Abstract
Background and Purpose
Over the last 50 years, chemolysis as a primary or adjuvant treatment for urinary stones has fallen in and out of favor. We review the literature for a historical perspective on the origins and chronology of Renacidin therapy, focusing on landmark studies and impracticalities that have seemingly condemned it to history.
Materials and Methods
A MEDLINE search was performed on the topic of chemolysis of urinary calculi. Historical literature was reviewed with regard to stone composition, treatment modalities, outcomes, and complications.
Results
A total of 61 articles were reviewed, 40 of which were case series, representing a total of 817 patients studied. Mulvaney first introduced Renacidin in 1959 as a modification of Suby and Albright's 1943 solution. Because of an overabundance of nonstandardized irrigation protocols, six deaths were reported in the early 1960s resulting in a Food and Drug Administration ban on the practice of upper urinary tract stone dissolution. Over time, Renacidin returned to the urologist's arsenal, appearing first as an adjunct to dissolve catheter and bladder calculi and later (1990) as an approved agent for renal pelvis and ureter use. This feat was almost single-handedly the result of a successful hemiacidrin case series published in 1971 by Nemoy and Stamey. By using daily urine cultures, prophylactic antibiotics, and meticulous intrarenal pressure monitoring, Nemoy and Stamey virtually eliminated all major irrigation complications, paving the way for a flurry of studies. More importantly, they established the link between residual struvite stones, persistent infection, and recurrent staghorn stone formation.
Conclusions
Dissolution of urinary calculi by chemolysis has been shown to be safe and effective if performed with sterile urine cultures, prophylactic antibiotics, and low intrapelvic pressures. The pioneers of this therapy are remembered for their attempts to develop an alternative to open surgery, and, in the process, solidified the “stone-free” concept for infection-based stones.
Introduction
“In the future, it is likely that irrigations of the urinary tract with various solutions designed to dissolve stones will be an important part of the practice of urology.”
—Arthur R. Israel, 19811
“Any urologist who treats stone disease should be familiar with the fundamentals of chemolysis and its potential role in the management of urinary calculi.”
—Arthur D. Smith, 20002
For years, urologists have dreamed of using chemicals and solutions to turn insoluble kidney and bladder stones into more water-soluble forms. This process, known as dissolution or chemolysis, seems plausible for a number of reasons. First, a variety of dissolution products have been found to be effective in laboratory and animal studies. Second, the urothelium of the renal pelvis and bladder is designed for fluid transit. Thus, as long as the system is adequately drained, the chemicals used for dissolution should pass out of the urinary tract with minimal absorption.3 Finally, open kidney stone surgical procedures of the mid-20th century were quite morbid. Stone dissolution would obviate the need for flank incisions to relieve renal colic and obstruction. With these factors in place, human trials of stone dissolution ensued.
The closest realization of dissolving stones came in the form of a solution called Renacidin (10% hemiacidrin). The triumphs, catastrophes, and controversy that surrounded Renacidin's use and seeming downfall will be chronicled in this review. In the peak of its popularity, Renacidin was being used to achieve stone-free status in recurrent struvite stone formers as a means of eradicating bacterial infection, a concept that is still relevant today. Although it is impossible to fully catalog 60 years of Renacidin's history, the novelty of its use in urology deserves mention and gives perspective for potential future therapies in the endourologists' armamentarium.
Materials and Methods
Relevant studies were searched from electronic databases including Cochrane Central Register of Controlled Trials (The Cochrane Library), MEDLINE, and EMBASE. Reference lists were also made from urology and nephrology textbooks and review articles. An on-line Google search was also used to identify legal documents and other pertinent materials. Search terms included all forms and abbreviations of renacidin, hemiacidrin, chemolysis, dissolution, urologic solution G, Suby's solution, ethylenediaminetetraacetic acid, and Guardian Chemical Corporation.
A total of 61 papers were reviewed. Of these, 21 were identified as basic science inquiries, case reports, or lower urinary tract investigations. The remaining 40 focused primarily on upper urinary tract stones, totaling 817 patients. All 40 upper tract stone studies were case series. Prospective clinical trials or cohort studies were not identified nor were any detailed Renacidin protocols before 1971. No data were present on patient selection criteria in these case series, and clear-cut variables (irrigation time, length of stay in hospital, stone analysis, etc.) were often lacking. From these 40 papers, 15 were chosen to be presented in tabular form.
The Beginning of Dissolution
The first reported case of stone dissolution was by Crowell4 in 1924. Using a primitive retrograde catheter, Crowell methodically filled a young cystine stone former's renal pelvis with an alkaline antiseptic lavage of mercurochrome every other day. Along with oral sodium bicarbonate for urinary alkalinization, Crowell repeated these weekly lavages for a total of 10 months until the roentgenogram was clear of stones. After this success, Hellstrom5 (1938) used 1% phosphoric acid with a mixture of boric acid and potassium permanganate to acidify alkaline salts associated with Staphylococcus-associated lithiasis.
