Abstract
The yeast cyclin-C–Cdk8p kinase complex represses the transcription of a subset of genes involved in the stress response. To relieve this repression, cyclin C is destroyed in cells exposed to H2O2 by the 26S proteasome. This report identifies Not4p as the ubiquitin ligase mediating H2O2-induced cyclin C destruction. Not4p is required for H2O2-induced cyclin C destruction in vivo and polyubiquitylates cyclin C in vitro by utilizing Lys48, a ubiquitin linkage associated with directing substrates to the 26S proteasome. Before its degradation, cyclin C, but not Cdk8p, translocates from the nucleus to the cytoplasm. This translocation requires both the cell-wall-integrity MAPK module and phospholipase C, and these signaling pathways are also required for cyclin C destruction. In addition, blocking cytoplasmic translocation slows the mRNA induction kinetics of two stress response genes repressed by cyclin C. Finally, a cyclin C derivative restricted to the cytoplasm is still subject to Not4p-dependent destruction, indicating that the degradation signal does not occur in the nucleus. These results identify a stress-induced proteolytic pathway regulating cyclin C that requires nuclear to cytoplasmic relocalization and Not4p-mediated ubiquitylation.
Key words: Mediator complex, Oxidative stress, Transcriptional repression, Ubiquitin protein degradation
Introduction
Exposure to environmental stress induces extensive remodeling of the transcriptome (reviewed by Mager and De Kruijff, 1995). The yeast cyclin-C–Cdk8p kinase complex is a highly conserved negative transcriptional regulator of many genes, including a subset of genes involved in the stress response (Conaway and Conaway, 2011). For example, cyclin-C–Cdk8p represses several stress response genes including the HSP70 chaperone SSA1 and catalase (Cooper et al., 1997; Holstege et al., 1998). Cyclin-C–Cdk8p associates with the RNA polymerase II holoenzyme mediator complex and has been reported to control transcription through modification of components of the transcription machinery. In yeast, both expression profiling and individual studies have indicated a predominantly repressive role for this complex (Carlson, 1997). By contrast, the human cyclin-C–CDK8 has been found to have a stress-induced co-activator function through transcription factors such as p53 (Belakavadi and Fondell, 2010; Donner et al., 2007; Meyer et al., 2008). However, in vitro transcription studies indicate that human cyclin-C–CDK8 can also have a negative role (Knuesel et al., 2009; Pavri et al., 2005) suggesting that cyclin-C–CDK8 plays a complex role in transcriptional control.
Several mechanisms have been identified that regulate transcription factors themselves. For example, the yeast Rlm1p is activated through phosphorylation by the mitogen-activated protein kinase (MAPK) Slt2p (also known as Mpk1p) (Watanabe et al., 1997). In addition, changes in subcellular localization represent an important mechanism that can control transcription factor activity. Stress-induced nuclear import of yeast Yap1p (Kuge et al., 2001) or Msn2p (Gorner et al., 1998) is required for their transcription activation function. Much less is known about the regulation of transcriptional repressors. Unlike the cyclins that regulate the cell cycle, cyclin C levels do not vary significantly during the cell cycle in yeast or human cells (Cooper et al., 1997; Lew et al., 1991). However, to relieve cyclin-C–Cdk8p-dependent repression in yeast, cyclin C is destroyed in cultures subjected to a variety of stressors (e.g. heat shock or oxidative stress) (Cooper et al., 1997; Cooper et al., 1999). This destruction is important as mutants lacking cyclin C are resistant to H2O2-induced programmed cell death (PCD) (Krasley et al., 2006). Oxidative-stress-induced cyclin C destruction requires the Slt2p MAPK cascade and the 26S proteasome (Cooper et al., 1999; Krasley et al., 2006) suggesting that this degradation is ubiquitin mediated.
Cellular stress also downregulates gene expression through several post-transcriptional mechanisms. For example, stress-induced mRNA decay is accelerated in specialized compartments termed P-bodies. In P-bodies, mRNAs are subjected to decapping and deadenylation by Dcp1p–Dcp2p and Ccr4p, respectively (reviewed by Eulalio et al., 2007; Parker and Sheth, 2007). Ccr4p is a component of a multi-subunit complex that also contains the Not4p ubiquitin ligase, which regulates diverse processes in both the nucleus and cytoplasm. Recent studies suggest that the mediator and Ccr4–Not complexes interact. In the nucleus, several components of the Ccr4–Not4 complex, when overexpressed, can associate with multiple members of the mediator complex including cyclin C and Cdk8p (Liu et al., 2001). The complexity of these interactions has made separating direct from indirect interactions difficult. In addition, genetic interactions have been observed between the Ccr4–Not complex and cyclin-C–Cdk8p. In the few examples described, components of the Ccr4–Not complex appear to either work in concert with the cyclin-C–Cdk8p mediator components (Liu et al., 2001) or in opposition (Lenssen et al., 2007) depending on the genes examined. In addition to its interactions with the mediator complex, nuclear Not4p enhances transcription by polyubiquitylating the histone demethylase Kdm5p (also known as Jhd2p) triggering its destruction (Mersman et al., 2009). By contrast, cytoplasmic Not4p alters the localization of the nascent-associated polypeptide complex component Egd2p (Panasenko et al., 2006). These findings indicate that Not4p ubiquitylation can regulate protein stability or localization. The present study describes a multi-step cyclin C destruction pathway involving MAPK pathway activation, nuclear to cytoplasmic relocalization and Not4p ubiquitin ligase activity.
Results
Not4p is required for oxidative-stress-induced cyclin C destruction
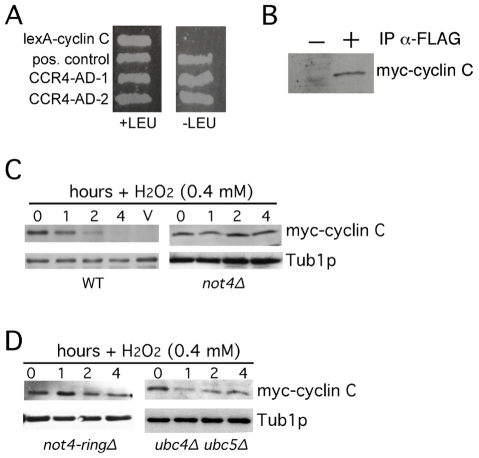
Our previous studies indicated that H2O2-induced destruction of the yeast cyclin C required the 26S proteasome but the ubiquitin ligase was unknown (Cooper et al., 1999; Krasley et al., 2006). To identify potential components of the cyclin C degradation pathway, two-hybrid studies were conducted with a cyclin C mutant (L28A) that no longer directly interacts with the RNA polymerase II holoenzyme (Cooper and Strich, 1999). When fused to lexA, this mutation prevents activation of the two-hybrid reporter gene by lexA–cyclin-C alone (Cohen et al., 2003; Cooper and Strich, 1999). DNA sequence analysis identified two clones containing the CCR4 gene as specific interactors with the lexA–cyclin-C bait (Fig. 1A). Ccr4p is the RNA deadenylase component of the multisubunit Ccr4–Not complex. This complex also contains the Not4p ubiquitin ligase that has been previously shown to genetically interact with Cdk8p (Liu et al., 2001). To determine whether Not4p associates with cyclin C in vivo, co-immunoprecipitation studies were performed in cells expressing epitope-tagged derivatives of cyclin C (myc–cyclin-C) and Not4p (FLAG–not4I37A). This not4 mutant harbors a mutation (I37A) that allows substrate association but not ubiquitylation (Laribee et al., 2007). An extract prepared from a log-phase culture was incubated with anti-FLAG antibodies and the presence of myc–cyclin-C in the immunoprecipitates was determined by western blotting. As anticipated, Not4p and cyclin C associated in a vegetative yeast culture (Fig. 1B). However, these experiments could not determine whether this interaction was direct or mediated through other proteins.
Fig. 1.
Not4p is required for cyclin C destruction. (A) Two-hybrid interactions between lexA–cyclin-C and two isolates of Ccr4–AD (activation domain) fusion proteins in EGy48 cells. Interactions are indicated by the ability to activate the lexAop-LEU2 growth on medium lacking leucine (–LEU). The positive control is lexA fused to the Gal4p activation domain. (B) An extract prepared from a mid-log-phase wild-type culture (RSY10) expressing myc–cyclin-C and FLAG–Not4pI37A was incubated with (+) or without (−) anti-FLAG antibody. The presence of myc–cyclin-C in the immunoprecipitates was determined by western blot analysis probing for myc. (C) Wild-type (RSY10) and not4Δ mutant (RSY1579) strains expressing myc–cyclin-C (pKC337) were grown to mid-log phase (0 hour) then treated with H2O2 for the indicated times. Cyclin C levels were determined by western blotting of immunoprecipitates. Tub1p levels were used as a loading control. (D) The experiment described in C was repeated with the not4Δ strain RSY1579 expressing Not4pringΔ on plasmid pN287 or the ubc4Δ ubc5Δ double mutant (MHY508).
