Abstract
Disrupted coordination of angiogenesis regulating signals, among them the vascular endothelial growth factor (VEGF) and angiopoietins (Angs), has been associated with abnormal angiogenesis and tumor progression. While VEGF induces endothelial cell proliferation, thereby initiating vessel formation, Angs are subsequently required for mural cell attachment, thus influencing remodeling and maturation of this vasculature. In addition to tumor cell, endothelial and mural cells, as well as myofibroblasts may also contribute to the secretion of these factors. In this study, we have analyzed by immunohistochemistry the expression of VEGF, Ang-1, Ang-2 and the Angs receptor Tie2 in both the stroma and tumor cells of mucoepidermoid carcinoma (MEC) of salivary gland. We have demonstrated that when myofibroblasts were detected adjacent to the cancer cells, they were frequently associated with intense positive staining for Ang-1 and Ang-2, and no reactivity to VEGF and Tie2. These myofibroblast-rich Ang-1 and Ang-2-stained areas were more commonly found in high-grade MEC cases than in low-grade ones. As for the malignant cells, they frequently expressed all proteins studied, but Ang-2 and VEGF were detected at higher levels compared to Ang-1 and Tie2. Our results indicate that the MEC environment favors cooperative activity between Angs and VEGF in modulating vascular growth and tumor aggressiveness.
Keywords: VEGF, Angiopoietins, Mucoepidermoid carcinoma, Salivary gland, Head and neck cancer
Introduction
Mucoepidermoid carcinoma (MEC) is a glandular epithelial malignant neoplasm characterized by mucous, intermediate and epidermoid cells [1]. As the most frequent primary salivary malignancy of both major and minor glands [1], it has been the focus of studies aimed at expanding the comprehension of the molecular alterations involved in its aggressiveness, which may be helpful in predicting the biological outcome. Investigating MEC metabolic status, we have recently demonstrated a correlation between Glucose Transporter Protein 1 (Glut-1) expression and MEC grade of malignancy, suggesting an adaptive strategy of the cells to survive in low oxygenation environments [2]. It is well known that this condition may stimulate angiogenesis, one of the six essential competences in cell physiology that cooperatively govern malignant growth [3]. This process normally requires strict coordination of the signals leading to endothelial sprouting, recruitment of contractile mural cells, and vascular maturation. One critical class of these signals is conveyed by soluble factors, such as vascular endothelial growth factor (VEGF) and angiopoietins (Angs) [4, 5]. Disrupted regulation of angiogenesis in tumors is frequently associated with excessive and continuous production of these vascular growth factors [4, 5].
The VEGF family members (VEGF A-D and PIGF) interact with closely related receptor tyrosine kinases (VEGFR1-3) found on the surface of endothelial cells to promote vascular permeability and also endothelial cell proliferation and survival [4, 5]. Compelling data from genetic and animal efficacy studies have validated VEGF as the best target for anti-angiogenesis therapies [4]. Nevertheless, in order to succeed in vascular formation, VEGF must act in concert with the angiopoietins, which are ligands for the specific tyrosine kinase receptor Tie2, also mostly found on endothelial cells [4, 5]. Ang-1 and Ang-2 are the best characterized members of the Ang family (Ang-1-4). Ang-1, mainly produced by mural cells (pericytes and smooth muscle cells), promotes Tie2 autophosphorylation upon binding, inducing endothelial cell survival and inhibition of vascular leakage and inflammation [4–6]. Ang-2, which is primarily produced by endothelial cells, also binds to Tie2 but does not induce receptor activation. Ang-2 induces dissociation of pericytes and smooth muscle cells from the endothelium, which, cooperatively with VEGF, stimulates the proliferation and migration of endothelial cells; however, in the absence of VEGF, it promotes vessel regression [4–6]. Consequently, while Ang-1 has been commonly associated with maintenance of vascular integrity in normal tissues, Ang-2 is correlated with vessel destabilization, a fundamental step in angiogenesis initiation [4–6]. Accumulating evidence suggests that temporal and spatial imbalance between Ang-1 and Ang-2, particularly higher relative levels of Ang-2, is implicated in the angiogenic switch that is critical for tumor progression [7]. In fact, overexpression of Ang-2 was reported in hepatocellular, renal, gastric, breast, colon, pancreas, and non-small lung cancers [7].
Besides tumor, endothelial, and mural cells, other cell types comprising the tumor microenvironment may also contribute to the secretion of vascular growth factors to support growth. Infiltration of fibroblasts and myofibroblasts (their differentiation derivatives expressing α-smooth muscle actin [α-SMA]) was reported to enable neovascularization by the release of proangiogenic factors that act in a paracrine fashion [8–12]. It has been demonstrated that endothelial cell infiltration into ovarian tumors was reliant on the presence of myofibroblasts [13].
