckoo along Europe (CH, Czech Republic; BV, Bavaria; RO, Romania; DK, Denmark; PO, Poland; SP, Spain; WP, Westphalia). Table shows data on cuckoo parasitism rate (%, with number of sampled nests in brackets), rejection rate of an experimentally added non-mimetic cuckoo-size egg (%, with number of sampled nests in brackets), number of clutches and cuckoo eggs in each population in which egg colour was measured with a spectrometer, degree of chromatic and achromatic matching between cuckoo and reed warbler eggs estimated with Vorobyev–Osorio model (see §2 for details), reed warbler and cuckoo EV (with number of eggs sampled in brackets), spring temperature and spring rainfall for the seven studied reed warbler populations. Owing to logistical problems, we could only measure with a spectrometer a random, but representative proportion (386 out of 501 clutches; i.e. 77%) of all the sampled nests for which we estimated cuckoo parasitism rate.
We analysed molecular markers for reed warblers in order to evaluate the genetic differentiation among the experimental populations and control for effects of gene flow in spatial analyses of the studied traits [34,35]. For the genetic analyses, we obtained reed warbler blood (5–15 μl) from the brachial vein either from nestlings (with only one sibling per nest included in analyses) or from mist-netted adults during the breeding season (21–54 individuals sampled per population, mean ± s.d. = 34.4 ± 10.6). Blood samples were stored in 96 per cent ethanol or Queen's lysis buffer until DNA extraction [34].
(b). Quantification of egg colour and volume in reed warblers and cuckoos
We estimated egg coloration (i.e. spectral reflectance at the 300–700 nm waveband) after reed warbler clutch completion with Ocean Optics spectrometer equipment: S2000 spectrometer connected to a deuterium–halogen light (D2-W, mini) by a coaxial reflectance probe (QR-400-7-UV-vis) and the OOIBase32 operating software (Ocean Optics, Inc., Dunedin, FL). Number of reed warbler clutches and cuckoo eggs measured are in figure 1. Reflectance was measured with the probe placed at a constant distance and a 45° angle. Measurements were relative to a standard white (WS-2) and to the dark, which was calibrated before measurement of each clutch. All measurements were performed in a dark room indoors to avoid an effect of ambient light on spectro-measurements. Previous studies using this calibration protocol have shown that spectrophotometry provides reliable estimates of egg coloration, and a high consistency between measures of eggs in a clutch of reed warblers [38].
Whole spectral reflectance data for cuckoo and reed warbler eggs was reduced by principal component analysis [38,50]. Together the three first components explained 99.1 per cent of the total variance in spectra of cuckoo and reed warbler eggs (see electronic supplementary material, figure S1). PC1 was approximately flat and described achromatic variation (brightness hereafter). PC2 had high positive loadings approximately at the blue (400–475 nm) and negative ones at the red (625–700 nm) wavelengths and thus could classify the sampled eggs along a gradient of blue–redness (blue–red chroma hereafter). PC3 had high negative loadings at short wavelengths (300–400 nm) and had high positive loadings approximately at the blue wavelengths (400–475 nm), and therefore we could classify the sampled eggs along a gradient of ultraviolet–blueness (UV–blue chroma hereafter; see electronic supplementary material, appendix figure S1). Egg length (EL) and breadth (EB) was estimated to the nearest 0.1 mm with digital callipers, and egg volume (EV) in cubic millimetres was calculated using the formula EV = 0.5 × EL × EB2 [51].
(c). Estimating cuckoo–reed warbler egg matching
The shape of a reflectance spectrum often does not directly equate to how the signal is processed by the receiver because of different sensitivity to different components of the spectrum. It is therefore important to use models of avian vision that account for what the receiver's (i.e. host's) eye actually can perceive as colour matching [52,53]. Here, we ran (Vorobyev–Osorio) physiological models [54] with Avicol software v. 3 [55] that reproduce host retinal functioning and that account for nest luminosity and bird sensitivity for estimating colour (i.e. chromatic and achromatic) matching from the perspective of a reed warbler host. Using these models, it is possible to integrate the reflectance spectra of cuckoo and reed warbler eggs and the ambient irradiance in the nests with published information for single and double cone photoreceptor spectral sensitivities, photoreceptor noise and the transmission properties of avian ocular media for the host receiver to generate biologically reliable colour-matching estimates [54]. Irradiance spectra at the nests of a typical open nester such as the reed warbler were extracted from Avilés et al. [56]. Spectral sensitivity has not been measured in the reed warbler; therefore, we used spectral sensitivity data from the blackbird Turdus merula [57,58], which is the closest relative of the reed warbler with known sensitivity information. The use of blackbird spectral sensitivity is justified given current evidence showing that visual sensitivities among higher passerines are highly conserved [59], and given negligible differences between model calculations obtained using spectral sensitivity data for different passerine species [60]. The model establishes a chromatic and achromatic (i.e. ΔS and ΔQ, respectively) distance, which describes the contrasts between two coloured eggs in a visual space that depends of the number of receptor types of the signal receiver. The units for ΔS and ΔQ are just noticeable differences (JNDs). Essentially, different eggs that appear similar to a reed warbler host (either because of the nature of their visual system or an absolutely small difference in the reflectance spectra of the eggs) result in small ΔS and ΔQ values, while eggs with a poor match have large ΔS and ΔQ values [61]. To investigate spatial variation in phenotypic matching between cuckoo eggs and those of the reed warbler hosts, we generated chromatic and achromatic contrasts (i.e. in JND) and absolute values of differences in EV between colour and volume averaged over reed warbler clutches in a population, and colour and volume for every cuckoo egg sampled in that population in the knowledge that high phenotype matching produces the most successful cuckoo parasitism rate [24].
