Abstract
Head and neck squamous cell carcinoma (HNSCC) often presents with cervical lymph node metastases and at times the primary tumor cannot be identified despite extensive workup. Lymphoma is the second most common neoplasm in the head and neck region but is seldom synchronous with HNSCC and rarely involves regional mucosal sites. We report herein a rare occurrence of tonsillar involvement by small lymphocytic lymphoma (SLL) incidentally detected during the workup for a cervical lymph node SCC metastasis of a 52-year-old non-smoker male. The microscopic human papillomavirus-positive SCC involving the tonsillar surface and crypts was obscured by SLL leading to the initial designation of ‘unknown primary’. The occult HNSCC are likely explained by small tumor size, quality and quantity of sampling, thoroughness of endoscopic, radiological and pathological assessment or a combination of the above. The coexistence of another tumor such as lymphoma has not yet been reported as a confounding factor in the workup for cervical SCC metastasis. Since oropharyngeal SCC can be very small and Waldeyer’s ring is a common site for lymphoma involvement, identification of such rare collision tumors requires pathologists’ awareness, extensive sampling and occasionally ancillary studies for the accurate diagnosis and staging essential for the correct management.
Keywords: Head and neck occult carcinoma, Tonsillar carcinoma, Small lymphocytic lymphoma, Collision tumor
Introduction
The incidence of oropharyngeal cancer has been increasing in the last three decades [1]. Occult head and neck squamous cell carcinoma, also referred to as “unknown primary” (HNSCCUP) are reported to arise in the tonsillar fossa in almost half of the cases with identified origin [2]. After HNSCC, lymphoma is the second most common cancer, representing 2–3% of regional malignant tumors [3] but the coexistence of HNSCC and lymphoma is rarely reported in the literature. Here we describe an unusual occurrence of SCC and small lymphocytic lymphoma concurrently involving the palatine tonsil and cervical lymph nodes, the latter obscuring the microscopic primary tumor, while the largest metastasis completely effaced the lymph node, precluding lymphoma identification at first diagnosis. Implications on the diagnosis and staging are discussed.
Materials and Methods
Biopsies were fixed in 10% formalin, embedded in paraffin and sectioned at 4 μm for hematoxylin-eosin staining and immunohistochemical analysis. Commercially available antibodies for CD5, CD20, CD23, AE1/3 (Dako, Carpinteria, CA), p16 (Santa Cruz Biotechnology, Santa Cruz, CA), cyclin D1 (Neomarker, Lab Vision Corp, Fremont, CA) and CD10 (Novocastra, Newcastle upon Tyne, UK) were used. In situ hybridization for human papillomavirus (HPV ISH) was performed using a commercially available probe for serotype 16/18 (Dako, Carpinteria, CA).
Case Presentation
A 52-year-old non-smoker male presented with an enlarged left cervical lymph node. He was otherwise asymptomatic and, after no improvement with antibiotherapy, a CT scan was performed revealing a 3.4 cm left anterior cervical node with ring enhancement, bilaterally enlarged cervical, anterior mediastinal, right paratracheal lymph nodes and an enlarged left tonsil. The excisional biopsy of the cervical lymph node performed at an outside institution revealed metastatic SCC.
Patient was referred to our institution where further workup was performed including a PET-CT scan which was negative for abnormal uptake of radioglucose in the lungs, neck or tonsils. No primary tumor was initially identified during panendoscopy when bilateral tonsillectomy, biopsies from base of tongue, hypopharynx, mediastinal nodes, and selective left neck dissection were performed. Due to extensive lymphoma involvement, the case was assigned to hematopathology for complete workup. At pathologic re-review before Head and Neck Tumor Board case presentation (case was listed as “HNSCCUP with SLL”), several suspicious foci were found and further workup (additional sections and immunohistochemistry) was performed identifying the tonsillar primary tumor. Except for the microscopic involvement of the left tonsil and 2 cervical nodes by SCC micrometastases, all the other tissues sampled were negative for SCC but diffusely involved by SLL. Blood cell counts were within normal limits and no bone marrow biopsy was performed.
