Abstract
The breast cancer 1 and 2, early onset (BRCA1 and BRCA2) genes are important for double-strand break repair by homologous recombination. Cells with inactivating mutations of the BRCA1 or BRCA2 tumor suppressor genes show increased sensitivity to Poly-ADP ribose polymerase (PARP)-inhibitors in vitro. Sporadic breast tumors with BRCA1 promoter hypermethylation show a similar phenotype to familial BRCA1 patient tumors termed “BRCAness.” Sporadic ovarian tumors with functional inactivation of BRCA1 by hypermethylation will also have the BRCA-deficiency phenocopy. The loss of BRCA1 expression associated with promoter hypermethylation will disrupt BRCA-associated DNA repair and may sensitize tumors to BRCA-directed therapies. Thus, the determination of methylation status of BRCA1 may be an important predictive classifier of response to PARP-inhibitor therapy. The methylation, and thereby functional, status of other genes implicated in the wider BRCA/homologous recombination (HR) pathway may also be relevant to the prediction of response to PARP-inhibitor therapy. Here, we describe the four optimal technologies for assaying the promoter methylation status of BRCA1 and/or other genes.
Keywords: BRCA1, Hypermethylation, PARP, Bisulfite sequencing, Pyrosequencing, Quantitative MSP, Methylation beadchip
1. Introduction
Cells with BRCA1 or BRCA2 dysfunction are extremely sensitized to the inhibition of PARP enzymatic activity either alone or when combined with DNA damaging agents, resulting from chromosomal instability, cell cycle arrest, and subsequent apoptosis. The levels of drug sensitivity are many folds higher than in isogenic cells with an intact BRCA pathway. The impairment of base excision repair by PARP-inhibitors is not lethal in cells with alternative mechanisms of DNA repair but proves insurmountable in cells deficient in homologous recombination (HR), such as is seen in tumors with loss of BRCA1 or BRCA2 function (1, 2), providing a therapeutic opportunity that has been demonstrated in large phase I studies of PARP-inhibitors (3).
Since the BRCA1 gene was first identified in 1994 (4), it has been shown that germline mutations are associated with inherited forms of breast cancer and ovarian cancer, but somatic point mutations are very rare in the sporadic forms of breast cancer and ovarian cancer (5, 6). However, several studies demonstrated a moderate frequency of loss of heterozygosity (LOH) (7, 8) and/or a reduced level or absence of BRCA1 mRNA and protein in a subset of sporadic breast cancer and ovarian cancer (9–11). These data suggested that transcriptional and/or posttranscriptional repression of BRCA1 may be involved in the development of sporadic breast cancer and ovarian cancer. One of the common mechanisms of functional inactivation of tumor suppressor genes in cancer cells is aberrant DNA hypermethylation of CpG islands in the promoter region of the gene that is associated with loss of gene expression (12).
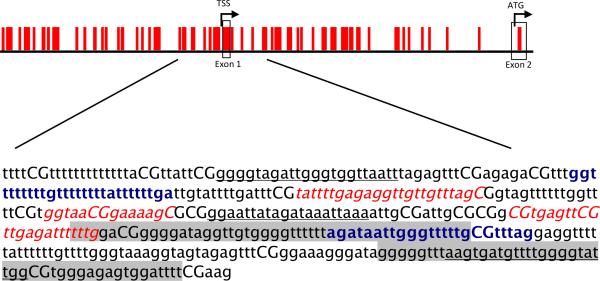
The CpG island in the promoter region of the BRCA1 gene is more than 2 kb in length (Fig. 1). The methylation status of this region has been broadly investigated in different sets of sporadic tumors, cell lines, and normal tissues from the breast and ovary. These studies demonstrated that BRCA1 methylation is tumor cell specific, being generally absent in normal cells (13, 14), and is usually accompanied by LOH (13). BRCA1 methylation is associated with decreased BRCA1 transcript in breast cancer (13, 15) and with decreased/absent level of BRCA1 protein by immunohistochemical analysis in breast cancer (16) and ovarian cancer (17). To date, BRCA1 methylation has been reported in sporadic breast cancer and ovarian cancer only. The frequency of methylation of the BRCA1 promoter is 10–15% in both breast and ovarian cancer (13, 15, 17).
Fig. 1.