(d). Genetic distance estimates
DNA was isolated from reed warbler blood using DNeasy Tissue Kit (Qiagen, Venlo, The Netherlands) or a Chelex (BioRad, Hercules, CA) resin-based extraction method [62]. Genotyping was performed using 10 polymorphic microsatellite loci originally developed for use in other passerine bird species: Aar4, Aar5, Aar8, Ase34, Ase58, FhU2, Pca3, Pdoμ1, POCC2 and Ppi2 [34]. Further details on allele diversity and amplification results for the primers used in this study are available in Prochazka et al. [34]. Allele frequencies for each population were calculated in FSAT v. 2.9.3.2 [63], and we obtained the paired FST-values between reed warbler populations as estimates of genetic distances.
(e). Climatic data
Previous work has shown that both spring temperature and accumulated rainfall correlated with cuckoo and reed warbler egg coloration at the population level, suggesting that climatic conditions during spring truly reflect the strength at which climate affects egg coloration in this system [45]. Therefore, we here relied on daily temperature and precipitation from 1 May to 30 June as local weather variables used to identify spatial patterns in cuckoo and reed warbler egg phenotypes mediated by environmental conditions. We selected this period because it was the period when all sampled clutches were laid [30,37]. Daily climatic data on temperature and precipitation in 2003 for the studied reed warbler populations were obtained from the European Climate Assessment and Dataset (ECA&D) project (http://eca.knmi.nl). The ECA&D contains series of daily observations at meteorological stations throughout Europe and the Mediterranean [64,65]. From these data, two indices of spring climatic conditions per population were calculated [66]: the average spring temperature (spring temperature hereafter) calculated from daily mean temperature data from 1 May to 30 June, and the accumulated rainfall (spring rainfall hereafter) during the same period (figure 1).
(f). Statistical analyses
In a first step, we calculated matrices of pair-wise differences between reed warbler populations in degree of parasite adaptation to reed warblers (i.e. chromatic, achromatic matching and absolute differences in volume), cuckoo and host egg phenotype (i.e. PC1, PC2 and PC3 colour scores and volume), degree of host adaptation to cuckoo parasitism (i.e. rejection rate of non-mimetic eggs), selective pressure exerted by cuckoos (i.e. parasitism rate), and environmental conditions (i.e. spring temperature and rainfall). Associations between matrices were then tested using partial Mantel's tests using the library ‘ecodist’ [67] implemented in the R package (R Development Core Team, Vienna, Austria). The significance of partial Mantel tests was tested using permutations with estimations of p-values after 2000 randomizations. The partial Mantel tests is a derived form of the Mantel test carried out using partial correlation statistics that quantifies the relationship between two distance matrices while controlling for the effects of other distance matrices. Mantel approaches are spatial methods explicitly developed for dealing with hypotheses under study that can only be formulated in terms of distances, as is often the case with genetic data and geographical distances [68,69].
The use of partial Mantel analyses implies performing many tests using the same data to investigate sets of hypotheses, which artificially increases the risk of drawing at least a false conclusion by chance [67]. We have corrected for multiple testing using the algorithm developed by Benjamini & Hochberg [70] to control the false discovery rate (FDR; further details in electronic supplementary material 2).
3. Results
(a). Association between degree of egg matching and parasitism rate, rejection rate and climatic conditions
Distance in parasitism and dissimilarities in cuckoo–host egg matching were not significantly associated when controlling for geographical and genetic distance and pair-wise differences in climatic condition (table 1). However, between-population differences in host defence (i.e. rejection rate) influenced egg phenotype matching by cuckoos when controlling for geographical and genetic distances and pair-wise differences in climatic conditions (table 1). Higher differences in rejection rate were associated with larger differences in volume match (table 1). However, distances in climatic conditions were not significantly associated with differences in cuckoo–host egg matching once the effect of parasitism-related variables and geographical and genetic distance were taken into account (table 1).
Table 1.
Partial Mantel tests for studying among-population associations between differences in cuckoo–host matching (chromatic, achromatic and volume) and differences in cuckoo parasitism rate, rejection rate, spring temperature and rainfall. The number of permutations was set to 2000 in all tests and variables in brackets indicate variables that are controlled in partial Mantel tests. Significant Mantel tests after correction for the false discovery rate are shown in bold.
| chromatic matching (ΔS) |
achromatic matching (ΔQ) |
volume matching |
||||
|---|---|---|---|---|---|---|
| Mantel r | p-value | Mantel r | p-value | Mantel r | p-value | |
| parasitism rate (geo-distance, genetic distance, temperature and rainfall) | −0.21 | 0.43 | 0.11 | 0.70 | −0.18 | 0.51 |
| rejection rate (geo-distance, genetic distance, temperature and rainfall) | −0.41 | 0.08 | −0.31 | 0.27 | 0.80 | 0.008 |
| temperature (geo-distance, genetic distance, parasitism rate, rejection rate and rainfall) | −0.09 | 0.74 | 0.47 | 0.07 | 0.25 | 0.36 |
| rainfall (geo-distance, genetic distance, parasitism rate, rejection rate and temperature) | 0.31 | 0.23 | 0 | 0.99 | 0.14 | 0.62 |
(b). Association between host egg phenotype and parasitism rate, rejection rate and climatic conditions
Parasitism rate influenced host egg phenotype, in that populations that differed more in parasitism rate differed more in host egg brightness and in their PC2 scores (i.e. were more different along the axis defining relative blueness versus redness) after controlling for geographical and genetic distance and pair-wise differences in climatic conditions (table 2). Population differences in rejection rate, however, did not significantly relate to population differences in reed warbler egg phenotype after controlling for geographical and genetic distance and pair-wise differences in climatic conditions (table 2).
Table 2.
