Abstract
Vascular endothelial growth factor (VEGF) plays a crucial role in developmental and pathological angiogenesis. Expression of VEGF in quiescent adult tissue suggests a potential role in the maintenance of mature blood vessels. We demonstrate, using a Vegf–lacZ reporter mouse model, that VEGF is expressed by arterial but not by venous or capillary endothelial cells (ECs) in vivo. Using an in vitro model, we show that arterial shear stress of human umbilical vein ECs (HUVECs) decreases apoptosis and increases VEGF expression, which is mediated by the induction of Krüppel-like factor 2 (KLF2). Additionally, shear stress stimulates the expression of VEGF receptor 2 (VEGFR2) and is associated with its activation. Knockdown of VEGF in shear stressed HUVECs blocks the protective effect of shear stress, resulting in EC apoptosis equivalent to that in control ECs cultured under static conditions. Similarly, treatment of ECs subjected to arterial shear stress with the VEGF receptor tyrosine kinase inhibitor SU1498, or VEGFR2 neutralizing antiserum, led to increased apoptosis, demonstrating that the mechanoprotection from increased shear is mediated by VEGFR2. Taken together, these studies suggest that arterial flow induces VEGF–VEGFR2 autocrine–juxtacrine signaling, which is a previously unidentified mechanism for vascular EC survival in adult arterial blood vessels.
Key words: Endothelial cells, Shear stress, VEGF, VEGFR2, KLF2
Introduction
Vascular endothelial growth factor (VEGF; also known as VEGF-A, VPF), a secreted, homodimeric, glycoprotein and potent angiogenic factor, was identified on the basis of its ability to induce blood vessel permeability and capillary endothelial cell (EC) proliferation (Senger et al., 1983; Ferrara and Henzel, 1989). VEGF also promotes EC migration and survival as well as the formation and maintenance of capillary fenestrations (Ferrara et al., 2003; Maharaj and D'Amore, 2007). During development, VEGF mediates both vasculogenesis, which is the de novo formation of blood vessels from endothelial precursors, and angiogenesis, which is the formation of new blood vessels from pre-existing venules and capillaries. In the adult, VEGF plays a major role in a variety of pathologies, including cancer and retinopathies, as well as in normal physiological processes, such as wound healing (Brown et al., 1992a; Frank et al., 1995) and in the female reproductive cycle (Otani et al., 1999). Although it is clear that VEGF is central to processes with active angiogenesis, less is known about its relevance in the normal adult. The continued expression of VEGF and the constitutive activation of the VEGF receptor 2 (VEGFR2) in virtually all quiescent adult tissues (Maharaj and D'Amore, 2007) support the concept that VEGF is essential for the integrity and/or maintenance of the adult vasculature.
The human VEGF gene contains eight exons that are alternatively spliced to generate multiple isoforms (Tischer et al., 1991), with VEGF121, VEGF165 and VEGF189 representing the major species. All VEGF isoforms bind with high affinity to two structurally related receptor tyrosine kinases, VEGFR1 (also known as Flt-1) and VEGFR2 (also known as KDR and Flk-1), which are expressed by ECs as well as by a variety of other cells (D'Amore, 2007). VEGF165 and VEGF189 also bind to neuropilin-1 (NRP-1) (Soker et al., 1998), which acts as a co-receptor. A majority of the biological effects of VEGF on ECs are mediated through VEGFR2 (Waltenberger et al., 1994; Clauss et al., 1996), which is increased during the angiogenic process and downregulated in quiescent endothelium (Millauer et al., 1993).
VEGF was initially considered to be an endothelial-specific mitogen, which is secreted by a variety of cell types, including epithelial cells (Berse et al., 1992; Brown et al., 1992b; Maharaj et al., 2006; Saint-Geniez et al., 2006) and vascular smooth muscle cells (SMCs) (Tischer et al., 1991; Brogi et al., 1994), and that acts in a paracrine manner on nearby ECs. It is well established that VEGF expression can be modulated by various stimuli (Loureiro and D'Amore, 2005), including growth factors, cytokines, hormones and hypoxia (Shweiki et al., 1992). In vitro studies demonstrate that biomechanical stimuli, such as cyclic stretch, cause increased VEGF expression by vascular SMCs (Quinn et al., 2002; Mata-Greenwood et al., 2005), as well as other cell types (Gruden et al., 1997; Seko et al., 1999b; Seko et al., 1999a; Muratore et al., 2000; Motokawa et al., 2005) including ECs (Suzuma et al., 2001; Zheng et al., 2001). More recently, fluid shear stress has been implicated in the upregulation of VEGF-A gene expression (Parmar et al., 2006; Thi et al., 2007; Goettsch et al., 2008; Li et al., 2009) although the role and relevance of shear-stress-induced VEGF expression has yet to be elucidated.
Members of the Krüppel-like factor (KLF) family of transcription factors, in particular KLF2, have received increased attention for their potential regulatory roles in the vasculature (Suzuki et al., 2005; Atkins and Jain, 2007). In situ hybridization of the developing mouse vasculature and adult human vascular tissue reveals that KLF2 expression is specific to the endothelium (Kuo et al., 1997; Dekker et al., 2002). In humans, for instance, KLF2 is expressed by the endothelium of the thoracic aorta (Dekker et al., 2002). Additionally, endothelial KLF2 is induced by prolonged shear stress (Dekker et al., 2002) and mediates the expression of genes important in inflammation, thrombosis and vessel tone (SenBanerjee et al., 2004; Dekker et al., 2005; Lin et al., 2005; Dekker et al., 2006; Parmar et al., 2006). It has been shown that 15.3% of genes induced by flow depend on KLF2 upregulation and 46% of the most highly flow-regulated genes require KLF2 induction (Parmar et al., 2006).
Our previous studies using a Vegf–lacZ reporter model to locate VEGF in the adult mouse revealed that VEGF is expressed by a number of cell types in various tissues, including podocytes, hepatocytes and adipocytes (Maharaj et al., 2006). Surprisingly, we also detected VEGF expression by the endothelium of some large vessels; specifically, VEGF expression was observed in the endothelium of the aorta, but not the inferior vena cava (Maharaj et al., 2006). Aside from their structural and functional differences, arteries and veins are exposed to distinct hemodynamic forces (dela Paz and D'Amore, 2008). Whereas cyclic stretch, or circumferential stress, is primarily experienced by the SMCs, fluid shear stress, the tangential component of hemodynamic forces, acts uniquely on the endothelium (Ballermann et al., 1998). To explore the possibility that differences in shear stress contribute to differential VEGF expression by the venous versus the arterial endothelium in vivo, we systematically examined VEGF expression by the endothelium using the Vegf–lacZ reporter mouse. We then studied the effects of arterial flow on endothelial VEGF expression and investigated the mechanism of this upregulation using the widely accepted in vitro model of human umbilical vein endothelial cells (HUVECs) exposed to shear stress.
Results
VEGF is expressed by arterial but not venous or capillary ECs in vivo
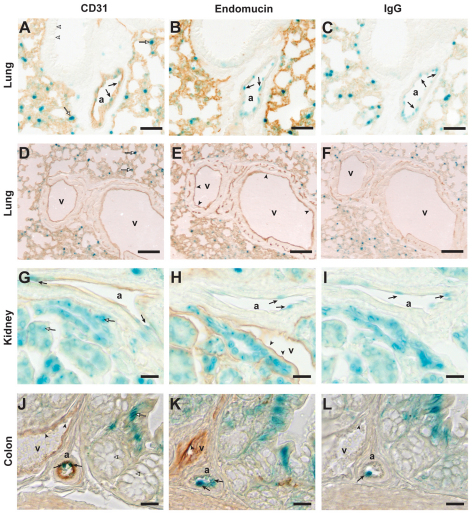
VEGF expression by vascular ECs was examined using adult Vegf–lacZ mice. The endothelium of veins and capillaries was distinguished from that of arteries by co-staining for CD31 (also known as platelet endothelial cell adhesion molecule; PECAM-1), a pan-EC marker, or endomucin, a specific marker of capillaries and veins (Kuhn et al., 2002; Samulowitz et al., 2002; dela Paz and D'Amore, 2008), in the same manner that ephrinB2 and EphB4 have been used to selectively mark arteries and veins, respectively, during vasculogenesis and angiogenesis. Consistent with our previous observations (Ng et al., 2001; Maharaj et al., 2006), VEGF was expressed by type II pneumocytes in the lung (Fig. 1A,D, open arrows), but was virtually absent in the bronchiolar epithelium (Fig. 1A, open arrowheads). VEGF was also expressed by the endothelium of some blood vessels (Fig. 1A–C, arrows). The ECs of pulmonary vessels that stained with X-gal (i.e. expressed VEGF) were positive for CD31 (Fig. 1A), but negative for endomucin (Fig. 1B), unambiguously identifying these vessels as arteries. By contrast, all endomucin-positive vessels were negative for β-galactosidase activity, indicating that the EC of neither veins nor capillaries in the lung express VEGF (Fig. 1E, arrowheads).