Trying to broaden our understanding of the molecular alterations involved in the aggressiveness of MEC, we have studied the expression of VEGF, Ang-1, Ang-2, and the Angs receptor Tie2, in the stroma as well as in the tumor cells of MEC presenting different histologic grades.
Materials and Methods
The present study protocol was approved by the Committee of Ethics of the São Leopoldo Mandic Institute and Research Center.
Eighteen tissue samples diagnosed as MEC were retrieved from the files of the Department of Pathology of the University of Campinas and from the files of the Laboratory of Pathology of the São Leopoldo Mandic Institute and Research Center. Tissue samples were available as formalin-fixed and paraffin-embedded material. Hematoxylin and eosin stained sections were examined and tumors were scored and graded by three experienced pathologists according to the World Health Organization’s grading system. This system establishes 3 grades of malignancy: low, intermediate, and high, based on: (1) the relative proportion of the cystic component in the entire tumor, (2) the number of mitotic figures per 10 high-power fields, (3) degree of anaplasia, (4) necrosis incidence and (5) neural involvement.
Serial sections, 3 μm in thickness, were obtained from paraffin-embedded samples, and the dewaxed sections were processed for antigen retrieval (Table 1). Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide. After washing, the sections were incubated with primary antibodies for Ang-1, Ang-2, VEGF, α-SMA and Tie2 (Table 1). Peroxidase-linked secondary antibodies and diaminobenzidine tetrahydrochloride (DAB; Envision HRP Kit, Dako Corp., Carpinteria, USA) were used to detect specific binding. The sections were counterstained with hematoxylin, dehydrated and mounted. Digital photomicrography used a Zeiss Axioskop 2 plus microscope equipped with AxioCam digital camera and Axiovision application software (Carl Zeiss, Gottingen, Germany).
Table 1.
Clone, pretreatment for epitope demasking, dilution, and incubation times for primary antibodies
| Antibody | Clone | Pretreatment | Dilution | Incubation time |
|---|---|---|---|---|
| Ang-1a | Polyclonal | Citrate 0,01 M; pH 6.0; 95°C; 30mim | 1:500 | 60 min |
| Ang-2a | Polyclonal | Citrate 0,01 M; pH 6.0; 95°C; 30mim | 1:500 | 60 min |
| Tie-2a | Monoclonal | Tris–EDTA; pH 9.0; 95°C; 30mim | 1:50 | Overnight |
| VEGFb | Monoclonal | Citrate 0,01 M; pH 6.0; 95°C; 30mim | 1:100 | Overnight |
| α-SMAc | A4 | 1:300 | 60 min |
aAbcam Inc., Cambridge, MA
bSanta Cruz Biotechnology Inc., Europe
cDako cytomation, Glostrup, Demark A/S
The endothelial cells of blood vessels present in tumoral specimens were used as positive controls and the primary antibody was omitted as a negative control.
For quantitative evaluation, scores for the expression of Ang-1, -2 and VEGF were assigned according to the percentage of stained tumor cells and stained desmoplastic areas within the tumor-associated stroma, from 1 to 4, as follows: 1, less than 10%; 2, staining of 10–25%; 3, staining of 25–50%; 4, staining of more than 50% of tumor cells or desmoplastic areas.
Results
In this investigation, the immunohistochemical expression of Ang-1 Ang-2, Tie2 and VEGF was evaluated according to the percentage of stained tumor cells and stained desmoplastic areas within the tumor-associated stroma of MEC cases presenting different histological grades (Table 2).
Table 2.