The patient underwent a concurrent chemoradiation regimen, receiving 66 gy total radiation and 2 doses of 100 mg/m2 cisplatin. SLL was initially followed without intervention. After 3 years, progressive lymphocytosis and increased adenopathy prompted the initiation of therapy with 6 cycles of fludarabin and rituximab over 5 months, with improvement of blood counts and remission of adenopathy. No evidence of SCC was seen at last follow up, 47 months after original diagnosis.
Pathology Findings
The excisional biopsy of the index lymph node showed a poorly differentiated, nonkeratinizing squamous cell carcinoma with areas of cystic degeneration and extensive fibrosis replacing most of the lymph node (Fig. 1a, b). An intraoperative consultation on the mediastinal lymph nodes revealed no carcinoma but prompted the lymphoma work up. Grossly, both tonsils were slightly enlarged (left more than right side), entirely submitted for microscopic evaluation and subsequently serially sectioned in several blocks. Both tonsils were diffusely involved by SLL with only small foci of residual uninvolved tonsillar lymphoid tissue. Classical diagnostic features (small, round lymphocytes, proliferation centers) were present (Fig. 2a) and neoplastic cells exhibited typical CLL/SLL immunoprofile (Fig. 2b): CD5+CD20+CD23+, negative for CD10 and cyclin D1. The left tonsil mucosa showed a single focus of severe dysplasia/squamous cell carcinoma in situ and several adjacent microscopic foci of invasive tumor partially obscured by the small lymphocytic proliferation, best highlighted by cytokeratin (Fig. 3a, b). SLL imparted a “lymphoepithelioma-like” morphology to few SCC foci (Fig. 4a), areas originally interpreted as crypt lymphoepithelium due to minimal cytologic atypia (Fig. 4a, inset). The tumor was present in two consecutive tissue sections (2/14) from the left tonsil, requiring serial sections through the block to reveal the tumor bulk (largest diameter 0.4 cm). Most clusters of malignant epithelial cells were budding from the surface mucosa but some were adjacent to the deep crypts. Mitotic figures were infrequent with a rare atypical mitosis identified. No significant keratin production or stromal response was identified. All cervical and mediastinal lymph nodes examined were involved by SLL, but only two showed microscopic deposits of SCC (0.3-cm the largest). SCC cells were positive for p16 (Fig. 4b) and HPV ISH both in the primary and metastatic foci.
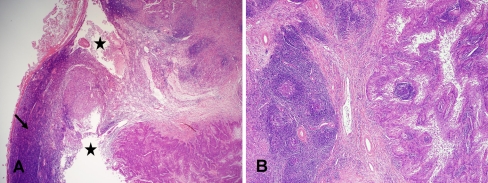
Fig. 1.
a, b Metastatic SCC involving the index cervical lymph node with areas of cystic degeneration (asterisk) and fibrosis (b) completely effacing nodal architecture and obscuring the coexistent SLL (arrow). (a HE, 20×; b HE, 40×)
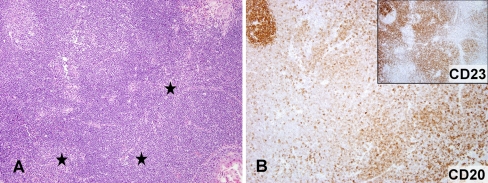
Fig. 2.
a, b Small cell lymphoma with proliferation centers (asterisk) and an uninvolved tonsillar germinal center (upper left corner) coexpressed CD20 and CD23 (inset). (a HE, 100×; b CD20, 100×, inset CD23, 100×)
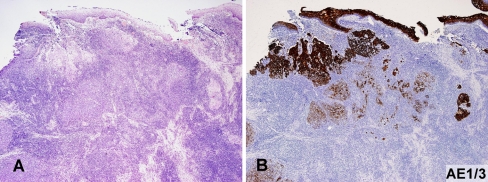
Fig. 3.