Fig. 1.
Arterial but not venous endothelium expresses VEGF in vivo. Tissues were dissected from adult Vegf–lacZ mice, fixed in 4% PFA, frozen in OCT and cut into 10 μm sections. Serial sections were assessed for β-galactosidase activity by staining with X-gal (blue). (A,D,G,J) Diaminobenzidine (DAB) staining (brown) of CD31 to identify all ECs. (B,E,H,K) Anti-endomucin IgG was used to identify venous ECs. (C,F,I,L) Sections incubated with pre-immune IgG, which served as negative controls. In the lung, kidney and colon, β-gal (as a reporter of VEGF expression) is visible in ECs that line the lumen of arteries (a; A–C,G–L, arrows) but not veins (v; E,H, J–L, arrowheads). Expression of VEGF was noted in type II pneumocytes of alveoli in the lung (A,D, open arrows), proximal convoluted tubule epithelium in the kidney (G, open arrows) and epithelial cells within the crypts of Lieberkühn in the colon (J, open arrows). VEGF expression was not detected in the bronchiolar epithelium in the lung (A, open arrowheads). Scale bars: 50 μm (A–C), 100 μm (D–F), 20 μm (G–L).
In the kidney, VEGF was expressed by the epithelium of the proximal convoluting tubule (Fig. 1G, open arrows), by podocytes (Maharaj et al., 2006) and by arterial ECs (Fig. 1G–I, arrows), but not by the venous endothelium (Fig. 1H, arrowheads). In the colon, VEGF expression was detected in some epithelial cells of the crypts of Lieberkühn (Fig. 1J, open arrows) and in the arterial endothelium (Fig. 1J–L, arrows). However, no VEGF expression was detected in the mucus-producing goblet cells (Fig. 1J, open arrowheads) or in the ECs of veins (Fig. 1J–L, arrowheads). Taken together, these results indicate that endothelial expression of VEGF is specific to the arterial branch of the vasculature.
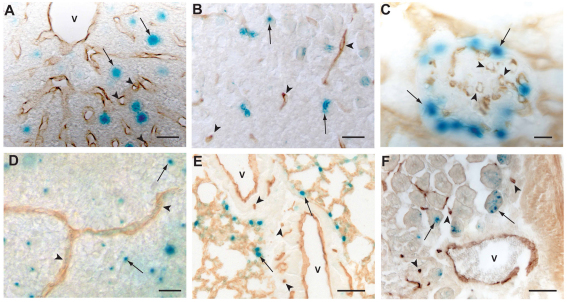
We next investigated whether VEGF is expressed by microvascular endothelium. In the liver, VEGF was expressed by the hepatocytes (Fig. 2A, arrows) (Maharaj et al., 2006), but not by the sinusoidal endothelium (Fig. 2A, arrowheads). In the brain, VEGF was expressed non-uniformly by the cerebral parenchyma (Fig. 2B, arrows), as was previously shown (Maharaj et al., 2006), but was undetectable in the capillaries (Fig. 2B, arrowheads). Similarly, the glomerular capillaries of the kidney were negative for VEGF expression (Fig. 2C, arrowheads), whereas the associated podocytes robustly expressed VEGF (Fig. 2C, arrows). In the adult retina, retinal pigment epithelium (Saint-Geniez et al., 2006), pericytes (Saint-Geniez et al., 2008), Müller cells and astrocytes (Fig. 2D, arrows) (Saint-Geniez et al., 2008) all expressed VEGF; however, the capillary endothelium, clearly visible in flat-mounted retinas, was negative for VEGF expression (Fig. 2D, arrowheads). Similarly, although VEGF was expressed by type II pneumocytes in the lung (Fig. 2E, arrows), it was not detected in the pulmonary capillaries (Fig. 2E, arrowheads). In the adult colon, VEGF expression was evident in epithelial cells (Fig. 2F, arrows) and arterial ECs (Fig. 1J–L, arrows), but not in capillary ECs. These findings indicate that normal, quiescent capillary endothelium does not express VEGF.
Fig. 2.
Capillary endothelium does not express VEGF in vivo. Tissues of adult Vegf–lacZ mice were sectioned and stained as in Fig. 1. The microvasculature of the liver (A), brain cerebrum (B), kidney (C), retina (D), lung (E) and colon (F) were all negative for VEGF expression (arrowheads). Among non-vascular cells within these same tissues, lacZ expression was detected in hepatocytes (A, arrows), cerebral parenchyma (D, arrows), renal podocytes (C, arrows), retinal astrocytes (D, arrows), type II pneumocytes of alveoli in the lung (E; arrows) and epithelial cells within the crypts of Lieberkühn in the colon (F; arrows). Scale bars: 20 μm (A,B,D), 10 μm (C), 50 μm (E,F).
Fluid shear stress induces VEGF expression by ECs
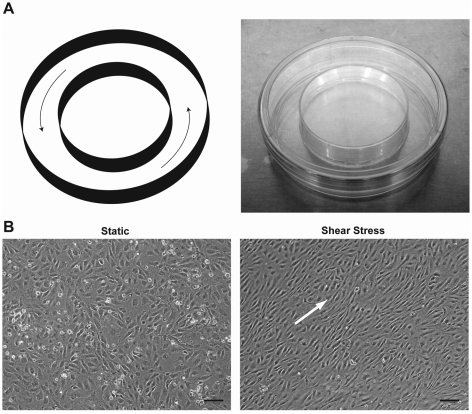
In light of major differences between venous and arterial blood flow and the associated shear stress, we examined whether shear stress might contribute to the observed difference in VEGF expression. To accomplish this, we adopted an in vitro model of laminar shear stress based on the orbital rotation of culture medium over a monolayer of ECs. Preliminary experiments, in which a pre-calculated orbital shear stress in the range of physiological arterial blood flow (6–40 dynes/cm2) was applied to cultured HUVECs for 24 hours, revealed a difference in morphology between the ECs at the periphery and those in the center of culture dishes. Cells at the periphery were elongated and aligned in the direction of flow, but those toward the center were not aligned and remained polygonal in shape, similar to cells cultured under static conditions (data not shown). These findings are consistent with a previous report that used optical Doppler velocimetry to measure the shear stress applied to bovine aortic ECs by orbital rotation and attributed differences in EC phenotype, which included morphology, to a substantially higher measured shear stress at the periphery than in the center (Dardik et al., 2005). To ensure that the cells in our studies were uniformly exposed to shear stress, we developed a modified 100-mm culture dish (Fig. 3A) that restricts cell attachment to the periphery of the dish. After 24 hours of shear stress in the modified culture dish, HUVECs were elongated and aligned, as opposed to cells under static conditions that retained their cobblestone appearance (Fig. 3B).
Fig. 3.
An in vitro model of arterial shear stress. (A) Schematic diagram (left) of arterial shear stress based on the orbital rotation of a modified 100-mm culture dish (right) designed for restricting the adhesion of ECs to the periphery. (B) Morphology of serum-starved HUVECs exposed for 24 hours to static (left) and orbital shear stress (right) conditions. The white arrow indicates the direction of shear stress. Scale bars: 100 μm.
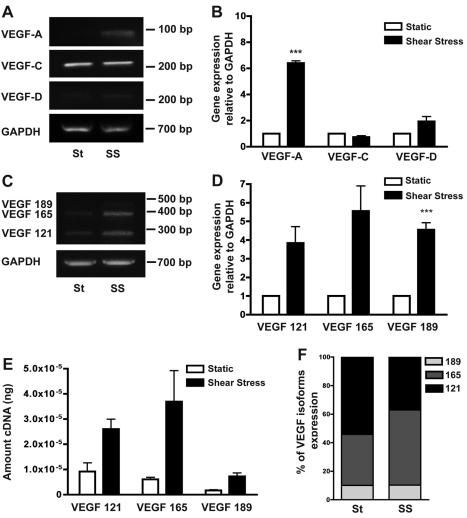
Using this model, we examined the effects of shear stress on VEGF expression. HUVECs exposed to arterial shear stress for 72 hours showed an increase in total VEGF-A mRNA expression compared with static control cells (Fig. 4A), and real-time PCR analysis revealed a more than sixfold increase in VEGF mRNA (Fig. 4B). Parallel experiments revealed that arterial shear stress had no significant effect on the expression of VEGF-C and VEGF-D, two additional members of the VEGF–PDGF family that can bind to and activate VEGFR2 (Fig. 4A,B), suggesting that shear stress-induced VEGF-A upregulation is specific.