We next determined whether Not4p regulated cyclin C stability. A previous study had found that loss of Not4p activity results in dramatic accumulation of the Kdm5p demethylase in vegetative cells (Mersman et al., 2009). Therefore, extracts were prepared from log-phase wild-type and not4Δ mutant cultures, and cyclin C levels were determined. Western blot analysis revealed that no significant difference was observed in the steady-state cyclin C levels in the wild-type or mutant strains (Fig. 1C, 0 hour timepoints). Next, we tested the requirement of Not4p for H2O2-induced destruction of cyclin C. The log-phase cultures described above were treated with H2O2 (0.4 mM) for the times indicated then subjected to western blot analysis probing for myc–cyclin-C. This revealed that Not4p is required for normal cyclin C destruction in response to oxidative stress (Fig. 1C). Not4p utilizes a ring finger domain to bind the Ubc4p and Ubc5p ubiquitin-conjugating enzymes necessary for substrate modification (Albert et al., 2002; Winkler et al., 2004). First, we established the requirement of the ring finger domain for cyclin C destruction. A plasmid expressing a Not4p derivative deleted for the ring finger domain (Not4pringΔ) was introduced into a not4Δ strain that also expressed myc–cyclin-C. A mid-log-phase culture was treated with H2O2 and cyclin C levels were determined by western blotting. As expected, Not4pringΔ was unable to mediate cyclin C destruction (Fig. 1D, left panel). Similarly, cyclin C was protected from destruction in a stressed mutant strain lacking both conjugating enzymes (ubc4Δ ubc5Δ; Fig. 1D, right panel) required for Not4p function. However, cyclin C levels were lower in this double-mutant compared with those in not4Δ strain suggesting that Not4p utilizes additional ubiquitin-conjugating enzymes. Our previous studies suggested that cyclin C destruction in response to heat shock is independent of the 26S proteasome (Cooper et al., 1999). Consistent with this possibility, Not4p was not required for cyclin C destruction in response to heat shock (supplementary material Fig. S1A,B). Taken together, these results indicate that Not4p is required for H2O2-induced cyclin C destruction.
Not4p polyubiquitylates cyclin C in vitro
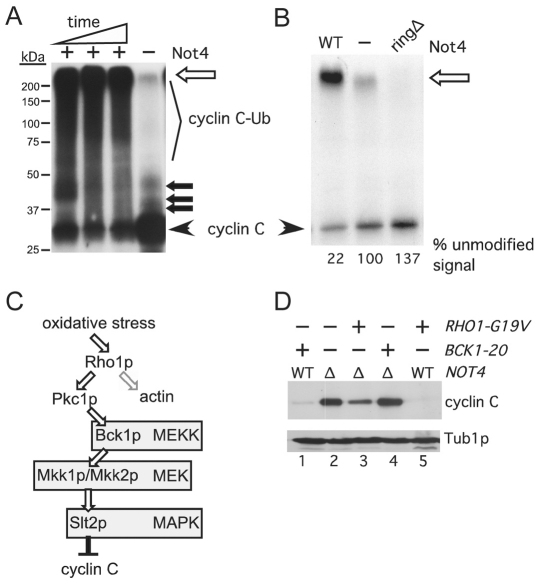
The ability of cyclin C and Not4p to associate in vivo suggested that the cyclin was a substrate of this ligase. To determine whether Not4p directly ubiquitylates cyclin C, in vitro ubiquitylation assays were performed. Not4p and [35S]cyclin C were prepared using in vitro transcription-coupled translation (see Materials and Methods). The products of the ubiquitylation assays were separated by SDS-PAGE and subjected to fluorography. Compared with unmodified cyclin C (Fig. 2A, arrowhead), a time-dependent increase in slower-migrating cyclin C that was dependent on the presence of Not4p was observed (representing the formation of ubiquitylated cyclin C, Fig. 2A, cyclin C-Ub). Much of the ubiquitylated cyclin C signal remained at the interface between the resolving and stacking gels (Fig. 2A, open arrow). This gel was overexposed to determine whether ubiquitin ligase activity could be detected in the concentrated transcription-coupled translation extract lacking Not4p. In this control, a modest amount of mono-, di- and tri-ubiquitylated cyclin C was observed as well as some larger species at the stacker-gel–resolving-gel interface. These signals suggest that some residual activity exists in the in vitro transcription-coupled translation extracts but this activity is significantly less than that observed for the Not4p-containing extract. These results indicate that Not4p is able to polyubiquitylate cyclin C in vitro.
Fig. 2.
Not4p ubiquitylates cyclin C in vitro. (A) In vitro ubiquitylation assays were performed as described in the Materials and Methods. The timepoints were 15, 30 and 90 minutes with a constant concentration of Not4p. The ubiquitylated (cyclin C-Ub) and unmodified (cyclin C) species are indicated. The open arrow indicates the boundary between the resolving and stacking gel. Closed arrows indicate predicted mono-, bi-, and tri-ubiquitylated cyclin C species. Molecular mass markers are indicated. (B) The experiment described in A was repeated with either Not4p (WT), Not4pringΔ (ringΔ) or no ligase (−) added. These reactions were carried out to completion (120 minutes). This gel was exposed to film for a shorter time to visualize the reduction in unmodified cyclin C in the Not4p reaction compared with that for Not4pringΔ. The unmodified signal was quantified by phosphorimaging with the no ligase control set at 100%. (C) Diagram of the cell-wall-integrity MAPK signal transduction pathway. Bifurcation of Rho1p signaling towards Pkc1p and actin organization is indicated. (D) Steady-state myc–cyclin-C levels were determined in non-stressed mid-log NOT4 (WT) or not4Δ (Δ) cultures expressing activated alleles of BCK1 or RHO1, as indicated. Tub1p levels were monitored as a loading control.
To determine whether cyclin C ubiquitylation was dependent on Not4p ubiquitin ligase activity, these experiments were repeated with the ring finger mutant. In this experiment, the reaction times were increased to allow the reactions to go to completion. This allowed us to monitor the loss of the unmodified cyclin C in order to better quantify the activity in each extract. Compared with the negative control (Fig. 2B, –), ~80% of the unmodified cyclin C was converted into a higher-molecular-mass species in the presence of Not4p (Fig. 2B, WT). Although synthesized to similar levels as the wild type in the in vitro transcription-coupled translation reaction (supplementary material Fig. S1C), the ringΔ mutant form of Not4p demonstrated less activity than the extract alone control. These findings suggest that the presence of Not4pringΔ leads to a dominant-negative activity with respect to endogenous ubiquitin ligases in the in vitro transcription-coupled translation extract. These results indicate that Not4p can ubiquitylate cyclin C in vitro and that this activity requires the conserved ring domain.
Not4p functions in the Slt2p MAPK-mediated cyclin C destruction pathway
Oxidative stress can activate several signaling pathways including the cell-wall-integrity signal transduction pathway through the small GTPase Rho1p. Among its several functions, Rho1p activates the control kinase Pkc1p (Levin, 2005). Pkc1p in turn stimulates a kinase cascade involving the MEK kinase Bck1p, the redundant MEKs Mkk1p and Mkk2p, and finally the Slt2p MAPK (Fig. 2C). This MAPK pathway is activated by oxidative stress (Lee et al., 1993; Nonaka et al., 1995) and is required for H2O2-, but not heat-shock-, induced cyclin C destruction (Krasley et al., 2006). Previous studies (Krasley et al., 2006) found that the presence of a constitutively active allele of BCK1 (BCK1-20) is sufficient to trigger cyclin C destruction in the absence of stress (Fig. 2D, compare lanes 1 and 2). In this experiment, we utilized the steady-state levels of cyclin C in the not4Δ strain to approximate normal cyclin C concentrations (Fig. 1C). Therefore, we next examined whether Not4p was required for Bck1-20p-induced destruction of cyclin C. A plasmid expressing BCK1-20 was introduced into the not4Δ mutant and steady-state cyclin C levels in a log-phase culture were determined by western blotting. These studies revealed that cyclin C is protected from destruction in the not4Δ strain (Fig. 2D, compare lanes 2 and 4). In addition to activating Pkc1p, the small G-protein Rho1p functions in other stress-related signaling pathways including heat shock (Delley and Hall, 1999; Harrison et al., 2004). Therefore, we next asked whether, similar to heat shock, constitutive activation of Rho1p could induce a Not4p-independent destruction of cyclin C. To test this possibility, a plasmid harboring a constitutively active allele of RHO1 (RHO1G19V) (Sekiya-Kawasaki et al., 2002) was introduced into a wild-type strain and cyclin C steady-state levels were determined in the absence of stress. Similar to strains expressing Bck1-20p, the presence of Rho1pG19V was also sufficient to induce cyclin C destruction (Fig. 2D, compare lanes 2 and 5). Interestingly, deleting NOT4 only partially rescued cyclin C degradation in the strain expressing Rho1pG19V (Fig. 2D, compare lanes 3 and 5). These results indicate that Rho1p activation can induce a cyclin C destruction pathway that is independent of the cell integrity pathway and Not4p. However, constitutively active Rho1p did not phenocopy heat shock as cyclin C destruction is completely independent of Not4p (supplementary material Fig. S1).