Clinicopathological and immunophenotypic features of MEC cases
| Patient no | Age (year) | Sex | Location | Histological grade | Ang-1 | Ang-2 | VEGF cells | ||
|---|---|---|---|---|---|---|---|---|---|
| Cells | Stroma | Cells | Stroma | ||||||
| 1 | 49 | M | Parotid | Low | 2 | 3 | 3 | 3 | 3 |
| 2 | 29 | F | Parotid | Low | 1 | 1 | 3 | 3 | 3 |
| 3 | 25 | M | Parotid | Low | 2 | 3 | 3 | 3 | 3 |
| 4 | 42 | F | Parotid | Low | 2 | 1 | 3 | 1 | 3 |
| 5 | 13 | M | Parotid | Low | 1 | 3 | 2 | 4 | 2 |
| 6 | 7 | F | Palate | Low | 2 | 2 | 3 | 1 | 2 |
| 7 | 32 | F | Palate | Low | 4 | 3 | 2 | 1 | 2 |
| 8 | 13 | M | Parotid | Low | 2 | 1 | 1 | 1 | 2 |
| 9 | 34 | M | Parotid | Low | 1 | 1 | 3 | 3 | 3 |
| 10 | 36 | M | Palate | Low | 1 | 1 | 3 | 1 | 3 |
| 11 | 55 | F | Palate | Intermediate | 4 | 1 | 4 | 1 | 3 |
| 12 | 25 | M | Parotid | Intermediate | 2 | 1 | 3 | 1 | 3 |
| 13 | 45 | F | Buccal Mucosa | Intermediate | 1 | 1 | 3 | 2 | 1 |
| 14 | 33 | M | Parotid | Intermediate | 2 | 3 | 3 | 1 | 2 |
| 15 | 65 | F | Sub- mandibular | High | 3 | 4 | 3 | 4 | 3 |
| 16 | 71 | M | Parotid | High | 1 | 2 | 2 | 2 | 2 |
| 17 | 51 | M | Parotid | High | 1 | 4 | 2 | 4 | 3 |
| 18 | 62 | M | Parotid | High | 2 | 4 | 2 | 2 | 3 |
We observed that tumor cells of all grades of MEC stained with Ang-1 and 2, although the expression of Ang-2 was detected at higher levels in most cases (Figs. 1b, 2b, representative for both Angs). Ang-1 and -2 were observed mainly in the solid component of the tumors, but not in the cells lining cystic spaces. Ang-1 staining was more intense in the central areas while higher staining intensity for Ang-2 was observed in the solid (higher grade) peripheral component. VEGF was expressed by tumor cells independent of the histologic grade, i.e. in the solid component as well as in the cystic areas (Figs. 1c, 2c).
Fig. 1.
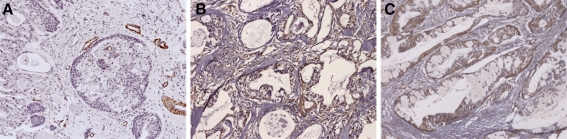
Immunohistochemical expression of α-SMA, Ang-2 and VEGF in low-grade salivary gland MEC. Malignant cell blocks in the representative area (patient number 8, Table 2) exhibit reactivity to Ang-2 (b) and VEGF (c). Scant occurrence of myofibroblasts in the tumor-associated stroma was detected by the positive expression of the α-SMA antibody (a) and these cells were positive for Ang-2 (b), but negative for VEGF (c). Original magnifications ×200
Fig. 2.
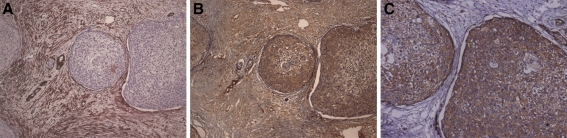
Immunohistochemical expression of α-SMA, Ang-2 and VEGF in high grade salivary gland MEC. Malignant cell blocks in the representative area (patient number 15, Table 2) exhibit intense reactivity to Ang-2 (b) and VEGF (c). Abundant occurrence of myofibroblasts in the tumor-associated stroma was detected by the positive expression of the α-SMA antibody (a) and these cells were intensely immunoreactive for Ang-2 (b), but not for VEGF (c). Original magnifications ×200
In the stroma, we detected that the myofibroblasts adjacent to the cancer cells, which characterize desmoplastic areas, were positively stained for Ang-1 and -2 (Figs. 1b, 2b, representative for both Angs), while the VEGF staining was negative in these areas (Figs. 1c, 2c). These Ang-1 and 2 stained desmoplastic areas were more frequently found in high grade tumors (Fig. 2b) than in low (Fig. 1b) and intermediate ones. The occurrence of myofibroblasts in desmoplastic areas was confirmed by the positive expression of the α-SMA antibody, abundant in high grade tumors (Fig. 2a) and scant in low grade ones (Fig. 1a).
The Tie2 receptor was slightly expressed, only by tumor cells independently of the tumor grade (not shown).
Discussion
In this study, we have shown the contribution of tumor cells and tumor-associated myofibroblasts, to the expression of the angiogenic growth factors VEGF, Ang-1, Ang-2 and the Angs receptor Tie2, in MEC of salivary gland. We have observed that when myofibroblasts were detected adjacent to the cancer cells, characterizing desmoplastic areas, they were frequently associated with intense positive staining for Ang-1 and Ang-2, and no reactivity to VEGF and Tie2 (Figs. 1, 2). As for the malignant cells, they commonly expressed all proteins studied, but Ang-2 and VEGF were detected at higher levels compared to Ang-1 and Tie2 (Figs. 1, 2).