Partial Mantel tests for studying among-population associations between differences in host egg phenotype (PC1, PC2, PC3 colour scores and EV) and differences in cuckoo parasitism rate, rejection rate spring temperature and rainfall. The number of permutations was set to 2000 in all tests and variables in brackets indicate variables that are controlled in partial Mantel tests. Significant Mantel tests after correction for the false discovery rate are shown in bold.
| PC1 colour score (brightness) |
PC2 colour score (blue–red) |
PC3 colour score (UV–blue) |
EV |
|||||
|---|---|---|---|---|---|---|---|---|
| Mantel r | p-value | Mantel r | p-value | Mantel r | p-value | Mantel r | p-value | |
| parasitism rate (geo-distance, genetic distance, temperature and rainfall) | 0.56 | 0.01 | 0.63 | 0.007 | 0.37 | 0.15 | 0.11 | 0.68 |
| rejection rate (geo-distance, genetic distance, temperature and rainfall) | −0.03 | 0.91 | −0.06 | 0.83 | −0.354 | 0.18 | 0.35 | 0.21 |
| temperature (geo-distance, genetic distance, parasitism rate, rejection rate and rainfall) | −0.57 | 0.03 | −0.35 | 0.21 | −0.45 | 0.09 | −0.60 | 0.01 |
| rainfall (geo-distance, genetic distance, parasitism rate, rejection rate and temperature) | −0.001 | 0.48 | 0.11 | 0.73 | −0.29 | 0.29 | 0.69 | 0.01 |
Differences in climatic conditions were related to differences in host egg phenotype after controlling for geographical and genetic distance, and differences in parasitism and rejection rate (table 2). Pair-wise differences in host egg brightness and in host EV increased with decreasing spring temperature differences between populations, whereas pair-wise differences in host EV increased with increasing spring rainfall differences between populations (table 2).
(c). Association between cuckoo egg phenotype and parasitism rate, rejection rate and climatic conditions
Host populations that differed more in parasitism rate were parasitized by cuckoo populations that differed more for PC2 (i.e. were more different along the axis defining relative blue–red reflection) after accounting for geographical and genetic distance and pair-wise differences in climatic conditions (table 3). Host populations that differed more in rejection rate were parasitized by cuckoo populations that differed less for PC2 and PC3, and more for cuckoo EV after simultaneously controlling for geographical and genetic distance and differences in climatic conditions (table 3).
Table 3.
Partial Mantel tests for studying among-population associations between differences in cuckoo egg phenotype (PC1, PC2, PC3 colour scores and EV) and differences in cuckoo parasitism rate, rejection rate and climatic conditions. The number of permutations was set to 2000 in all tests and variables in brackets indicate variables that are controlled in partial Mantel tests. Significant Mantel tests after correction for the false discovery rate are shown in bold.
| PC1 colour score (brightness) |
PC2 colour score (blue–red) |
PC3 colour score (UV–blue) |
EV |
|||||
|---|---|---|---|---|---|---|---|---|
| Mantel r | p-value | Mantel r | p-value | Mantel r | p-value | Mantel r | p-value | |
| parasitism rate (geo-distance, genetic distance, temperature and rainfall) | 0.37 | 0.14 | 0.75 | 0.003 | 0.49 | 0.04 | −0.19 | 0.46 |
| rejection rate (geo-distance, genetic distance, temperature and rainfall) | 0.30 | 0.38 | −0.73 | 0.001 | −0.62 | 0.016 | 0.80 | 0.007 |
| temperature (geo-distance, genetic distance, parasitism rate, rejection rate and rainfall) | −0.64 | 0.01 | 0.42 | 0.09 | 0.18 | 0.53 | −0.01 | 0.92 |
| rainfall (geo-distance, genetic distance, parasitism rate, rejection rate and temperature) | 0.52 | 0.14 | −0.87 | 0.001 | 0.22 | 0.44 | 0.42 | 0.10 |
Differences in climatic conditions explained differences in cuckoo egg phenotype between populations after accounting for cuckoo parasitism-related distances and geographical and genetic distances (table 3). Differences in temperature between populations were negatively correlated with differences in brightness, whereas differences in rainfall were positively correlated with differences in the blue–red axis of cuckoo eggs (i.e. PC2 colour scores; table 3).
4. Discussion
(a). Host defence and cuckoo host egg phenotype matching
Common cuckoos parasitizing different reed warbler populations are exposed to different levels of defence by hosts [30] (table 1). Differences in host defences explained spatial variation in the strength of the coevolutionary interaction across reed warbler populations once gene flow between populations, geographic distance and environmental conditions (i.e. spring temperature and rainfall) were taken into account. Indeed, a variable strongly related to the level of host-specific defences against cuckoos in the different populations (i.e. rejection rate) was associated with the relative level of parasite counter-adaptation (i.e. host egg phenotype matching by cuckoos) in different populations. In a previous study, in which we considered reed warbler populations sympatric and allopatric with the cuckoo in Europe, it was shown that geographical distances between populations did not explain differences in rejection rate between reed warbler populations [30]. Furthermore, genetic distance did not explain rejection differences in the set of seven sympatric populations considered in the present study (Mantel r = 0.24, p = 0.27). Thus, the found pattern was independent of genetic and geographical differences between populations, suggesting that an important part of variance in cuckoo level of counter-adaptation to reed warbler hosts is due to current selection pressure exerted by host defences. As far as we are aware, our results provide the first evidence that the geographically structured pattern of host resistance in response to cuckoo parasitism in Europe [30] may also affect the spatial dynamics of cuckoo counter-adaptation in terms of egg phenotype matching.
The across-population correlation between cuckoo egg phenotype matching and rejection found here cannot be unequivocally considered as evidence of coevolution, however [13,71]. Theoretical models have shown that trait correlation across sites could potentially arise if the interaction has potent fitness consequences only for one of the two species or if the outcome of the interactions depends on the phenotype of one of the two species [13]. Detailed mechanistic studies on cuckoo–reed warbler interactions have shown that cuckoo parasitism imposes strong fitness costs on the reed warbler and that egg rejection by reed warbler imposes large costs on the cuckoo [21]. Furthermore, mimicry (i.e. trait matching) in the cuckoo–reed warbler interaction depends on both host and parasite egg phenotypes [21]. Thus, the cuckoo–reed warbler interaction does not fulfil the one-way interaction condition that can create trait correlation virtually indistinguishable from that evolved due to a coevolutionary process.