A year later, Albright and coworkers6 dissolved human calcium phosphate bladder stones using isotonic citrate solutions (pH=4.0).6 This acidic solution, however, caused painful mucosal bleeding for the patient, so a rabbit model was developed to study dissolution speed using decalcified teeth. Their work culminated in 1943, when Drs. Suby and Albright7 reported a reduction in bladder mucosal irritation and bleeding by adding magnesium to the citric acid solution (Table 1).7 This product (isotonic citrate, magnesium oxide, and sodium carbonate) became known as urologic solution G or Suby solution (Table 2).7 Although effective, the use of this solution was soon to be overshadowed by the establishment of Renacidin.
Table 1.
Landmark Dissolution Cases
| Author, Year | N | Agent | Delivery method | Composition (n) | Mean duration | Dissolution results | Mean F/U | Notes |
|---|---|---|---|---|---|---|---|---|
| Suby and Albright 19437 | 7 | Solution G | Intermittent PNT±UC | CaP (2) Mixed (2) NR (3) |
45 days | Complete: 4/7 Partial: 2/7 Failure: 1/7 |
NR | + hematuria and lower urinary tract symptoms; air pyelography used to ensure stone-solution contact |
| Mulvaney 19599 | 3 | Renacidin | Intermittent PNT, SPT, Foley | NR | NR | Complete: 3/3 | NR | This report includes results of in vitro dissolution of 50 stones |
| Mulvaney 196015 | 46 | Renacidin | Continuous PNT+UC, Foley | Mixed | NR | Success: -Upper 11/21 -Bladder 5/10 -Catheter 15/15 |
NR | First report of staghorn stone dissolution; success defined as stone reduction or disappearance on radiography |
| Timmerman and Kallistratos 196642 | 260 | EDTA | Continuous PNT | 90% calcium | NR | Complete:145/260 Partial: 113/260 No response: 2/260 |
NR | Largest reported dissolution series; success defined as reduction or disappearance of stone on radiography; DVT rate, 3.8% |
| Comarr et al 197118 | 119 | Renacidin | Intermittent Two UC | Struvite | 70 days | Complete: 51/119 Partial: 26/119 No response: 37/119 Stopped early: 5/119 |
NR | Series consists of spinal cord injury patients only |
| Nemoy and Stamey197114 | 14 | Renacidin (8); Antibiotics (14) | Continuous PNT (5) UC (3) |
Struvite | 11 days | Complete: 8/8 | 3 years | Standardized dissolution protocols, antibiotics, and sterile urine; no stone recurrence in follow-up |
Before the 1970s, all percutaneous nephrostomy tubes were placed open or at the time of open lithotomy.
PNT=percutaneous nephrostomy tube; UC=ureteral catheter; CaP=calcium phosphate; NR=not recorded; SPT=suprapubic tube; EDTA=ethylenediaminetetraacetic acid.
Table 2.
Comparison of Renacidin with Urologic Solution G
| Attribute | Renacidin (10% hemiacidrin) | Urologic solution G (Suby) |
|---|---|---|
| First urology publication | Mulvaney19599 | Suby and Albright 19437 |
| pH | 3.9 | 4.0 |
| Manufacturer | Guardian Lab (Hauppage, NY) | Baxter International, Inc (Deerfield, IL) |
| Constituent (g in 1 L water) | ||
| Citric acid | 28.2 | 32.4 |
| Magnesium carbonate | 14.8 | 0 |
| D-gluconic acid | 5.0 | 0 |
| Sodium carbonate | 0 | 4.3 |
| Magnesium oxide | 0 | 3.8 |
| Magnesium acid-citrate | 2.5 | 0 |
| Calcium carbonate | 1.0 | 0 |
Development and Introduction of Hemiacidrin (Renacidin)
In 1955, Guardian Chemical Corporation began manufacturing a liquid product to dissolve calcium deposits that frequently clogged milk pasteurizing equipment and tubing. In 1957, Dr. William P. Mulvaney (urologist at the University of Cincinnati) approached Dr. Alfred E. Globus (founder of Guardian Co. and biochemist who developed the solution) and suggested that the product may be useful in dissolving urinary catheter calcium deposits and encrustation.
After the product was renamed Renacidin, Dr. Mulvaney presented his preliminary data at the 1957 American Urological Association meeting. The solution itself was similar in pH and buffering capacity to the Suby G solution but contained malonic and gluconic acids (Table 2). The protons of these acids were believed to complex with phosphate (phosphoric acid) and calcium (calcium citrate) to form soluble compounds at a pH of 4.0. Because struvite stone solubility increased at pH <5.5, Dr. Mulvaney believed Renacidin would be useful in dissolving struvite stones. For commercial purposes, Guardian began producing the product in powder form, where 100 g Renacidin could be reconstituted in 1000 mL distilled water. Once in solution, Renacidin was stable for long periods and could be autoclaved or boiled without losing its potency.8
In 1959, Dr. Mulvaney published the first study describing the in vitro properties of Renacidin (10% hemiacidrin) on 50 human calculi as well as in three human case reports.9 He and his colleagues found that Renacidin sequestered 400% more mineral than any single organic acid alone, such as citric acid.10 They thought that the added magnesium salts (roughly four times the elemental magnesium of the Suby G, Table 2) provided ion exchange with stone calcium and enhanced dissolution with the added benefit of decreased mucosal irritation.9,11–14 It appeared to act as an excellent solvent for calcium phosphate, calcium carbonate, and magnesium ammonium phosphate stones. Cholesterol, uric acid, and calcium oxalate stones, however, were relatively insoluble to Renacidin.