Previous reports have associated a relatively higher level of Ang-1 with a stable vasculature in normal tissue, while greater Ang-2 expression has been linked to tumor angiogenesis and growth [4, 5]. By inducing detachment of pericytes, Ang-2 would allow the interaction of endothelial cells with other angiogenic factors, such as VEGF, thus promoting vessel sprouting and growth, whereas covered vessels would be less receptive to growth stimulus. With this in mind, our results of slightly higher Ang-2 expression compared to Ang-1 could indicate a trend in tumor angiogenesis. However, conflicting results regarding the association of Ang-1/Ang-2 expression and tumor progression have been described. Tumor malignancy was correlated not only with Ang-1 overexpression (in high-grade gliomas, non-small cell lung carcinoma, plasmacytomas, and ovarian, breast, and gastric carcinomas) but also with overexpression of Ang-2 (in hepatocellular carcinoma, angiosarcoma, pancreatic adenocarcinoma, gastric, colon, breast, prostate and non-small cell lung cancer) [6, 7]. Moreover, ectopic expression of Ang-1 and Ang-2 was demonstrated to promote seemingly contradicting vascular outcomes in a variety of tumor models [6]. It was argued that, by promoting vessel coverage and maturation, Ang-1 allowed for better efficiency in nutrient exchange to the previously dysfunctional vasculature [14], thereby exerting a prosurvival function on endothelial cells. In this manner, Ang-1 would favor angiogenic sprouting, rather than restrict tumor progression. This effect would be particularly important during the plastic phase resulting from the presence of Ang-2 and VEGF, wherein uncovered vessels would be more prone to apoptosis [4]. Making the effects of Ang-1-Tie2 interaction even more complex, it was demonstrated that Ang-1 may favor blood vessel quiescence, acting as a bridge to Tie2 at cell–cell contacts; however, in contrast, in isolated cells, extracellular matrix-bound Ang-1 anchors Tie2 at cell-substratum contacts, signaling for angiogenesis [15, 16]. The collagen binding capacity of Angs explains our observation of intense staining of MEC extracellular matrix for these growth factors (Figs. 1b, 2b).
Fibroblasts, the predominant cells of the stromal compartment, have been assumed to have a profound influence in the progression of carcinomas. As neoplastic cells activate the stroma, fibroblasts may differentiate into myofibroblasts, acquiring the contractile function provided by myofilaments of smooth-muscle cells. In this manner, besides functioning in collagen deposition in the extracellular matrix, these cells become tissue remodeling effectors. Thanks to these actions, myofibroblasts were initially regarded as a barrier which confines the developing tumor. However, recent findings argue that myofibroblasts may actively participate in tumor invasion [17] and metastasis by secretion of proteolytic enzymes, growth factors and cytokines [18, 19]. It was shown that cancer cell-derived transforming growth factor (TGFβ1) modulates myofibroblast differentiation from the local fibroblast population, and that these myofibroblasts release paracrine signals that ultimately promote invasion of colon, breast and squamous carcinoma cells [19–21]. Myofibroblasts present in human breast carcinomas overexpress CXCL12, a chemokine able to recruit endothelial progenitor cells from the bone marrow, playing an important role in angiogenesis [11]. In addition, the production of Ang-1 and Ang-2 by fibroblasts and myofibroblasts recruited to tumor stroma was shown to support vascular stability, and subsequent switch, leading to ovarian tumors’ exit from dormancy [22]. Our observation in MEC cases of the association of myofibroblasts with positive Ang-1 and Ang-2 staining suggested that the presence of these cells could indicate regions where sprouting angiogenesis would be favored. Interestingly, these myofibroblast-rich Ang-1 and Ang-2-stained areas were more frequently found in high-grade MEC cases (Fig. 2b) than in low-grade ones (Fig. 1b), which could suggest a contribution of these cells and their derived signals to the aggressiveness of salivary gland MEC. This result is in agreement with those obtained in previous studies, employing the same MEC samples, which demonstrated that samples classified as high-grade presented considerable inferior microvessel density (MVD) [23] and higher glucose transporter protein 1 (Glut-1) expression, compared with those of lower grades. Altogether, these results support a speculation of hypoxic stimulation of glycolytic metabolism and angiogenesis. Additionally, the angiogenesis-supportive environment provided by myofibroblasts has been associated with more aggressive tumor phenotypes [17–22, 24].