Distribution of CpG sites and position of oligonucleotide primers or probes in the promoter region of the BRCA1 gene. Modified methylated sequence of the positive strand is shown with CG dinucleotides in capitals. Nucleotide 1,388 to 1,779 based on GenBank accession number U37574 are shown. Primers for direct bisulfite sequencing are bolded and in blue. Pyrosequencing amplification and sequencing primers are underlined. qMSP Taqman probe and primer set are in italics and red. Two oligonucleotide probes on the Infinium array are shown with a gray background. A third is outside the sequence shown.
Similarities between the histopathology and molecular biology of sporadic breast cancers with hypermethylation of BRCA1 and inherited BRCA1 breast cancer led to the concept of “BRCAness” (18). Both familial BRCA1 breast tumors and sporadic breast tumors with BRCA1 methylation are associated with lack of estrogen and progesterone receptor expression, the medullary and mucinous subtypes, amplification of MYC but not the HER2/neu proto-oncogene, and have a similar global gene expression profile (18). BRCAness in sporadic ovarian cancer is less well-studied and consequently, there is less evidence. Interestingly, carriers of BRCA1 or BRCA2 mutation with ovarian cancer appear to respond better to carboplatin than patients with sporadic ovarian cancer (19, 20).
The model for sensitivity to PARP inhibition is dependent upon HR deficiency rather than inherited BRCA mutation. Therefore, PARP inhibitors can be applicable to sporadic tumors with loss of function of BRCA1 or BRCA2 or other impairments of the HR pathway (1, 2, 21]. It follows that the methylation status of other genes involved in the BRCA/HR pathway is also relevant to PARP-inhibitor response. The BRCA2 gene appears to be unmethylated or very rarely methylated in breast, ovarian, or other cancer types (22–24). The partner and localizer of BRCA2 (PALB2) gene is also a Fanconi's Anemia gene (FANC-N), a breast cancer susceptibility gene as well as a component of the BRCA1/BRCA2 DNA repair pathway and has aberrant promoter hypermethylation associated with loss of expression in 5–10% of breast and ovarian tumors (25).
Since hypermethylation is associated with loss of expression, BRCA1 mRNA transcript or BRCA1 protein levels could be used as a readout for PARP-inhibitor response. mRNA expression as a readout can be complicated by isoforms and by the normal cells in the tumor biopsy. Antibody specificity is an issue in immunohistochemistry. Furthermore, aberrant hypermethylation in tumor cells is a positive alteration which facilitates detection. Perhaps, most importantly, while sporadic tumors with BRCA1 methylation show BRCAness (18), it is unclear whether sporadic tumors with an unmethylated BRCA1 promoter and decreased BRCA1 expression do.
An estimate of the tumor cell content of the biopsy and quantitative analysis of methylation are important since both the density of methylation within a gene promoter as well as intratumor heterogeneity might be expected to be related to response to PARPtherapy. Direct bisulfite sequencing reads the greatest number of CpG sites, and is semiquantitative, e.g., 20, 50 or 80%, etc. Pyrosequencing is more quantitative but provides a short read only. qMSP is rapid and reliable, is most sensitive for tumor-cell poor biopsies, or body fluids but also only interrogates several CpG sites (26). The Infinium HumanMethylation27 beadchip permits simultaneous assessment of many genes but currently includes only several selected CpG sites from the promoter region (although a further generation beadchip will likely address this point) and is the most expensive in cost. In this chapter, we describe these four assays that allow investigators to determine the methylation status of the critical region of the BRCA1 promoter CpG island associated with loss of expression, and thereby response to PARP-inhibitor therapy (Figs. 2–5).
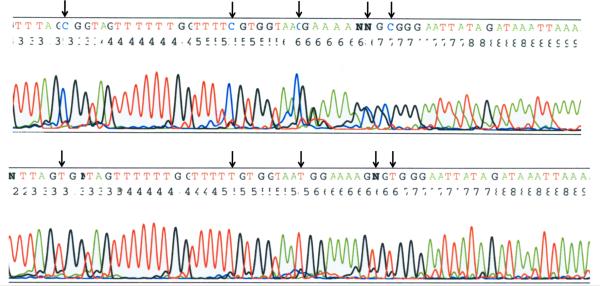
Fig. 2.
Direct bisulfite sequencing of a region (nt 1,521–1,583 GenBank U37574) of the BRCA1 promoter in bisulfite-modified tumor DNA. (Top) Tumor is methylated. (Bottom) Tumor is unmethylated. Black arrows indicate position of cytosine of CG dinucleotide.