a, b Microscopic focus of invasive SCC in tonsil, obscured by diffuse involvement by SLL (a) is best highlighted by AE1/3 staining (b) (a HE, 40×; b AE1/3, 40×)
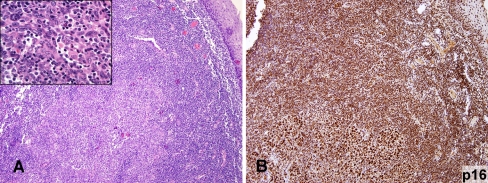
Fig. 4.
a, b The tonsillar SCC had focally lymphoepithelioma-like morphology a and diffusely and strongly expressed nuclear p16 (b). (a HE, 100×, inset HE, 600×; b p16, 100×)
Discussion
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most common adult leukemia in the western hemisphere, with a worldwide incidence estimated between 1 and 5.5 per 100,000 [4]. It is an indolent systemic neoplasm with marrow, peripheral blood involvement and/or disseminated lymphadenopathy occurring in patients with a median age of 64–70 [4]. When presenting with nodal or extranodal tissue involvement but no marrow or blood lymphocytosis, the disease is conventionally referred to as small lymphocytic lymphoma (SLL) [5]. Head and neck lymphomas represent 2–3% of all regional malignant tumors, the second most common neoplasms after SCC [3]. Regional lymph node presentation occurs in 90% of head and neck lymphoma cases, the remainder involving extranodal sites such as Waldeyer’s ring [6], with palatine tonsils accounting for more than a third of these cases [7]. Although almost all types of lymphoma of Waldeyer’s ring have been reported [8, 9], CLL/SLL with tonsillar involvement is a relatively rare occurrence [10], but is likely under-recognized. Approximately 10–20% of CLL/SLL patients develop second primary malignancies, which in two-thirds of the cases are the cause of their demise [11]. Head and neck neoplasms are common solid tumors occurring either synchronously or subsequently in CLL/SLL patients, possibly due to immune dysregulation [12–14]. A recent study showed high HPV prevalence in various second solid neoplasms in CLL patients [15], further supporting this hypothesis.
SCC is the most common cancer of head and neck and often presents with cervical lymph node metastasis without a detected primary despite workup, also referred to as “occult” or “SCC with unknown primary” (HNSCCUP), with a reported incidence of 5% [16]. Approximately one-third to one half of these tumors will be found to originate in the tonsil or tonsillar fossa [2, 17]. Although recent molecular studies showed promising results for identifying the primary site [18], the current standard HNSCCUP workup includes a combination of CT or MRI, positron emission tomography (PET), and panendoscopy (nasopharyngoscopy, laryngoscopy, bronchoscopy and esophagoscopy) with biopsies from several mucosal sites (base of tongue, nasopharynx, piriform sinus). The rate of success in identifying the primary tumor is variable, likely related to the diagnostic workup strategy, primary tumor size, radiological and pathological thoroughness, the quantity and quality of the tissue sampled and, even in recent studies, it was reported to be only 45–59% [19, 20]. In both series, the most commonly identified tumors after complete workup were in oropharynx. Although panendoscopy is standard in HNSCCUP workup, there is no general consensus on the sampling modality of the palatine tonsils. Recent studies provide compelling support for unilateral [21] or even bilateral [22] tonsillectomy, which, in the experience of several groups, provided a significantly higher yield in detecting occult tumors than deep tonsillar biopsy [2, 16, 23, 24]. The morphologic findings in our case further support this clinical approach: the few microscopic SCC foci might have been missed, had only tonsillar biopsies been obtained. In addition, the minute tumor might not have been sampled if only representative sections were submitted. In our case, SLL imparted a lymphoepithelial morphology to the tumor, mimicking normal tonsillar mucosa. The small size of tonsillar tumors, often discrepant to the neck metastasis, warrants complete embedding of all tonsillar tissue for microscopic evaluation.