H2O2 exposure induces cyclin C cytoplasmic relocalization
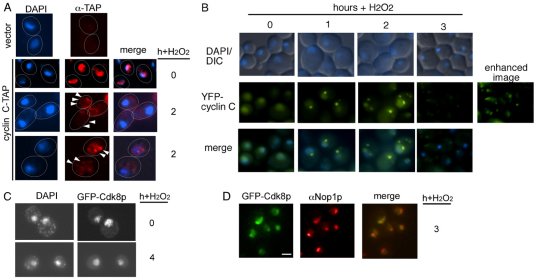
Previous studies have found that transcription factor destruction can occur either in the nucleus (Ryo et al., 2003) or the cytoplasm (Swanson et al., 2001). Therefore, indirect immunofluorescence was employed to follow the subcellular localization of a functional chromosomally tandem affinity purification (TAP)-tagged cyclin C (cyclin-C–TAP) in stressed and unstressed cultures (see the Materials and Methods). As expected, cyclin-C–TAP was predominantly nuclear before stress treatment, consistent with its role as a transcriptional repressor (Fig. 3A). However, the cyclin-C–TAP signal re-distributed to the cytoplasm in the majority of cells following 2 hours of exposure to H2O2. The cytoplasmic cyclin C did not represent newly synthesized protein, as a similar result was obtained in cycloheximide-treated cells (data not shown). Closer examination revealed that cytoplasmic cyclin C formed multiple punctate foci in the cytoplasm (arrowheads). These results suggest that cyclin C relocalizes from the nucleus to the cytoplasm following H2O2 exposure.
Fig. 3.
Stress-induced cyclin C cytoplasmic relocalization. (A) Cyclin-C-–TAP subcellular localization was monitored by indirect immunofluorescence before (0 hour) and at 2 hours following exposure to H2O2 (0.4 mM). Nuclear location was determined by DAPI staining. The vector strain (top panels) was a control for non-specific binding of the anti-TAP antibody. (B) A mid-log-phase culture (RSY10) harboring pBK38 (YFP–cyclin-C) was exposed to H2O2 (0.4 mM) for the times indicated. The position of the nucleus (DAPI) and GFP–cyclin-C (pBK1) was determined by fluorescence microscopy of living cells. The enhanced panel was produced by increasing the gain of the image. (C) GFP–Cdk8p subcellular localization was determined by fluorescence microscopy of fixed cells before and following H2O2 treatment (0.4 mM). DAPI staining was used to indicate nucleasr location. (D) Indirect immunofluorescence using Nop1p antibodies was used to visualize the nucleolus 3 hours following H2O2 treatment (0.4 mM). GFP–Cdk8p was visualized by fluorescence microscopy. Scale bar: 5 μm.
To verify that these results were not an artefact of cell fixation or the presence of the TAP moiety, these experiments were repeated in living cells expressing an YFP–cyclin-C fusion protein. YFP–cyclin-C represents a useful reporter for cyclin C as it is nuclear under normal growing conditions (Fig. 3B; see supplementary material Fig. S2A for a GFP–cyclin-C derivative) and complements the aberrant expression of a spo13-lacZ reporter gene in a cnc1Δ mutant strain (supplementary material Fig. S2B). Finally, it is destroyed in response to H2O2 treatment (compare supplementary material Fig. S2C, Fig. S2D). Fluorescence microscopy of non-fixed cells revealed that the YFP–cyclin-C localization also changed from diffuse nuclear to cytoplasmic foci following H2O2 exposure (Fig. 3B). Compared with GFP–cyclin-C decay kinetics (supplementary material Fig. S2C), the appearance of cytoplasmic foci occurred within the timeframe of cyclin C destruction. By 3 hours, the YFP–cyclin-C signal was difficult to detect with the same exposure times used in the previous timepoints. Visualization of the 3-hour timepoint required a longer exposure to clearly identify the foci. These foci were not due to the reporter protein as GFP expressed by itself did not demonstrate stress-induced aggregates (supplementary material Fig. S2E). Taken together, these results indicate that cyclin C distribution changes from nuclear to cytoplasmic following stress treatment.
GFP–Cdk8p relocalizes to the nucleolus following H2O2 exposure.
The stress-induced cytoplasmic foci formation of cyclin C prompted the question of whether Cdk8p also exhibited subcellular relocalization. To examine this question, a functional GFP–CDK8-encoding fusion gene was constructed and placed under the control of the GAL1 promoter (see Materials and Methods for details). In cultures grown on galactose (which induces expression of the fusion gene), diffuse nuclear GFP staining signal was observed (Fig. 3C) that was absent in repressing dextrose-containing medium (supplementary material Fig. S2E). When exposed to 0.4 mM H2O2, the GFP–Cdk8p signal remained nuclear indicating that Cdk8p did not stay associated with cyclin C when it translocated to the cytoplasm. One explanation for cyclin C relocalization in response to H2O2 stress is that there is a general loss of nuclear integrity. Although GFP–Cdk8p remained nuclear following stress, it is significantly larger (89 kDa) than GFP–cyclin-C (59 kDa) or cyclin-C–TAP (48 kDa) so perhaps is subjected to a restriction of non-specific movement. Therefore, we probed nuclear integrity further by following the localization of Nop1p, a 34-kDa protein associated with the nucleolus. The experiment was repeated and Nop1p subcellular localization was determined by indirect immunofluorescence. The experiments revealed that GFP–Cdk8p and Nop1 colocalized in yeast cells before and following exposure to H2O2 (Fig. 3D) indicating that nuclear integrity remains intact during this stage in the stress response. Taken together, these findings indicate that cyclin C and Cdk8p separate in the nucleus following stress treatment and that cyclin C, but not Cdk8p, translocates to the cytoplasm.
The cell-wall-integrity MAPK pathway is required for normal cytoplasmic foci formation
As discussed above, the Slt2p MAPK pathway is required for H2O2-, but not heat-shock-, induced cyclin C destruction. To test whether this pathway also mediates stress-induced cyclin C relocalization, a mutant deleted for the SLT2 MAPK (slt2Δ) and expressing GFP–cyclin-C was exposed to H2O2 (0.4 mM) for 4 hours. Fluorescence microscopy revealed that GFP–cyclin-C remained mostly nuclear in the slt2Δ mutant although some cytoplasmic foci were still observed (Fig. 4A, arrows in bottom panels). These results indicate that Slt2p is required for normal H2O2-induced cyclin C cytoplasmic foci formation. To determine whether Slt2p activation is sufficient to induce cyclin C translocation, the constitutively active MEK kinase allele BCK1-20 was introduced into a wild-type strain also expressing GFP–cyclin-C. Although the GFP–cyclin-C signal intensity was substantially reduced in the BCK1-20-expressing cells, increased exposure revealed the presence of cytoplasmic foci in unstressed cells (Fig. 4B). These results indicate that ectopic activation of the cell-wall-integrity pathway is sufficient to induce cyclin C relocalization and destruction. Finally, we previously reported that phospholipase C, a signaling protein not connected to the cell-wall-integrity pathway, is required for H2O2-induced cyclin C destruction (Cooper et al., 1999). A mutant lacking Plc1p demonstrated a severe defect in cytoplasmic foci formation following H2O2 exposure (Fig. 4C). These results indicate that the multiple signaling pathways that are required for H2O2-induced cyclin C destruction also mediate its nuclear to cytoplasmic relocalization.
Fig. 4.