Their “proof of concept” experiment was a small case series of three patients who had significant kidney and bladder stones along with catheter encrustation. Despite a reported 100% success rate (Table 1), this series was limited by lack of stone composition, follow-up data, and the need for indefinite daily 10% hemiacidrin catheter irrigation to ensure removal of calcifications.9,10
Based on Dr. Mulvaney's initial reports, several large case series with modest results were published using Renacidin irrigations to treat catheter encrustation and a variety of renal and bladder stones compositions, not just struvite (Table 1).8,13,15–20 This increase in clinical use also unveiled some limitations. First, because Renacidin solution contained high levels of magnesium and phosphate, its use was contraindicated in patients with advanced renal disease (creatinine clearance <10 mL/min).21–27 Second, as if foreshadowing future catastrophies, Mulvaney reported in 1960 that debris and sand tended to obstruct single ureteral catheters, resulting in patient discomfort and obstruction.15 Years later, the development of percutaneous nephrostomy puncture techniques would lead to improved irrigation and drainage (Fig. 1).28
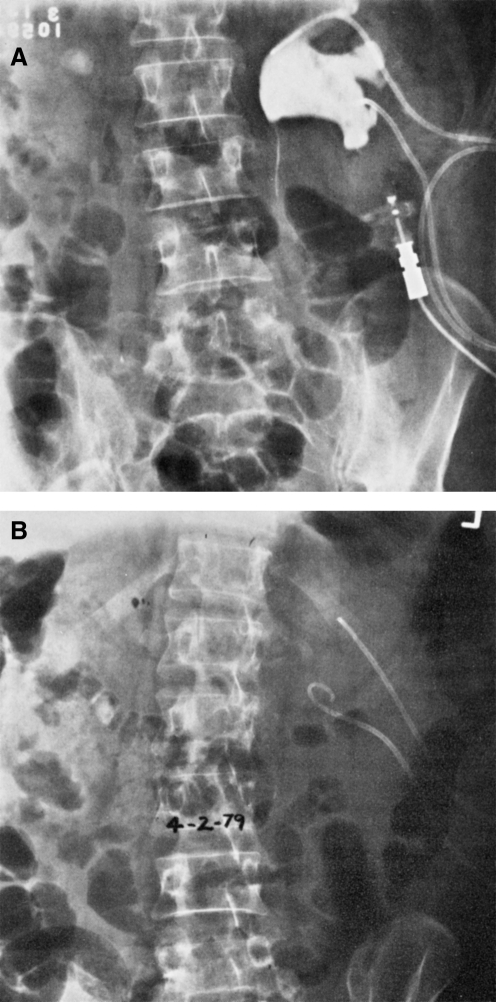
FIG. 1.
(A) Left-sided struvite kidney stone by plain film with indwelling nephrostomy tubes; (B) plain film after 17 days of Renacidin irrigation showing complete stone dissolution. Reprinted with permission from Massaro and colleague. Pharmacotherapy.27
In the early 1960s, however, haphazard Renacidin delivery protocols through ureteral catheters were associated with six patient deaths that would take Renacidin almost 30 years to surmount.29,30
Banned by the Food and Drug Administration (FDA)
The safety of Renacidin irrigation first came into question when Kohler30 reported a patient mortality during irrigation use. He described postmortem renal infarction, necrosis, and purulent kidney infection in a stone patient with high inflow pressures over 80 mm Hg during Renacidin irrigation therapy. Fostvedt and Barnes29 added four cases of sudden death from suspected pyelovenous backflow with postmortem findings ranging from papillary necrosis to cerebral edema.29 Finally, Auerbach and associates31 followed with a case of severe pyelonephritis, ureteritis, and chemical pyelitis in a patient with bilateral renal calculi. The patient's ultimate cause of death was ruled pulmonary embolus, but the striking renal findings were attributed to Renacidin.31
Based on these six deaths, on June 13, 1963, the FDA banned the use of Renacidin irrigation for the upper urinary tract and bladder.28,32 The FDA acknowledged that inconsistencies in protocols and user mistakes likely led to the Renacidin mortalities and pointed to sepsis, rather than direct toxicity of the agent, as the cause of death in all cases. Not surprisingly, Mulvaney came out in defense of the solution, attributing the deaths to obstructed ureteral catheters leading to increased intrapelvic pressures and sepsis. He pointed to the safety of the procedure at renal pressures below 24 to 30 cm H20 and even advocated adding neomycin directly to the solution to prevent infectious complications.19 The intrapelvic pressures he described were subsequently validated by other investigators to be high enough for stone fragmentation but low enough to minimize pyelovenous backflow.33 Finally, Mulvaney noted that most adverse events seemed to occur at night when nursing care was scarce, and his safety frustrations were echoed by multiple other authors.3,8,17,23,34
Based on Mulvaney's comments, on August 8, 1963, less than 2 months after their first decision, the FDA approved Renacidin to “prevent formation of and to dissolve calcifications in catheters in the urinary bladder.” Interestingly, the ruling on the upper urinary tract remained in place until October 1990, when Renacidin was approved as an “orphan drug” for the treatment of renal and bladder calculi of the apatite or struvite variety (United-Guardian Inc., U.S. patent #4,962,208). The term orphan drug refers to a drug or biologic intended for use in a rare disease or condition (defined as affecting fewer than 200,000 Americans) whose sponsor receives certain governmental benefits in exchange for drug development. Therefore, for almost 30 years, Renacidin's use in the upper tract was classified as investigational and needed informed patient consent, approval by a hospital human ethics committee, and occasionally permission from the FDA.18,35 Undoubtedly, this drug would have retreated into history had it not been for two advocating urologists and a new protocol.