Fig. 4.
Effect of arterial shear stress on VEGF expression by HUVEC in vitro. (A) RT-PCR analysis of the effect of static (St) versus shear stress (SS) on EC expression of VEGF family members. VEGF-A was induced by 72 hours of shear stress (representative image of three independent experiments). (B) Relative expression of VEGF family members measured by real-time PCR and normalized to GAPDH mRNA levels. ***P<0.001. (C) Representative gel showing increased expression of all VEGF isoforms by shear-stress-induced HUVECs compared with static control cells as measured by RT-PCR. (D) Relative expression of VEGF isoforms as measured by real-time PCR and normalized to GAPDH mRNA levels. ***P<0.001. (E) Quantification of VEGF isoforms expression by HUVECs exposed to static and shear stress conditions as measured by real-time PCR. The amount of cDNA was normalized using GAPDH levels and quantified using a standard curve for each VEGF isoform. (F) Percentage expression of VEGF isoforms, with total VEGF expression set to 100%, in static and shear stress conditions.
To determine the effect of arterial shear stress on VEGF-A isoform expression, semi-quantitative RT-PCR was performed using a primer pair that simultaneously amplifies VEGF121, VEGF165 and VEGF189. All three VEGF isoforms were upregulated in HUVECs exposed to shear stress (Fig. 4C), and this was confirmed by real-time PCR (Fig. 4D). Although similar increases in relative gene expression were observed for VEGF121 (~4-fold), VEGF165 (~5.5-fold) and VEGF189 (~4.5-fold), the absolute levels of the isoforms differed, with VEGF189 the least abundant under both static and shear stress conditions (Fig. 4E). Shear stress resulted in a modest shift in VEGF isoforms distribution with VEGF121 decreasing from 54% to 37% and VEGF165 increasing from 36% to 53% of total VEGF mRNA (Fig. 4F).
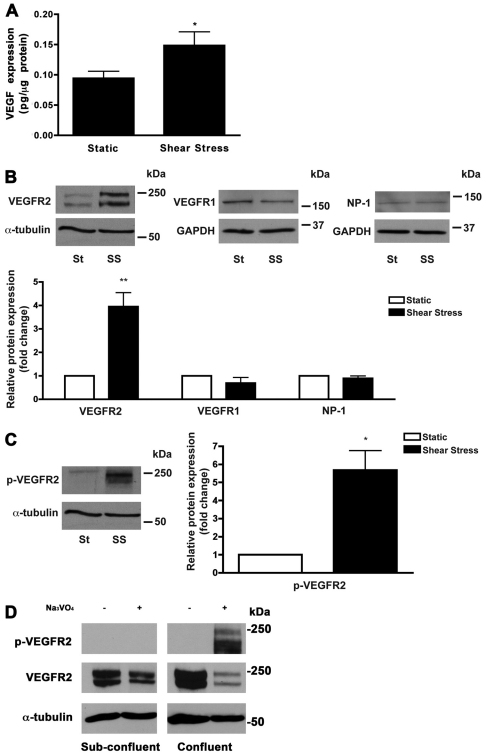
Shear stress leads to increased VEGF protein expression and VEGFR2 expression and activation
To confirm that changes in VEGF expression occur at the protein level, we performed ELISA. Analysis of VEGF protein expression showed a significant (P=0.0234) increase in shear-stress-induced HUVECs compared with static cultures (Fig. 5A). To determine the effect of arterial shear stress on the level of VEGF receptors, western blot analysis was performed on lysates of HUVECs exposed to static or shear stress for 72 hours. VEGFR2 levels were markedly increased in cells exposed to shear stress (Fig. 5B), with a fourfold increase compared with cells cultured under static conditions. VEGFR2 phosphorylation was also significantly increased (approximately sixfold; P=0.0119) in cells exposed to shear stress (Fig. 5C). By contrast, the levels of the two other VEGF-A receptors, VEGFR1 and neuropilin-1, remained unchanged in cells exposed to shear stress (Fig. 5B). Interestingly, VEGFR2 phosphorylation was markedly reduced in sub-confluent HUVECs (Fig. 5D). Together, these results indicate that VEGF signaling, specifically through VEGFR2, is increased in cells exposed to arterial shear stress.
Fig. 5.
Shear stress increases VEGF and VEGFR2 protein expression. (A) Quantification of total VEGF protein expression by static and shear stress-induced HUVECs as determined by ELISA. Each sample was normalized to total protein content (pg/μg protein). *P<0.05. (B) Western blot analysis of lysates from HUVECs exposed to static (St) and shear stress (SS) conditions for 72 hours. Shear stress induced VEGFR2 expression, but had no effect on VEGFR1 or neuropilin-1 (NP-1) expression. (C) Phosphorylated VEGFR2 (p-VEGFR2) was increased in HUVECs grown under shear stress. Images shown are representative blots from three independent experiments. Quantification of protein expression is shown in the bar graphs as fold-change over static control levels, normalized to α-tubulin or GAPDH. *P<0.05. (D) Cell lysates from confluent or sub-confluent cultures of HUVECs were prepared in the presence of Na3VO4, an inhibitor of tyrosine phosphatase activity. Representative blot depicts an absence of VEGFR2 phosphorylation in sparse HUVECs. Total VEGFR2 and α-tubulin levels were also assessed as controls for protein loading.
Arterial shear stress is protective for ECs in vitro
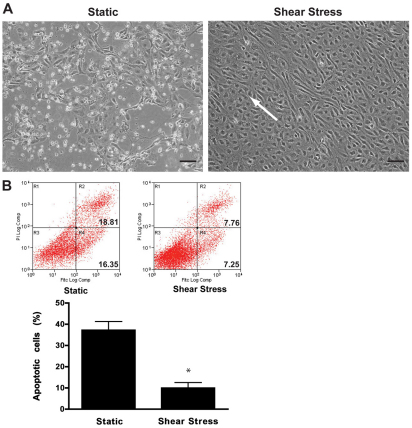
Although HUVECs grown in low serum and exposed to continuous shear stress for 72 hours were elongated, aligned and healthy (Fig. 6A), HUVECs cultured under static conditions showed signs of cellular stress and death. To quantify the differences between HUVECs grown under static conditions and shear stress, apoptosis was assessed. Exposure of HUVECs to shear stress led to a significant (P=0.0443) decrease in the percentage of apoptotic cells compared with cells grown under static conditions (Fig. 6B), indicating that arterial shear stress was protective against apoptosis.
Fig. 6.
Arterial shear stress protects ECs against apoptosis. (A) Morphology of serum-starved HUVECs after 72 hours exposure to static (left) and shear stress (right) conditions. White arrow indicates EC alignment in the direction of flow. Scale bars: 100 μm. (B) Flow cytometric analysis of apoptosis following annexin V and PI staining of HUVECs grown under static and shear stress conditions. A representative set of scatter dot plots (top) shows the percentage of early and late stage apoptosis of static and shear-stress-induced HUVECs. Exposure to shear stress led to a significant decrease in the percentage of apoptotic cells, as quantified in the bar graph (bottom). EC apoptosis is expressed as means ± s.e.m. (n=3), *P<0.05.
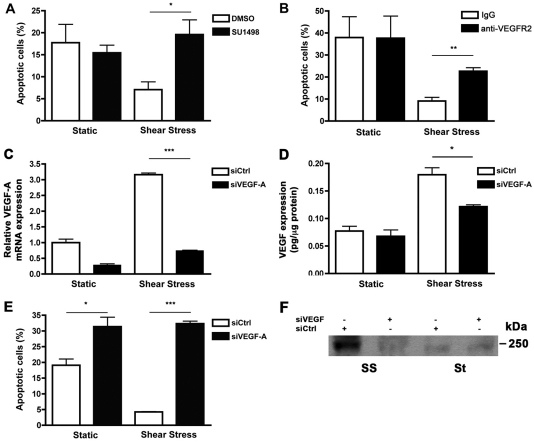
To determine whether shear stress-induced EC survival was mediated by VEGFR2 signaling, the effect of the receptor tyrosine kinase inhibitor SU1498 (Strawn et al., 1996; Wang et al., 2002; Boguslawski et al., 2004; Masumura et al., 2009) on apoptosis of cells cultured under static and flow conditions was assessed. SU1498 did not lead to a significant change in the percentage of apoptotic HUVECs cultured under static conditions compared with untreated cells (Fig. 7A). By contrast, SU1498 treatment of HUVECs exposed to shear stress blocked the protective effect of shear stress, leading to increased apoptosis compared with cells exposed to shear stress in the absence of the inhibitor. Similar results were obtained when VEGFR2 signaling was inhibited using a VEGFR2 neutralizing antibody (Fig. 7B).