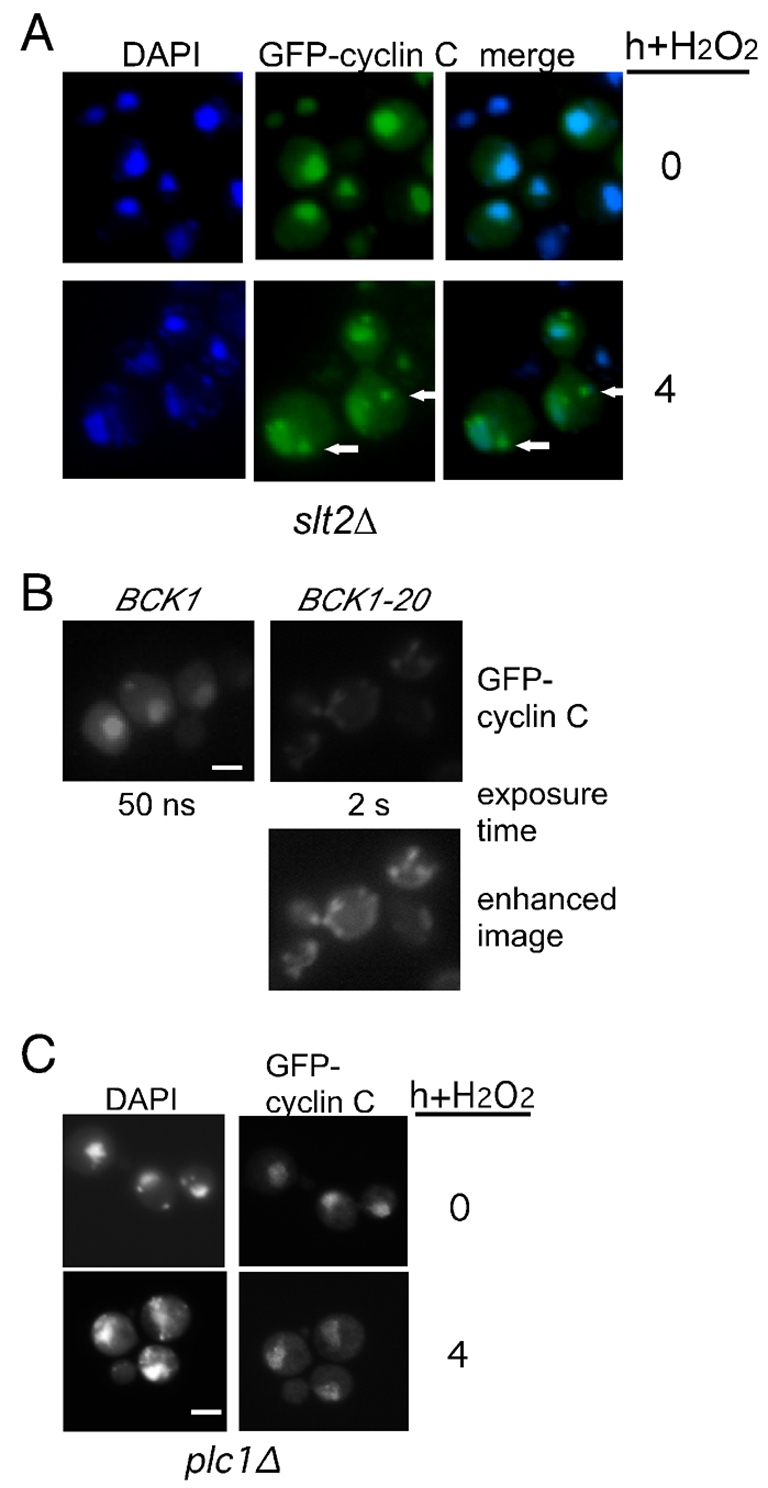
The Slt2p MAPK pathway is required for cyclin C cytoplasmic translocation. (A) An slt2Δ mutant strain (RSY1057) expressing GFP–cyclin-C (pBK1) was examined by fluorescence microscopy following heat shock (top panels) or 0.4 mM H2O2 treatment (bottom panels). Arrows indicate observed cytoplasmic foci. Nuclear location was followed with DAPI staining. (B) A wild-type strain (RSY10) expressing GFP–cyclin-C (pBK1) and either the constitutively active (BCK1-20) or the wild-type (BCK1) MEK kinase allele, were grown to mid-log-phase under non-stress conditions. The nuclei and GFP–cyclin-C were visualized as described for A. The exposure times for the top panels are indicated. The bottom panel was enhanced to more fully reveal the GFP–cyclin-C signal in the BCK1-20 expressing cells. (C) GFP–cyclin-C localization was monitored in a plc1Δ strain (RSY531) before and 4 hours following H2O2 exposure (0.4 mM). Exposure times were identical for visualizing the GFP–cyclin-C signal before and after stress application.
Cytoplasmic relocalization correlates with cyclin C destruction
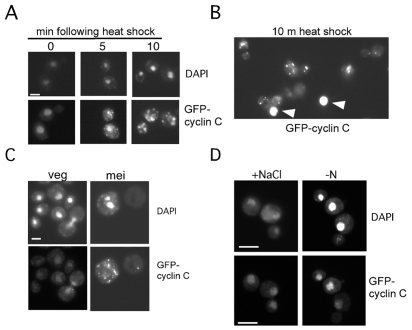
Previous studies revealed that cyclin C is destroyed in response to some, but not all, stressors (Cooper et al., 1997; Cooper et al., 1999). To determine whether cyclin C cytoplasmic relocalization occurs in response to all stresses, or only those that evoke its destruction, a wild-type strain expressing GFP–cyclin-C was subjected to a series of stress protocols. First, cyclin C is destroyed in response to heat shock or when cells enter meiotic development (Cooper et al., 1997). Therefore, GFP–cyclin-C was visualized in cells either following heat stress or after transfer to sporulation medium. Fluorescence microscopy revealed that GFP–cyclin-C formed punctate cytoplasmic foci following heat shock (Fig. 5A). As with cells expressing BCK1-20, the reduced GFP–cyclin-C signal following heat shock required longer exposure to allow visualization. For example, nuclear GFP–cyclin-C signals are overexposed (Fig. 5B, arrowheads) when exposure times are adjusted to clearly visualize cytoplasmic foci. Cytoplasmic foci were observed 2 hours following transfer to sporulation medium (Fig. 5C, mei), corresponding to approximately the time in meiosis when cyclin C is destroyed (Cooper et al., 1997). Conversely, two conditions that do not induce cyclin C destruction, high osmolarity and nitrogen starvation (Cooper et al., 1999), did not induce a change in the diffuse nuclear GFP signal (Fig. 5D). These findings indicate that cytoplasmic foci formation correlates with cyclin C destruction.
Fig. 5.
GFP–cyclin-C relocalizes in response to stressors that induce destruction. (A) A wild-type strain (RSY10) harboring pBK1 (GFP–cyclin-C) was subjected to heat shock (37°C) for the times indicated. Nuclear (DAPI) and GFP–cyclin-C signals were determined by fluorescence microscopy. (B) Comparison of GFP–cyclin-C nuclear and cytoplasmic foci signals following heat shock. Arrowheads indicate nuclear signals with the same exposure required to detect cytoplasmic foci. (C) Wild-type diploid culture (RSY335) harboring pBK1 was grown in synthetic acetate medium (veg) then transferred to sporulation medium for 6 hours (mei). The position of the nucleus (DAPI) and GFP–cyclin-C are indicated. (D) GFP–cyclin-C was visualized in a wild-type strain (RSY10) subjected to either high salt (0.4 M NaCl) for 30 minutes or starved for nitrogen (−N) for 2 hours. The position of the nucleus (DAPI) and GFP–cyclin-C are indicated. Scale bars: 5 μm.
Cytoplasmic relocalization of cyclin C is required for normal stress response gene mRNA induction
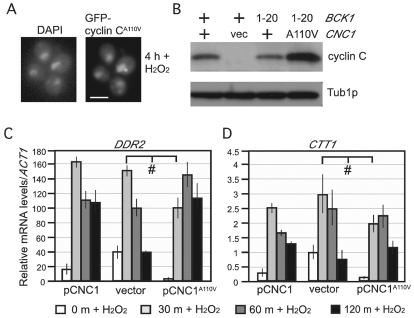
Cytoplasmic relocalization might represent one step to inactivate the cyclin C repressor function. To test this possibility, we utilized a single amino acid substitution mutation (A110V) that protects cyclin C from destruction in response to H2O2 or meiosis (Cooper et al., 1997; Cooper et al., 1999). First, we determined whether this single amino acid substitution affected cyclin C relocalization. The A110V mutation was introduced in the GFP–CNC1-encoding reporter gene and the localization of GFP–cyclin-CA110V was determined following exposure to H2O2. Fluorescence microscopy revealed that the A110V mutation blocked the majority of cytoplasmic relocalization although some foci were still observed (Fig. 6A). These findings indicate that both cis and trans mutations that prevent cyclin C destruction also prevent normal cytoplasmic foci formation. To determine whether this relocalization domain functioned in the cell-wall-integrity signaling pathway, the levels of cyclin CA110V were monitored in a strain expressing the activated BCK1-20 allele. These experiments demonstrated that the A110V mutation is epistatic to BCK1-20 (Fig. 6B).
Fig. 6.
Cytoplasmic relocalization is required for normal stress response gene induction. (A) A wild-type strain (RSY10) harboring a CNC1A110V expression plasmid was exposed to H2O2 (0.4 mM) for 4 hours. The positions of the nuclei (DAPI) and GFP–cyclin-CA110V are indicated. (B) Wild-type strains (RSY10) harboring plasmids expressing wild type (+) or the indicated alleles of CNC1 or BCK1. cyclin C and cyclinCA110V levels were monitored in log-phase cultures by western blotting. Tub1p served as a loading control. The vector (vec) lane controls for specificity for the myc-tagged alleles of CNC1 and CNC1A110V with anti-myc antibody. A log-phase wild-type strain (RSY10) was treated with H2O2 (0.4 mM) for the indicated times and the levels of DDR2 (C) and CTT1 (D) mRNA were determined by RT-PCR. Transcript levels are given relative to the internal NUP85 mRNA control. Error bars indicate the s.d. from the mean of two independent cultures assayed in triplicate. #P<0.05.
As the A110V mutation does not reduce cyclin C repressor function (Cooper et al., 1997), we tested the importance of nuclear to cytoplasmic relocalization on cyclin C activity. Two genes (DDR2 and CTT1) were identified that are repressed by cyclin-C–Cdk8p (Holstege et al., 1998) and induced in response to oxidative stress (Martinez-Pastor et al., 1996). In these experiments, a cnc1-null strain (cnc1Δ) was transformed with plasmids expressing wild-type cyclin C, the A110V derivative or a vector control. Quantitative PCR was utilized to examine the mRNA induction kinetics of these two genes before and following exposure to 0.4 mM H2O2. As expected, the mRNA levels of both genes were elevated in the vector control strain compared with either cyclin-C-expressing culture before the addition of H2O2 (Fig. 6C,D, compare 0 minute timepoints). These results confirm that cyclin-C–Cdk8p repress these loci under non-stress conditions. Following H2O2 treatment, the strain expressing the wild-type cyclin C or the vector control displayed a rapid increase in mRNA levels, peaking at 30 minutes, for both genes. Interestingly, although similar maximum mRNA levels were achieved in cyclin-C- and cyclin-CA110V-expressing strains, both DDR2 and CTT1 peak mRNA levels appeared 30 minutes later in cyclin CA110V cells. This result is consistent with our previous finding that cyclin CA110V reduced, but did not eliminate, the mRNA induction of the early meiotic gene SPO13 (Cooper et al., 1997). These findings indicate that cyclin C relocalization is required for the normal induction kinetics of DDR2 and CTT1 mRNA following H2O2 stress but that normal (or nearly normal) expression is eventually observed.