The levels of Ang-1 and Ang-2 provided by tumor cells and by myofibroblasts, in addition to those of VEGF produced by the tumor cells, could reflect a propensity to an active angiogenic phase in our MEC samples, favored by the migrating, sprouting, and antiapoptotic effects of Ang/VEGF cooperative signaling. In agreement with this cooperation, it was demonstrated that tumor-derived VEGF was able to induce angiogenesis in ovarian carcinoma, but not sufficient for vascular maintenance. This was only achieved with the infiltration of myofibroblasts expressing Ang-1 and Ang-2 [25]. The complementary activity of Angs and VEGF was also narrowed by the demonstration that hyperpermeable vessels in VEGF-overexpressing mice were restituted by Ang-1 [26]. In accordance to previous reports, we also detected Tie2 expression in the tumor cells, but the meaning of this is largely unknown. Even so, it was suggested that extraendothelial Tie2 receptors could sequestrate Ang-1 from susceptible endothelial cells [6].
In conclusion, the study reported here shows the contribution to expression of angiogenic growth factors by tumor cells and tumor-associated myofibroblasts in MEC, suggesting that the malignant milieu favors a cooperative activity between Angs and VEGF in modulating vascular growth and tumor aggressiveness.
Acknowledgments
This work was supported by the Brazilian Agencies FAPESP and CNPq.
References
- 1.Gnepp DR, Brandwein-Gensler MS, El-Naggar AK, Nagao T. Mucoepidermoid carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and Genetics. Head and Neck Tumous. 1st ed. Lyon: IARC Press; 2005. p. 219–220.
- 2.Demasi AP, Costa AF, Altemani A, et al. Glucose transporter protein 1 expression in mucoepidermoid carcinoma of salivary gland: correlation with grade of malignancy. Int J Exp Pathol. 2010;91:107–113. doi: 10.1111/j.1365-2613.2009.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 5.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008;73:751–762. doi: 10.1134/S0006297908070031. [DOI] [PubMed] [Google Scholar]
- 6.Shim WS, Ho IA, Wong PE. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Mol Cancer Res. 2007;5:655–665. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- 7.Bach F, Uddin FJ, Burke D. Angiopoietins in malignancy. Eur J Surg Oncol. 2007;33:7–15. doi: 10.1016/j.ejso.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Orimo A, Tomioka Y, Shimizu Y, et al. Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin Cancer Res. 2001;7:3097–3105. [PubMed] [Google Scholar]
- 9.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilead A, Meir G, Neeman M. The role of angiogenesis, vascular maturation, regression and stroma infiltration in dormancy and growth of implanted MLS ovarian carcinoma spheroids. Int J Cancer. 2004;108:524–531. doi: 10.1002/ijc.11583. [DOI] [PubMed] [Google Scholar]
- 11.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Walter-Yohrling J, Pratt BM, Ledbetter S, et al. Myofibroblasts enable invasion of endothelial cells into three-dimensional tumor cell clusters: a novel in vitro tumor model. Cancer Chemother Pharmacol. 2003;52:263–269. doi: 10.1007/s00280-003-0664-2. [DOI] [PubMed] [Google Scholar]
- 14.Machein MR, Knedla A, Knoth R, et al. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557–1570. doi: 10.1016/S0002-9440(10)63413-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuhara S, Sako K, Minami T, et al. Differential function of Tie2 at cell–cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 16.Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 17.Araujo VC, Furuse C, Cury PR, et al. Desmoplasia in different degrees of invasion of carcinoma ex-pleomorphic adenoma. Head Neck Pathol. 2007;1:112–117. doi: 10.1007/s12105-007-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 19.Wever O, Nguyen QD, Hoorde L, et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 20.Lewis MP, Lygoe KA, Nystrom ML, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey TM, Eneman J, Crocker A, et al. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat. 2008;110:39–49. doi: 10.1007/s10549-007-9684-7. [DOI] [PubMed] [Google Scholar]
- 22.Gilad AA, Israely T, Dafni H, et al. Functional and molecular mapping of uncoupling between vascular permeability and loss of vascular maturation in ovarian carcinoma xenografts: the role of stroma cells in tumor angiogenesis. Int J Cancer. 2005;117:202–211. doi: 10.1002/ijc.21179. [DOI] [PubMed] [Google Scholar]
- 23.Costa AF, Demasi AP, Bonfitto VL, et al. Angiogenesis in salivary carcinomas with and without myoepithelial differentiation. Virchows Arch. 2008;453:359–367. doi: 10.1007/s00428-008-0664-z. [DOI] [PubMed] [Google Scholar]
- 24.Wever O, Demetter P, Mareel M, et al. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 25.Granot D, Addadi Y, Kalchenko V, et al. In vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors. Cancer Res. 2007;67:9180–9189. doi: 10.1158/0008-5472.CAN-07-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]