Fig. 7.
VEGFR2 signaling is involved in shear-stress-induced EC survival. (A) HUVECs were exposed to static or shear stress conditions in the presence of SU1498 or DMSO. Flow cytometric analysis of apoptosis revealed a marked increase in the percentage of apoptotic cells when HUVECs were exposed to shear stress in the presence of SU1498. Apoptosis of static cells treated with SU1498 was unaffected. *P<0.05. (B) HUVECs were exposed to static or shear stress conditions in the presence of anti-VEGFR2 or pre-immune IgG. Flow cytometric analysis revealed a marked increase in the percentage of apoptotic cells when VEGFR2 signaling was inhibited by antibody neutralization in HUVECs exposed to shear stress. Apoptosis of static cells was unaffected by the presence of anti-VEGR2. **P<0.01. (C) HUVECs were transfected with non-targeting siRNA oligonucleotides or siRNA oligonucleotides directed against VEGF for 48 hours, followed by exposure to static or shear stress conditions for an additional 24 hours. Real-time PCR analysis was performed to validate the knockdown of VEGF expression. Gene expression, which was normalized to that of GAPDH, is relative to that in HUVECs transfected with control siRNA. ***P<0.001. (D) Quantification of total VEGF protein expression by siRNA-transfected HUVECs subjected to static or shear stress conditions as determined by ELISA. Each sample was normalized to total protein content (pg/μg protein). *P<0.05. (E) Flow cytometry showed a dramatic increase in apoptosis when sheared HUVECs were transfected with siVEGF compared with siCtrl. ***P<0.001. (F) HUVECs were transfected with non-targeting siRNA oligonucleotides (siCtrl) or siRNA oligonucleotides directed against VEGF (siVEGF) for 48 hours prior to exposure to static (St) or shear stress (SS) conditions for 24 hours. Representative blot showing reduced levels of shear-stress-induced VEGFR2 phosphorylation in the presence of siVEGF compared with siCtrl.
Neutralization of the VEGF ligand using bevacizumab had a variable effect on EC survival; in some experiments the VEGF blockade led to increased apoptosis of shear-stress-induced HUVECs, whereas in others it had no effect (data not shown). Because HUVECs subjected to shear stress express both VEGF and VEGFR2, we speculate that this variability is due to the fact that the confluent HUVECs signal very efficiently in a juxtacrine manner, making the VEGF inaccessible to neutralization. Therefore, to confirm that shear-stress-induced EC survival was due to increased VEGF expression, we examined apoptosis of HUVECs transfected with siRNA oligonucleotides designed to knock down VEGF (siVEGF). As a control, HUVECs were transfected with non-targeting siRNA oligonucleotides (siCtrl). Static and shear-stress-induced VEGF mRNA expression levels were decreased by 71% and 77%, respectively, in cells transfected with siRNA against VEGF compared with non-specific control siRNA (Fig. 7C). Similarly, transfection of siVEGF resulted in decreased VEGF protein expression in both static and sheared HUVECs (Fig. 7D). siCtrl-transfected HUVECs were protected by shear stress (Fig. 7E). However, there was a significant increase in apoptosis in HUVECs that were transfected with siVEGF prior to shear stress, indicating that protection by shear stress is mediated by increased VEGF expression. Western blot analysis also showed that silencing of VEGF prevents phosphorylation of VEGFR2 in response to shear stress (Fig. 7F).
Shear stress-induced VEGF upregulation is mediated by KLF2
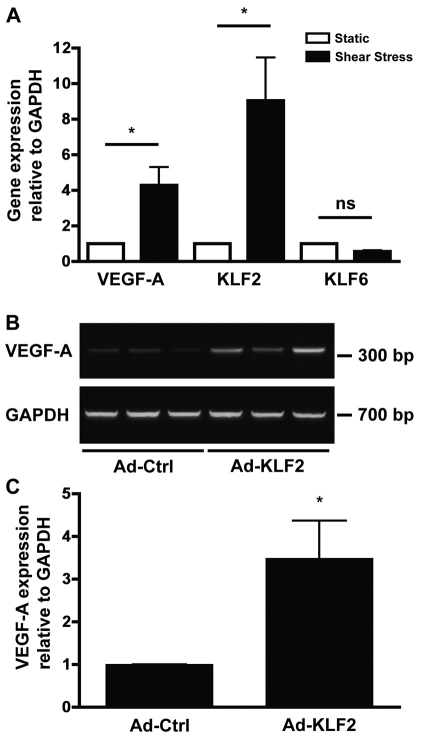
Because KLF proteins have been shown to mediate the increased expression of a number of shear stress-induced genes, we investigated a possible role for KLF proteins in shear stress-induced VEGF expression. We first examined the expression of KLF2 and KLF6 and observed that the expression level of KLF2 (approximately ninefold) was markedly increased in shear-stress-cultured HUVECs compared with static cells (Fig. 8A). Expression of KLF6, however, remained unchanged (Fig. 8A).
Fig. 8.
VEGF-A upregulation is associated with increased KLF2 expression. (A) Real-time PCR analysis of the expression of VEGF-A and KLF family members in HUVECs in response to 24 hours of shear stress. Gene expression was calculated relative to that of static-cultured HUVECs and normalized to that of GAPDH. *P<0.05. (B) Representative gel showing a marked increase in VEGF-A mRNA expression in Ad-KLF2-infected HUVECs compared with Ad-Ctrl-infected controls performed in triplicate. (C) Relative expression of VEGF-A mRNA in Ad-Ctrl- vs. Ad-KLF-2-infected HUVECs measured by real-time PCR and normalized to GAPDH mRNA levels. *P<0.05.
To determine whether increased VEGF is associated with KLF2 upregulation, we performed overexpression studies in static-cultured HUVECs, which have very low levels of VEGF. HUVECs infected with an adenovirus expressing KLF2 (Ad-KLF2) exhibited an increase in VEGF mRNA expression compared with cells that were infected with a control GFP virus (Ad-GFP; Fig. 8B). Quantification, using real-time PCR analysis, revealed a more than threefold increase in VEGF expression (Fig. 8C).
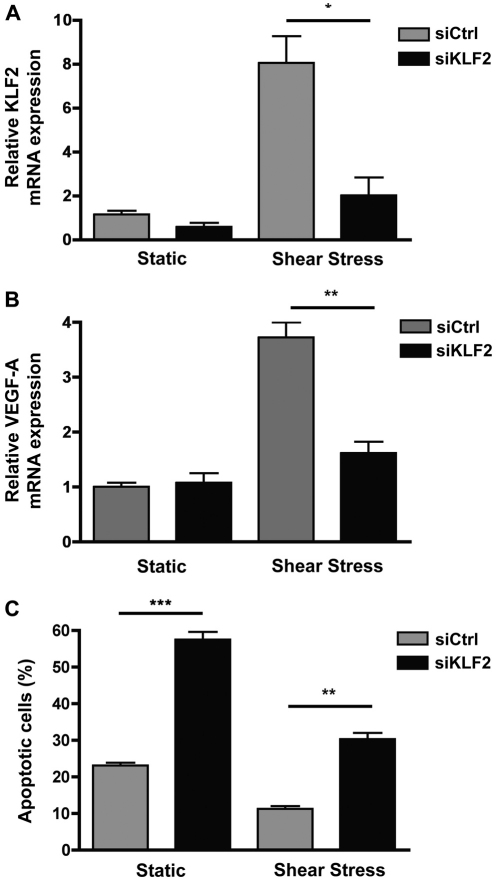
In order to assess whether KLF2 is necessary for the observed increase in VEGF expression in ECs grown under shear stress, we performed siRNA knockdown experiments. Using an siRNA designed to be specific for KLF2 (siKLF2), we knocked down shear-stress-induced KLF2 expression by 75% compared with HUVECs exposed to shear stress and transfected with a non-targeting siRNA oligonucleotide (siCtrl; Fig. 9A), which was close to the level observed in static-cultured HUVECs. siRNA knockdown of KLF2 resulted in a dramatic reduction in VEGF expression (~60%) in cells exposed to shear stress (Fig. 9B). VEGF expression in siKLF2-transfected HUVEC grown under static conditions remained unchanged, suggesting that KLF2 specifically mediates shear-stress-induced VEGF expression.
Fig. 9.