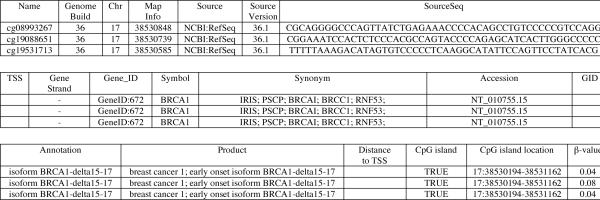
Fig. 5.
Example of Infinium HumanMethylation27 BeadChip annotation and β-value of BRCA1 after hybridization of bisulfite-modified normal lymphocyte DNA. β, an estimate of the proportion of cells in which the corresponding gene is methylated; values of β close to 1 indicate high levels of methylation while values near 0 are unmethylated.
2. Materials
2.1. DNA Extraction
2.1.1. Fresh, Frozen Tissue Specimens
Proteinase K buffer: 0.075 M NaCl, 0.024 M EDTA, pH 8.0.
10% SDS.
Proteinase K.
Phase lock gel tubes (5 Prime, Gaithersburg, MD) or regular 1.7 ml microcentrifuge tubes.
Phenol:chloroform:isoamyl alcohol (25:24:1).
10 M ammonium acetate.
Glycogen.
100% Ethanol (ice cold).
70% Ethanol.
ddH2O.
Nanodrop or spectrophotometer.
2.1.2. Paraffin-Embedded Tissue Specimens
Paraffin-embedded tissue sections on glass slide.
Razor blades.
Xylene.
100% Ethanol.
2.2. M.SssI Treatment for in vitro methylation
CpG Methyltransferase (M.SssI) is supplied with 10× NEBuffer and 32 mM S-adenosyl methionine (SAM) (New England Biolabs, Ipswich, MA).
Normal DNA (e.g., lymphocytes from peripheral blood of a healthy individual).
2.3. Bisulfite Modification of DNA
The following reagents are required for manual modification. Alternatively, use commercially available kits (e.g., EZ-DNA Methylation Kit, Zymo Research, Orange, CA) according to the manufacturer's instructions.
2 M and 3 M NaOH.
3 M NaHSO3 pH 5.0.
10 mM Hydroquinone.
DNA cleanup kit (e.g., Wizard DNA Clean-Up System, Promega, Madison, WI).
80% Isopropanol.
10 M Ammonium acetate.
Glycogen.
Vacuum manifold (Promega).
100% Ethanol (ice cold).
70% Ethanol.
ddH2O (warm).
2.4 Direct Bisulfite Sequencing
Primers for direct bisulfite sequencing, see Table 1 for BRCA1, or design primers as described in ref. 27.
Taq DNA polymerase.
10 mM dNTP mix.
10× PCR buffer (100 ml): 16.6 ml of 1 M (NH4)2SO4, 33.5 ml of 2 M Tris–HCl (pH 8.8), 6.7 ml of 1 M MgCl2, 700 μl of 14.4 M of β-mercaptoethanol, and 42.5 ml of ddH2O. Final concentrations are: (NH4)2SO4 (166 mM), Tris–HCl (670 mM), MgCl2 (67 mM), and β-mercaptoethanol (100 nM).
ddH2O.
Thermocycler (e.g., Eppendorf Mastercycler® Thermal Cycler, Eppendorf, Hauppauge, NY).
Mineral oil
NuSieve 3:1 agarose.
Running buffer 10× TBE.
Ethidium bromide.
Gel loading buffer: 300 μl glycerol, 1.5 g Ficoll PM400, 0.025 g bromophenol blue, 600 μl 10× TBE, adjust final volume to 10 ml with ddH2O.
Gel imaging system (e.g., model TVC-512R, Spectroline, Ultraviolet Transilluminator, Spectronics Corporation, Westbury, NY)
MiniElute Gel Extraction kit (e.g., Qiagen, Valencia, CA)
ABI 3100A capillary genetic analyzer.
Sequencher software version 4.2.2.
Table 1.