Not4p mediates cyclin C destruction, not its relocalization, in response to H2O2 stress
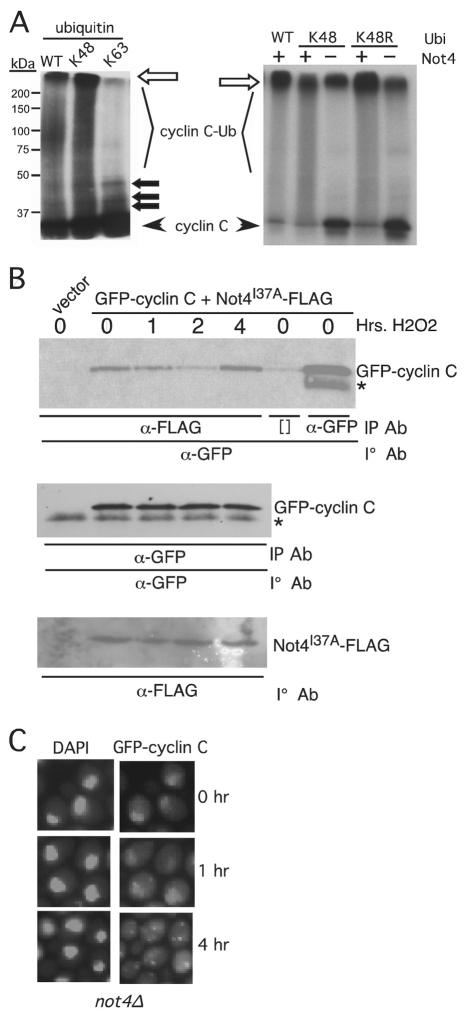
Not4p can regulate either stability or localization of a protein. Our data indicate that cyclin C relocalization is important for its destruction, consistent with either model for Not4p function. In addition, the Ccr4–Not complex is located in both the nucleus and cytoplasm (Laribee et al., 2007; Mulder et al., 2007; Panasenko et al., 2006). Therefore, Not4p modification could direct cyclin C to a specific cytoplasmic address where it is acted upon by another factor. Alternatively, Not4p could be modifying cyclin C in the cytoplasm to stimulate 26S-proteasome-dependent degradation. To investigate these possibilities, we further probed Not4p-dependent cyclin C polyubiquitylation. Polyubiquitin chains are recognized differently by the cell depending on their specific linkage (Komander, 2009). Ubiquitin chains formed through lysine 48 or 29 are associated with enhanced protein degradation through the 26S proteasome, whereas Lys63 linkage confers many cellular functions, including changes in protein localization. To determine which ubiquitin linkage is involved in Not4p-dependent ubiquitylation of cyclin C, the in vitro reactions described in Fig. 2A were repeated with wild-type ubiquitin or mutant proteins containing only Lys48 or Lys63. As before, the addition of ubiquitin induces the formation of higher-molecular-mass species of cyclin C (Fig. 7A, left panel). Similarly, the reaction containing the Lys48-only ubiquitin derivative also formed higher-molecular-mass forms of cyclin C indicating that Not4p can utilize this residue for chain formation. Reactions containing only ubiquitin Lys63 produced mono-, bi- and tri-ubiquitylated cyclin C (closed arrows) similar to the background activity shown in Fig. 2A. Taken together, these results indicate that Not4p can directly polyubiquitylate cyclin C through Lys48 linkage. To determine whether Not4p exclusively utilizes Lys48 linkages or can recognize other lysine linkages that mediate proteolysis (e.g. Lys29 or Lys11), these experiments were repeated with a ubiquitin derivative mutated only for Lys48 (K48R). These studies found significant activity with the K48R mutant (Fig. 7A, right panel) indicating that Not4p can generate polyubiquitin linkages at more than one lysine. These results are consistent with a model that Not4p regulates cyclin C destruction but not its relocalization.
Fig. 7.
Not4p functions in the cytoplasm to promote cyclin C destruction. (A) The in vitro ubiquitylation experiments described in Fig. 2 were repeated with wild-type ubiquitin or derivatives containing only lysine 48 (K48) or lysine 63 (K63) (left panel). The right panel shows ubiquitin in derivatives mutated only for Lys48 (K48R). The unmodified 35S-labeled cyclin C input and ubiquitylated cyclin C species (cyclin C-Ub) are indicated. The open arrow indicates the boundary between the resolving and stacking gel. Closed arrows indicate predicted mono-, bi- and tri-ubiquitylated cyclin C species. The positions of molecular mass markers (kDa) are shown on the left. (B) A wild-type strain (RSY10) expressing GFP–cyclin-C and Not4I37A–FLAG was grown to mid-log-phase and subjected to oxidative stress for the times indicated. Extracts prepared from these samples were immunoprecipitated with anti-FLAG or -GFP monoclonal antibody as indicated and the immunoprecipitates (IP) subjected to western blot analysis probing for GFP–cyclin-C. The input control western blots monitoring GFP–cyclin-C and Not4I37A–FLAG are depicted in the two lower panels. The asterisk indicates a non-specific crossreactive signal. Brackets ([]) indicate the no antibody control lanes. (C) A mutant strain deleted for NOT4 (not4Δ) and expressing GFP–cyclin-C (pBK1) was subjected to H2O2 treatment for the times indicated. The position of the nucleus was determined by DAPI staining.
If Not4p mediates cyclin C destruction, not localization, then two predictions can be made. First, Not4p should associate with cyclin C in the cytoplasm. Second, Not4p would not be required for cyclin C relocalization. To test the first prediction, co-immunoprecipitation studies were conducted to determine whether the Not4p–FLAG ring mutant (Not4pI37A; described in Fig. 1) and GFP–cyclin-C interacted following stress exposure. GFP–cyclin-C was chosen so we could follow the movement to the cytoplasm in the experimental cultures. Extracts prepared from the culture before and following addition of H2O2 were immunoprecipitated with anti-FLAG antibody and the immunoprecipitates were subjected to western blot analysis probing for the presence of GFP–cyclin-C. GFP–cyclin-C was detected in the immunoprecipitates even 4 hours after stress (Fig. 7B). These results are consistent with the possibility that Not4p ubiquitylates cyclin C in the cytoplasm. Next, we determined whether Not4p was required for GFP–cyclin-C cytoplasmic foci formation. GFP–cyclin-C localization was monitored in a not4-null (not4Δ) mutant before and after H2O2 addition as described above. Cytoplasmic GFP–cyclin-C foci were readily detected by 4 hours (Fig. 7C) as observed previously (Fig. 3B). Taken together, these results point to a cytoplasmic role for Not4p in mediating cyclin C destruction.
Nuclear localization is not required for H2O2-induced cyclin C destruction
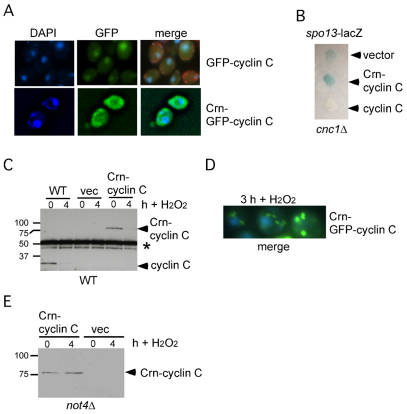
The studies just described indicate a cytoplasmic role for Not4p in mediating cyclin C destruction. If correct, then cyclin C nuclear localization might not be necessary for Not4p-dependent destruction. To address this question, the N-terminal portion of Crn1p, a protein associated with actin rafts at the plasma membrane (Humphries et al., 2002), was fused to the N-terminus of cyclin C and GFP–cyclin-C (see Materials and Methods for details). Previous studies have found that this region of Crn1p was sufficient to target the ubiquitin ligase Doa10p to the cytoplasm (Deng and Hochstrasser, 2006). To verify that the Crn1p domain was functioning as expected, two experiments were performed. First, the subcellular localization of Crn–GFP–cyclin-C was monitored by fluorescence microscopy. As anticipated, this fusion protein localized to the cytoplasm and not the nucleus (Fig. 8A). Second, the ability of Crn–cyclin-C to repress transcription of the meiotic reporter gene spo13-lacZ was tested. Overlay plate assays (see Materials and Methods for details) revealed that Crn–cyclin-C was not able to repress spo13-lacZ expression (Fig. 8B) as would be expected if the protein were directed to the cytoplasm.
Fig. 8.