New Life for Renacidin
In 1971, Nemoy and Stamey14 published a landmark article on the adjuvant use of Renacidin through percutaneous nephrostomy tubes (PNT) after open pyelolithotomy in patients harboring infectious stones, specifically Proteus mirabilis and Klebsiella.14 More than any previous dissolution article, Nemoy and Stamey highlighted the concept of a formal protocol for dissolution therapy in the setting of infectious stones after open stone surgery (Table 3). First, they outlined absolute contraindications to Renacidin, including infected urine, fever, or persistent flank discomfort. Their pre-surgical guidelines emphasized daily urine cultures for proper antibacterial selection and confirmation of sterility before surgery or irrigation.14
Table 3.
Abbreviated Nemoy and Stamey Protocol for Renacidin Irrigation (1971)14
| Protocol | Contraindications and requirements |
|---|---|
| 1. Preoperative laboratory studies: blood count, metabolic panel, calcium, uric acid, liver function tests. | Absolute contraindications: UTI, fever, and/or persistent flank discomfort. |
| 2. Abdominal x-ray, IVP. | 1. One week preoperative penicillin G or ampicillin. Sterile urine during irrigation. |
| 3. Urine culture by renal pelvis needle aspiration before surgery. | 2. Daily nephrostomy tube urine cultures. |
| 4. Copious irrigation during open stone surgery. | 3. Halt irrigation if nephrostomy tube site leaks. |
| 5. Low pressure saline irrigation on post-operative day 4 to test for leak. If negative, start 10% Renacidin at 120 mL/hr through nephrostomy tube. | 4. Patients disconnect inflow tube at the first sign of flank discomfort, even before notifying the nurse. |
| 6. Once tomograms are negative, continue irrigations for an additional 24–48 hours. | 5. For patients with vesical irritation, reduce Renacidin to 50 mL/hr or alternate Renacidin and saline solution (1 liter) each hour and place a urinary catheter. |
UTI=urinary tract infection; IVP=intravenous pyelography.
During open surgery, the renal pelvis and collecting system were irrigated copiously with saline to flush out stone fragments. Renacidin irrigation began on the fourth or fifth postoperative day with saline irrigation to test for complete healing of the pyelotomy incision. If no leakage, fever, or flank discomfort occurred, 10% Renacidin was then started at 120 ml/h through a nephrostomy tube. Once there was absence of visible particles on tomography, the irrigations would cease after an additional 24 to 48 hours. Interestingly, they even allowed patients to have control over their irrigations by instructing them on “… how to disconnect the inflow tube at the first sign of flank discomfort, even before notifying the nurse. This important precaution allows immediate reduction of intrarenal pressure in the presence of temporary outflow obstruction.”14
In response to the controversy surrounding Renacidin-associated deaths, Nemoy and Stamey reasoned they were secondary to pyelovenous backflow of untreated urinary tract infections and stated, “It is abundantly clear from these reports that if the physician is unwilling to assume responsibility for a sterile renal urine prior to and during irrigation … he should not attempt dissolution of infection stones.”14
With safe and effective protocols in place, clinical applications for Renacidin irrigation and struvite stones intensified (Fig. 2). During the next 15 years (1973 to 1988), Renacidin reached its pinnacle of publications with its use described through PNT after open lithotomy, through ureteral catheters for chronically ill or spinal cord injury patients, and even as primary therapy through fluoroscopic PNT techniques (Table 4).3,33,35–38 Many of these investigators also contributed refinements to the protocol, such as irrigating for an additional 48 hours after radiography has shown disappearance of stones or halting irrigation altogether if a week of treatment did not result in a 50% reduction in size.3,33,36–38
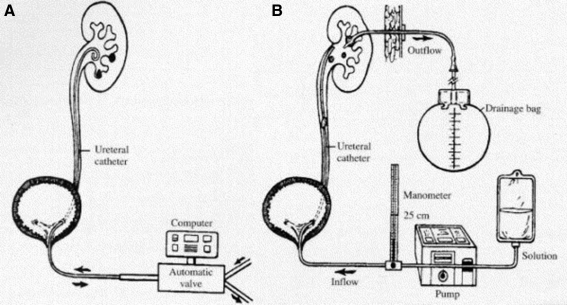
FIG. 2.