Shear-stress-induced VEGF-A expression is mediated by KLF2. (A) Real-time PCR analysis to validate the knockdown of KLF2 expression in HUVECs transfected with siRNA oligonucleotides directed against KLF2 for 48 hours, followed by exposure to static or flow conditions for an additional 24 hours. Gene expression, which is normalized to that of GAPDH, is relative to the expression in HUVECs transfected with control siRNA. *P<0.05. (B) Relative expression of VEGF-A in static versus sheared HUVECs transfected with siKLF2 compared with siCtrl measured by real-time PCR and normalized to the expression of GAPDH. **P<0.01. (C) Flow cytometric analysis of apoptosis revealed a marked increase in the percentage of apoptotic cells when HUVECs were transfected with siKLF2 prior to exposure to shear stress. Apoptosis of static cells transfected with siKLF2 was also increased. **P<0.01, ***P<0.001.
We next examined whether the reduction in VEGF expression caused by KLF2 knockdown affected cell survival. siRNA-mediated suppression of KLF2 in HUVECs under shear stress led to increased apoptosis (~30%) compared with sheared control cells (~11%; Fig. 9C). Interestingly, apoptosis of siKLF2-transfected cells under static conditions was also increased, suggesting that KLF2 regulates other shear-independent genes.
Discussion
We previously observed that VEGF is expressed by the endothelium lining the aorta but not the adjacent inferior vena cava (Maharaj et al., 2006). Although a recent study confirmed our initial findings (Lee et al., 2007), this report concluded that all endothelium, including large and small vessels, express VEGF. However, the latter study did not distinguish between arterial, venous and capillary ECs. Because we had observed the absence of VEGF expression by the endothelium of the inferior vena cava, we hypothesized that the profile of VEGF expression is more limited and is restricted to arterial ECs. Further, we proposed that arterial VEGF expression is mediated, at least in part, by shear stress, and that shear-stress-induced VEGF expression represents an important mechanism for arterial EC homeostasis.
Our findings using Vegf–lacZ mice demonstrate that expression of VEGF in the adult vascular endothelium is indeed specific to the arterial endothelium; all ECs positive for endomucin, a known marker of veins and capillaries (Kuhn et al., 2002; Samulowitz et al., 2002; dela Paz and D'Amore, 2008), were negative for β-galactosidase activity. Although VEGF expression was not observed in arterial ECs of all tissues examined, our data suggest that under normal conditions only arterial ECs synthesize VEGF. Differences in the degree of shear stress, circumferential stretch and/or tissue microenvironment (Suzuma et al., 2001; Zheng et al., 2001) could account for the variation in VEGF expression along the arterial tree.
We previously reported VEGF expression by SMCs of the inferior vena cava (Maharaj et al., 2006) and by retinal capillary pericytes (Saint-Geniez et al., 2008) as well as by non-vascular cells that are in close proximity to the microvasculature in different tissues, such as type II pneumocytes in the lung, podocytes in the kidney and hepatocytes in the liver [this paper and Maharaj et al. (Maharaj et al., 2006)]. We speculate that although venous and capillary ECs do not express VEGF, their proximity to VEGF-producing cells and their architecture provide easy access for the venous and capillary endothelia to VEGF produced by adjacent cells. In support of this concept, all fenestrated capillaries are near a VEGF-producing epithelium (e.g. podocytes in the glomerulus and epithelium in the choroid plexus) and it has been well documented that neutralization of VEGF leads to regression of these fenestrated capillary beds (Baffert et al., 2006; Kamba et al., 2006). By contrast, arterial ECs produce their own VEGF because the internal elastic lamina is a substantial physical barrier to VEGF produced by the underlying SMC. Induction of VEGF expression by shear stress would address this issue, providing a potential autocrine–juxtacrine signaling pathway. In addition, the potential damage caused by the relatively high shear stress and circumferential stretch in the arterial tree could lead arterial ECs to require a higher level of VEGF for survival.
One can argue that the shear stress observed in the microcirculation in vivo is at least within the range of that of arterioles (Lipowsky et al., 1978; Papaioannou and Stefanadis, 2005) and therefore is actually higher than the model of shear stress we adapted for our in vitro studies. Although shear stress clearly represents a major regulator, as our in vitro studies have shown, we acknowledge that it is not the only factor contributing to the differences in VEGF expression we observed in vivo. It is conceivable that there are negative feedback loops caused by VEGF secreted from adjacent cells that prevent venous or capillary ECs from making their own VEGF.
Both pulsatile flow (Li et al., 2009) and laminar shear stress (Goettsch et al., 2008) have been reported to increase VEGF expression; however, the effect of shear stress on isoform distribution has not been examined. We show that HUVECs grown under static culture express all three major VEGF isoforms, with VEGF121 the predominant (54%) and VEGF189 the least abundant (10%) form. Although the levels of all three isoforms were similarly increased with shear stress, the level of VEGF121 relative to total VEGF expression decreased while VEGF165 increased. VEGF isoforms are generated from a single gene by alternative splicing and differ in their inclusion of exons 6 and 7 that encode for domains that mediate VEGF binding to heparan sulfate (Houck et al., 1991; Houck et al., 1992) and neuropilin (Soker et al., 1998) and therefore determine their localization upon secretion. Whereas VEGF121, which lacks these two domains, would be the most diffusible, VEGF189, which includes both domains, is expected to be sequestered locally, with VEGF165 having intermediate properties. Thus, expression of sequestered VEGF isoforms by ECs exposed to shear stress might reflect the need for VEGF to be localized in the face of arterial flow. In addition, the synthesis of the soluble isoforms by arterial ECs suggests that diffusibility is important for their functions. Thus, in addition to a role for VEGF in EC survival, the arterial endothelium could be the source of the low levels of circulating VEGF that have been shown to be necessary for the maintenance of vessel tone (Hood et al., 1998; Walshe et al., 2009).
The existence of an autocrine–juxtacrine signaling pathway in arterial endothelium is supported by our observation that VEGFR2 expression and activation is increased in HUVECs exposed to arterial shear stress. This is consistent with previous reports of flow upregulating VEGFR2 expression by ECs in culture (Abumiya et al., 2002; Shay-Salit et al., 2002; Urbich et al., 2003), but more importantly corroborates our observation of constitutive VEGFR2 phosphorylation in aortic endothelium in vivo (Maharaj et al., 2006). Considering the role of VEGF in pathological neovascularization, continuous VEGFR2 activation might be expected to lead to EC hyperproliferation, such as seen in hemangiomas. However, it is important to note that the action of VEGF in the adult vasculature is balanced by the effects of other factors such as angiopoietin-1 and TGF-β1. In support of this notion, we have previously shown that whereas the addition of VEGF alone induces EC division in a three-dimensional model in vitro and TGF-β1 alone leads to apoptosis, the simultaneous administration of VEGF and TGF-β1 results in the suppression of both proliferation and apoptosis (Ramsauer and D'Amore, 2007).
Others have suggested that the action of VEGF on ECs in vivo is through an intracrine mechanism (Lee et al., 2007), a conclusion based on the fact that the survival effects of VEGF could only be blocked using small molecule antagonists but not by extracellular blockade of VEGF. Two lines of evidence argue against this interpretation. First, we have shown that suppression of VEGF can be achieved in vitro with VEGFR2 neutralizing antisera. Second, the close proximity of ECs to one another and their production of predominantly cell-associated VEGF would permit VEGF signaling in an autocrine–juxtacrine manner, i.e. VEGF produced by one EC would signal itself or a neighboring EC, making the VEGF largely inaccessible to exogenous VEGF-neutralizing antisera. In support of this concept, VEGFR2 phosphorylation in sparse HUVECs was lower than in confluent cells (Fig. 5D). A previous report demonstrated that phosphorylation of VEGFR2 was higher in sparse than in confluent HUVECs (Grazia Lampugnani et al., 2003). Our studies differ from this previous report in that we examined activation of VEGFR2 in the absence of exogenously added VEGF whereas the previous report examined the effect of exogenously added VEGF. We interpret our result as indicating that the VEGF produced by the ECs in confluent cultures has ready access to VEGFR2 on the adjacent cells, but not in sparse cultures.
We and others (Dimmeler et al., 1996) have demonstrated that shear stress protects HUVECs cultured in low serum from the apoptosis seen in cells cultured under static conditions. Our observation that blockade with the tyrosine kinase inhibitor SU1498 or with anti-VEGFR2 prevents the shear-stress-mediated survival indicates that this mechanoprotection is mediated by VEGFR2 signaling. Evidence obtained using ECs transfected with siRNA directed against VEGF indicates that the activation of VEGFR2 in response to shear stress is mediated by increased VEGF expression rather than by direct mechanical forces on the cells. VEGF-C and VEGF-D have been shown to activate VEGFR2 in cultured ECs (Joukov et al., 1996; Achen et al., 1998), but neither has been shown to be expressed by vascular ECs in vivo. Furthermore, the fact that VEGF-C and VEGF-D mRNA expression was not significantly increased in HUVECs exposed to shear stress makes it unlikely that either ligand is responsible for the increased VEGFR2 activation in ECs exposed to shear stress.