Cytoplasmic cyclin C is destroyed in response to H2O2. (A) GFP–cyclin-C (pBK1, top panels) or Crn–GFP–cyclin-C (pBK16, bottom panels) localization was followed by fluorescence microscopy in log-phase wild-type cultures. Nuclear localization was followed by DAPI staining and the merge of the two panels is shown on the right. In the GFP–cyclin-C sample, the mitochondria are also stained using Mito-red (Molecular Probes). (B) spo13-lacZ expression was followed in a cnc1Δ mutant (RSY391) expressing Crn-cyclin C (pLR145), cyclin C (pKC337) or vector control. X-Gal cleavage (blue color) indicates spo13-lacZ expression. (C) Steady state myc–cyclin-C (pKC337) or Crn–myc–cyclin C (pLR145) levels (arrowheads) were determined in mid-log-phase cultures before (0 hour) and 4 hours following H2O2 treatment (0.4 mM) by western blot analysis of immunoprecipitates. The parental vector (vec) serves as a negative control for the anti-myc antibody. Asterisks indicate heavy chain crossreactivity to the secondary antibody. (D) A merged DAPI and GFP image from the strain described in A at 3 hours following H2O2 treatment (0.4 mM). (E) Crn–cyclin-C levels were monitored in a not4Δ strain by western blotting before and 4 hours after H2O2 treatment (0.4 mM). The vector (vec) strain controls for non-specific binding of the anti-myc antibody. Molecular mass standards (kDa) are given on the left.
As both cyclin C and Crn–cyclin-C contain a single myc epitope, and were expressed from the same promoter on a single-copy plasmid, we were able to directly compare their steady-state levels. Extracts prepared from mid-log-phase cultures expressing cyclin C or Crn–cyclin-C were subjected to immunoprecipitation followed by western blotting. These studies revealed that steady-state Crn–cyclin-C levels were similar to those of cyclin C (Fig. 8C, compare 0 hour lanes). These results indicate that simply mislocalizing cyclin C to the cytoplasm is not sufficient to alter its stability. Next, we asked whether nuclear localization was necessary for cyclin C destruction following H2O2 treatment. The mid-log-phase cultures just described were treated with H2O2 for 4 hours and cyclin C and Crn–cyclin-C levels were determined by immunoprecipitation followed by western blotting. These studies revealed that both proteins were destroyed, indicating that nuclear localization is not a prerequisite for stress-induced cyclin C destruction. Consistent with this conclusion, Crn–GFP–cyclin-C produced discrete cytoplasmic foci following stress (Fig. 8D). Finally, we tested whether H2O2-induced Crn–cyclin-C destruction required Not4p. The Crn–cyclin-C expression plasmid was introduced into the not4Δ mutant strain and the experiment described in Fig. 7C was repeated. These studies revealed that Not4p is indeed required for Crn–cyclin-C destruction (Fig. 8E). Taken together, these studies indicate that cyclin C localization in the cytoplasm is sufficient to permit H2O2-dependent Not4p destruction.
Discussion
The cyclin-C–Cdk8 complex is a conserved transcriptional regulator that plays a positive and negative role in controlling gene expression. In all systems examined, the cyclin-C–Cdk8 kinase associates with the mediator complex and is proposed to regulate transcription through direct modification of the basal transcription machinery, other mediator components or histones (Akoulitchev et al., 2000; Hirst et al., 1999; Knuesel et al., 2009). However, little is known about how the individual components of the mediator themselves are controlled. In yeast, one mechanism to remove cyclin-C–Cdk8-dependent repression is through stress-induced cyclin C destruction, which occurs through a ubiquitin-mediated process (Cooper et al., 1999; Krasley et al., 2006). This present study reveals new aspects of cyclin C regulation. First, we demonstrate that cyclin C undergoes stress-induced nuclear to cytoplasmic relocalization. Second, this translocation requires activation of the cell-wall-integrity MAPK pathway. Finally, we provide both genetic and biochemical evidence that the ubiquitin ligase Not4p mediates H2O2-induced destruction of cyclin C. These results describe a stress-induced degradation pathway for cyclin C that involves nuclear to cytoplasmic translocation and the Not4p ubiquitin ligase.
The finding that Not4p is required for stress-induced cyclin C destruction was surprising given the two-hybrid and immunoprecipitation studies in this report and elsewhere indicating that these two proteins associate in the absence of stress when the cyclin is stable. Normally, the association of ligase with substrate will induce polyubiquitylation and ultimately destruction. Rather, our results support a model that cyclin C is ubiquitylated by Not4p in the cytoplasm. One possible explanation is that, similar to MAPK cascades, enzymes and substrates can be positioned together but are not active until the correct signal is applied. Alternatively, the inability of Not4p to ubiquitylate cyclin C might be owing to the nature of the complexes to which each belongs. Both the Ccr4–Not complex and the mediator complex are composed of multiple components that probably associate with other surrounding factors found at promoters (Collart, 2003; Conaway and Conaway, 2011). Therefore, the interactions we and others detect in non-stressed cells might actually be indirect and mediated through one or more additional proteins.
Our results indicate that H2O2-induced relocalization of cyclin C requires activation of a MAPK pathway. Phosphorylation is a well-known modification controlling subcellular localization. For example, the human cyclin D1 is imported into the nucleus during G1 where it activates CDK4 thus promoting cell cycle progression (Baldin et al., 1993). Phosphorylation of cyclin D1 by GSK-3β induces disassociation from Cdk4 and subsequent nuclear export by CRM1 (also known as XPO1) (Diehl et al., 1998). In agreement with this overall strategy, we found that Cdk8p remains nuclear (or nucleolar) following cyclin C translocation to the cytoplasm. A simple model to explain our results is that Slt2p directly phosphorylates cyclin C, triggering its translocation. However, the only potential Slt2p phosphorylation site on the yeast cyclin C did not alter its relocalization or destruction kinetics (data not shown). These results suggest that MAPK modification of another factor(s) represents the signal to initiate cyclin C relocalization. In yeast, one possible intermediate is Ask10p. This protein associates with cyclin C, is required for its destruction and is phosphorylated in response to oxidative stress (Cohen et al., 2003). Other potential regulators are Med12p and Med13p, two conserved factors that make up the rest of the Cdk8 module. Analyzing the role of these factors in cyclin C localization might provide insight into the regulatory relationships between the components of this complex.
Not4p is a component of a large and multifunctional complex that is involved in many cellular functions. Originally identified as a negative transcriptional regulator (Cade and Errede, 1994), other studies have also indicated a transactivator role for Not4p (Cui et al., 2008). Not4p ubiquitylates the histone demethylase Jhd2p (Kdm5p) leading to its destruction (Mersman et al., 2009). Kdm5p demethylates trimethyl and dimethyl histone H3 lysine 4, which is associated with transcriptional repression (Dehe and Geli, 2006). Similarly, we demonstrate that cyclin C destruction is mediated by Not4p. These findings suggest that Not4p can play a positive role in gene expression through targeted destruction of negative factors. Our data suggest that Not4p ubiquitylates cyclin C in the cytoplasm. However, aberrantly targeting cyclin C to the cytoplasm is not sufficient to trigger Not4p-dependent destruction in the absence of stress. These results indicate that a signal in addition to cytoplasmic localization is necessary for Not4p to recognize cyclin C as a substrate. This suggests that Not4p and/or cyclin C has to undergo some type of H2O2-induced ‘activation’ to promote ubiquitylation. Alternatively, a specific cytoplasmic address might be required for the Not4p–cyclin-C interaction to occur in a manner that is directed by a stress-induced pathway and is not provided in the Crn–cyclin-C fusion protein. Determining whether cyclin C associates with a specific organelle or other cytoplasmic compartment will help answer this question.
Normally, directing a transcription factor out of the nucleus is sufficient to inhibit its activity. We provide evidence that cyclin C relocalization is required for normal mRNA induction of two oxidative-stress-induced genes, CTT1 and DDR2. However, retaining cyclin C in the nucleus did not significantly reduce the eventual level of induction. These results suggest that the strong transactivators associated with stress gene induction can eventually overcome the presence of cyclin-C–Cdk8p. Alternatively, cyclin-C–Cdk8p might be removed from the individual promoters rendering the kinase inactive even when cyclin C does not translocate to the cytoplasm. The additional observation that cyclin C not only becomes cytoplasmic, but is also destroyed, adds an additional level of regulation. As either cytoplasmic localization or proteolysis within the nucleus would be sufficient to inactivate cyclin-C–Cdk8p, it is not clear what the cell gains by doing both. One possibility is that destroying cyclin C requires new protein synthesis to re-establish the repression of genes it controls. Given that a hallmark of the stress response is a prohibition of translation of non-stress-related proteins (de Haro et al., 1996), this mechanism would insure that only when the damage has been repaired, and the stressing agent removed, would cyclin C be translated again. Consistent with this model, we have demonstrated that cyclin C levels fall below the limits of detection when cultures are subjected to a mild (37°) heat shock (Cooper et al., 1997). As the cells adapt to the higher temperature, the stress response is suppressed and cell division returns. Simultaneously with restarting cell cycle progression, cyclin C levels returned to pre-heat shock levels. By making cyclin C re-synthesis dependent on the return to growth, this system would ensure that the reestablishment of stress gene repression would not happen until the cell has adapted to the adverse conditions or the stressor has been removed.