Irrigation equipment configurations for direct chemolysis I. (A) Retrograde ureteral catheter with computer-driven inflow/outflow; (B) retrograde ureteral catheter with computer inflow and nephrostomy outflow. Reprinted with permission from Bernardo and Smith. Urol Clin North Am.2
Table 4.
Contemporary Renacidin Dissolution Cases
| Author, Year | N | Delivery method (n) | Stone composition (n) | Mean duration (d) | Dissolution responses | Reported complications | F/U (mos) | Notes |
|---|---|---|---|---|---|---|---|---|
| Blaivas et al 19753 | 9 | PNT | Struvite (8) Apatite (1) |
11 | Complete 6/9 Partial 2/9 Failure 1/9 |
UTI 4/19 Fever 3/9 Pain 3/9 |
12 | Intrapelvic pressure kept below 25 cm H2O by Y-tubing between nephrostomy, irrigation, and manometer; no recurrence during short follow-up |
| Fam et al 197636 | 11 | PNT after OL (6); UC (5) | Struvite/apatite | OL 12–120 UC 14–115 |
Complete: -UC 4/5 -PNT 4/6 |
Fever 4/11 Hematuria 3/11 Recurrence 2/11 |
12 | 10/11 patients had spinal cord injuries; stone sizes for UC and residual stones all <2 cm |
| Jacobs and Gittes 197637 | 11 | PNT after OL | Struvite (7) Calcium (2) |
7 | Complete 9/11 Failure 2/11 |
Fever 2/11 | 17 | Adjuvant therapy after open lithotomy in 11 patients; dissolution failed in calcium-based stones |
| Dretler et al 197928 | 8 | PNT | Struvite (presumed) | 16 | Complete 6/8 Partial 2/8 |
Flank pain 3/8 Fever 3/8 |
NR | Patient allowed to disconnect PNT; 2/8 patients needed additional PNT because of ureteral obstruction |
| Sant et al 198335 | 21 | PNT after OL (19); PNT (2) | Struvite | 13 | Complete 18/21 Partial 2/21 Failure 1/21 |
Back pain 9/21 Recurrence 2/21 |
66 | Consecutive open series with adjuvant Renacidin that included two patients with nonsurgical PNT dissolution; long follow-up |
| Silverman and Stamey 198340 | 46 | PNT after OL (46) | Struvite/apatite (46) | 4.5 | Complete 40/46 Failure 6/46 |
NR | 84 | All patients placed on Renacidin irrigation after OL regardless of stone status; 1/46 patients had documented stone recurrence in mean 7 years |
| Dretler and Pfister 198448 | 28 | PNT | Struvite (presumed) | 2–30 | Complete 19/28 Partial 6/28 Failure 3/28 |
Fever 8/28 Candiduria 6/28 Sepsis 1/28 |
3–84 | Hospital stay 5–60 days; stone recurrence documented in 3/19 patients complete stone clearance |
| Palmer et al 198746 | 15 | PNT (1); PNT after OL (6) or MIS (8) | Struvite (12); apatite (3) | 28 | Complete 11/15 Partial 1/15 Failure 3/15 |
Leakage 15/15 Fever 14/15 Admission 3/15 Pain 2/15 |
NR | Highly motivated patients who performed outpatient PNT irrigation after stone removal; stones recurred in 3/15; cost approximated a 1-day inpatient hospitalization |
| Spirnak et al 198845 | 11 | PNT after SWL | Struvite (presumed) | 6 | Complete 9/11 Partial 2/11 |
Pyelo 2/11 Candiduria 2/11 Edema and PNT 1/11 |
NR | Case series of complex struvite stone patients managed by irrigation after SWL; average 12-day hospital stay |
PNT=percutaneous nephrostomy tube; UTI=urinary tract infection; OL=open lithotomy; UC=ureteral catheter; NR=not recorded; MIS=minimally invasive surgery; SWL=shockwave lithotripsy.
More than their attention to dissolution safety, Nemoy and Stamey14 are credited with observing the relationship between small residual stones, incomplete infection eradication, and high struvite recurrence rate. They theorized that urea-splitting organisms were deeply embedded within struvite stones, protecting them from the action of antibacterial agents. Unlike many others, they stated that small stones could lead to persistent infection and stone recurrence and strongly advocated for complete stone-free results after pyelolithotomy for struvite stones.39 Silverman and Stamey40 went on to prove the theory by placing 46 struvite stone patients with bacteriuria on a “total therapeutic program.” (Table 4) After open surgical debulking and culture-sensitive antimicrobial therapy, they placed all patients on adjuvant dissolution therapy with Renacidin through a Hemovac tube for at least 48 hours or until stone fragments were dissolved. With years more follow-up than any other series, Stamey boasted a 2.5% stone recurrence rate while his peers at that time reported stone recurrences of 30% in 6 years.41 Stamey concluded that “… the urologist has no choice but to use Renacidin in the kidney when residual struvite or apatite fragments are left in the kidney postoperatively.”