Our results demonstrate a role for KLF2 in mediating shear stress induction of VEGF. Although a specific consensus KLF2 binding site has yet to be identified, different KLF family members can bind a general GC-rich binding motif, referred to as the ‘CACCC’ element or ‘GT box’. Thrombomodulin and endothelial nitric oxide synthase genes are among the best-characterized direct targets of KLF2 that contain one or more CACCC elements; interestingly, both of these genes are involved in endothelial homeostasis. Analysis of the sequence of the VEGF gene promoter reveals the presence of several CACCC or GGGTG motifs, indicating that VEGF could be another direct homeostatic target of KLF2.
Taken together, our data indicate that VEGF is essential for the survival of the arterial endothelium. This finding adds to the growing body of evidence that supports a role for VEGF expression in cell survival and homeostasis in the adult and thus has implications for chronic VEGF neutralization. Anti-VEGF therapies are being widely utilized for the treatment of a variety of cancers (Yang et al., 2003; Hurwitz et al., 2004; Willett et al., 2004). In the absence of the ability to specifically target pathological VEGF, chronic anti-VEGF therapy should be administered with caution.
Materials and Methods
Animals
Adult heterozygous mice generated by breeding Vegf–lacZ mice (generously provided by Andras Nagy, University of Toronto, Toronto, ON, Canada) (Miquerol et al., 1999) to either Swiss Webster or C57BL/6 background mice were used for all histological studies. All animal experiments were performed according to the relevant regulatory standards.
Histological analysis and microscopy
Tissues were dissected from adult mice that were anesthetized with ketamine–xylazine and perfused with PBS through the aorta. After overnight fixation at 4°C with 4% paraformaldehyde in PBS, tissues were washed with PBS and cryoprotected for 1 hour each at room temperature (RT) with 2:1, 1:1 and 1:2 mixtures of 5% and 20% sucrose in PBS followed by 2 hours at RT with 20% sucrose in PBS. Tissues were then infiltrated overnight at 4°C and embedded the next day with the same 2:1 mixture of 20% sucrose and OCT compound before finally being frozen on dry ice.
For visualization of VEGF expression, 10 μm-thick serial sections from Vegf–lacZ mice were cut on a cryostat (Leica CM1900) and stained overnight at 37°C with X-gal using the In Situ β-Galactosidase Staining Kit (Stratagene). For identification of vascular cell type, tissue sections were subjected to immunoperoxidase staining. After washing with PBS and permeabilization with PBS containing 0.2% Tween 20 (PBST), endogenous peroxidase activity was blocked by incubation with 1% H2O2 in PBS for 10 minutes. Non-specific binding was blocked by incubation with blocking buffer (3% rabbit serum in PBST) for 30 minutes at RT. Tissue sections were incubated with either rat anti-mouse CD31 (1:250, BD Pharmingen) or rat anti-mouse endomucin (1:500, mAb V.7C7 generously provided by Dietmar Vestweber, Max-Planck-Institute, Münster, Germany) diluted in blocking buffer overnight at 4°C. Each experiment included a section incubated with rat IgG as a negative control. For staining of flat-mounted retinas, 0.1% saponin was added to PBST during permeabilization. Slides were examined and images were captured using a Zeiss Axioskop 2 MOT microscope with a mounted AxioCam digital camera under both 20× and 40× objective lenses. Post-acquisition processing of images was done using Adobe Photoshop.
Cell culture
Human umbilical vein endothelial cells (HUVECs) (kindly provided by Kay Case, Center for Excellence in Vascular Biology, Brigham and Women's Hospital, Boston, MA) were cultured in EGM-2 (endothelial growth medium 2; EBM-2 SingleQuots excluding VEGF; Lonza, Walkersville, MD) supplemented with 20% fetal bovine serum, L-glutamine and penicillin–streptomycin. Before each experiment, cells were grown to confluence and synchronized by culture for 24 hours in starvation medium (EBM-2, 0.5% fetal bovine serum, 25 mM HEPES, penicillin–streptomycin). For experiments in which inhibition of VEGF–VEGFR2 signaling was investigated, SU1498 (Sigma) was added to the starvation medium at a final concentration of 10 nmol/ml.
Shear stress model
HUVECs were seeded in the periphery of modified 100-mm culture dishes (Fig. 3A) that were made by bonding the bottoms of 60-mm culture dishes upside down into the center of 100-mm culture dishes using medical adhesive (Silastic; Factor II, Inc., Lakeside, AZ). Dishes were sterilized under UV light for 15 minutes and then coated with 0.1% gelatin for 15 minutes prior to plating of cells. Confluent monolayers were subjected to orbital shear stress for 72 hours at 37°C in the presence of CO2 using an orbital shaker (Bellco Biotechnology, Vineland, NJ) positioned inside an incubator as previously described (Ley et al., 1989). Because cells are restricted to the periphery in these modified culture dishes, the shear stress across each monolayer was approximated as the maximal wall shear stress: τmax=a√ρη(2πf)3, where a is the radius of orbital rotation (1.25 cm), ρ is the density of the medium (1.0 g/ml), η is the viscosity of the medium (7.5×10−3 dynes·s/cm2) and f is the frequency of rotation (rotations/second). Using this equation, a shear stress of 10 dynes/cm2 is achieved at a rotating frequency of 195 rpm, which is within the range of physiological arterial shear stress (~6–40 dynes/cm2). HUVECs from the same passage, which were not subjected to shear stress, were kept in the same incubator and served as the static control. Alignment of ECs was visualized using a Nikon Eclipse TE2000-S microscope with a SPOT digital camera system under a 10× objective.
RNA isolation and reverse transcription-PCR analysis
Total RNA was isolated (RNAqueous-4PCR Kit; Ambion, Inc.) according to the manufacturer's protocol. Contaminating DNA was removed by treatment with DNase 1 (Ambion, Inc.) for 30 minutes at 37°C. First-strand cDNA was synthesized from 1 μg total RNA with oligo(dT)12–18 and reverse transcriptase (SuperScript III; Invitrogen) in a 20-μl reaction for 50 minutes at 50°C. RNA template was subsequently removed by digestion with 2 IU RNase H (Invitrogen) for 20 minutes at 37°C. A template of 1 μl cDNA was used in each amplification reaction. For standard PCR, 200 μM dNTPs (Roche), 1 IU Taq DNA polymerase (Denville Scientific) and 0.2 μM of the appropriate primer pair (supplementary material Table S1) were used. For real-time PCR, reactions were performed on the ABI Prism 9700 Sequence Detection System (Applied Biosystems) using 0.4 μM primers (supplementary material Table S1) and the SYBR Green PCR Master Mix (Applied Biosystems), according to the manufacturer's instructions. Amplification of GAPDH mRNA was performed on each sample as a control for normalization. Quantification of VEGF isoform expression was based on standard curves (10−3–10−7 ng DNA) of plasmids encoding VEGF121, VEGF165 and VEGF189.
Western blot analysis
Cells were collected in PBS and disrupted in NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM EDTA) at 4°C. Protease (Complete, Mini, EDTA-free; Roche) and phosphatase inhibitors (Phosphatase Inhibitor Cocktail 2; Sigma) were added immediately before cell lysis. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce). Equal amounts of protein (20 μg) were separated by SDS-PAGE and transferred to PVDF membranes (Immobilin-P; Millipore). After blocking with 1× TBS containing 0.1% Tween 20 and 5% nonfat dry milk, membranes were probed with primary antibody, detected with horseradish-conjugated secondary antibody (GE Healthcare) and developed with ECL or ECL Plus (GE Healthcare). The following primary antibodies were used: rabbit anti-VEGFR1 (1:2000; Abcam), rabbit anti-VEGFR2 (1:500; Cell Signaling), rabbit anti-phosphorylated VEGFR2 (1:2000; Invitrogen), rabbit anti-neuropilin (1:1000; Santa Cruz Biotechnology), rabbit anti-GAPDH (1:500; Santa Cruz Biotechnology) and mouse anti-α-tubulin (1:1000; Calbiochem). When necessary, membranes were stripped in 62.5 mM Tris-HCl, pH 6.8, 2% SDS and 100 mM β-mercaptoethanol for 30 minutes at 55°C, and re-probed.
ELISA
Total VEGF protein expression from conditioned medium and lysates of static control and sheared cells was determined using the Quantikine Human VEGF Immunoassay ELISA kit (R&D Systems), according to the manufacturer's protocol. Absorbance readings minus background were converted to picograms using standard curves from the kit. Results were normalized to total protein levels and represented as pg/mg of protein.