Materials and Methods
Yeast strains, plasmids and medium
The strains used in this study are derived from a W303-related strain RSY10 (Strich et al., 1989) and are listed in supplementary material Table S1. In accordance with the mediator nomenclature unification effort (Bourbon et al., 2004), the cyclin-C–Cdk8p kinase has CNC1 (also known as SSN8, SRB11, UME3) and CDK8 (also known as SSN3, SRB10, UME5) gene designations, respectively. The slt2Δ (RSY1057) and cnc1Δ (RSY391) mutant strains were described previously (Krasley et al., 2006). The ubc4Δ ubc5Δ strain (MHY508) was previously described (Chen et al., 1993). The not4Δ deletion strain (RSY1595) is an ADE2 derivative of KMY1 (Mulder et al., 2005) in the W303 background. The CNC1-TAP W303a strain was provided by N. van Berkum. Plasmids pKC337 (ADH1-myc-CNC1 CEN) (Cooper et al., 1997) and pBW2 (spo13-lacZ reporter) (Wang et al., 1987) have been described previously. The chromosomal GAL1-GFP-cyclin C (RSY997) and GAL1-GFP-CDK8 (RSY998) fusion genes were constructed as described previously (Longtine et al., 1998). The GFP-CNC1 fusion expression plasmid (pBK1) was constructed by amplifying the GFP-CNC1 allele from RSY997 and inserting the gel-purified fragment into the single-copy plasmid pBC102 (ADH1 promoter, CYC2 terminator, CEN). The YFP-CNC1 reporter gene was constructed by amplifying yEcitrine from pKT175 (EUROSCARF) using oligonucleotides 5′-GCTAGCATGTCTAAAGGAGAAGAA-3′ and 5′-GCTAGCTTTGTATAGTTCATCCAT-3′ and inserted into the myc-epitope tagged CNC1 open reading frame in pBC102. The first 400 amino acids of Crn1p was amplified with oligonucleotides 5′-CCGCGGATGAGTGGAAAATTTGTTCGTGC-3′ and 5′-CCGCGGCATACTAACGAGAATTGGACC-3′ and inserted into pKC337 to make the single-copy Crn-myc-CNC1 plasmid (pLR145). The 1.2 Kbp SacII fragment from pBK15 containing the Crn1 amino terminal domain was cloned into pBK14 to generate Crn-GFP-cyclin C (pBK16). The NOT4-FLAG and not4ringΔ (pN287) expression plasmids were gifts from Nicholas Laribee (Laribee et al., 2007). The I37A mutation was introduced into the Ring domain by site directed mutagenesis (5′-GATTATTGCCCTCCTGCTGAGCCAATGGATATT-3′) forming pKC606. The not4ringΔ mutant was placed under the control of the T7 promoter for in vitro transcription coupled translation reactions by inserting the XbaI-HindIII fragment from pN287 into the same restriction endonuclease sites in pET28(a) (Novagen) forming pMS2. All site directed mutagenesis was verified by DNA sequence analysis. The BCK1-20 and RHO1G19V expression plasmids were gifts from David Levin and Yoshikazu Ohya, respectively.
Reporter gene functionality tests
The ability of cyclin C derivatives to repress spo13-lacZ reporter gene expression was accomplished as described previously (Strich et al., 1989) with the following modifications. Yeast strain RSY391 (cnc1Δ) transformed with either cyclin C, Crn–cyclin-C, GFP–cyclin-C or GFP–Cdk8p expression plasmids, or vector alone, was spotted onto sterile filter paper (Whatmann), placed on solid selective minimal medium then incubated for 2 days. The filters were lifted, rapidly lysed by submersion in liquid nitrogen, then overlain with 0.7 % agar, 0.1 M NaPO4 pH 7.0 and 40 μM X-Gal.
RT-PCR assays
Quantitative PCR was conducted using Taqman™ protocols (Applied Biosystems) with the following oligonucleotides. CTT1, 5′-CGCCGCTCCATACCAGAAT-3′, 5′-CGGTGGAAAAACGAACAAGAC-3′; DDR2, 5′-CTGTCTTCGGCCTCGCTACT-3′, 5′-GCGTTACTCGTGGTGTTGGA-3′; NUP85, 5′-TTCGCGAAGGAGCATAATGC-3′, 5′-ACACTTCCAATTCATTCAGAATCG-3′. Two independent cultures were subjected to H2O2 stress (0.4 mM) for the times indicated in the text. Total RNA prepared from each timepoint was assayed in triplicate and all values are expressed relative to the internal NUP85 control. The standard deviation from three replicate reactions is indicated in the figures.
Microscopy and cell analysis
Yeast cells were fixed with 4% paraformaldehyde and 3.4% sucrose for 1 hour at room temperature, washed three times in water and prepared for fluorescence microscopy as described previously (Guacci et al., 1997). Mounting medium (10 mg/ml p-phenylenediamine, 1× PBS) containing 50 ng/ml DAPI was used to stain nuclei and prevent photo bleaching. Images were obtained using a Nikon microscope (model E800) with an 100× objective (Plan Fluor Oil, NA 1.3) and a CCD camera (Hamamatsu C4742). Data were collected and processed using Image Pro software. For immunostaining studies, antibodies directed against the TAP affinity purification moiety (Thermo Scientific) and Alexa-Fluor-594-conjugated secondary antibody (Molecular Probes) were used according to supplier's recommendations.
In vitro ubiquitylation assays
In vitro ubiquitylation assays were conducted as described previously (Mersman et al., 2009) with the following modifications. Not4p and cyclin C were synthesized using a cell free transcription coupled translation system as described by the supplier (Promega). Cyclin C was metabolically labeled by addition of 35S-methionine to the reaction. Ubc4p ubiquitin conjugating enzyme was prepared using an E. coli expression system as described previously (Burton et al., 2005). Reactions were stopped by boiling in 2× sample buffer, separated using SDS-PAGE and then subjected to fluorography.
Western blot analysis
Extracts to analyze myc–cyclin-C levels were prepared from mid-log-phase cultures (5×106 cells per ml in minimal medium) as described previously (Cooper et al., 1997). Although expressed from the ADH1 promoter, myc–cyclin-C levels are still below the limits of detection using standard western blot protocols. Therefore, 250 μg of soluble extract were subjected to immunoprecipitation and the immunoprecipitates were subjected to western blot studies using antibodies directed against myc (Roche). The 12G10 monoclonal anti-Tub1p antibody (Williams et al., 1995) was obtained from the Developmental Studies Hybridoma Bank, University of Iowa. Western blot signals were detected using goat anti-mouse-IgG secondary antibodies conjugated to alkaline phosphatase (Sigma) and the CDP-Star chemiluminescence kit (Tropix). Signals were quantified by phosphorimaging (Kodak Inc.). Half-lives were calculated by linear regression analysis with curves possessing r values >0.9.
Acknowledgements
We thank D. Levin, M. Solomon, F. Luca, M. Hochstrasser, N. van Berkum, D. Levin, Y. Ohya, J. H. Hegemann, D. Cao and R. Laribee for strains, antibodies, strains and plasmids. We also thank F. Luca, J. Broach and M. Hochstrasser for helpful discussions and M. Henry for critical reading of this manuscript.