Primers and probes for BRCA1. All sequence is given 5' – 3'. R = degenerate G or A. The qMSP primers and probe for the control ACTINβ gene are given in Subheading 2.5.
| Assay | Oligonucleotide | Sequence | Product |
|---|---|---|---|
| Direct sequencing | Amplification F | GGTTTTTTTTGTTTTTTTTATTTTTTGA | 215bp |
| Amplification R | CTAAACRCAAAAACCCAATTATCT | ||
| Sequencing | Use either Amp F or R above (see Note 1) | ||
| Pyrosequencing | Amplification F | GGGGTAGATTGGGTGGTTAATT | 340bp |
| Amplification R | CCAATACCCCAAAACATCACTT | ||
| Sequencing R | TTTAATTTATCTATAATTCC | ||
| qMSP | Amplification F | TATTTTGAGAGGTTGTTGTTTAGC | 119bp |
| Amplification R | CAAAAAATCTCAACGAACTCACG | ||
| Probe | FAM-GGTAACGGAAAAGC-MGBNFQ |
2.5 Pyrosequencing
For primers for amplification and sequencing, see Table 1 for BRCA1 or design using Biotage software (Biotage AB, Uppsala, Sweden) or as (28)
For preparation of sample and calibration standards, see 2.1 (DNA Extraction), 2.2 (M.SssI Treatment) and 2.3 (Sodium Bisulfite Modification of Genomic DNA).
For PCR amplification and gel electrophoresis, see 2.4.
For pyrosequencing analysis, use the Pyro Gold reagent Kit, Pyrosequencing PSQ 96MA genetic analysis system, and PSQ 96MA2.1 software or as described in ref. 28.
2.6 Quantitative real time MSP
For primer and probe design, use commercial Primer Express software (Applied Biosystems, Carlsbad, CA) as described in ref. 29.
M.SssI-treated DNA.
TaqMan® Universal PCR Master Mix, No AmpErase® UNG (Applied Biosystems, Carlsbad, CA).
-
Primers: ACTINβ (see Note 2)
Forward: 5'-TGGTGATGGAGGAGGTTTAGTAAGT-3'
Reverse: 5'-AACCAATAAAACCTACTCCTCCCTTAA-3'
Probe: 6FAM – ACCACCACCCAACACACAATAACAAACACA – TAMRA: BRCA1: see Table 1
ddH2O
Standard optical 96-well plate (Applied Biosystems).
MicroAmp® optical adhesive film (Applied Biosystems).
Centrifuge with 96-well plate holder.
Real-Time PCR system (e.g., 7500 Real-Time PCR System, Applied Biosystems).
Nanodrop or spectrophotometer.
2.7. Infinium HumanMethylation27 BeadChip
Bisulfite treatment: EZ-DNA Methylation Kit (Zymo Research) according to the manufacturer's instructions (note special requirements for Infinium BeadChip).
Assay: Infinium HumanMethylation27 DNA Analysis Kit (WG-311-2201, Illumina Inc., San Diego, CA) www.illumina.com/methylation
3. Methods
3.1. DNA Extraction
3.1.1. Fresh, Frozen Tissue Specimens
With a sterile blade cut tissue into small pieces and transfer to sterile 1.7 ml microcentrifuge tube.
Add 350 μl 1× Proteinase K buffer, 40 μl 10% SDS, 10 μl Proteinase K (10 mg/ml solution) and incubate sample at 37°C overnight (see Note 3).
Add 400 μl of phenol:chloroform:isoamyl alcohol (25:24:1)
Mix 5 min on rotator, then centrifuge for 5 min at 14000g.
Transfer top layer containing DNA to a phase lock gel tube.
Repeat 3–5.
Add 400 μl chloroform and repeat 4–5.
Precipitate DNA by adding 10 M ammonium acetate (1/10 of total volume), 2 μl of glycogen, and 2.5× volume of 100% ethanol (ice-cold).
Remove DNA pellet with sterile pipette tip, allow to air-dry and resuspend in ddH2O.
Quantify DNA yield using a Nanodrop or other spectrophotometer.
3.1.2. Paraffin-Embedded Tissue Specimens
With sterile blade scrape tissue section from slide and place into 1.7 ml microcentrifuge tube.
Add 1 ml xylene. Invert several times and incubate for 10 min at room temperature.
Centrifuge for 5 min at 14000g.
Discard xylene and repeat 2–4 until complete removal of paraffin.
Add 1 ml of 100% ethanol, mix, and incubate at room temperature for 30 min.
Centrifuge for 5 min at 14000g and discard ethanol.