A second non-coevolutionary process that theoretical models have suggested may readily lead to correlated traits between interacting species is stabilizing selection with correlated optimal phenotypes [13]. Such a scenario is likely to arise if traits involved in interactions are affected by similar abiotic variables. Previous studies reported that environmental gradients influenced the magnitude of trait correlations in prey–predator evolutionary interactions [14]. Here, we explored the influence of differences in spring temperature and rainfall (as a measure for differences in breeding abiotic environments experienced by cuckoos and hosts) on the relationship between host level of defences and cuckoo–host egg phenotype matching. Population differences in spring climatic conditions explained differences in cuckoo and host egg phenotypes (see below). However, differences in host rejection rate still explained differences in cuckoo egg phenotype matching once we controlled for differences in spring climatic conditions, which suggests that selection exerted by host defences must be strong relative to stabilizing selection imposed by abiotic environment in cuckoo and reed warbler egg phenotypes.
EV, but not chromaticity and brightness, was the component of cuckoo host egg phenotype matching associated with egg rejection of cuckoo eggs. Analyses of cuckoo egg phenotype confirmed this finding since spatial variation in volume of cuckoo eggs was positively associated with spatial variation in rejection rate irrespective of genetic and geographical distances between populations. Comparative studies have shown that parasitic cuckoo species have smaller eggs relative to body size than non-parasitic ones [72,73]. Indeed, long-term specialization of the cuckoo on one or a few favourite hosts with variable egg size has resulted in host-specific egg size polymorphism in the cuckoo [31,74]. It has been suggested that laying a small egg that matches the host eggs in size might benefit the cuckoo both by increasing the chance of acceptance and by facilitating incubation [72]. Experimental studies have confirmed the adaptive value of laying small eggs for cuckoos parasitizing reed warbler hosts since reed warblers can more easily discriminate against foreign eggs larger than their own [21]. Therefore, our results suggest a main role of current level of host defence on the evolution of cuckoo egg phenotype over the analysed geographical scale. Interestingly, we also found that chromaticity of host egg phenotype was most influenced by cuckoo parasitism. In cuckoo–host systems, it is well established that rejection behaviour of hosts has led to egg mimicry by cuckoos [23–24,60,75]. Selection for discrimination of mimetic cuckoo eggs is expected to be facilitated by low variation in host egg appearance within a clutch [76–82], which will increase variation between clutches laid by different host females at the population level [24,77]. Increased host phenotypic polymorphism renders it less likely that a cuckoo can accurately match any one individual's clutch in the population, thus facilitating egg recognition and rejection [83,84]. In agreement with this scenario of cuckoo parasitism favouring host egg polymorphism at the population level, we have found that larger differences in chromaticity were associated with larger differences in cuckoo parasitism between populations (table 2), which provides a new source of evidence that cuckoo parasitism may exert reciprocal selection on the morphology of host eggs.
(b). Climatic conditions and cuckoo host egg phenotype matching
Previous studies have revealed the influence of climatological factors on different features of the arms race between cuckoos and their hosts in a single population (spring climate influences cuckoo–host egg phenotype matching [45]; climate change affects cuckoo–host mismatching in phenology [85]). Whenever traits involved in spatial coevolutionary interactions are influenced by similar abiotic and/or biotic factors, stabilizing selection towards correlated optimal phenotypes may readily lead to correlation between traits [13]. Our results revealed that differences in spring climatic conditions between the different populations did not explain a significant proportion of between-population differences in cuckoo–host egg phenotype matching once host level of defences was held constant (table 1).
Interestingly, spatial analyses performed separately on host and cuckoo egg phenotypes revealed that host and cuckoo egg phenotypes were more dissimilar in populations that were more dissimilar in spring climatic conditions irrespective of between-population differences in parasitism and host defence. A previous long-term study in one population revealed that blueness of cuckoo and reed warbler eggs were affected by different climatic components, which suggests an effect of abiotic factors on cuckoo–host phenotypes through a mechanism of species-specific susceptibility to environmental effects [45]. Our results over a large geographical scale confirm these findings, since we have detected that spring climatic factors affected different egg features for cuckoos and reed warblers in Europe. In cuckoos, climatic effects were evident on egg brightness and coloration, whereas in hosts spring climatic conditions influenced brightness and volume (tables 2 and 3). Differences in diet between cuckoos and reed warblers would predict species-specific susceptibility to environmental factors affecting food availability. Adult cuckoos mostly feed on caterpillars, which are avoided by reed warblers [19], while adult reed warblers feed on other insects, spiders and small snails captured mostly in reeds [32]. Abundance of these prey types for cuckoos and reed warblers can be affected by climatological conditions [47,48]. Alternatively, direct effects of environmental factors may also induce species-specific environmental effects on egg colour if cuckoo and reed warbler females had different oxidative costs associated with the inclusion of blue–green-related pigments in their eggs. Indeed, pigments used to colour the eggs cannot be used to prevent cellular oxidative stress processes [86], and there exists evidence suggesting a limitation of blue–green-related pigment to colour the eggs [44]. In addition, cuckoos lay many more eggs than non-parasitic birds [72], and thus this limitation could be important for cuckoo females. Importantly, although our results demonstrated species-specific climatic effects on cuckoos and their reed warbler hosts, differences in cuckoo–host egg phenotype matching were not explained by climatic conditions when the effect of host level of defence was considered in the same analysis, which suggests that selection arising from environmental factors should be low relative to selection exerted by host defences in this putative arms race.
5. Conclusion
Two important consequences emerge from our study. First, through the association between cuckoo level of counter-adaptation measured as phenotype matching in a genetically determined trait (i.e. egg appearance [40]) and a genetically determined component of host defence against cuckoos (i.e. rejection rate [87]), we have found differences in the strength of the coevolutionary interactions between common cuckoos and reed warblers at a European geographical scale. Second, for species interactions mediated by a mechanism of phenotype matching (e.g. similarity in egg appearance of cuckoos and host eggs) that occur over a gradient of environmental conditions, stabilizing selection imposed by the abiotic environment may differ for the two species, and/or sometimes be strong relative to reciprocal selection, leading to apparent imperfect cuckoo–host egg phenotype matching. Our results therefore highlight the importance of considering environmental gradients as well as species-specific environmental susceptibility of traits when studying the spatial dynamic of coevolutionary adaptations.