Footnotes
Funding
This work was supported by Public Health Service [grant number CA-099003]; the National Institutes of Health [grant number GM086788] to R.S.; and the W. W. Smith Charitable Trust [grant number CO 604 to K.F.C.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.096479/-/DC1
References
- Akoulitchev S., Chuikov S., Reinberg D. (2000). TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407, 102-106 [DOI] [PubMed] [Google Scholar]
- Albert T. K., Hanzawa H., Legtenberg Y. I., de Ruwe M. J., van den Heuvel F. A., Collart M. A., Boelens R., Timmers H. T. (2002). Identification of a ubiquitin-protein ligase subunit within the CCR4-NOT transcription repressor complex. EMBO J. 21, 355-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. (1993). Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7, 812-821 [DOI] [PubMed] [Google Scholar]
- Belakavadi M., Fondell J. D. (2010). Cyclin-dependent kinase 8 positively cooperates with Mediator to promote thyroid hormone receptor-dependent transcriptional activation. Mol. Cell. Biol. 30, 2437-2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H. M., Aguilera A., Ansari A. Z., Asturias F. J., Berk A. J., Bjorklund S., Blackwell T. K., Borggrefe T., Carey M., Carlson M., et al. (2004). A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol. Cell 14, 553-557 [DOI] [PubMed] [Google Scholar]
- Burton J. L., Tsakraklides V., Solomon M. J. (2005). Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol. Cell 18, 533-542 [DOI] [PubMed] [Google Scholar]
- Cade R. M., Errede B. (1994). MOT2 encodes a negative regulator of gene expression that affects basal expression of pheromone-responsive genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 14, 3139-3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. (1997). Genetics of transcriptional regulation in yeast: connection of the RNA polymerase II CTD. Annu. Rev. Cell Dev. Biol. 13, 1-23 [DOI] [PubMed] [Google Scholar]
- Chen P., Johnson P., Sommer T., Jentsch S., Hochstrasser M. (1993). Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MAT α2 repressor. Cell 74, 357-369 [DOI] [PubMed] [Google Scholar]
- Cohen T. J., Lee K., Rutkowski L. H., Strich R. (2003). Ask10p mediates the oxidative stress-induced destruction of the Saccharomyces cerevisiae C-type cyclin Ume3p/Srb11p. Eukaryot. Cell 2, 962-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M. A. (2003). Global control of gene expression in yeast by the Ccr4-Not complex. Gene 313, 1-16 [DOI] [PubMed] [Google Scholar]
- Conaway R. C., Conaway J. W. (2011). Function and regulation of the Mediator complex. Curr. Opin. Genet. Dev. 21, 225-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. F., Strich R. (1999). Functional analysis of the yeast C-type cyclin Ume3p/Srb11p-RNA polymerase II holoenzyme interaction. Gene Expr. 8, 43-57 [PMC free article] [PubMed] [Google Scholar]
- Cooper K. F., Mallory M. J., Smith J. S., Strich R. (1997). Stress and developmental regulation of the yeast C-type cyclin UME3 (SRB11/SSN8). EMBO J. 16, 4665-4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. F., Mallory M. J., Strich R. (1999). Oxidative stress-induced destruction of the yeast C-type cyclin Ume3p requires the phosphatidylinositol-specific phospholipase C and the 26S proteasome. Mol. Cell. Biol. 19, 3338-3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Ramnarain D. B., Chiang Y. C., Ding L. H., McMahon J. S., Denis C. L. (2008). Genome wide expression analysis of the CCR4-NOT complex indicates that it consists of three modules with the NOT module controlling SAGA-responsive genes. Mol. Genet. Genomics 279, 323-337 [DOI] [PubMed] [Google Scholar]
- de Haro C., Mendez R., Santoyo J. (1996). The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 10, 1378-1387 [DOI] [PubMed] [Google Scholar]
- Dehe P. M., Geli V. (2006). The multiple faces of Set1. Biochem. Cell Biol. 84, 536-548 [DOI] [PubMed] [Google Scholar]
- Delley P. A., Hall M. N. (1999). Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147, 163-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M., Hochstrasser M. (2006). Spatially regulated ubiquitin ligation by an ER/nuclear membrane ligase. Nature 443, 827-831 [DOI] [PubMed] [Google Scholar]
- Diehl J. A., Cheng M., Roussel M. F., Sherr C. J. (1998). Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12, 3499-3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner A. J., Szostek S., Hoover J. M., Espinosa J. M. (2007). CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol. Cell 27, 121-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. (2007). P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8, 9-22 [DOI] [PubMed] [Google Scholar]
- Gorner W., Durchschlag E., Martinez-Pastor M. T., Estruch F., Ammerer G., Hamilton B., Ruis H., Schuller C. (1998). Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12, 586-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci V., Koshland D., Strunnikov A. (1997). A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91, 47-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J., Golemis E. A., Chertkov H., Brent R. (1993). Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell 75, 791-803 [DOI] [PubMed] [Google Scholar]
- Harrison J. C., Zyla T. R., Bardes E. S., Lew D. J. (2004). Stress-specific activation mechanisms for the “cell integrity” MAPK pathway. J. Biol. Chem. 279, 2616-2622 [DOI] [PubMed] [Google Scholar]
- Hirst M., Kobor M. S., Kuriakose N., Greenblatt J., Sadowski I. (1999). GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol. Cell 3, 673-678 [DOI] [PubMed] [Google Scholar]
- Holstege F. C., Jennings E. G., Wyrick J. J., Lee T. I., Hengartner C. J., Green M. R., Golub T. R., Lander E. S., Young R. A. (1998). Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95, 717-728 [DOI] [PubMed] [Google Scholar]
- Humphries C. L., Balcer H. I., D'Agostino J. L., Winsor B., Drubin D. G., Barnes G., Andrews B. J., Goode B. L. (2002). Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159, 993-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel M. T., Meyer K. D., Donner A. J., Espinosa J. M., Taatjes D. J. (2009). The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of mediator. Mol. Cell. Biol. 29, 650-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D. (2009). The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 37, 937-953 [DOI] [PubMed] [Google Scholar]
- Krasley E., Cooper K. F., Mallory M. J., Dunbrack R. L., Jr, Strich R. (2006). Regulation of the oxidative stress response through Slt2p-dependent destruction of Cyclin C in S. cerevisiae. Genetics 172, 1477-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Arita M., Murayama A., Maeta K., Izawa S., Inoue Y., Nomoto A. (2001). Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol. Cell. Biol. 21, 6139-6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laribee R. N., Shibata Y., Mersman D. P., Collins S. R., Kemmeren P., Roguev A., Weissman J. S., Briggs S. D., Krogan N. J., Strahl B. D. (2007). CCR4/NOT complex associates with the proteasome and regulates histone methylation. Proc. Natl. Acad. Sci. USA 104, 5836-5841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Irie K., Gotoh Y., Watanabe Y., Araki H., Nishida E., Matsumoto K., Levin D. E. (1993). A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol. 13, 3067-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenssen E., Azzouz N., Michel A., Landrieux E., Collart M. A. (2007). The Ccr4-not complex regulates Skn7 through Srb10 kinase. Eukaryot. Cell 6, 2251-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E. (2005). Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69, 262-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Dulic V., Reed S. I. (1991). Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66, 1197-1206 [DOI] [PubMed] [Google Scholar]
- Liu H., Chiang Y., Pan J., Chen J., Salvadore C., Audino D., Badarinarayana V., Palaniswamy V., Anderson B., Denis C. (2001). Characterization of CAF4 and CAF16 reveals a functional connection between the CCR4-NOT complex and a subset of SRB proteins of the RNA polymerase II holoenzyme. J. Biol. Chem. 276, 7541-7548 [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., III, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961 [DOI] [PubMed] [Google Scholar]
- Mager W. H., De Kruijff A. J. J. (1995). Stress-induced transcriptional activation. Micro. Rev. 59, 506-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor M. T., Marchler G., Schuller C., Marchler-Bauer A., Ruis H., Estruch F. (1996). The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15, 2227-2235 [PMC free article] [PubMed] [Google Scholar]
- Mersman D. P., Du H. N., Fingerman I. M., South P. F., Briggs S. D. (2009). Polyubiquitination of the demethylase Jhd2 controls histone methylation and gene expression. Genes Dev. 23, 951-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. D., Donner A. J., Knuesel M. T., York A. G., Espinosa J. M., Taatjes D. J. (2008). Cooperative activity of cdk8 and GCN5L within Mediator directs tandem phosphoacetylation of histone H3. EMBO J. 27, 1447-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K. W., Winkler G. S., Timmers H. T. (2005). DNA damage and replication stress induced transcription of RNR genes is dependent on the Ccr4-Not complex. Nucleic Acids Res. 33, 6384-6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K. W., Brenkman A. B., Inagaki A., van den Broek N. J., Timmers H. T. (2007). Regulation of histone H3K4 tri-methylation and PAF complex recruitment by the Ccr4-Not complex. Nucleic Acids Res. 35, 2428-2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal R. K., Riles L., Johnston M., Hegemann J. H. (1996). Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast 12, 773-786 [DOI] [PubMed] [Google Scholar]
- Nonaka H., Tanaka K., Hirano H., Fujiwara T., Kohno H., Umikawa M., Mino A., Takai Y. (1995). A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14, 5931-5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko O., Landrieux E., Feuermann M., Finka A., Paquet N., Collart M. A. (2006). The yeast Ccr4-Not complex controls ubiquitination of the nascent-associated polypeptide (NAC-EGD) complex. J. Biol. Chem. 281, 31389-31398 [DOI] [PubMed] [Google Scholar]
- Parker R., Sheth U. (2007). P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635-646 [DOI] [PubMed] [Google Scholar]
- Pavri R., Lewis B., Kim T. K., Dilworth F. J., Erdjument-Bromage H., Tempst P., de Murcia G., Evans R., Chambon P., Reinberg D. (2005). PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol. Cell 18, 83-96 [DOI] [PubMed] [Google Scholar]
- Ryo A., Suizu F., Yoshida Y., Perrem K., Liou Y., Wulf G., Rottapel R., Yamaoka S., Lu K. (2003). Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell. 12, 1413-1426 [DOI] [PubMed] [Google Scholar]
- Sekiya-Kawasaki M., Abe M., Saka A., Watanabe D., Kono K., Minemura-Asakawa M., Ishihara S., Watanabe T., Ohya Y. (2002). Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genet. 162, 663-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich R., Slater M. R., Esposito R. E. (1989). Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc. Natl. Acad. Sci. USA 86, 10018-10022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Locher M., Hochstrasser M. (2001). A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 15, 2660-2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. T., Frackman S., Kowalisyn J., Esposito R. E., Elder R. (1987). Developmental regulation of SPO13; a gene required for separation of homologous chromosomes at meiosis I. Mol. Cell. Biol. 7, 1425-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Takaesu G., Hagiwara M., Irie K., Matsumoto K. (1997). Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17, 2615-2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. E., Honts J. E., Dress V. M., Nelsen E. M., Frankel J. (1995). Monoclonal antibodies reveal complex structure in the membrane skeleton of Tetrahymena. J. Eukaryot. Microbiol. 42, 422-427 [DOI] [PubMed] [Google Scholar]
- Winkler G. S., Albert T. K., Dominguez C., Legtenberg Y. I., Boelens R., Timmers H. T. (2004). An altered-specificity ubiquitin-conjugating enzyme/ubiquitin-protein ligase pair. J. Mol. Biol. 337, 157-165 [DOI] [PubMed] [Google Scholar]