Detection of apoptotic cells
In vitro apoptosis was measured by staining cells for annexin V and propidium iodide (PI) using the Vybrant Apoptosis Assay Kit #2 (Invitrogen) followed by fluorescence-activated cell sorting (FACS) analysis using a flow cytometer (Becton Dickinson FACScan) as previously described (Bryan et al., 2008). Cells that were annexin positive and PI negative (early) or positive (late) were designated as apoptotic.
Adenoviral-mediated infection
HUVECs were seeded to 75–80% confluency a day before adenoviral infection. Cells were infected with Ad-GFP or Ad-mKLF2 (multiplicity of infection=10) for 24 hours (both generous gifts from Guillermo García-Cardeña, Center for Excellence in Vascular Biology, Brigham and Women's Hospital, Boston, MA). Cells were then washed with 1× PBS and processed for RNA.
siRNA-mediated knockdown
HUVECs were seeded to 75–80% confluence a day before transfection. Cells were then washed with 1× PBS to remove residual antibiotics and equilibrated in OPTI-MEM. siRNA was incubated with Oligofectamine in OPTI-MEM (both from Invitrogen) at RT for 30 minutes to form complexes, then added to the cells at a final siRNA concentration of 200 nmol/l. After 6 hours, complete growth medium without antibiotics was added. At 24 hours post-transfection, cells were washed with 1× PBS and complete growth medium was added. At 48 hours post-transfection, cells were placed into serum-starved conditions (EBM-2 + 0.5% FBS + P/S) and subjected to shear stress for 24 hours. siRNAs directed against VEGF and KLF2 were purchased as SMART pools (L-003550-00-0005 and L-006928-00-0005, respectively, from Dharmacon). Non-targeting control siRNA (D-001810-01-05) was also obtained from Dharmacon.
Statistical analysis
All experimental data, unless otherwise specified, are expressed as means ± s.e.m. from at least three independent experiments. Single comparisons were performed using Student's t-test. Multiple comparisons were performed using ANOVA with individual significant differences determined by post-hoc analysis using Student's t-test with Bonferroni correction. P-values of <0.05 were taken to indicate statistical significance.
Acknowledgements
The authors thank Guillermo García-Cardeña for his helpful discussions and for providing valuable reagents.
Footnotes
Funding
This work was supported by National Institutes of Health [grant numbers EY05318 and EY015435 to P.A.D.]; and by a National Research Service Award Institutional Research Training Grant [grant number T32 HL076115 to N.G.P.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.084301/-/DC1
References
- Abumiya T., Sasaguri T., Taba Y., Miwa Y., Miyagi M. (2002). Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler. Thromb. Vasc. Biol. 22, 907-913 [DOI] [PubMed] [Google Scholar]
- Achen M. G., Jeltsch M., Kukk E., Mäkinen T., Vitali A., Wilks A. F., Alitalo K., Stacker S. A. (1998). Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 95, 548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G. B., Jain M. K. (2007). Role of Kruppel-like transcription factors in endothelial biology. Circ. Res. 100, 1686-1695 [DOI] [PubMed] [Google Scholar]
- Baffert F., Le T., Sennino B., Thurston G., Kuo C. J., Hu-Lowe D., McDonald D. M. (2006). Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol. 290, H547-H559 [DOI] [PubMed] [Google Scholar]
- Ballermann B. J., Dardik A., Eng E., Liu A. (1998). Shear stress and the endothelium. Kidney Int. Suppl. 67, S100-S108 [DOI] [PubMed] [Google Scholar]
- Berse B., Brown L. F., Van de Water L., Dvorak H. F., Senger D. R. (1992). Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 3, 211-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski G., McGlynn P. W., Harvey K. A., Kovala A. T. (2004). SU1498, an inhibitor of vascular endothelial growth factor receptor 2, causes accumulation of phosphorylated ERK kinases and inhibits their activity in vivo and in vitro. J. Biol. Chem. 279, 5716-5724 [DOI] [PubMed] [Google Scholar]
- Brogi E., Wu T., Namiki A., Isner J. M. (1994). Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation 90, 649-652 [DOI] [PubMed] [Google Scholar]
- Brown L. F., Yeo K. T., Berse B., Yeo T. K., Senger D. R., Dvorak H. F., van de Water L. (1992a). Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 176, 1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Tognazzi K., Manseau E. J., Van de Water L., Senger D. R., Dvorak H. F., Rosen S. (1992b). Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 42, 1457-1461 [DOI] [PubMed] [Google Scholar]
- Bryan B. A., Walshe T. E., Mitchell D. C., Havumaki J. S., Saint-Geniez M., Maharaj A. S., Maldonado A. E., D'Amore P. A. (2008). Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol. Biol. Cell 19, 994-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M., Weich H., Breier G., Knies U., Röckl W., Waltenberger J., Risau W. (1996). The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 271, 17629-17634 [DOI] [PubMed] [Google Scholar]
- D'Amore P. (2007). Vascular endothelial cell growth factor-A: Not just for endothelial cells anymore. Am. J. Pathol. 171, 14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardik A., Chen L., Frattini J., Asada H., Aziz F., Kudo F. A., Sumpio B. E. (2005). Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg. 41, 869-880 [DOI] [PubMed] [Google Scholar]
- Dekker R. J., van Soest S., Fontijn R. D., Salamanca S., de Groot P. G., VanBavel E., Pannekoek H., Horrevoets A. J. (2002). Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100, 1689-1698 [DOI] [PubMed] [Google Scholar]
- Dekker R. J., van Thienen J. V., Rohlena J., de Jager S. C., Elderkamp Y. W., Seppen J., de Vries C. J., Biessen E. A., van Berkel T. J., Pannekoek H., et al. (2005). Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am. J. Pathol. 167, 609-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R. J., Boon R. A., Rondaij M. G., Kragt A., Volger O. L., Elderkamp Y. W., Meijers J. C., Voorberg J., Pannekoek H., Horrevoets A. J. (2006). KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 107, 4354-4363 [DOI] [PubMed] [Google Scholar]
- dela Paz N. G., D'Amore P. A. (2008). Arterial versus venous endothelial cells. Cell Tissue Res. 335, 5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S., Haendeler J., Rippmann V., Nehls M., Zeiher A. M. (1996). Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 399, 71-74 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W. J. (1989). Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161, 851-858 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Gerber H. P., LeCouter J. (2003). The biology of VEGF and its receptors. Nat. Med. 9, 669-676 [DOI] [PubMed] [Google Scholar]
- Frank S., Hübner G., Breier G., Longaker M. T., Greenhalgh D. G., Werner S. (1995). Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 270, 12607-12613 [DOI] [PubMed] [Google Scholar]
- Goettsch W., Gryczka C., Korff T., Ernst E., Goettsch C., Seebach J., Schnittler H. J., Augustin H. G., Morawietz H. (2008). Flow-dependent regulation of angiopoietin-2. J. Cell. Physiol. 214, 491-503 [DOI] [PubMed] [Google Scholar]
- Grazia Lampugnani M., Zanetti A., Corada M., Takahashi T., Balconi G., Breviario F., Orsenigo F., Cattelino A., Kemler R., Daniel T. O., et al. (2003). Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell. Biol. 161, 793-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G., Thomas S., Burt D., Lane S., Chusney G., Sacks S., Viberti G. (1997). Mechanical stretch induces vascular permeability factor in human mesangial cells: mechanisms of signal transduction. Proc. Natl. Acad. Sci. USA 94, 12112-12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood J. D., Meininger C. J., Ziche M., Granger H. J. (1998). VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol. 274, H1054-H1058 [DOI] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. (1991). The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 5, 1806-1814 [DOI] [PubMed] [Google Scholar]
- Houck K. A., Leung D. W., Rowland A. M., Winer J., Ferrara N. (1992). Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 267, 26031-26037 [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335-2342 [DOI] [PubMed] [Google Scholar]
- Joukov V., Pajusola K., Kaipainen A., Chilov D., Lahtinen I., Kukk E., Saksela O., Kalkkinen N., Alitalo K. (1996). A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 290-298 [PMC free article] [PubMed] [Google Scholar]
- Kamba T., Tam B. Y. Y., Hashizume H., Haskell A., Sennino B., Mancuso M. R., Norberg S. M., O'Brien S. M., Davis R. B., Gowen L. C., et al. (2006). VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560-H576 [DOI] [PubMed] [Google Scholar]
- Kuhn A., Brachtendorf G., Kurth F., Sonntag M., Samulowitz U., Metze D., Vestweber D. (2002). Expression of endomucin, a novel endothelial sialomucin, in normal and diseased human skin. J. Invest. Dermatol. 119, 1388-1393 [DOI] [PubMed] [Google Scholar]