Acknowledgements
We thank Johannes Bang, Josef Beier, David Bigas Campàs, Andrzej Dyrcz, Klaus Ernst, Jon Fjeldså, Inge Hafstad, Marcel Honza, Bernd Leisler, Péter L. Pap, Stefani Pleines, Geir Rudolfsen, Karl Schulze-Hagen, Petr Prochazka, Henrik Jensen and Georg Sennert for various contributions. We acknowledge the E-OBS dataset from the EU-FP6 project ENSEMBLES (http://ensembles-eu.metoffice.com) and the data providers in the ECA&D project (http://eca.knmi.nl) for kindly providing climatic data for the studied populations. During manuscript preparation J.M.A. was funded by CAS and the Spanish Ministry of Education and Science (CGL2008-00718). Fieldwork was supported for F.F., A.M., E.R. and B.G.S. by a Norwegian Research Council fellowship, while J.R.V. was funded by a grant from the Faculty of Science and Technology, NTNU. Ben Ridenhour, Judith L. Bronstein, Elizabeth Adkins-Regan and two anonymous referees made very useful suggestions on previous drafts of the manuscript.
References
- 1.Thompson J. N. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Thompson J. N. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 3.Hochberg M. E., van Baalen M. 1998. Antagonistic coevolution over productivity gradients. Am. Nat. 152, 620–634 10.1086/286194 (doi:10.1086/286194) [DOI] [PubMed] [Google Scholar]
- 4.Gomulkiewicz R., Thompson J. N., Holt R. D., Nuismer S. L., Hochberg M. E. 2000. Hot spots, cold spots, and the geographic mosaic theory of coevolution. Am. Nat. 156, 156–174 10.1086/303382 (doi:10.1086/303382) [DOI] [PubMed] [Google Scholar]
- 5.Gomulkiewicz R., Drown D. M., Dybdahl M. F., Godsoe W., Nuismer S. L., Pepin K. M., Ridenhour B. J., Smith C. I., Yoder J. B. 2007. Dos and don'ts of testing the geographic mosaic theory of coevolution. Heredity 98, 249–258 10.1038/sj.hdy.6800949 (doi:10.1038/sj.hdy.6800949) [DOI] [PubMed] [Google Scholar]
- 6.Lively C. M., Jokela J. 1996. Clinal variation for local adaptation in a host–parasite interaction. Proc. R. Soc. Lond. B 263, 891–897 10.1098/rspb.1996.0132 (doi:10.1098/rspb.1996.0132) [DOI] [Google Scholar]
- 7.Benkman C. W., Holimon W. C., Smith J. W. 2001. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution 55, 282–294 [DOI] [PubMed] [Google Scholar]
- 8.Toju H., Sota T. 2006. Imbalance of predator and prey armament: geographic clines in phenotypic interface and natural selection. Am. Nat. 167, 105–117 10.1086/498277 (doi:10.1086/498277) [DOI] [PubMed] [Google Scholar]
- 9.Hanifin C. T., Brodie E. D., Brodie E. D. 2008. Phenotypic mismatches reveal escape from arms-race coevolution. Public Libr. Sci. 6, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson B., Terblanche J. S., Ellis A. G. 2010. Predictable patterns of trait mismatches between interacting plants and insects. BMC Evol. Biol. 10, 204. 10.1186/1471-2148-10-204 (doi:10.1186/1471-2148-10-204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodie E. D., Ridenhour B. J., Brodie E. D. 2002. The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 56, 2067–2082 [DOI] [PubMed] [Google Scholar]
- 12.Anderson B., Johnson S. D. 2008. The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution 62, 220–225 10.1111/j.1558-5646.2007.00275.x (doi:10.1111/j.1558-5646.2007.00275.x) [DOI] [PubMed] [Google Scholar]
- 13.Nuismer S. L., Gomulkiewicz R., Ridenhour B. J. 2010. When is correlation coevolution? Am. Nat. 175, 525–537 10.1086/651591 (doi:10.1086/651591) [DOI] [PubMed] [Google Scholar]
- 14.Toju H. 2008. Fine-scale local adaptation of weevil mouthpart length and camellia pericarp thickness: altitudinal gradient of a putative arms race. Evolution 62, 1086–1102 10.1111/j.1558-5646.2008.00341.x (doi:10.1111/j.1558-5646.2008.00341.x) [DOI] [PubMed] [Google Scholar]
- 15.Thomas M. B., Blanford S. 2003. Thermal biology in insect–parasite interactions. Trends Ecol. Evol. 18, 344–350 10.1016/S0169-5347(03)00069-7 (doi:10.1016/S0169-5347(03)00069-7) [DOI] [Google Scholar]
- 16.Blanford S., Thomas M. B., Pugh C., Pell J. K. 2003. Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecol. Lett. 6, 2–5 10.1046/j.1461-0248.2003.00387.x (doi:10.1046/j.1461-0248.2003.00387.x) [DOI] [Google Scholar]
- 17.Zhang Q. G., Buckling A. 2011. Antagonistic coevolution limits population persistence of a virus in a thermally deteriorating environment. Ecol. Lett. 14, 282–288 10.1111/j.1461-0248.2010.01586.x (doi:10.1111/j.1461-0248.2010.01586.x) [DOI] [PubMed] [Google Scholar]
- 18.Davies N. B. 2000. Cuckoos, cowbirds and other cheats. London: T. A. D. Poyser [Google Scholar]
- 19.Wyllie I. 1981. The cuckoo. London: Batsford [Google Scholar]
- 20.Kruger O. 2007. Cuckoos, cowbirds and hosts: adaptations, trade-offs and constraints. Phil. Trans. R. Soc. B 362 1873–1886 10.1098/rstb.2006.1849 (doi:10.1098/rstb.2006.1849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies N. B., Brooke M. D. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284 10.1016/S0003-3472(88)80269-0 (doi:10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 22.Rothstein S. I. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Evol. Syst. 21, 481–508 10.1146/annurev.es.21.110190.002405 (doi:10.1146/annurev.es.21.110190.002405) [DOI] [Google Scholar]