Air-dry pellet at room temperature.
Continue with step 2 Section 3.1.1.
3.2. M.SssI Treatment
Add 2.5 μl of 32 mM SAM, 6.25 μl of CpG Methyltransferase (M.SssI), 25 μl 10× NEBuffer to 20 μg of normal DNA in a sterile 1.7 ml microcentrifuge tube and adjust the total volume to 250 μl with ddH2O.qq
Incubate at 37°C for 4 hours.
Add 3.13 μl (12.5u) of M.SssI enzyme (4000u/ml) and 5 μl SAM.
Incubate at 37°C for 4 hours.
Add 250 μl of phenol:chloroform:isoamyl alcohol (25:24:1).
Mix 5 min on rotator, then centrifuge for 5 min at 14000g at room temperature.
Transfer top layer containing DNA to a 1.7 ml phase lock gel tube.
Repeat 5–7.
Add 250 μl chloroform and repeat 6 and 7.
Precipitate DNA by adding 10 M ammonium acetate (1/10 of total volume), 2 μl of glycogen, and 2.5× volume of 100% ethanol (ice-cold).
Centrifuge for 30 min at 14000g at room temperature.
Discard the supernatant and wash the pellet with 70% ethanol (ice-cold).
Centrifuge for 5 min at 14000g, carefully discard the supernatant, and allow the pellet to air-dry.
Resuspend DNA pellet in ddH2O.
Quantify DNA yield using a Nanodrop or spectrophotometer.
Bisulfite modify M.SssI-treated DNA as needed
3.3. Bisulfite Modification of DNA
3.3.1. Day 1
-
1
Dilute 1 μg of genomic DNA in 50 μl ddH2O
-
2
Add 5.7 μl of 2 M NaOH. Vortex and incubate 10 min at 37°C to denaturate the DNA.
-
3
Add 33 μl of freshly prepared 10 M hydroquinone and 525 μl of 3 M NaHSO3, vortex.
-
4
Add 25 μl of mineral oil and incubate at 50°C for 16–18 hours.
3.3.2. Day 2
-
5
Prepare mini-columns for each sample. Label both the column and syringe barrel. Connect columns to vacuum manifold.
-
6
Add 1 ml Wizard DNA Clean-Up Resin to the microcentrifuge tube below the oil. Mix up and down well and transfer the sample to the corresponding column. Apply vacuum.
-
7
Fill barrel with 80% isopropanol and apply vacuum.
-
8
Transfer empty columns into new labeled microcentrifuge tubes.
-
9
Add 50 μl of warm ddH2O. Incubate 1 min and centrifuge 1 min at 14000g.
-
10
Discard the columns.
-
11
Add 5.5 μl 3 M NaOH, vortex, incubate for 10 min at room temperature.
-
12
Add 17 μl of 10 M ammonium acetate, 1 μl of glycogen, and 400 μl of ice-cold 100% ethanol to the each sample.
-
13
Keep overnight at −20°C.
3.3.3. Day 3
-
14
Centrifuge samples for 20–30 min at 14000g. Discard supernatant.
-
15
Add 400 μl of 70% ethanol.
-
16
Centrifuge for 5 min at 14000g. Discard supernatant.
-
17
Dry pellet and resuspend in 25 μl of ddH2O.
-
18
Store bisulfite-modified DNA at −20°C or longer term at −80°C.
3.4 Direct Bisulfite Sequencing
3.4.1. PCR amplification
-
1
Determine the number of samples including controls (see Note 4). Prepare PCR master mix. The following is the amount for one sample and should be multiplied by the total number of samples.
10× PCR buffer 2 μl 10 mM dNTP mix 1 μl 100 ng/μl forward primer 1.5 μl 100 ng/μl reverse primer 1.5 μl Taq DNA polymerase 0.5 μl ddH2O 10.5 μl -
2
Aliquot 17 μl of PCR master mix into separate tubes.
-
3
Add 3 μl of bisulfite-treated DNA. Add 1–2 drops of mineral oil if thermocycler does not have a heated lid.
-
4
Amplification program: 95°C – 5 min; 1× (95°C – 30 sec, 64°C – 1 min, 72°C – 30 sec); 1× (95°C – 30 sec, 63°C – 1 min, 72°C – 30 sec); 35× (95°C – 30 sec, 62°C – 1 min, 72°C – 30 sec). Final step: 72°C – 10 min and hold at 4°C.