The incidence of HPV-positive oropharyngeal SCC has been increasing in the last three decades and is considered a distinct SCC subtype, with a better prognosis than HPV-negative tumors [25]. 50–90% of the tonsillar SCCs are associated with HPV and HPV-positive status in HNSCCUP was proposed as surrogate marker for oropharyngeal origin, where a small primary should be intensively sought [26]. Cystic degeneration is commonly seen in cervical metastases of tonsillar HPV-positive tumors [27] and, together with the usual phenotype (poorly differentiated, mostly nonkeratinizing, basaloid features), can offer another morphologic clue to the likely origin of select HNSCCUP.
PET scan has a reported sensitivity and specificity in the HNSCCUP workup of 88 and 75%, respectively [28]. Microscopic primary tumors as the one presented here likely account for many PET false negatives. The reported PET accuracy in detecting CLL/SLL is even lower [29]. None of the two neoplasms were identified by PET in our patient, either at the primary site or in the cervical lymph nodes. Conversely, the CT scan findings lead to additional sampling of mediastinal contralateral paratracheal lymph nodes, and rendered an initial clinical higher stage. Since metastatic SCC can extensively replace the lymph node architecture and, often in clinical practice, the diagnosis is confirmed using small samples (fine needle aspiration of the neck metastasis being the most common), the risk of falsely upstaging the SCC patients by the nodal lymphoma is not just theoretic and may lead to unnecessary therapeutic procedures. In our case, the main metastasis subtotally replaced the lymph node architecture and, although SLL was focally present, it was originally overlooked in the excisional material. No further workup was performed at the time, but all the other cervical nodes in the selective neck dissection were involved by SLL.
Identifying the origin of HNSCCUP is extremely important for proper management by better targeting the radiation field and sparing other mucosal sites from unnecessary radiation and its related morbidity which includes xerostomia, dysphagia, lymphedema and neck stiffness.
The coexistence of oropharyngeal SCC and lymphoma is rare but the latter might obscure the primary SCC and falsely upstage the patient, leading to unnecessary therapy. Proper diagnosis and staging require a high index of suspicion, extensive sampling and, occasionally, ancillary studies.
Footnotes
E. C. Minca—Visiting physician in the Department of Pathology.
References
- 1.Auluck A, Hislop G, Bajdik C, et al. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)-related and HPV-unrelated sites in a multicultural population: the British Columbia experience. Cancer. 2010;116:2635–2644. doi: 10.1002/cncr.25087. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Mancuso AA, Parsons JT, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Head Neck. 1998;20:739–744. doi: 10.1002/(SICI)1097-0347(199812)20:8<739::AID-HED13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.DePeña CA, Tassel P, Lee YY. Lymphoma of the head and neck. Radiol Clin North Am. 1990;28:723–743. [PubMed] [Google Scholar]
- 4.Redaelli A, Laskin BL, Stephens JM, et al. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13:279–287. doi: 10.1111/j.1365-2354.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 5.Müller-Hermelink KH, Montserrat E, Catovsky D, Campo E, Harris NL, Stein H, et al. Chronic lymphocytic leukaemia/small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008. pp. 180–184. [Google Scholar]
- 6.Hanna E, Wanamaker J, Adelstein D, et al. Extranodal lymphomas of the head and neck. A 20-year experience. Arch Otolaryngol Head Neck Surg. 1997;123:1318–1323. doi: 10.1001/archotol.1997.01900120068011. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs C, Hoppe RT. Non-Hodgkin’s lymphomas of head and neck extranodal sites. Int J Radiat Oncol Biol Phys. 1985;11:357–364. doi: 10.1016/0360-3016(85)90158-0. [DOI] [PubMed] [Google Scholar]