Declining Popularity
Even in light of Nemoy and Stamey's protocols, the shortcomings of Renacidin seemed to outweigh its advantages (Table 5). First, stone analysis was necessary for the proper selection of the irrigant, and this was not always available. Second, vigilance in maintaining flow and proper equipment was frustrating. Constant nursing care was needed for most patients, and placement of additional ureteral catheters or PNT was needed if obstruction occurred. The protocols were labor intensive, necessitating prolonged antibiotics, daily urine cultures, serum magnesium levels, biweekly radiography, and complete absence of fever or pain. Deep venous thrombosis prophylaxis was considered mandatory because of patient immobility during therapy.28,31,42 In 1979, Dretler and colleagues28 (Table 4) chronicled these concerns by reporting frequent irrigation cessations for elevated intrapelvic pressures, repeated fluoroscopy trips to radiology, and psychologically taxing lengths of immobility and hospitalization in patients on Renacidin irrigation.
Table 5.
Reasons for the Decline in Renacidin Therapy
| Cost |
| Intense irrigation protocols |
| Narrow clinical stone population |
| Advanced monitoring equipment |
| Advent of minimally invasive therapies |
| Patient noncompliance and disinterest |
| Additional ureteral and nephrostomy tubes |
| Prolonged immobility and hospitalizations |
| Repeated fluoroscopy to verify effectiveness |
With the introduction of percutaneous nephrolithotomy (PCNL) in 197643 and shockwave lithotripsy (SWL) in 1980,44 the era of minimally invasive surgery (MIS) for stone disease began. Urologists were eager to learn new procedures that reduced patient morbidity and, most importantly, were not banned by the FDA. As MIS popularity expanded, Renacidin began to be used in an adjuvant setting. Spirnak and coworkers45 described an 80% stone-free rate when Renacidin was combined with SWL in a group of patients with complex struvite stones. Palmer and colleagues46 detailed a group of patients who completed months of daily outpatient renacidin irrigations through a PNT after PCNL or SWL with only three patients needing inpatient hospital admission during 365 total outpatient days (Table 4). Although they reported total outpatient daily cost equal to that of one hospital day stay, several others documented that adjuvant Renacidin added 11 days to hospital stay and up to 3 months of outpatient therapy.3,30,33
In October 1990, the ban on Renacidin's upper tract use was lifted, but it was too little, too late. Since 1990, only 20 Renacidin articles have been published, and most of these are in vitro studies. The era of managed care and cost containment did to Renacidin what a 30-year FDA ban could not. Perhaps Rodman47 was right when he wrote these sentiments, “One has to wonder whether the advent of managed care and the consequent emphasis on shortening hospital stays did not adversely affect the management of struvite stone disease.”
Renacidin is not completely gone. United-Guardian Inc. continues to report annual Renacidin U.S. sales from $1.2 to 1.5 million, primarily from lower urinary and catheter irrigation use. As recent as Medicare contract year 2010, Renacidin has a Healthcare Common Procedure Coding System code and continues to be listed in the Federal Register as a paid outpatient service to Medicare beneficiaries. Certainly, stone dissolution by Renacidin is not a panacea, and discretion must be exercised when considering its use. After reviewing the literature, Renacidin appears to be a valuable addition to the urologic armamentarium if used within its narrow clinical setting and with cautious vigilance.
Conclusions
Renacidin has had a complex history marked with promise and frustration. Although it survived an FDA ban, it could not survive the era of MIS, cost containment, and managed care. Renacidin will be remembered as a novel therapy for patients who were poor surgical candidates and made headway in struvite stone formers, sparing them morbidity and stone recurrence after primary surgical interventions. For patients with colonized urinary tracts and infectious stones, postoperative and prophylactic irrigations with Renacidin were shown to eliminate the nidus for recurrent infection and serve as a potential long-lasting cure. This, at least in theory, should be considered when its higher healthcare costs are discussed.
The importance of Renacidin in the discovery and achievement of stone-free status for the infected stone patient cannot be overstated. Patients with complex struvite stone disease who are not cured by stone removal alone still exist. In these patients, it may be time to reconsider the role of Renacidin irrigation therapy.
Abbreviations Used
- FDA
Food and Drug Administration
- MIS
minimally invasive surgery
- PCNL
percutaneous nephrolithotomy
- PNT
percutaneous nephrostomy tube
- SWL
shockwave lithotripsy
Disclosure Statement
No competing financial interests exist. Funding for this study was obtained through grant R01 DK061666-06 (Canales).