3.4.2. Gel Electrophoresis
Prepare 2% agarose gel. Dissolve 5 g of agarose gel in 250 ml 1× TBE buffer, boil in microwave oven for 1.5 min, add 15 μl of ethidium bromide.
Mix PCR products with loading buffer and run on agarose gel with size markers at 100 V for 1 h.
Use UV-based gel imaging system to visualize the amplicon and cut out gel slice containing the amplified PCR product.
Purify PCR product using Qiagen's MiniElute Gel Extraction kit according to the manufacturer's instructions.
3.4.3. Sequencing
Send purified PCR product along with either forward or reverse primers to local sequencing facility. DNA sequence is determined using the ABI 3100A capillary genetic analyzer.
DNA sequence data are analyzed using Sequencher Software, Version 4.2.2.
3.5. Pyrosequencing
3.5.1. Preparation of sample and calibration standards
Extract genomic DNA as in 3.1.1. for fresh or frozen tissue samples or 3.1.2. for paraffin-embedded tissue. Treat normal DNA with CpG Methyltransferase SssI (see 3.2.). Use normal lymphocyte DNA or commercially available unmethylated DNA as unmethylated template. Treat sample and standards with sodium bisulfite (see 3.3.).
3.5.2. PCR amplification
Prepare master mix (see 3.4.1.). Amplify bisulfite-treated DNA with one or two biotinylated primers using the following program: 95°C – 5 min; 40× (95°C – 30 sec, 59°C – 1 min, 72°C – 30 sec). Final step: 72°C – 10 min and hold at 4°C.
3.5.3. Gel electrophoresis and purification of PCR product
See 3.4.2.
3.5.4. Pyrosequencing Analysis
Send purified PCR product and pyrosequence primers to local pyrosequencing facility. DNA sequence is determined by the Pyrosequencing PSQ 96MA genetic analysis system using the Pyro Gold Reagent Kit according to manufacturer's instructions.
DNA sequence data are analyzed using the PSQ 96MA 2.1 software.
3.6 Quantitative Real-Time MSP
3.6.1. Preparation of standard dilutions
For absolute quantification, use a standard curve constructed by amplifying known amounts of standard DNA (4 tenfold, 10–104 copies per microliter, serial dilutions of bisulfite-modified M.SssI-treated DNA) in a parallel group of reactions run under the same conditions as the sample of interest.
3.6.2. PCR amplification
-
1
Keep the Taqman probe on ice and protected from light. Determine the total number of bisulfite-modified specimen DNA samples, standards, and no template controls to be analyzed. Prepare separate PCR master mixes for the gene of interest (e.g., BRCA1) and the reference gene (e.g., ACTINβ). The following amounts are for one sample and should be multiplied by the total number of samples with an additional 10% for pipette error.
TaqMan Universal PCR Master Mix (2×) 12.5 μl 60 ng/μl Forward primer 1.5 μl 60 ng/μl Reverse primer 1.5 μl 20 pmol/μl TaqMan probe 0.25 μl ddH2O 6.25 μl -
2
Add 22 μl aliquot of each PCR master mix into an individual well of a standard optical 96-well plate. Perform each reaction (as well as each control reaction) in duplicate.
-
3
Add 3 μl of bisulfite-modified M.SssI-treated DNA to each standard and sample well and 3 μl of ddH2O to no template control.
-
4
Cover plate with adhesive film and centrifuge plate at 800g for 2 min at +4°C or room temperature.
-
5
Insert plate into real-time PCR machine. PCR conditions are 95°C − 10 min, 50× (95°C – 15 sec, 60°C – 1 min, 72°C – 15 sec).
-
6
Assess methylation status by threshold cycle (Ct) and/or quantity (see Note 5)
3.7 Infinium HumanMethylation27 BeadChip
The specimen DNA is bisulfite modified with the EZ-DNA Methylation Kit according to manufacturer's instructions (note special requirements for Infinium BeadChip).
Bisulfite-treated DNA is isothermally amplified overnight and enzymatically fragmented.
Fragmented DNA samples are applied to the BeadChip. During hybridization, single-stranded DNA anneals to locus-specific DNA oligomers linked to individual bead types. Each bead type corresponds to each CpG locus – one to the methylated and the other to the unmethylated state. Allele-specific primer annealing is followed by single-base extension using dinitrophenyl (DNP)- and Biotin-labeled ddNTPs.