- 23.Moksnes A., Røskaft E., Braa A. T. 1991. Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk 108, 348–354 [Google Scholar]
- 24.Brooke M. D., Davies N. B. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632 10.1038/335630a0 (doi:10.1038/335630a0) [DOI] [Google Scholar]
- 25.Davies N. B., Brooke M. D. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 58, 207–224 10.2307/4995 (doi:10.2307/4995) [DOI] [Google Scholar]
- 26.Soler M., Møller A. P. 1990. Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature 343, 748–750 10.1038/343748a0 (doi:10.1038/343748a0) [DOI] [Google Scholar]
- 27.Briskie J. V., Sealy S. G., Hobson K. A. 1992. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 46, 334–340 10.2307/2409854 (doi:10.2307/2409854) [DOI] [PubMed] [Google Scholar]
- 28.Soler J. J., Martinez J. G., Soler M., Møller A. P. 2001. Coevolutionary interactions in a host–parasite system. Ecol. Lett. 4, 470–476 10.1046/j.1461-0248.2001.00247.x (doi:10.1046/j.1461-0248.2001.00247.x) [DOI] [Google Scholar]
- 29.Martin-Galvez D., Soler J. J., Martinez J. G., Krupa A. P., Soler M., Burke T. 2007. Cuckoo parasitism and productivity in different magpie subpopulations predict frequencies of the 457 bp allele: a mosaic of coevolution at a small geographic scale. Evolution 61, 2340–2348 10.1111/j.1558-5646.2007.00194.x (doi:10.1111/j.1558-5646.2007.00194.x) [DOI] [PubMed] [Google Scholar]
- 30.Stokke B. G., Hafstad I., Rudolfsen G., Moksnes A., Møller A. P., Røskaft E., Soler M. 2008. Predictors of resistance to brood parasitism within and among reed warbler populations. Behav. Ecol. 19, 612–620 10.1093/beheco/arn007 (doi:10.1093/beheco/arn007) [DOI] [Google Scholar]
- 31.Moksnes A., Røskaft E. 1995. Egg-morphs and host preference in the common cuckoo Cuculus canorus: an analysis of cuckoo and host eggs from European museum collections. J. Zool. Lond. 236, 625–648 10.1111/j.1469-7998.1995.tb02736.x (doi:10.1111/j.1469-7998.1995.tb02736.x) [DOI] [Google Scholar]
- 32.Cramp S. 1998. The complete birds of the Western palearctic. Oxford, UK: Oxford University Press [Google Scholar]
- 33.Foppen R. P. B., Chardon J. P., Liefveld W. 2000. Understanding the role of sink patches in source-sink metapopulations: reed warbler in an agricultural landscape. Conserv. Biol. 14, 1881–1892 10.1046/j.1523-1739.2000.99022.x (doi:10.1046/j.1523-1739.2000.99022.x) [DOI] [PubMed] [Google Scholar]
- 34.Prochazka P., Stokke B. G., Jensen H., Fainova D., Bellinvia E., Føssoy F., Vikan J. R., Bryja J., Soler M. 2011. Low genetic differentiation among reed warbler Acrocephalus scirpaceus populations across Europe. J. Avian Biol. 42, 103–113 10.1111/j.1600-048X.2010.05161.x (doi:10.1111/j.1600-048X.2010.05161.x) [DOI] [Google Scholar]
- 35.Avilés J. M., et al. 2011. The common cuckoo Cuculus canorus is not locally adapted to its reed warbler Acrocephalus scirpaceus host. J. Evol. Biol. 24, 314–325 10.1111/j.1420-9101.2010.02168.x (doi:10.1111/j.1420-9101.2010.02168.x) [DOI] [PubMed] [Google Scholar]
- 36.Lindholm A. K. 1999. Blood parasitism by the cuckoo on patchy reed warbler populations in Britain. J. Anim. Ecol. 68, 293–309 10.1046/j.1365-2656.1999.00286.x (doi:10.1046/j.1365-2656.1999.00286.x) [DOI] [Google Scholar]
- 37.Stokke B. G., et al. 2007. Host density predicts presence of cuckoo parasitism in reed warblers. Oikos 116, 913–922 [Google Scholar]
- 38.Avilés J. M., Stokke B. G., Moksnes A., Røskaft E., Asmul M., Møller A. P. 2006. Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J. Evol. Biol. 19, 1901–1910 10.1111/j.1420-9101.2006.01166.x (doi:10.1111/j.1420-9101.2006.01166.x) [DOI] [PubMed] [Google Scholar]
- 39.Collias E. C. 1993. Inheritance of egg-color polymorphism in the village weaver (Ploceus cucullatus). Auk 110, 683–692 [Google Scholar]
- 40.Gosler A. G., Barnett P. R., Reynolds S. J. 2000. Inheritance and variation in eggshell patterning in the great tit Parus major. Proc. R. Soc. Lond. B 267, 2469–2473 10.1098/rspb.2000.1307 (doi:10.1098/rspb.2000.1307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes B. O., Gilbert A. B., Brown M. F. 1986. Categorization and causes of abnormal eggshells: relationship with stress. Br. Poult. Sci. 27, 325–337 10.1080/00071668608416885 (doi:10.1080/00071668608416885) [DOI] [PubMed] [Google Scholar]
- 42.Joseph N. S., Robinson N. A., Renema R. A., Robinson F. E. 1999. Shell quality and color variation in broiler breeder eggs. J. Appl. Poult. Res. 8, 70–74 [Google Scholar]
- 43.Mertens K., et al. 2010. The transmission color value: a novel egg quality measure for recording shell color used for monitoring the stress and health status of a brown layer flock. Poult. Sci. 89, 609–617 10.3382/ps.2009-00261 (doi:10.3382/ps.2009-00261) [DOI] [PubMed] [Google Scholar]