References
- 1.Rodman JS. Reckler JM. Israel AR. Hemiacidrin irrigations to dissolve stone remnants after nephrolithotomy. Problems with solution flow. Urology. 1981;18:127–130. doi: 10.1016/0090-4295(81)90420-9. [DOI] [PubMed] [Google Scholar]
- 2.Bernardo NO. Smith AD. Chemolysis of urinary calculi. Urol Clin North Am. 2000;27:355–365. doi: 10.1016/s0094-0143(05)70264-0. [DOI] [PubMed] [Google Scholar]
- 3.Blaivas JG. Pais VM. Spellman RM. Chemolysis of residual stone fragments after extensive surgery for staghorn calculi. Urology. 1975;6:680–686. doi: 10.1016/0090-4295(75)90794-3. [DOI] [PubMed] [Google Scholar]
- 4.Crowell A. Cystine nephrolithiasis. Surg Gynecol Obstet. 1924;38:87–91. [Google Scholar]
- 5.Hellstrom J. The significance of staphylococci in the development and treatment of renal and ureteral stones. BJU. 1938;10:348–372. [Google Scholar]
- 6.Albright F. Sulkowitch HW. Chute R. Nonsurgical aspects of the kidney stone problem. JAMA. 1939;113:2049–2053. [Google Scholar]
- 7.Suby HI. Albright F. Dissolution of phosphatic urinary calculi by the retrograde introduction of a citrate solution containing magnesium. NEJM. 1943;228:81–91. [Google Scholar]
- 8.Ries S. Malament M. Renacidin: A urinary calculi solvent. J Urol. 1962;87:657–661. doi: 10.1016/S0022-5347(17)65022-5. [DOI] [PubMed] [Google Scholar]
- 9.Mulvaney W. A new solvent for certain urinary calculi: A preliminary report. J Urol. 1959;82:546–548. doi: 10.1016/S0022-5347(17)65927-5. [DOI] [PubMed] [Google Scholar]
- 10.Mulvaney WP. Ibanez JG. Ratledge HW. The use of renacidin in preventing calcification of indwelling catheters. Surgery. 1960;48:584–587. [PubMed] [Google Scholar]
- 11.Shortliffe LM. Spigelman SS. Infection stones. Evaluation and management. Urol Clin North Am. 1986;13:717–726. [PubMed] [Google Scholar]
- 12.Dretler SP. Ureteral stone disease. Options for management. Urol Clin North Am. 1990;17:217–230. [PubMed] [Google Scholar]
- 13.Mischol HR. Wildbolz E. Instrumental chemolysis of renal calculi: Indications and dangers. J Urol. 1971;105:607–610. doi: 10.1016/s0022-5347(17)61587-8. [DOI] [PubMed] [Google Scholar]
- 14.Nemoy NJ. Stamey TA. Surgical, bacteriological, and biochemical management of “infection stones”. JAMA. 1971;215:1470–1476. [PubMed] [Google Scholar]
- 15.Mulvaney W. The clinical use of renacidin in urinary calcifications. J Urol. 1960;84:206–212. doi: 10.1016/S0022-5347(17)65519-8. [DOI] [PubMed] [Google Scholar]
- 16.Hepworth RG. Hemiacidrin (renacidin): A study of its use in 40 patients with bladder calculi. Can J Surg. 1963;6:276–280. [PubMed] [Google Scholar]
- 17.Russell M. Dissolution of bilateral renal staghorn calculi with renacidin. J Urol. 1962;88:141–144. doi: 10.1016/S0022-5347(17)64754-2. [DOI] [PubMed] [Google Scholar]
- 18.Comarr AE. Kawaichi GK. Morris L. Bors E. Dissolution of renal stones by renacidin in patients with spinal cord injury. Proc Veterans Adm Spinal Cord Inj Conf. 1971;18:174–179. [PubMed] [Google Scholar]
- 19.Mulvaney WP. Henning DC. Solvent treatment of urinary calculi: Refinements in technique. J Urol. 1962;88:145–149. doi: 10.1016/S0022-5347(17)64755-4. [DOI] [PubMed] [Google Scholar]
- 20.Bundrick WS. Culkin DJ. Mata JA. Venable DD. Unresectable calcified bladder tumor: Hemiacidrin irrigation as an adjunct to resection. J Urol. 1991;145:142–143. doi: 10.1016/s0022-5347(17)38272-1. [DOI] [PubMed] [Google Scholar]
- 21.Rosen D. Nemoy N. Wolf P, et al. Intravenous infusion of renacidin in dogs. Invest Urol. 1971;9:31–33. [PubMed] [Google Scholar]
- 22.Cato A. Tulloch A. Hypermagnesemia in a uremic patient during renal pelvis irrigation with renacidin. J Urol. 1974;111:313–314. doi: 10.1016/s0022-5347(17)59955-3. [DOI] [PubMed] [Google Scholar]
- 23.Davis TA. New method of intrarenal irrigation to dissolve calculi. J Urol. 1964;92:599–602. doi: 10.1016/S0022-5347(17)64018-7. [DOI] [PubMed] [Google Scholar]
- 24.Thompson IM., Jr Mora RV. Hypermagnesemia associated with hemiacidrin irrigation. J Urol. 1984;132:741–742. doi: 10.1016/s0022-5347(17)49851-x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson C. Azmy A. Beattie TJ. Murphy AV. Hypermagnesemia and progression of renal failure associated with renacidin therapy. Clin Nephrol. 1986;25:266–267. [PubMed] [Google Scholar]