Fluorescent staining. The intensities of the beads' fluorescence are detected by the Illumina BeadArray Reader, and analyzed using Illumina's BeadStudio software.
DNA methylation values, described as beta-values, vary between 0 (unmethylated) and 1 (fully methylated), representing the ratio of the intensity of the methylation bead type to the combined locus intensity (see Note 6).
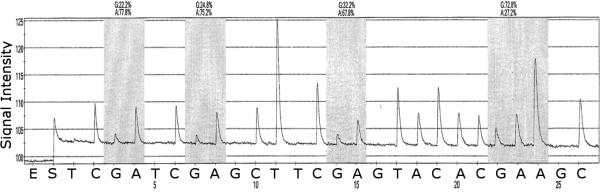
Fig. 3.
A pyrogram of a tumor DNA showing heterogeneous levels of methylation at 4 CG sites in the CpG island of the BRCA1 gene promoter (nt 1539–1563 Genbank U37574). The y-axis represents the signal intensity in arbitrary units, the x-axis shows the dispensation sequence. The sequence reads 3' CGCGCTTTTCCGTTACCACGAAAAC 5'
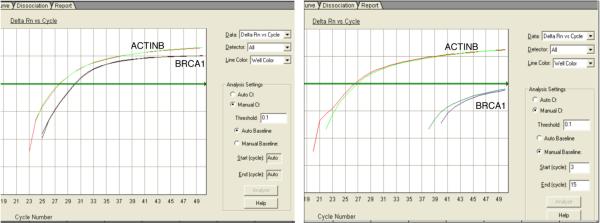
Fig. 4.
qMSP amplification plot of a tumor DNA ran in duplicate with methylated BRCA1 (left) and unmethylated BRCA1 (right). The horizontal green line is the threshold.
Acknowledgements
This work was supported by the Ovarian Cancer SPORE at Fox Chase Cancer Center (P50 CA083638).
Footnotes
Approximately, the first 35–50 bp of sequence from the 3'end of the sequencing primer cannot be read. If the reverse amplification primer is used as a sequencing primer, then G indicates the presence of methylation and A an unmethylated cytosine in a CG dinucleotide. If an amplification primer is also used as a sequencing primer, the resulting sequence may be less clean depending on the efficiency of the cleanup of excess dNTPs and primer dimers.
To save time and money, the unmethylated sequence of the target gene can be replaced by a gene unmethylated in normal and cancer cells, e.g., ACTINβ. To minimize and correct sample-to-sample variation caused by any difference in the amount of starting material between samples is to amplify, simultaneously with target gene of interest, gene of reference (i.e. ACTINβ) against which the gene of interest can be normalized. This method is based on the assumption that the copy number of the normalizing gene (ACTINβ on chromosome 7p) is normal or known.
Alternatively, incubate at 50°C for 3–4 hours. Inverting the Eppendorf tube by hand several times and/or adding additional proteinase K will aid digestion.
The considerable sequence difference between completely methylated and unmethylated DNA after bisulfite modification results in a difference in melting and annealing characteristics that may lead to preferential amplification of the unmethylated strand (PCR amplification bias) (30). It is very important to detect any PCR bias. This can be achieved by using a mixture of completely methylated and unmethylated DNA in different ratios or at a minimum 50:50.
The formula to calculate PMA represents the ratio between the PCR amplification product of the gene of interest and the reference gene multiplied by 100%. PMA usually is given as log value Ln (%+1).
The Infinium beadchip also contains CG sites located outside the promoter region of a gene. The Infinium data for a given tumor specimen should be interrogated as to the position of the methylated CG site with regard to the promoter region, then compared to normal progenitor cell DNA for verification that the methylation is aberrant.