- 44.Soler J. J., Navarro C., Pérez-Contreras T., Avilés J. M., Cuervo J. J. 2008. Sexually selected egg coloration in spotless starlings. Am. Nat. 171, 183–194 10.1086/524958 (doi:10.1086/524958) [DOI] [PubMed] [Google Scholar]
- 45.Avilés J. M., Stokke B. G., Moksnes A., Røskaft E., Møller A. P. 2007. Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav. Ecol. Sociobiol. 61, 475–485 10.1007/s00265-006-0275-0 (doi:10.1007/s00265-006-0275-0) [DOI] [Google Scholar]
- 46.Turner J. R. G., Gatehouse C. M., Corey A. C. 1987. Does solar energy control organic diversity? Butterflies, moths and the British climate. Oikos 48, 195–205 10.2307/3565855 (doi:10.2307/3565855) [DOI] [Google Scholar]
- 47.Polis G. A., Hurd S. D., Jackson C. T., Pinero F. S. 1997. El Nino effects on the dynamics and control of an island ecosystem in the Gulf of California. Ecology 78, 1884–1897 [Google Scholar]
- 48.Sillett T. S., Holmes R. T., Sherry T. W. 2000. Impacts of a global climate cycle on population dynamics of a migratory songbird. Science 288, 2040–2042 10.1126/science.288.5473.2040 (doi:10.1126/science.288.5473.2040) [DOI] [PubMed] [Google Scholar]
- 49.Soler J. J., Martínez J. G., Soler M., Møller A. P. 1999. Genetic and geographic variation in rejection behavior of cuckoo eggs by European magpie populations: an experimental test of rejecter-gene flow. Evolution 53, 947–956 10.2307/2640734 (doi:10.2307/2640734) [DOI] [PubMed] [Google Scholar]
- 50.Cuthill I. C., Bennett A. T. D., Partridge J. C., Maier E. J. 1999. Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 153, 183–200 10.1086/303160 (doi:10.1086/303160) [DOI] [PubMed] [Google Scholar]
- 51.Hoyt D. F. 1979. Practical methods of estimating volume and fresh weight of bird eggs. Auk 96, 73–77 [Google Scholar]
- 52.Cassey P., Grim T., Honza M., Hauber M. E. 2008. The modeling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol. Lett. 4, 515–517 10.1098/rsbl.2008.0279 (doi:10.1098/rsbl.2008.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avilés J. M. 2008. Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc. R. Soc. B 275, 2345–2352 10.1098/rspb.2008.0720 (doi:10.1098/rspb.2008.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vorobyev M., Osorio D., Bennett A. T. D., Marshall N. J., Cuthill I. C. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 183, 621–633 10.1007/s003590050286 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 55.Gomez D. 2006. Avicol: a program to analyse spectrometric data, vol. 1. See http://sites.google.com/site/avicolprogram.
- 56.Avilés J. M., Perez-Contreras T., Navarro C., Soler J. J. 2008. Dark nests and conspicuousness in color patterns of nestlings of altricial birds. Am. Nat. 171, 327–338 10.1086/527493 (doi:10.1086/527493) [DOI] [PubMed] [Google Scholar]
- 57.Hart N. S., Partridge J. C., Cuthill I. C., Bennett A. T. D. 2000. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit Parus caeruleus L. and the blackbird Turdus merula L. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 186, 375–387 10.1007/s003590050437 (doi:10.1007/s003590050437) [DOI] [PubMed] [Google Scholar]
- 58.Hart N. S., Vorobyev M. 2005. Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 191, 381–392 10.1007/s00359-004-0595-3 (doi:10.1007/s00359-004-0595-3) [DOI] [PubMed] [Google Scholar]
- 59.Ödeen A., Håstad O. 2003. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 20, 855–861 10.1093/molbev/msg108 (doi:10.1093/molbev/msg108) [DOI] [PubMed] [Google Scholar]
- 60.Spottiswoode C. N., Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676 10.1073/pnas.0910486107 (doi:10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osorio D., Vorobyev M. 1996. Colour vision as an adaptation to frugivory in primates. Proc. R. Soc. Lond. B 263, 593–599 10.1098/rspb.1996.0089 (doi:10.1098/rspb.1996.0089) [DOI] [PubMed] [Google Scholar]
- 62.Walsh P. S., Metzger D. A., Higuchi R. 1991. Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10, 506–513 [PubMed] [Google Scholar]
- 63.Goudet J. 2001. FSTAT, a program to estimate and test gene diversities and fixation indices version 2.9.3. Université de Lausanne, Lausanne, Switzerland [Google Scholar]
- 64.Haylock M. R., Hofstra N., Klein A. M. G., Tank E. J., Klok P., Jones D., New M. 2008. A European daily high-resolution gridded dataset of surface temperature and precipitation. J. Geophys. Res. (Atmos.) 113, D20119. 10.1029/2008JD010201 (doi:10.1029/2008JD010201) [DOI] [Google Scholar]
- 65.van den Besselaar E. J. M., Haylock M. R., van der Schrier G., Klein Tank A. M. G. 2011. A European daily high-resolution observational gridded data set of sea level pressure. J. Geophys. Res. 111, D11110. 10.1029/2010JD015468 (doi:10.1029/2010JD015468) [DOI] [Google Scholar]
- 66.Torti V. M., Dunn P. O. 2005. Variable effects of climate change on six species of North American birds. Oecologia 145, 486–495 10.1007/s00442-005-0175-4 (doi:10.1007/s00442-005-0175-4) [DOI] [PubMed] [Google Scholar]
- 67.Goslee S. C., Urban D. L. 2007. The Ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 22, 1–19 [Google Scholar]