- 8.Menárguez J, Mollejo M, Carrión R, et al. Waldeyer’s ring lymphomas. a clinicopathological study of 79 cases. Histopathology. 1994;24:13–22. doi: 10.1111/j.1365-2559.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 9.Bajetta E, Buzzoni R, Rilke F, et al. Non-Hodgkin’s lymphomas of Waldeyer’s ring. Tumori. 1983;69:129–136. doi: 10.1177/030089168306900208. [DOI] [PubMed] [Google Scholar]
- 10.Kaur P, Nazeer T. B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma presenting in the tonsil: a case report and review of literature. Am J Otolaryngol. 2004;25:121–125. doi: 10.1016/j.amjoto.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Kyasa MJ, Hazlett L, Parrish RS, et al. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leuk Lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- 12.Santoro A, Rilke F, Franchi F, et al. Primary malignant neoplasms associated with chronic lymphocytic leukemia. Tumori. 1980;66:431–437. doi: 10.1177/030089168006600404. [DOI] [PubMed] [Google Scholar]
- 13.Hisada M, Biggar RJ, Greene MH, et al. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–1981. doi: 10.1182/blood.V98.6.1979. [DOI] [PubMed] [Google Scholar]
- 14.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–269. doi: 10.1001/jama.1975.03250030023012. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JM, Andritsos L, Lucas D, et al. Second malignancies in B-cell chronic lymphocytic leukaemia: possible association with human papilloma virus. Br J Haematol. 2010;149:388–390. doi: 10.1111/j.1365-2141.2010.08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall DA, Johnstone PA, Foss RD, et al. Tonsillectomy in diagnosis of the unknown primary tumor of the head and neck. Otolaryngol Head Neck Surg. 2000;122:52–55. doi: 10.1016/S0194-5998(00)70143-4. [DOI] [PubMed] [Google Scholar]
- 17.Guntinas-Lichius O, Peter Klussmann J, Dinh S, et al. Diagnostic work-up and outcome of cervical metastases from an unknown primary. Acta Otolaryngol. 2006;126:536–544. doi: 10.1080/00016480500417304. [DOI] [PubMed] [Google Scholar]
- 18.Barker EV, Cervigne NK, Reis PP, et al. MicroRNA evaluation of unknown primary lesions in the head and neck. Mol Cancer. 2009;8:127. doi: 10.1186/1476-4598-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waltonen JD, Ozer E, Hall NC, et al. Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg. 2009;135:1024–1029. doi: 10.1001/archoto.2009.145. [DOI] [PubMed] [Google Scholar]
- 20.Cianchetti M, Mancuso AA, Amdur RJ, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119:2348–2354. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- 21.Righi PD, Sofferman RA. Screening unilateral tonsillectomy in the unknown primary. Laryngoscope. 1995;105:548–550. doi: 10.1288/00005537-199505000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Koch WM, Bhatti N, Williams MF, et al. Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg. 2001;124:331–333. doi: 10.1067/mhn.2001.114309. [DOI] [PubMed] [Google Scholar]
- 23.Kothari P, Randhawa PS, Farrell R. Role of tonsillectomy in the search for a squamous cell carcinoma from an unknown primary in the head and neck. Br J Oral Maxillofac Surg. 2008;46:283–287. doi: 10.1016/j.bjoms.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Waltonen JD, Ozer E, Schuller DE, et al. Tonsillectomy vs. deep tonsil biopsies in detecting occult tonsil tumorsx. Laryngoscope. 2009;119:102–106. doi: 10.1002/lary.20017. [DOI] [PubMed] [Google Scholar]
- 25.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 26.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2:163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 28.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101:2641–2649. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 29.Karam M, Novak L, Cyriac J, et al. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer. 2006;107:175–183. doi: 10.1002/cncr.21967. [DOI] [PubMed] [Google Scholar]