References
- 1.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 2.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 3.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 4.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 5.Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266:120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- 6.Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Futreal PA, Soderkvist P, Marks JR, Iglehart JD, Cochran C, Barrett JC, et al. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992;52:2624–2627. [PubMed] [Google Scholar]
- 8.Russell SE, Hickey GI, Lowry WS, White P, Atkinson RJ. Allele loss from chromosome 17 in ovarian cancer. Oncogene. 1990;5:1581–1583. [PubMed] [Google Scholar]
- 9.Russell PA, Pharoah PD, De Foy K, Ramus SJ, Symmonds I, Wilson A, et al. Frequent loss of BRCA1 mRNA and protein expression in sporadic ovarian cancers. Int J Cancer. 2000;87:317–321. doi: 10.1002/1097-0215(20000801)87:3<317::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 12.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 14.Mancini DN, Rodenhiser DI, Ainsworth PJ, O'Malley FP, Singh SM, Xing W, et al. CpG methylation within the 5′ regulatory region of the BRCA1 gene is tumor specific and includes a putative CREB binding site. Oncogene. 1998;16:1161–1169. doi: 10.1038/sj.onc.1201630. [DOI] [PubMed] [Google Scholar]
- 15.Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21:1761–1765. doi: 10.1093/carcin/21.9.1761. [DOI] [PubMed] [Google Scholar]
- 16.Matros E, Wang ZC, Lodeiro G, Miron A, Iglehart JD, Richardson AL. BRCA1 promoter methylation in sporadic breast tumors: relationship to gene expression profiles. Breast Cancer Res Treat. 2005;91:179–186. doi: 10.1007/s10549-004-7603-8. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–5333. [PubMed] [Google Scholar]
- 18.Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 19.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 20.Pal T, Permuth-Wey J, Kapoor R, Cantor A, Sutphen R. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer. 2007;6:113–119. doi: 10.1007/s10689-006-9112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 22.Collins N, Wooster R, Stratton MR. Absence of methylation of CpG dinucleotides within the promoter of the breast cancer susceptibility gene BRCA2 in normal tissues and in breast and ovarian cancers. Br J Cancer. 1997;76:1150–1156. doi: 10.1038/bjc.1997.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gras E, Cortes J, Diez O, Alonso C, Matias-Guiu X, Baiget M, et al. Loss of heterozygosity on chromosome 13q12–q14, BRCA-2 mutations and lack of BRCA-2 promoter hypermethylation in sporadic epithelial ovarian tumors. Cancer. 2001;92:787–795. doi: 10.1002/1097-0142(20010815)92:4<787::aid-cncr1384>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Hilton JL, Geisler JP, Rathe JA, Hattermann-Zogg MA, DeYoung B, Buller RE. Inactivation of BRCA1 and BRCA2 in ovarian cancer. J Natl Cancer Inst. 2002;94:1396–1406. doi: 10.1093/jnci/94.18.1396. [DOI] [PubMed] [Google Scholar]
- 25.Potapova A, Hoffman AM, Godwin AK, Al-Saleem T, Cairns P. Promoter hypermethylation of the PALB2 susceptibility gene in inherited and sporadic breast and ovarian cancer. Cancer Res. 2008;68:998–1002. doi: 10.1158/0008-5472.CAN-07-2418. [DOI] [PubMed] [Google Scholar]
- 26.Kagan J, Srivastava S, Barker PE, Belinsky SA, Cairns P. Towards Clinical Application of Methylated DNA Sequences as Cancer Biomarkers: A Joint NCI's EDRN and NIST Workshop on Standards, Methods, Assays, Reagents and Tools. Cancer Res. 2007;67:4545–4549. doi: 10.1158/0008-5472.CAN-06-2888. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Rohde C, Tierling S, Stamerjohanns H, Reinhardt R, Walter J, et al. DNA methylation analysis by bisulfite conversion, cloning, and sequencing of individual clones. Methods Mol Biol. 2009;507:177–187. doi: 10.1007/978-1-59745-522-0_14. [DOI] [PubMed] [Google Scholar]
- 28.Dejeux E, El abdalaoui H, Gut IG, Tost J. Identification and quantification of differentially methylated loci by the pyrosequencing technology. Methods Mol Biol. 2009;507:189–205. doi: 10.1007/978-1-59745-522-0_15. [DOI] [PubMed] [Google Scholar]
- 29.Licchesi JD, Herman JG. Methylation-specific PCR. Methods Mol Biol. 2009;507:305–323. doi: 10.1007/978-1-59745-522-0_22. [DOI] [PubMed] [Google Scholar]
- 30.Warnecke PM, Stirzaker C, Melki JR, Millar DS, Paul CL, Clark SJ. Detection and measurement of PCR bias in quantitative methylation analysis of bisulphite-treated DNA. Nucleic Acids Res. 1997;25:4422–4426. doi: 10.1093/nar/25.21.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]