- 68.Fortin M. J., Gurevitch J. 2001. Mantel tests: spatial structure in field experiments. In Design and analysis of ecological experiments (eds Scheiner S. M., Gurevitch J.), pp. 308–326, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- 69.Legendre P., Fortin M. J. 2010. Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol. Ecol. Res. 10, 831–844 10.1111/j.1755-0998.2010.02866.x (doi:10.1111/j.1755-0998.2010.02866.x) [DOI] [PubMed] [Google Scholar]
- 70.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 [Google Scholar]
- 71.Janzen D. H. 1980. When is it coevolution? Evolution 34, 611–612 10.2307/2408229 (doi:10.2307/2408229) [DOI] [PubMed] [Google Scholar]
- 72.Payne R. B. 1974. Evolution of clutch size and reproductive rates in parasitic cuckoos. Evolution 28, 169–181 10.2307/2407320 (doi:10.2307/2407320) [DOI] [PubMed] [Google Scholar]
- 73.Kruger O., Davies N. B. 2004. The evolution of egg size in the brood parasitic cuckoos. Behav. Ecol. 15, 210–218 10.1093/beheco/arg104 (doi:10.1093/beheco/arg104) [DOI] [Google Scholar]
- 74.Antonov A., Stokke B. G., Vikan J. R., Fossoy F., Ranke P. S., Røskaft E., Moksnes A., Møller A. P., Shykoff J. A. 2010. Egg phenotype differentiation in sympatric cuckoo Cuculus canorus gentes. J. Evol. Biol. 23, 1170–1182 10.1111/j.1420-9101.2010.01982.x (doi:10.1111/j.1420-9101.2010.01982.x) [DOI] [PubMed] [Google Scholar]
- 75.Avilés J. M., Vikan J. R., Fossoy F., Antonov A., Moksnes A., Røskaft E., Stokke B. G. 2010. Avian color perception predicts behavioral responses to experimental brood parasitism in chaffinches. J. Evol. Biol. 23, 293–301 10.1111/j.1420-9101.2009.01898.x (doi:10.1111/j.1420-9101.2009.01898.x) [DOI] [PubMed] [Google Scholar]
- 76.Øien I. J., Moksnes A., Røskaft E. 1995. Evolution of variation in egg color and marking pattern in European passerines: adaptations in a coevolutionary arms race with the cuckoo, Cuculus canorus. Behav. Ecol. 6, 166–174 10.1093/beheco/6.2.166 (doi:10.1093/beheco/6.2.166) [DOI] [Google Scholar]
- 77.Soler J. J., Møller A. P. 1996. A comparative analysis of the evolution of variation in appearance of eggs of European passerines in relation to brood parasitism. Behav. Ecol. 7, 89–94 10.1093/beheco/7.1.89 (doi:10.1093/beheco/7.1.89) [DOI] [Google Scholar]
- 78.Stokke B. G., Moksnes A., Røskaft E., Rudolfsen G., Honza M. 1999. Rejection of artificial cuckoo (Cuculus canorus) eggs in relation to variation in egg appearance among reed warblers (Acrocephalus scirpaceus). Proc. R. Soc. Lond. B 266, 1483–1488 10.1098/rspb.1999.0804 (doi:10.1098/rspb.1999.0804) [DOI] [Google Scholar]
- 79.Stokke B. G., Moksnes A., Røskaft E. 2002. Obligate brood parasites as selective agents for evolution of egg appearances in passerine birds. Evolution 56, 199–205 [DOI] [PubMed] [Google Scholar]
- 80.Stokke B. G., Takasu F., Moksnes A., Roskaft E. 2007. The importance of clutch characteristics and learning for antiparasite adaptations in hosts of avian brood parasites. Evolution 61, 2212–2228 10.1111/j.1558-5646.2007.00176.x (doi:10.1111/j.1558-5646.2007.00176.x) [DOI] [PubMed] [Google Scholar]
- 81.Avilés J. M., Møller A. P. 2003. Meadow pipit (Anthus pratensis) egg appearance in cuckoo (Cuculus canorus) sympatric and allopatric populations. Biol. J. Linnean Soc. 79, 543–549 10.1046/j.1095-8312.2003.00208.x (doi:10.1046/j.1095-8312.2003.00208.x) [DOI] [Google Scholar]
- 82.Moskát C., Avilés J. M., Bán M., Hargitai R., Zölei A. 2008. Experimental support for the use of egg uniformity in parasite egg discrimination by cuckoo hosts. Behav. Ecol. Sociobiol. 62, 1885–1890 10.1007/s00265-008-0618-0 (doi:10.1007/s00265-008-0618-0) [DOI] [Google Scholar]
- 83.Victoria J. K. 1972. Clutch characteristics and egg discriminative ability of the African village weaverbird Ploceus cucullatus. Ibis 114, 367–376 10.1111/j.1474-919X.1972.tb00834.x (doi:10.1111/j.1474-919X.1972.tb00834.x) [DOI] [Google Scholar]
- 84.Lahti D. C. 2006. Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 60, 157–168 10.1554/05-052.1 (doi:10.1554/05-052.1) [DOI] [PubMed] [Google Scholar]
- 85.Saino N., Rubolini D., Lehikoinen E., Sokolov L. V., Bonisoli-Alquati A., Ambrosini R., Boncoraglio G., Møller A. P. 2009. Climate change effects on migration phenology may mismatch brood parasitic cuckoos and their hosts. Biol. Lett. 5, 539–541 10.1098/rsbl.2009.0312 (doi:10.1098/rsbl.2009.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moreno J., Osorno J. L. 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol. Lett. 6, 803–806 10.1046/j.1461-0248.2003.00505.x (doi:10.1046/j.1461-0248.2003.00505.x) [DOI] [Google Scholar]
- 87.Martin-Galvez D., Soler J. J., Martinez J. G., Krupa A. P., Richard M., Soler M., Møller A. P., Burke T. 2006. A quantitative trait locus for recognition of foreign eggs in the host of a brood parasite. J. Evol. Biol. 19, 543–550 10.1111/j.1420-9101.2005.01002.x (doi:10.1111/j.1420-9101.2005.01002.x) [DOI] [PubMed] [Google Scholar]