- Kuo C. T., Veselits M. L., Barton K. P., Lu M. M., Clendenin C., Leiden J. M. (1997). The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11, 2996-3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chen T. T., Barber C. L., Jordan M. C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K. P., Iruela-Arispe M. L. (2007). Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Lundgren E., Berger E., Arfors K. E. (1989). Shear-dependent inhibition of granulocyte adhesion to cultured endothelium by dextran sulfate. Blood 73, 1324-1330 [PubMed] [Google Scholar]
- Li M., Scott D. E., Shandas R., Stenmark K. R., Tan W. (2009). High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann. Biomed. Eng. 37, 1082-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Kumar A., SenBanerjee S., Staniszewski K., Parmar K., Vaughan D. E., Gimbrone M. A., Jr, Balasubramanian V., Garcia-Cardena G., Jain M. K. (2005). Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 96, e48-e57 [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Kovalcheck S., Zweifach B. W. (1978). The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ. Res. 43, 738-749 [DOI] [PubMed] [Google Scholar]
- Loureiro R., D'Amore P. (2005). Transcriptional regulation of vascular endothelial growth factor in cancer. Cytokine Growth Factor Rev. 16, 77-89 [DOI] [PubMed] [Google Scholar]
- Maharaj A. S., D'Amore P. A. (2007). Roles for VEGF in the adult. Microvasc. Res. 74, 100-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj A. S., Saint-Geniez M., Maldonado A. E., D'Amore P. A. (2006). Vascular endothelial growth factor localization in the adult. Am. J. Pathol. 168, 639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumura T., Yamamoto K., Shimizu N., Obi S., Ando J. (2009). Shear stress increases expression of the arterial endothelial marker EphrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler. Thromb. Vasc. Biol. 29, 2125-2131 [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E., Grobe A., Kumar S., Noskina Y., Black S. M. (2005). Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-beta1 and reactive oxygen species: a requirement for NAD(P)H oxidase. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L288-L289 [DOI] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. (1993). High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72, 835-846 [DOI] [PubMed] [Google Scholar]
- Miquerol L., Gertsenstein M., Harpal K., Rossant J., Nagy A. (1999). Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev. Biol. 212, 307-322 [DOI] [PubMed] [Google Scholar]
- Motokawa M., Kaku M., Tohma Y., Kawata T., Fujita T., Kohno S., Tsutsui K., Ohtani J., Tenjo K., Shigekawa M., et al. (2005). Effects of cyclic tensile forces on the expression of vascular endothelial growth factor (VEGF) and macrophage-colony-stimulating factor (M-CSF) in murine osteoblastic MC3T3-E1 cells. J. Dent. Res. 84, 422-427 [DOI] [PubMed] [Google Scholar]
- Muratore C. S., Nguyen H. T., Ziegler M. M., Wilson J. M. (2000). Stretch-induced upregulation of VEGF gene expression in murine pulmonary culture: a role for angiogenesis in lung development. J. Pediatr. Surg. 35, 906-912; discussion 912-903 [DOI] [PubMed] [Google Scholar]
- Ng Y. S., Rohan R., Sunday M. E., Demello D. E., D'Amore P. A. (2001). Differential expression of VEGF isoforms in mouse during development and in the adult. Dev. Dyn. 220, 112-121 [DOI] [PubMed] [Google Scholar]
- Otani N., Minami S., Yamoto M., Shikone T., Otani H., Nishiyama R., Otani T., Nakano R. (1999). The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J. Clin. Endocrinol. Metab. 84, 3845-3851 [DOI] [PubMed] [Google Scholar]
- Papaioannou T. G., Stefanadis C. (2005). Vascular wall shear stress: basic principles and methods. Hellenic J. Cardiol. 46, 9-15 [PubMed] [Google Scholar]
- Parmar K. M., Larman H. B., Dai G., Zhang Y., Wang E. T., Moorthy S. N., Kratz J. R., Lin Z., Jain M. K., Gimbrone M. A., Jr., et al. (2006). Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. P., Schlueter M., Soifer S. J., Gutierrez J. A. (2002). Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L897-L903 [DOI] [PubMed] [Google Scholar]
- Ramsauer M., D'Amore P. A. (2007). Contextual role for angiopoietins and TGFbeta1 in blood vessel stabilization. J. Cell. Sci. 120, 1810-1817 [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M., Maldonado A. E., D'Amore P. A. (2006). VEGF expression and receptor activation in the choroid during development and in the adult. Invest. Ophthalmol. Vis. Sci. 47, 3135-3142 [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M., Maharaj A., Walshe T., Tucker B., Sekiyama E., Kurihara T., Darland D., Young M., D'Amore P., Chan-Ling T. (2008). Endogenous VEGF is required for visual function: Evidence for a survival role on Müller cells and photoreceptors. PLoS ONE 3, e3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulowitz U., Kuhn A., Brachtendorf G., Nawroth R., Braun A., Bankfalvi A., Böcker W., Vestweber D. (2002). Human endomucin: distribution pattern, expression on high endothelial venules, and decoration with the MECA-79 epitope. Am. J. Pathol. 160, 1669-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko Y., Seko Y., Takahashi N., Shibuya M., Yazaki Y. (1999a). Pulsatile stretch stimulates vascular endothelial growth factor (VEGF) secretion by cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 254, 462-465 [DOI] [PubMed] [Google Scholar]
- Seko Y., Seko Y., Fujikura H., Pang J., Tokoro T., Shimokawa H. (1999b). Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest. Ophthalmol. Vis. Sci. 40, 3287-3291 [PubMed] [Google Scholar]
- SenBanerjee S., Lin Z., Atkins G. B., Greif D. M., Rao R. M., Kumar A., Feinberg M. W., Chen Z., Simon D. I., Luscinskas F. W., et al. (2004). KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199, 1305-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. (1983). Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983-985 [DOI] [PubMed] [Google Scholar]
- Shay-Salit A., Shushy M., Wolfovitz E., Yahav H., Breviario F., Dejana E., Resnick N. (2002). VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 99, 9462-9467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. (1992). Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843-845 [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735-745 [DOI] [PubMed] [Google Scholar]
- Strawn L. M., McMahon G., App H., Schreck R., Kuchler W. R., Longhi M. P., Hui T. H., Tang C., Levitzki A., Gazit A., et al. (1996). Flk-1 as a target for tumor growth inhibition. Cancer Res. 56, 3540-3545 [PubMed] [Google Scholar]
- Suzuki T., Aizawa K., Matsumura T., Nagai R. (2005). Vascular implications of the Kruppel-like family of transcription factors. Arterioscler. Thromb. Vasc. Biol. 25, 1135-1141 [DOI] [PubMed] [Google Scholar]
- Suzuma I., Hata Y., Clermont A., Pokras F., Rook S. L., Suzuma K., Feener E. P., Aiello L. P. (2001). Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 50, 444-454 [DOI] [PubMed] [Google Scholar]
- Thi M. M., Iacobas D. A., Iacobas S., Spray D. C. (2007). Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Ann. N.Y. Acad. Sci. 1117, 73-81 [DOI] [PubMed] [Google Scholar]
- Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. (1991). The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 266, 11947-11954 [PubMed] [Google Scholar]
- Urbich C., Stein M., Reisinger K., Kaufmann R., Dimmeler S., Gille J. (2003). Fluid shear stress-induced transcriptional activation of the vascular endothelial growth factor receptor-2 gene requires Sp1-dependent DNA binding. FEBS Lett. 535, 87-93 [DOI] [PubMed] [Google Scholar]
- Walshe T., Saint-Geniez M., Maharaj A., Sekiyama E., Maldonado A., D'Amore P., Eickelberg O. (2009). TGF-ββ is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS ONE 4, e5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C. H. (1994). Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 269, 26988-26995 [PubMed] [Google Scholar]
- Wang Y., Miao H., Li S., Chen K. D., Li Y. S., Yuan S., Shyy J. Y., Chien S. (2002). Interplay between integrins and FLK-1 in shear stress-induced signaling. Am. J. Physiol. Cell Physiol. 283, C1540-C1547 [DOI] [PubMed] [Google Scholar]
- Willett C. G., Boucher Y., di Tomaso E., Duda D. G., Munn L. L., Tong R. T., Chung D. C., Sahani D. V., Kalva S. P., Kozin S. V., et al. (2004). Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 10, 145-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. C., Haworth L., Sherry R. M., Hwu P., Schwartzentruber D. J., Topalian S. L., Steinberg S. M., Chen H. X., Rosenberg S. A. (2003). A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349, 427-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Seftor E. A., Meininger C. J., Hendrix M. J., Tomanek R. J. (2001). Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-beta. Am. J. Physiol. Heart Circ. Physiol. 280, H909-H917 [DOI] [PubMed] [Google Scholar]