- 26.Brock WA. Nachtsheim DA. Parsons CL. Hemiacidrin irrigation of renal pelvic calculi in patients with ileal conduit urinary diversion. J Urol. 1980;123:345–347. doi: 10.1016/s0022-5347(17)55927-3. [DOI] [PubMed] [Google Scholar]
- 27.Massaro FJ. Weiner B. Sant GR. Meares EM., Jr Dissolution of struvite calculi by hemiacidrin solution. Pharmacotherapy. 1983;3:344–348. doi: 10.1002/j.1875-9114.1983.tb03300.x. [DOI] [PubMed] [Google Scholar]
- 28.Dretler SP. Pfister RC. Newhouse JH. Renal-stone dissolution via percutaneous nephrostomy. N Engl J Med. 1979;300:341–343. doi: 10.1056/NEJM197902153000704. [DOI] [PubMed] [Google Scholar]
- 29.Fostvedt GA. Barnes RW. Complications during lavage therapy for renal calculi. J Urol. 1963;89:329–331. doi: 10.1016/S0022-5347(17)64553-1. [DOI] [PubMed] [Google Scholar]
- 30.Kohler FP. Renacidin and tissue reaction. J Urol. 1962;87:102–108. doi: 10.1016/S0022-5347(17)64915-2. [DOI] [PubMed] [Google Scholar]
- 31.Auerbach S. Mainwaring R. Schwarz F. Renal and ureteral damage following clinical use of Renacidin. JAMA. 1963;183:61–63. doi: 10.1001/jama.1963.63700010030020a. [DOI] [PubMed] [Google Scholar]
- 32.Ebel A. Kuo J. Post mortem findings in a patient treated with renacidin for unilateral renal calculosis. Proc Annu Clin Spinal Cord Inj Conf. 1964;13:93–97. [PubMed] [Google Scholar]
- 33.Dretler SP. Pfister RC. Primary dissolution therapy of struvite calculi. J Urol. 1984;131:861–863. doi: 10.1016/s0022-5347(17)50683-7. [DOI] [PubMed] [Google Scholar]
- 34.Mulvaney W. The hydrodynamics of renal irrigations: With reference to calculus solvents. J Urol. 1963;89:765–768. doi: 10.1016/S0022-5347(17)64643-3. [DOI] [PubMed] [Google Scholar]
- 35.Sant GR. Blaivas JG. Meares EM., Jr Hemiacidrin irrigation in the management of struvite calculi: Long-term results. J Urol. 1983;130:1048–1050. doi: 10.1016/s0022-5347(17)51676-6. [DOI] [PubMed] [Google Scholar]
- 36.Fam B. Rossier AB. Yalla S. Berg S. The role of hemiacidrin in the management of renal stones in spinal cord injury patients. J Urol. 1976;116:696–698. doi: 10.1016/s0022-5347(17)58975-2. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs SC. Gittes RF. Dissolution of residual renal calculi with hemiacidrin. J Urol. 1976;115:2–4. doi: 10.1016/s0022-5347(17)59046-1. [DOI] [PubMed] [Google Scholar]
- 38.Sheldon CA. Smith AD. Chemolysis of calculi. Urol Clin North Am. 1982;9:121–130. [PubMed] [Google Scholar]
- 39.Lerner SP. Gleeson MJ. Griffith DP. Infection stones. J Urol. 1989;141:753–758. doi: 10.1016/s0022-5347(17)41002-0. [DOI] [PubMed] [Google Scholar]
- 40.Silverman DE. Stamey TA. Management of infection stones: The Stanford experience. Medicine (Baltimore) 1983;62:44–51. doi: 10.1097/00005792-198301000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Griffith DP. Struvite stones. Kidney Int. 1978;13:372–382. doi: 10.1038/ki.1978.55. [DOI] [PubMed] [Google Scholar]
- 42.Timmermann A. Kallistratos G. Modern aspects of chemical dissolution of human renal calculi by irrigation. J Urol. 1966;95:469–475. doi: 10.1016/S0022-5347(17)63480-3. [DOI] [PubMed] [Google Scholar]
- 43.Fernström I. Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol. 1976;10:257–259. doi: 10.1080/21681805.1976.11882084. [DOI] [PubMed] [Google Scholar]
- 44.Chaussy C. Brendel W. Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2:1265–1268. doi: 10.1016/s0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- 45.Spirnak JP. DeBaz BP. Green HY. Resnick MI. Complex struvite calculi treated by primary extracorporeal shock wave lithotripsy and chemolysis with hemiacidrin irrigation. J Urol. 1988;140:1356–1359. doi: 10.1016/s0022-5347(17)42043-x. [DOI] [PubMed] [Google Scholar]
- 46.Palmer JM. Bishai MB. Mallon DS. Outpatient irrigation of the renal collecting system with 10 per cent hemiacidrin: Cumulative experience of 365 days in 13 patients. J Urol. 1987;138:262–265. doi: 10.1016/s0022-5347(17)43113-2. [DOI] [PubMed] [Google Scholar]
- 47.Rodman JS. Struvite stones. Nephron. 1999;81(suppl 1):50–59. doi: 10.1159/000046299. [DOI] [PubMed] [Google Scholar]
- 48.Dretler SP. Pfister RC. Newhouse JH. Prien EL., Jr Percutaneous catheter dissolution of cystine calculi. J Urol. 1984;131:216–219. doi: 10.1016/s0022-5347(17)50312-2. [DOI] [PubMed] [Google Scholar]